Abstract
Receptors belonging to the TNF-receptor (TNF-R) superfamily include important costimulatory molecules, many of which specifically affect T cell activation. Tumor necrosis factor receptor-associated factors (TRAFs) are recruited to many TNF-R superfamily members and are important modulators of the proximal signaling events that occur at the time of receptor engagement and activation. TRAF5 has been shown to be a positive regulator of a number of these receptors that are involved in T cell costimulation. However, the potential importance of TRAF5 in cellular immune responses to infection or in T cell expansion and memory have not been studied. We report here that TRAF5 was required for optimal CD8+ T cell responses following infection with Listeria monocytogenes expressing ovalbumin (LM-OVA). TRAF5 was necessary for optimal T cell expansion following primary infection with LM-OVA, and its absence resulted in fewer memory CD8+ T cells following LM-OVA infection, together with higher bacterial loads in the liver. The effect of TRAF5 on CD8+ T cell expansion was T cell intrinsic and not due to effects of TRAF5 deficiency on antigen presenting cells. Although their proliferative ability remained intact, CD8+ T cells from TRAF5-/- mice were more sensitive to apoptosis and were unresponsive to the pro-survival effects of the TNF-R superfamily costimulator CD27. Collectively, these studies identify TRAF5 as an important positive signaling element that enhances T cell expansion and pathogen containment by providing a survival advantage to responding antigen-specific CD8+ T cells during infection.
Keywords: TRAF, Cell Activation, T cell, Apoptosis
Introduction
The quality and magnitude of T cell memory is modulated by costimulation via CD28/B7 interactions (1, 2) and the engagement of TNF-R superfamily members such as CD27 (3) and OX40 (4). This family of costimulators is integral to T cell function and its members are expressed at different stages during T cell activation (5). CD27, for example, is most highly expressed by naïve and memory T cells (6, 7). The surface expression of OX40 and 4-1BB, by contrast, is induced by T cell receptor (TCR) stimulation and further enhanced by CD28 costimulation (8, 9). Individual members of the TRAF family of cytoplasmic adapter proteins play various roles in signaling by TNF-R superfamily members, which are dependent upon the specific receptor, cell type, and context (10). Many studies have been published to date about the multiple roles played by TRAFs 2 and 6 in immune cell function (10-12) and the diverse roles played by TRAF3 in TLR signaling and lymphocyte survival are becoming increasingly appreciated (13-15). However, the biological role of TRAF5 in vivo has remained unclear. Initially, the functions of TRAFs 5 and 2 were thought to be redundant, based upon results of studies using transiently overexpressed TRAFs in non-immune, transformed cell lines (16). Additionally, experiments characterizing the TRAF5 deficient mouse did not conclusively demonstrate a clear and distinct role for TRAF5 in immunity (17). In these studies, the effects of TRAF5 deficiency were determined by measuring the in vitro activation of early signaling events, and did not address how the absence of TRAF5 affects the in vivo immune response to infection.
TRAF5 has been shown to associate with several TNF-R superfamily members involved in T cell costimulation, including CD27 (16), OX40 (18), GITR (19, 20) and 4-1BB (21). There are several hypotheses regarding the mechanism of T cell costimulation by these receptors, including the promotion of survival during expansion (4, 5, 22) and/or enhancement of proliferation (5, 23, 24). CD27 has been shown to use TRAF5 as a positive regulator of NF-κB and JNK activation in vitro (16), suggesting that TRAF5 may be an important mediator of T cell costimulation. Additionally, the T cell costimulatory molecules OX40 (25) and GITR (19, 20) have been reported to require TRAF5 for the activation of various signal transduction pathways. However, the significance of TRAF5 in T cell immunity in vivo has not been previously tested.
We hypothesized that TRAF5 acts as a positive regulator of T cell costimulation, and that it facilitates cell survival signals delivered via TNF-R superfamily members. To address this hypothesis, we employed the Listeria monocytogenes-ovalbumin (LM-OVA) infection model (26, 27). We found that in the absence of TRAF5, infected mice demonstrated a defect in CD8+ T cell expansion, leading to lower levels of antigen-specific CD8+ T cells during primary expansion, memory and secondary expansion. This decreased expansion of antigen-specific CD8+ T cells resulted in reduced bacterial clearance from the liver early during secondary challenge. The decreased CD8+ T cell expansion observed in TRAF5-/- mice was paralleled by decreased in vitro survival capacity of TRAF5-deficient T cells following activation through the TCR. This report presents the first evidence for a biologically important role for TRAF5 in CD8+ T cell responses to infection in vivo.
Materials and Methods
Mice
TRAF5-/- mice and littermate controls were obtained from Dr. Michael Croft (La Jolla Institute for Allergy & Immunology, La Jolla, CA) and were created by Dr. Hiroyasu Nakano (17). OT-1 TRAF5-/- mice were created by breeding OT-1 TCR transgenic mice (Thy1.1 or Thy1.2) obtained from Dr. John Harty (University of Iowa, Iowa City, IA) to TRAF5-/- mice (Thy1.2). The OT-1 transgene was detected through the use of anti-Vβ5.1 mAb (eBiosciences, San Diego, CA) and PCR using mouse tail DNA as a source of template. PCR was used to screen for the existence of the targeted and germline TRAF5 allele(s). CD45.1+ C57BL/6 mice were obtained from Jackson Laboratories, Bar Harbor, ME. Mice were maintained under pathogen-free conditions at the University of Iowa. Use of mice in this study was according to a protocol approved by The University of Iowa Animal Care and Use Committee.
Listeria monocytogenes infection
8-12 week-old mice were infected i.v. with 0.1 LD50 (1×104 CFU) virulent LM-OVA (Listeria monocytogenes expressing the gene encoding chicken ovalbumin) (28). At day 7 (primary response) or 40 (memory response) post infection (p.i.), spleens and livers were collected to determine bacterial load, as detailed below. At day 43 p.i. mice were infected i.v. with 5 LD50 (5×105 CFU) virulent LM-OVA. At days 3 and 5 post secondary challenge spleens and livers were collected to determine bacterial load. Livers were homogenized in 10mL 0.2% IGEPAL in H2O and spleens in 7mL 0.2% IGEPAL in H2O. Organ homogenates were subjected to serial dilutions and plated on TSB-streptomycin agar plates to determine CFUs of LM-OVA in liver and spleen. Splenocytes were also collected at the time points listed above for flow cytometric analysis of OVA-specific T lymphocytes.
Enumerating OVA-specific T lymphocytes by intracellular staining for IFN-γ
Spleens were harvested from infected mice and erythrocytes were depleted by hypotonic lysis. Splenocytes were washed and resuspended in fresh medium (RPMI 1640, 8% FCS, Pen/Strep, and β2-mercapoethanol). Total splenocytes were counted on a hemocytometer, and 200μL of this suspension was transferred to sterile plastic tubes. 200μL of medium plus 2μL/mL GolgiPlug™ (brefeldin A; BD Pharmingen), with or without 1μM of purified OVA peptide (SIINFEKL), were then added to the tubes, which were incubated at 37°C for 6 hours prior to staining. CD4+ T cells were stimulated using the same method with the exception that the stimulatory peptide was 5μM of listeriolysin O antigen LLO190-201. Splenocytes were surface-stained with α-Thy1.2-APC (clone 53-2.1, BD Pharmingen) and α-CD8α-PerCP (clone 53-6.7, BD Pharmingen) or α-CD4-PerCP (clone RM4-5, BD Pharmingen) mAbs prior to fixation and intracellular cytokine staining. Staining was completed using α-IFN-γ-phycoerythrin (PE) labeled mAbs (clone XMG1.2, Biolegend). In some cases cells were stained with fluorescein isothyocyanate (FITC)-labeled α-IFN-γ and were costained with α-IL-2-PE (clone JES6-5H4, BD Pharmingen) or α-TNF-α-PE (clone MP6-XT22, Biolegend). T cell surface phenotype was determined using α-CD43-PE (clone R2/60, eBiosciences), α-CD44-PE(clone IM7, eBiosciences), and α-CD127-PE (clone A7R34, eBiosciences) mAbs.
Detection of OVA257-264-specific CD8+ T cells
MHC class I tetramers were prepared following published protocols (29). Staining with APC-conjugated tetramers was followed with surface staining for CD8.
Dendritic cell (DC) vaccinations
DC vaccinations were performed as previously described (30). Cells were activated with LPS (100ng/mL; Sigma, St. Louis, MO), CpG-B oligodeoxynucleotide 2084 (100ng/mL) (Integrated DNA Technologies, Coralville, IA), 5μg/mL α-CD40 mAb (clone HM40.3, eBioscience) or both CpG 2084 and α-CD40 mAb for 48 hours. Activation status was determined by flow cytometric staining for CD11c, CD86 and MHC-class II expression. DCs were harvested, washed and coated with SIINFEKL peptide (1μM) for 3 hours at 37°C, then washed and resuspended in sterile PBS. 5×105 activated, peptide coated DCs were injected i.v. into recipients (TRAF5-/- mice or littermate controls).
OT-1 adoptive transfer
T cells were obtained from the peripheral blood of TCR transgenic OT-1 TRAF5-/- and OT-1 TRAF5+/+ mice. Erythrocytes were lysed by hypotonic solution and OT-1 T cells enumerated by flow cytometry. Suspensions of OT-1 T cells from TRAF5-/- mice (CD45.2, Thy1.2) and OT-1 T cells from TRAF5+/+ mice (CD45.2, Thy1.1/Thy1.2) were mixed 1:1 (500 TRAF5-/- OT-1 T cells and 500 TRAF5+/+ OT-1 T cells) in sterile PBS and injected i.v. into CD45.1 C57BL/6 recipients. On the following day recipient mice were infected with 0.1 LD50 (1×104 CFU) virulent LM-OVA.
CFSE assays
CD8+ T cells were purified from the spleens of TRAF5-/- or control mice by negative selection (EasySep, Stem Cell Technologies, Vancouver, Canada) and labeled with carboxyfluoroscein succinimidyl ester (CFSE) from Sigma-Aldrich (1μM). CFSE labeled cells were plated at 3×105 cells/well in 48 well plates coated with 0.5μg/mL α-CD3 mAb (clone 145-2C11, eBioscience, San Deigo, CA) with or without 10μg/mL soluble α-CD28 mAb (clone 37.51, eBioscience,) or 10μg/mL plate-bound α-CD27 mAb (clone LG.3A10, BD Pharmingen, San Deigo, CA) for 24, 48 and 72 hours. Cell division was determined by CFSE dilution via flow cytometry.
Propidium iodide staining
CD8+ T cells were purified from the spleens of TRAF5-/- or control mice by negative selection and stimulated as in the CFSE and caspase 3 activity assays. Following stimulation, cells were fixed with ice cold 70% ethanol and stained with 10μg/mL propidium iodide for 30 minutes prior to FACS analysis.
Caspase 3 activity
Splenocytes were washed and resuspended in fresh medium (RPMI 1640, 8% FCS, Pen/Strep, and 10-5M 2-mercapoethanol). Splenocyte suspensions were stimulated with SIINFEKL peptide (1μM) with α-Fas mAb (Jo2, BD Pharmingen) for 48 hours. Costimulation was provided with Hi5 insect cells expressing membrane-bound CD70 via a recombinant baculovirus. Insect cells grow at room temperature, and lyse to form membrane fragments within 18 hrs. when cultured at 37°C. Following stimulation, the cells were transferred to 1.5mL tubes and centrifuged for 1 minute at 2000 rpm (380g). To each pellet, 75μL of caspase 3 substrate (PhiPhiLux-G1D2, OncoImmunin, Gaithersburg, MD) was added and pellets were incubated for 1 hour in a 37°C water bath. Cells were then washed and stained with α-CD8 PerCP mAbs. CD8-negative cells were excluded by gating and caspase 3 activity was observed using flow cytometry.
Statistical analysis
P values were generated using Student's t test (unpaired, two-tailed, at 95% confidence interval).
Results
Expansion and memory levels of antigen (Ag)-specific CD8+ T cells in TRAF5-/- mice following primary infection with Listeria monocytogenes
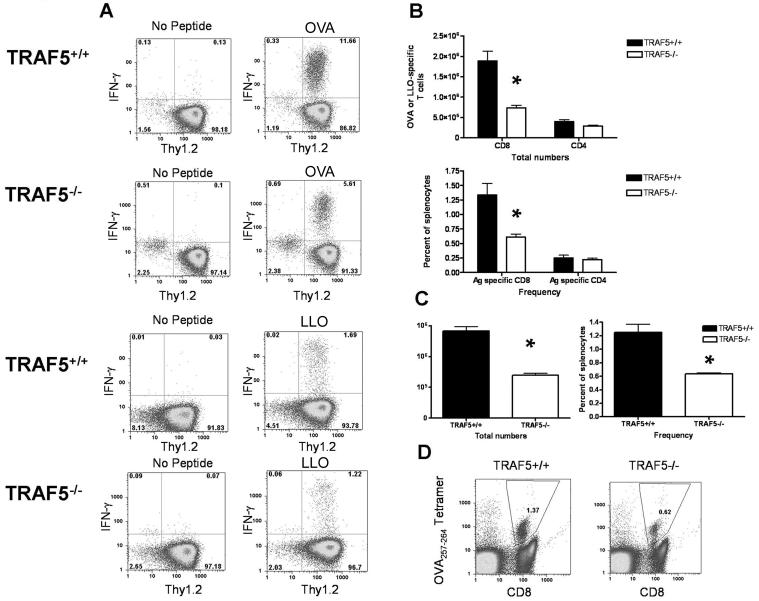
TRAF5 has been implicated as a positive regulator of TNF-R superfamily members known to function as T cell costimulators (17, 18, 20). If TRAF5 is important to T cell costimulation, this predicts a decrease in T cell expansion and memory following infection of TRAF5-/- mice with a pathogen. TRAF5-/- mice and littermate controls were infected i.v. with 0.1LD50 (1×104 CFU) virulent LM-OVA. At day 7 p.i., Ag-specific T cells were detected using intracellular cytokine staining (ICS) for IFN-γ following in vitro stimulation with OVA257-264 (CD8+) or LLO190-201(CD4+). During the primary infection, there were significantly fewer Ag-specific CD8+ T cells in TRAF5-/- mice, both in frequency and total numbers (Fig. 1A & B). There was no statistically significant difference in the number of Ag-specific CD4+ T cells between TRAF5-/- mice and littermates. There were, on average, 60% fewer Ag-specific CD8+ T cells in TRAF5-/- mice and 30% fewer CD4+ T cells than found in littermate controls (Fig. 1B). No differences were observed between TRAF5-/- mice or littermate controls in IL-2 or TNF-α production by Ag-specific CD8+ or CD4+ T cells, nor were there any differences in CD43, CD44 or CD127 expression (Fig. S1A-S1D).
Figure 1. The effects of TRAF5 deficiency on T cell expansion in response to LM-OVA.
(A) TRAF5-/- mice (□) and littermate controls (TRAF5+/+) (■) were infected i.v. with a sub-lethal dose of virulent LM-OVA (0.1XLD50 or 1000 CFU). (C & D) CD8+ T cell expansion in response to the primary infection was measured using OVA-peptide-MHC class I tetramer staining at day 7. * represents P<0.001 in a paired students t test (95% confidence interval) on the composite data of 3 independent experiments (N= 8 mice per group) for ICS experiments and 2 independent experiments (N= 7 littermate control mice and N= 4 TRAF5-/- mice) for tetramer experiments).
Because Ag-specific T cells were enumerated by counting T cells producing IFN-γ in response to stimulation by peptide, defects in IFN-γ production could explain the differences observed in the TRAF5-/- mice. To test this possibility, we repeated LM-OVA infection and enumerated Ag-specific CD8 T cells using OVA257-264-specific MHC class I tetramers. Tetramer-positive OVA-specific CD8+ T cells at day 7 p.i. yielded similar results to those obtained by intracellular cytokine staining for IFN-γ (Fig. 1C & D).
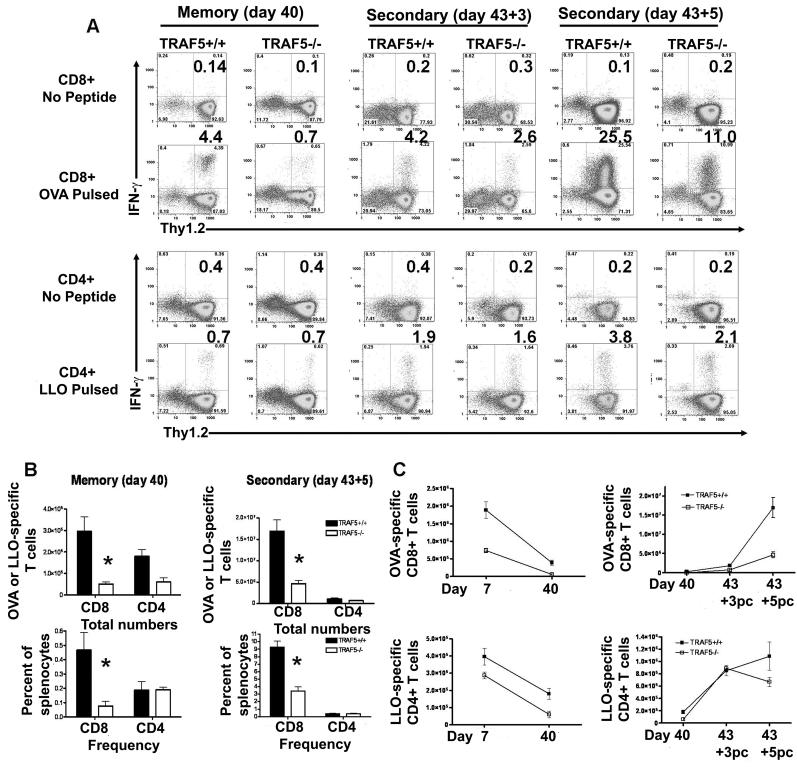
The results indicated a defect in primary expansion of CD8+ T cells in the TRAF5-/- mice following infection with LM-OVA. To determine if this effect was restricted to the primary response or affected the memory T cell pool as well, we specifically tested the effect of TRAF5 deficiency on memory T cell levels following LM-OVA infection. On day 40 p.i. with 0.1LD50 LM-OVA, the number of memory CD8+ T cells in TRAF5-/- mice was on average only 15% of the level found in the littermate controls (Fig. 2A & B). CD8+ T cells from TRAF5-/- mice exhibited a higher degree of contraction (93%) than their littermates (80%) (Fig. 2C). Interestingly, total numbers but not frequencies of memory CD4+ T cells in TRAF5-/- mice were also negatively affected (Fig 2B). These data demonstrate that TRAF5-/- mice have a defect in CD8+ T cell expansion following infection with LM-OVA, and this defect resulted in a significantly decreased memory CD8+ T cell pool. As in the primary infection, IL-2 and TNF-α production were not affected, nor was the expression of CD43, CD44 or CD127 on CD4+ and CD8+ T cells (Fig. S1E-S1H). These data exclude deficiencies in T cell IL-2 and TNF-α production as a cause for this expansion defect, and demonstrate that phenotypic markers of activation and memory are normal in TRAF5-/- mice (Fig. S1A-S1H). We have previously examined purified T cells from TRAF5 deficient mice and littermate controls stimulated with CD27L (CD70) and found no differences in the ability to activate NF-κB, JNK, ERK and p38 (data not shown).
Figure 2. The effects of TRAF5 deficiency on T cell memory and secondary T cell expansion.
(A &B) Memory levels of CD8+ and CD4+ T cells were measured at day 40 p.i., by IFN-g ICS (Composite data from 3 independent experiments where N= 8 mice per group). T cell expansion in response to a secondary infection (i.v.) of LM-OVA (5XLD50 or 5×105 CFU) was observed using ICS for IFN-γ at day 5 post challenge (Composite data from 3 independent experiments where N= 7 mice per group). (C) These methods were also used to measure contraction of both CD8+ and CD4+ T cells. Expansion from memory to the peak secondary response was also measured for CD8+ and CD4+ T cells. * represents P<0.05 in a paired students t test (95% confidence interval).
Expansion of antigen-specific CD8+ T cells in TRAF5-/- mice following secondary challenge with Listeria monocytogenes
TRAF5-/- mice and littermate controls previously infected with 0.1 LD50 LM-OVA were challenged with 5 LD50 LM-OVA at day 43 p.i. Ag-specific T cells were measured via IFN-γ ICS on days 3 and 5 post challenge, to test the effect of TRAF5 deficiency on the ability of memory T cells to undergo expansion following secondary infection. Decreased expansion of Ag-specific CD8+ T cells was observed at both time points in TRAF5-/- mice (Fig. 2A & B). The total number of antigen-specific CD8+ T cells at day 5 post challenge was only ~30% that of littermate controls (Fig. 2B), indicating a defect in secondary expansion. IL-2, TNF-α, CD43, CD44, CD27 and CD127 production and expression were unaffected (Fig. S1I-SIL).
To investigate the effect of TRAF5 on the protective capacity of the secondary response to infection, TRAF5-/- mice and littermates were primed with 0.1 LD50 LM-OVA and challenged 43 days later with 5 LD50 LM-OVA. Naïve mice were challenged with 5 LD50 LM-OVA as a positive control to ensure that unimmunized mice were not protected from infection, which was evaluated by monitoring clearance of the bacteria from spleens and livers. Naive controls had nearly 1×1010 CFU/gram of liver and 1×109 CFU/spleen at day 3 post challenge (Fig. 3A & B). By comparison, littermates primed with LM-OVA had an ~ 7 log decrease in bacterial load in both organs. LM-OVA-primed TRAF5-/- mice could clear a large proportion of LM-OVA from the spleen, although their spleens contained ~92% more bacteria than did littermates (Fig. 3A). Interestingly, 7 out of 9 TRAF5-/- mice (pooled data from two experiments) still had between 1×106 and 1×107 CFU/gram of liver at day 3 post challenge, indicating that the negative effect on CD8+ T cell expansion observed in the TRAF5-/- mice resulted in a delayed clearance of bacteria from the liver (Fig. 3B).
Figure 3. Protection from secondary infection.
TRAF5-/- mice and littermate controls (TRAF5+/+) mice were infected i.v. with a sub-lethal dose of virulent LM-OVA (0.1XLD50 or 1000 CFU). At day 43 p.i. TRAF5-/- and littermate controls were challenged with a lethal dose (5XLD50 or 5×105 CFU) of LM-OVA. Naïve mice were also infected as unimmunized controls. Spleens (A) and livers (B) were harvested on day 3 and 5 post challenge. Cumulative data are from 2 independent experiments.
T-cell intrinsic nature of the expansion defect in TRAF5-/- mice
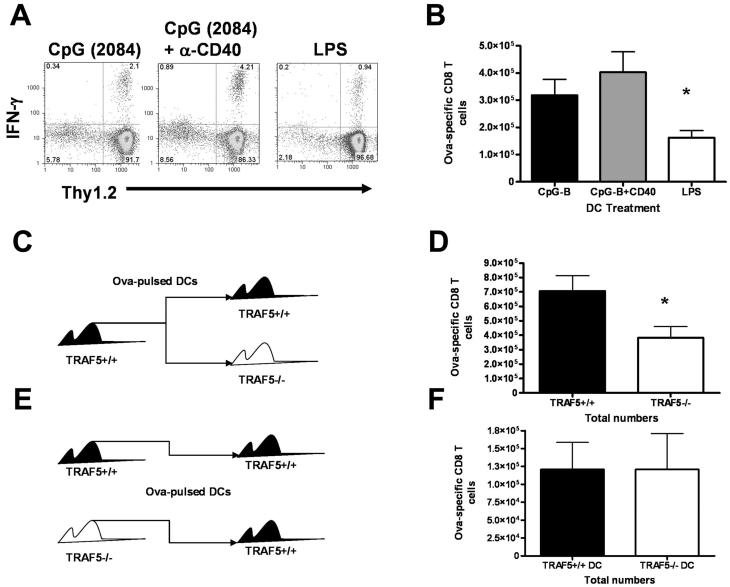
TRAF5 is a potential positive regulator of T cell costimulatory molecules such as CD27 (16). Additionally, TRAF5 may positively regulate CD40 signaling (17, 31). Because CD40 is a potent activator of dendritic cells (DC), it was important to know whether TRAF5 was influencing CD8+ T cell expansion directly through T cell costimulation, and/or if the effect was mediated by enhancing DC activation via CD40 signaling. To test the importance of TRAF5 as a T cell intrinsic factor in T cell expansion we employed a DC vaccination strategy to control whether the T cells or the DCs were TRAF5 deficient (32). TRAF5-/- mice and littermates were given OVA257-264 peptide-loaded bone marrow derived DC (BMDC) from wild type mice. At day 7 post immunization, OVA257-264-specific T cells were detected using ICS for IFN-γ. When TRAF5-sufficient DCs were used to immunize TRAF5-/- mice and littermates, significantly fewer OVA257-264-specific CD8+ T cells were generated in the TRAF5-/- mice (54% of littermate levels, Fig. 4C & 4E). To test the effect of TRAF5 deficiency on the ability of DCs to prime CD8+ T cell expansion, we used DCs generated from the bone marrow of TRAF5-/- mice or littermate controls to immunize C57Bl/6 mice (Fig 4D & 4F). When Wt mice immunized with DCs from TRAF5-/- mice were compared with those immunized with DCs from littermates, we found no difference in the expansion of Ag-specific CD8+ T cells, indicating that TRAF5 deficiency in DCs had no effect on CD8+ T cell expansion (Fig. 4E & 4F). Thus, the defect in CD8+ T cell expansion in TRAF5-/- mice is T cell intrinsic.
Figure 4. Primary response in mice receiving dendritic cell (DC) vaccination.
C57BL\6 mice were immunized with 5×105 bone marrow-derived DCs from TRAF5+/+ mice. DCs were matured with 100ng/mL CpG-B oligonucleotide (2084), 100ng/mL CpG-B (2084) oligonucleotide plus 5μg/mL anti-CD40 mAb (HM-40.1) or 100ng/mL LPS for 48 hours prior to immunization (A & B). TRAF5-/- mice and littermate controls (TRAF5+/+) were immunized with 5×105 bone marrow-derived DCs from TRAF5+/+ mice or (C) TRAF5-/- DCs were used to immunize TRAF5+/+ mice, while TRAF5+/+ DCs were used to immunize TRAF5+/+ mice as a control (D). Ag-specific CD8+ T cells were measured by ICS for IFN-γ at day 7 post immunization (E). * represents P<0.05 in a paired students t test (95% confidence interval). Data in (C) are cumulative from 3 independent experiments where N= 8 mice per group. Data in (D) are cumulative from 2 independent experiments where N= 4 mice per group.
Other activation parameters were measured in DCs from TRAF5-/- mice, including expression of OX40L, CD27L (CD70), CD86, and IL-12 production. DCs from TRAF5-/- mice did not show any defects in the ability to upregulate costimulatory ligands or produce IL-12 when stimulated through CD40 and TLR9 (Fig. S2 and data not shown). However, optimal expression of CD70 on the surface of DCs from TRAF5-/- mice and littermate controls was dependent on the stimulation given. LPS was insufficient for inducing CD70 expression on BMDC. TLR9 plus CD40 stimulation resulted in better CD70 expression and produced more Ag-specific CD8+ T cells than those vaccinations using DCs stimulated with LPS alone (Fig. 4A & B). Taken together, these data indicate that the defect in T cell expansion and the downstream decrease in CD8+ T cell memory numbers in TRAF5-/- mice is not a result of defects in TRAF5-/- DC.
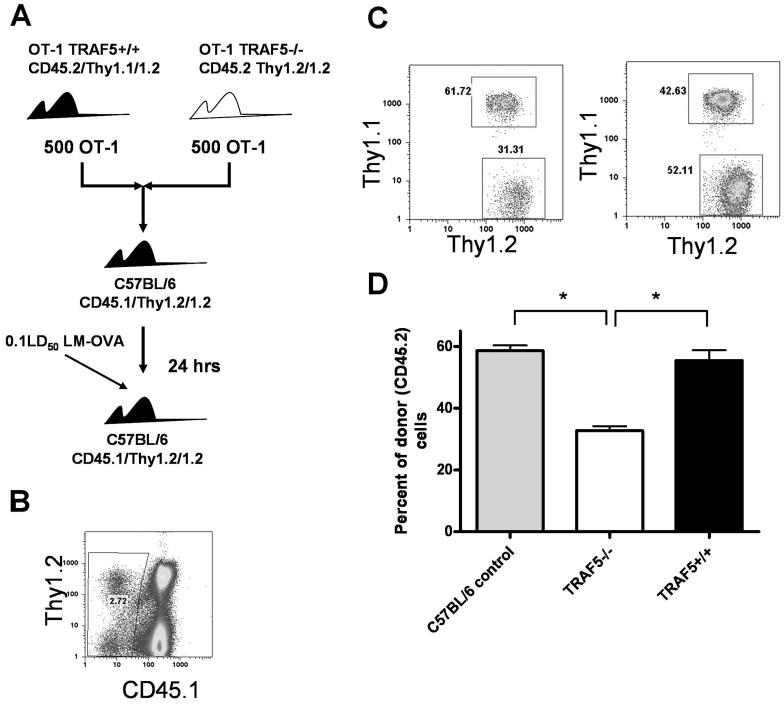
Next, we asked if unequal numbers of OVA-specific precursors in uninfected mice could explain the observed decrease in Ag-specific T cell expansion following infection. The possibility that naive TRAF5-/- mice have a lower precursor frequency of OVA-specific T cells is small, because the frequency of both CD4+ and CD8+ T cells in naive TRAF5-/- mice is normal (17) and was found in our experiments to be identical to littermate controls and C57Bl/6 mice (data not shown). However, to normalize the numbers of Ag-specific T cells prior to infection, OT-1 T cells from TRAF5-/- and TRAF5+/+ mice were used as donor cells for adoptive transfer into congenic (CD45.1) recipients in the LM-OVA infection model. By mixing donor cells in a 1:1 ratio we equalized the precursor frequency of TRAF5-/- and TRAF5+/+ cells prior to adoptive transfer, and their expansion was monitored within the same recipient (Fig. 5A). At 24 hours post transfer, mice were infected with 0.1LD50 LM-OVA. On day 7 p.i. spleens were harvested and the frequencies of CD45.1-/Thy1.1+/1.2+ (TRAF5+/+) and CD45.1-/Thy1.2+/1.2+ (TRAF5-/-) CD8+ T cells were determined by FACS (Fig. 5C). Following infection, there were consistently 50% fewer TRAF5-/- OT-1 T cells than TRAF5+/+ OT-1 T cells recovered from recipient mice (Fig. 5D). These results were remarkably similar to the results observed during the LM-OVA infection experiments shown in Fig. 1 and DC vaccination experiments (Fig. 4). Results confirm that the expansion defect observed in TRAF5-/- CD8+ T cells is not due to precursor frequency differences or defects in DC function.
Figure 5. Intrinsic nature of the expansion defect in TRAF5 deficient mice.
CD45.2/Thy1.2/1.2 OT-1 TRAF5-/- T cells were mixed 1:1 (1000 total CD8+ T cells) with T cells from littermate controls (CD45.2/Thy1.1/1.2 OT-1 TRAF5+/+) and transferred i.v. into naïve CD45.1/Thy1.2/1.2 mice. Recipient mice were infected with 0.1XLD50 virulent LM-OVA 24 hrs later (A). At day 7 CD45.1 was used to exclude T cells from recipient mice (B) and OT-1 TRAF5-/- CD8+ T cells and littermate OT-1 CD8 + T cells were measured as Thy1.1 versus Thy1.2 (C & D). * represents P<0.001 in a paired students t test (95% confidence interval). This figure contains cumulative data from 2 independent experiments where N= 8 mice per group.
CD8+ T cell survival vs. proliferation in TRAF5-/- mice
Decreased expansion of CD8+ T cells in TRAF5-/- mice could be due to an increased sensitivity to apoptosis, defective proliferation, or both. Many of the TNF-R superfamily members expressed by T cells that bind TRAF5 are involved in T cell costimulation (33) and could potentially affect proliferation and/or cell survival. To ask whether TRAF5 plays a positive role in cell proliferation, purified CD8+ T cells from TRAF5-/- mice or littermate controls were labeled with the fluorescent dye CFSE and stimulated with plate-bound α-CD3 Ab for 72 hours. Costimulation was provided in some wells via soluble α-CD28 Ab or plate-bound α-CD27 Ab. Signaling through CD28 does not require TRAF5 (34) and was a positive control for T cell costimulation. CFSE dilution showed that naïve CD8+ T cells from TRAF5-/- mice proliferated to the same degree as those from littermates following α-CD3 stimulation, whether they received costimulation through CD28 or CD27 (Fig. 6A).
Figure 6. Proliferation and apoptosis in CD8+ T cells.
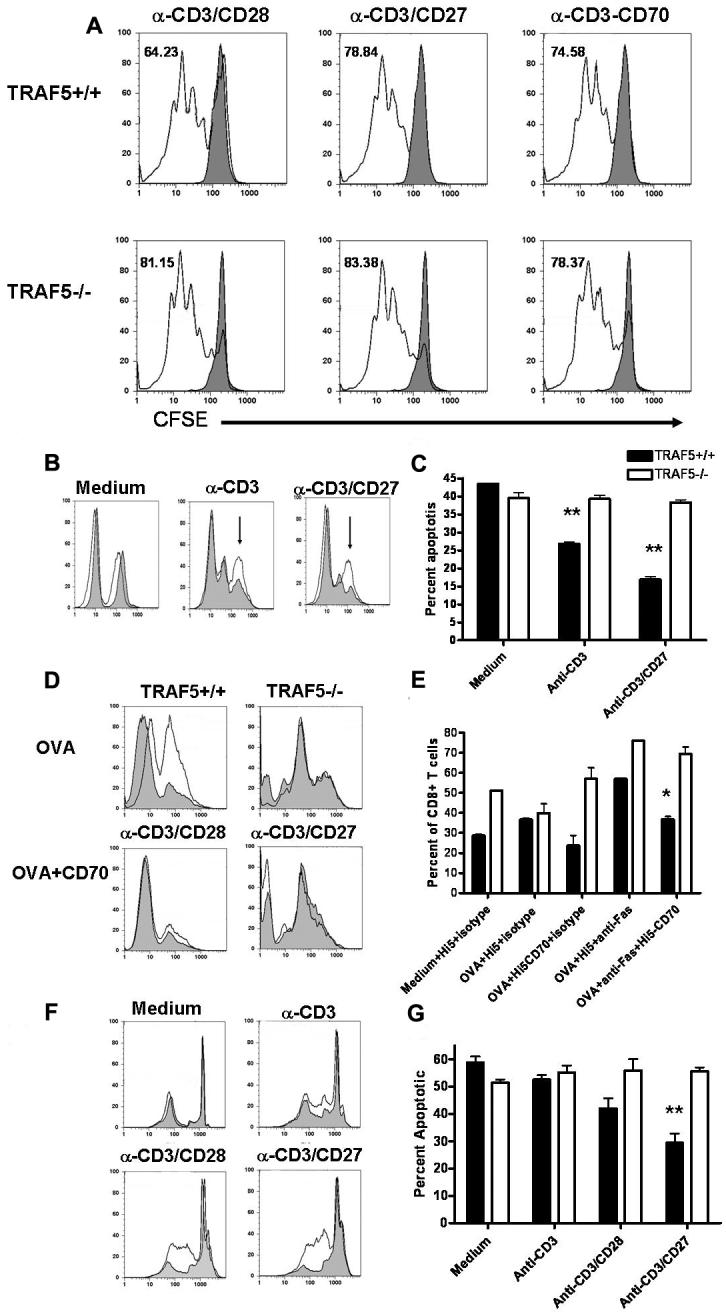
Purified CD8+ T cells were labeled with CFSE prior to stimulation. 3×105 CD8+ T cells/well were stimulated with plate bound anti-CD3 mAb (0.5ug/mL) with or without soluble anti-CD28 (10ug/mL) or anti-CD27 (10ug/mL) mAbs, or insect cells infected with CD70-producing baculovirus for 72 hours. Filled histograms represent control wells where cells were treated with a sub-optimal concentration of plate bound anti-CD3 mAb and isotype controls for anti-CD28 and anti-CD27 mAbs or insect cells infected with wild type baculovirus (A). Apoptosis was also measured as caspase 3 activation using the same stimulation parameters (TRAF5-/- CD8+ T cells (□) and littermate control CD8+ T cells (■) (C). Open histograms represent TRAF5-/- CD8+ T cell and filled histograms represent littermate control CD8+ T cells (B). Splenocytes from OT-1 TRAF5-/- CD8+ T cells (□) and littermate OT-1 CD8+ T cells (■) were pulsed with SIINFEKL peptide with or without anti-Fas/CD95 mAb, Hi5 insect cells expressing CD70 or anti-Fas + Hi5 CD70. Apoptosis was measured as an increase in caspase 3 activity in CD8+ cells (D & E). Filled histograms represent cells treated with isotype control mAb for anti-Fas/CD95 (Jo2 mAb) and open histograms represent cells treated with anti-Fas/CD95 mAb (D). CD8+ T cells were fixed and stained with propidium iodide following 48 hour stimulations (TRAF5-/- CD8+ T cells (□) and littermate CD8+ T cells (■) (F & G). Open histograms represent TRAF5-/- CD8+ T cell and filled histograms represent littermate control CD8+ T cells (F) Data are representative of 3 independent experiments with similar results. *= P<0.05 and ** =P<0.001 in a paired students t test (95% confidence interval).
To test sensitivity to apoptosis, we stimulated CD8+ T cells in vitro and measured caspase 3 activity. At 48 hours following stimulation, CD8+ T cells from TRAF5-/- mice contained 25% more caspase 3 activity than did CD8+ T cells from littermate controls (Fig. 6B & C). Using agonistic anti-CD3 mAbs to stimulate purified CD8+ T cells can result in low-level activation of caspase 3. However, this peak is lower in MFI intensity and resides between the peaks of live and apoptotic cells. For these experiments only the caspase 3 high peak (shown by the arrows in Fig 6B) was counted in the analysis. Interestingly, this peak was not observed when using OT-1 T cells activated with OVA257-264. Recent analysis of a CD27 knockout mouse shows that CD27 deficient CD8+ T cells have increased sensitivity to Fas/CD95-mediated apoptosis (22). To test whether the CD8+ T cells from TRAF5-/- mice were also more sensitive to CD95 killing, OT-1 T cells from TRAF5-/- mice were stimulated in vitro with Ag (OVA257-264) rather than anti-CD3 mAb. Stimulation of the TCR with anti-CD3 mAb or OVA peptide is used to upregulate CD95 on the cell surface. By using OVA peptide to stimulate OT-1 T cells within a whole splenocyte culture we could use more physiologic TCR stimulation conditions and determine whether CD27 signaling could rescue CD8+ T cells from CD95 mediated apoptosis. Splenocytes were treated with OVA257-264, α-CD95 mAb (Jo2) or isotype control mAb, with or without membrane-bound CD27 ligand (CD70) for 48 hours. Apoptotic CD8+ T cells were identified by staining for CD8 together with a reagent (PhiPhiLux-G1D2) that detects activated caspase 3. In this way, caspase 3 activity could be quantitated in CD8+ T cells alone in cultures containing whole splenocytes. As with the purified CD8+ T cells stimulated with agonistic antibodies to the TCR complex, peptide-stimulated T cells from OT-1 TRAF5-/- mice that were treated with α-CD95 mAb consistently resulted in higher caspase 3 activity than T cells from the littermates (Fig. 6D & 6E). Interestingly, membrane bound CD70 (CD27L) reduced caspase 3 activity in CD8+ T cells from littermate controls but had no effect on caspase 3 activity in CD8+ T cells from OT-1 TRAF5-/- (Fig. 6D & E) Results were confirmed using propidium iodide staining for DNA fragmentation (Fig. 6F & 6G) and using PhiPhiLux® concomitantly with YOPRO-1, which accurately stains apoptotic cells (data not shown) (35). These findings support our hypothesis that the CD8+ T cell expansion defect observed in TRAF5-/- mice was due to increased propensity of activated CD8+ T cells to undergo apoptosis, and not to impaired proliferation.
Interestingly, we observed no detectable differences in levels of phosphorylated JNK, p38 or ERK between TRAF5+/+ and TRAF5-/- T cells stimulated with membrane bound CD70 (Fig. S3A). We also observed no difference in IκBα degradation between TRAF5+/+ and TRAF5-/- T cells stimulated with membrane bound CD70 (Fig. S3A) or cross-linked α-CD27 agonistic mAb (Fig. S3B). Our findings regarding NF-κB are not surprising, considering the well documented redundancy between TRAF2 and TRAF5 in the activation of NF-κB (16, 17) and the results published by Nakano and colleagues demonstrating no effect of TRAF5 deficiency on the activation of JNK and NF-κB in primary thymocytes from TRAF5-/- mice stimulated via CD27 (17).
Discussion
The power of TNF-R superfamily members to regulate T cell costimulation has been demonstrated in infection (3, 22), tumor immunotherapy (36) and autoimmunity (25). However, the role of TRAFs in this process remains poorly defined. TRAF5 binds to several TNF-R superfamily members that regulate T cell function (16, 18, 21). This, together with published data demonstrating that TRAF5 has functional importance for OX40 (25) and GITR (20) suggests a link between TRAF5, T cell costimulation and responses to infection.
LM-OVA infection of TRAF5 deficient mice revealed a specific role for TRAF5 in CD8+ T cell expansion, resulting in significantly fewer OVA-specific CD8+ T cells in both the primary and secondary CD8+ T cell response. CD8+ T cells from TRAF5-/- mice also showed much reduced memory responses following infection. Although complete ablation of CD8+ T cell expansion in TRAF5 deficient mice was not seen, this was not surprising, because other costimulators such as CD28 that do not require TRAF5 are still functional in these mice.
An important question raised by our results is which events in the T cell response to an infectious pathogen were compromised by TRAF5 deficiency. While the effect of TRAF5 deficiency on CD8+ T cell expansion was striking, we did not observe defects in T cell proliferation, cytokine production or activation phenotype. This indicates that the role of TRAF5 in the CD8+ T cell response to infection is specific to cell fitness during clonal expansion and is separate from cytokine production or the differentiation of activated T cells to effector and memory subsets. Our results from LM-OVA infections of TRAF5-/- mice are strikingly similar to those obtained from influenza virus infection of CD27-/- mice. CD27-/- mice also exhibit defects in primary expansion of CD8+ T cells, resulting in a diminished memory pool (3, 37). We have also observed that as in the CD27-/- mouse, the TRAF5-/- mouse appears to have defects in CD8+ T cell survival rather than proliferation (3). Recent work by Dolfi and colleagues has shown that CD8+ T cell resistance to apoptosis is acquired late in the primary response and is required at this stage to generate a memory pool similar in cell number, but more effective in response to secondary challenge (22). Our results demonstrate that CD8+ T cells from TRAF5+/+ mice were protected from CD95- mediated apoptosis when stimulated with membrane-bound CD70, while those from TRAF5-/- mice were not. However, in contrast to Dolfi et al., we observed a reduction in the number of Ag-specific CD8+ T cells in the memory pool. This is not necessarily surprising, because absence of TRAF5 potentially affects multiple TNF-R superfamily members involved in CD8+ T cell expansion, whereas deficiency of CD27, or CD70 blockade affects only one of these pathways. It will be interesting to determine if and how additional T cell costimulatory TNF-R superfamily members are influenced by TRAF5.
TRAF5 deficiency might alternatively have indirect effects on the LM-OVA T cell response, via potential defects in CD40 signaling to antigen presenting cells (17). The optimal surface expression of many T cell costimulatory TNF-R superfamily member ligands by APCs is positively influenced by CD40 and TLR signaling (38). We found that DCs from TRAF5 deficient mice, stimulated with CpG and agonistic anti-CD40 antibodies, displayed identical surface expression of the costimulatory ligands OX40L, CD70 and CD86, compared to littermate control DCs, and also had comparable production of the proinflammatory cytokine, IL-12. TRAF5-/- DCs had no defects in elicitation of OVA-specific T cell expansion. Thus, while it is likely that TRAF5 plays specific roles in signaling by TNFR superfamily molecules to APC, its major role in CD8+ T cell responses to infection is in directly delivering signals to the T cell.
Our data demonstrated that the expansion defect observed in TRAF5-/- mice was not due to a defect in numbers of OVA-specific T cell precursors. Thus the absence of TRAF5 led to a T-cell intrinsic defect in the immune response to a pathogenic microbe. The defect in T cell expansion observed could result from either defective proliferation, or increased susceptibility to apoptosis during expansion. There is some evidence that costimulation by TNF-R superfamily members can promote cell proliferation (39, 40) However, the majority of published work has suggested that T cell costimulatory TNF-R superfamily molecules act to promote survival during clonal expansion by modulating the function of pro and anti-apoptotic proteins (4, 22). CD27 protects CD8+ T cells from CD95-mediated apoptosis during the primary response to influenza virus infection (22), and our results demonstrate that apoptosis resistance in TRAF5-sufficient mice was enhanced by the TRAF5-binding receptor CD27. However, while TRAF5-/- T cells showed normal proliferative capacity, they displayed increased caspase 3 activity, enhanced susceptibility to apoptosis and reduced responsiveness to CD27 stimulation.
The underlying mechanism of the enhanced susceptibility to apoptosis in CD8+ T cells from TRAF5-/- mice remains unclear. We did not observed any reproducible differences caused by TRAF5 deficiency in the activation of NF-κB, p38, ERK or JNK (Figs. S3A and S3B). We also found no detectable differences in the expression of the Bcl-2 family members, Bcl-2, Bcl-XL and Bim (data not shown). It is likely that there is some overlap between TRAF2 and TRAF5 in some functions. Evidence of TRAF5/TRAF2 redundancy in NF-κB and JNK activation was observed in both B and T lymphocytes in the first description of the TRAF5-/- mice by Nakano in 1999 (17), and is consistent with our present findings. We hypothesize that TRAF5 affects survival through a separate, non-redundant pathway that is at least partially independent of NF-κB.
Although priming with a sublethal dose of LM-OVA protected even the TRAF5-/- mice from a subsequent lethal dose, TRAF5-/- mice consistently showed several orders of magnitude higher bacterial loads in their livers at day 3 post challenge. That this effect was not as pronounced in the spleen may be due to decreased clearance of LM-OVA in liver in TRAF5-/- mice or a defect in T cell migration in TRAF5-deficient mice. It is also possible that the reduction in expanded CD8+ T cells in TRAF5-/- leaves enough CD8+ T cells to reduce bacterial loads in the spleen, but produces insufficient numbers to readily eliminate the bacteria from peripheral organs, resulting in a delay in bacterial clearance from the liver.
Our data suggest that TRAF5 plays an important supporting role, allowing optimal expansion of CD8 T cells after infection, resulting in the generation of a memory pool sufficient to provide protection against secondary challenge. Thus, the deletion of one receptor (e.g. CD27) or one adaptor molecule (such as TRAF5) results in reduction but not complete ablation of T cell expansion and cellular immunity. Reduction in T cell expansion in TRAF5-/- mice results in delayed but not ablated clearance of LM. This may have a modest effect on the survival of laboratory mice in germ-free and predator-free laboratory environments. However, the optimal CD8+ T cell response, which includes TRAF5 contributions to costimulatory signaling, may make the difference between the survival or death of a mouse in a natural setting. The information gained by understanding the effects of costimulatory TNF-R superfamily members may also be used in designing new vaccines and tumor therapies through precise manipulation of T cell costimulation.
In summary, we have defined a novel role for TRAF5 as a positive regulator of CD8+ T cell survival via costimulatory TNF-R superfamily members. This work reveals the importance of TRAF5 in T cell costimulation and enhanced T cell survival during expansion, and identifies a specific functional role for TRAF5 in T cell responses to infection.
Supplementary Material
Acknowledgements
The authors thank Dr. Michael Croft for the TRAF5 deficient mice as well as Drs. John Harty and Kelly Messingham for reagents, protocols, and advice.
This work is supported by a pre-doctoral fellowship awarded by the American Heart Association 0715658Z (Z.J.K) and by grants from the National Institute of Allergy and Infectious Disease, the National Cancer Institute and the Veteran's Administration (G.A.B).
Footnotes
Disclosures The authors have no conflicting financial interests.
Online Supplemental Material Fig. S1A, E and I show the levels of IL-2 produced by antigen-specific CD8+ and CD4+ T cells from TRAF5-/- mice and littermate control mice during the primary infection, memory response and secondary infection respectively. Fig. S1B, F and J show the levels of TNF-α produced by antigen-specific CD4+ T cells from TRAF5-/- mice and littermate control mice during the primary infection, memory response and secondary infection respectively. Fig. S1C, G and K show the surface expression of CD44 and CD127 by antigen-specific CD4+ T cells from TRAF5-/- mice and littermate control mice during the primary infection, memory and secondary infection respectively. Fig. S1D, H and L show the surface expression of CD44, CD43, CD127 and CD27 by antigen-specific CD8+ T cells from TRAF5-/- mice and littermate control mice during the primary infection, memory and secondary infection respectively. Fig. S2 shows the expression of OX40L and CD70 on bone marrow-derived DCs from TRAF5-/- mice and littermate control mice activated with TLR ligands with or without anti-CD40 treatment.
References
- 1.Suresh M, Whitmire JK, Harrington LE, Larsen CP, Pearson TC, Altman JD, Ahmed R. Role of CD28-B7 interactions in generation and maintenance of CD8 T cell memory. J Immunol. 2001;167:5565–5573. doi: 10.4049/jimmunol.167.10.5565. [DOI] [PubMed] [Google Scholar]
- 2.Flynn K, Mullbacher A. The generation of memory antigen-specific cytotoxic T cell responses by CD28/CD80 interactions in the absence of antigen. Eur J Immunol. 1997;27:456–462. doi: 10.1002/eji.1830270216. [DOI] [PubMed] [Google Scholar]
- 3.Hendriks J, Gravestein LA, Tesselaar K, van Lier RA, Schumacher TN, Borst J. CD27 is required for generation and long-term maintenance of T cell immunity. Nat Immunol. 2000;1:433–440. doi: 10.1038/80877. [DOI] [PubMed] [Google Scholar]
- 4.Rogers PR, Song J, Gramaglia I, Killeen N, Croft M. OX40 Promotes Bcl-xL and Bcl-2 Expression and Is Essential for Long-Term Survival of CD4 T Cells. Immunity. 2001;15:445–455. doi: 10.1016/s1074-7613(01)00191-1. [DOI] [PubMed] [Google Scholar]
- 5.Croft M. Costimulation of T cells by OX40, 4-1BB, and CD27. Cytokine & Growth Factor Reviews The TNF Superfamily. 2003;14:265–273. doi: 10.1016/s1359-6101(03)00025-x. [DOI] [PubMed] [Google Scholar]
- 6.Hendriks J, Xiao Y, Rossen JW, van der Sluijs KF, Sugamura K, Ishii N, Borst J. During viral infection of the respiratory tract, CD27, 4-1BB, and OX40 collectively determine formation of CD8+ memory T cells and their capacity for secondary expansion. J Immunol. 2005;175:1665–1676. doi: 10.4049/jimmunol.175.3.1665. [DOI] [PubMed] [Google Scholar]
- 7.Lens SMA, Tesselaar K, van Oers MHJ, van Lier RAW. Control of lymphocyte function through CD27-CD70 interactions. Seminars in Immunology. 1998;10:491–499. doi: 10.1006/smim.1998.0154. [DOI] [PubMed] [Google Scholar]
- 8.Pollok K, Kim Y, Zhou Z, Hurtado J, Kim K, Pickard R, Kwon B. Inducible T cell antigen 4-1BB. Analysis of expression and function. J Immunol. 1993;150:771–781. [PubMed] [Google Scholar]
- 9.Gramaglia I, Weinberg AD, Lemon M, Croft M. Ox-40 Ligand: A Potent Costimulatory Molecule for Sustaining Primary CD4 T Cell Responses. J Immunol. 1998;161:6510–6517. [PubMed] [Google Scholar]
- 10.Bishop GA. The multifaceted roles of TRAFs in the regulation of B-cell function. 2004;4:775–786. doi: 10.1038/nri1462. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi T, Walsh MC, Choi Y. The role of TRAF6 in signal transduction and the immune response. Microbes and Infection. 2004;6:1333–1338. doi: 10.1016/j.micinf.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Hostager BS. Roles of TRAF6 in CD40 signaling. Immunol Res. 2007;39:105–114. doi: 10.1007/s12026-007-0082-3. [DOI] [PubMed] [Google Scholar]
- 13.Hacker H, Redecke V, Blagoev B, Kratchmarova I, Hsu L-C, Wang GG, Kamps MP, Raz E, Wagner H, Hacker G, Mann M, Karin M. Specificity in Toll-like receptor signalling through distinct effector functions of TRAF3 and TRAF6. 2006;439:204–207. doi: 10.1038/nature04369. [DOI] [PubMed] [Google Scholar]
- 14.Oganesyan G, Saha SK, Guo B, He JQ, Shahangian A, Zarnegar B, Perry A, Cheng G. Critical role of TRAF3 in the Toll-like receptor-dependent and -independent antiviral response. Nature. 2006;439:208–211. doi: 10.1038/nature04374. [DOI] [PubMed] [Google Scholar]
- 15.Xie P, Stunz LL, Larison KD, Yang B, Bishop GA. Tumor Necrosis Factor Receptor-Associated Factor 3 Is a Critical Regulator of B Cell Homeostasis in Secondary Lymphoid Organs. Immunity. 2007;27:253–267. doi: 10.1016/j.immuni.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akiba H, Nakano H, Nishinaka S, Shindo M, Kobata T, Atsuta M, Morimoto C, Ware CF, Malinin NL, Wallach D, Yagita H, Okumura K. CD27, a member of the tumor necrosis factor receptor superfamily, activates NF-kappaB and stress-activated protein kinase/c-Jun N-terminal kinase via TRAF2, TRAF5, and NF-kappaB-inducing kinase. J Biol Chem. 1998;273:13353–13358. doi: 10.1074/jbc.273.21.13353. [DOI] [PubMed] [Google Scholar]
- 17.Nakano H, Sakon S, Koseki H, Takemori T, Tada K, Matsumoto M, Munechika E, Sakai T, Shirasawa T, Akiba H, Kobata T, Santee SM, Ware CF, Rennert PD, Taniguchi M, Yagita H, Okumura K. Targeted disruption of Traf5 gene causes defects in CD40- and CD27-mediated lymphocyte activation. Proc Natl Acad Sci U S A. 1999;96:9803–9808. doi: 10.1073/pnas.96.17.9803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawamata S, Hori T, Imura A, Takaori-Kondo A, Uchiyama T. Activation of OX40 Signal Transduction Pathways Leads to Tumor Necrosis Factor Receptor-associated Factor (TRAF) 2- and TRAF5-mediated NF-kappa B Activation. J. Biol. Chem. 1998;273:5808–5814. doi: 10.1074/jbc.273.10.5808. [DOI] [PubMed] [Google Scholar]
- 19.Esparza EM, Arch RH. Glucocorticoid-Induced TNF Receptor Functions as a Costimulatory Receptor That Promotes Survival in Early Phases of T Cell Activation. J Immunol. 2005;174:7869–7874. doi: 10.4049/jimmunol.174.12.7869. [DOI] [PubMed] [Google Scholar]
- 20.Esparza EM, Lindsten T, Stockhausen JM, Arch RH. Tumor Necrosis Factor Receptor (TNFR)-associated Factor 5 Is a Critical Intermediate of Costimulatory Signaling Pathways Triggered by Glucocorticoid-induced TNFR in T Cells. J. Biol. Chem. 2006;281:8559–8564. doi: 10.1074/jbc.M512915200. [DOI] [PubMed] [Google Scholar]
- 21.Hauer J, Puschner S, Ramakrishnan P, Simon U, Bongers M, Federle C, Engelmann H. TNF receptor (TNFR)-associated factor (TRAF) 3 serves as an inhibitor of TRAF2/5-mediated activation of the noncanonical NF-kappaB pathway by TRAF-binding TNFRs. Proc Natl Acad Sci U S A. 2005;102:2874–2879. doi: 10.1073/pnas.0500187102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dolfi DV, Boesteanu AC, Petrovas C, Xia D, Butz EA, Katsikis PD. Late Signals from CD27 Prevent Fas-Dependent Apoptosis of Primary CD8+ T Cells. J Immunol. 2008;180:2912–2921. doi: 10.4049/jimmunol.180.5.2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ronchetti S, Nocentini G, Bianchini R, Krausz LT, Migliorati G, Riccardi C. Glucocorticoid-Induced TNFR-Related Protein Lowers the Threshold of CD28 Costimulation in CD8+ T Cells. J Immunol. 2007;179:5916–5926. doi: 10.4049/jimmunol.179.9.5916. [DOI] [PubMed] [Google Scholar]
- 24.Habib-Agahi M, Phan TT, Searle PF. Co-stimulation with 4-1BB ligand allows extended T-cell proliferation, synergizes with CD80/CD86 and can reactivate anergic T cells. Int Immunol. 2007;19:1383–1394. doi: 10.1093/intimm/dxm106. [DOI] [PubMed] [Google Scholar]
- 25.So T, Salek-Ardakani S, Nakano H, Ware CF, Croft M. TNF Receptor-Associated Factor 5 Limits the Induction of Th2 Immune Responses. J Immunol. 2004;172:4292–4297. doi: 10.4049/jimmunol.172.7.4292. [DOI] [PubMed] [Google Scholar]
- 26.Shen H, Miller JF, Fan X, Kolwyck D, Ahmed R, Harty JT. Compartmentalization of Bacterial Antigens: Differential Effects on Priming of CD8 T Cells and Protective Immunity. Cell. 1998;92:535–545. doi: 10.1016/s0092-8674(00)80946-0. [DOI] [PubMed] [Google Scholar]
- 27.Harty JT, Lenz LL, Bevan MJ. Primary and secondary immune responses to Listeria monocytogenes. Curr Opin Immunol. 1996;8:526–530. doi: 10.1016/s0952-7915(96)80041-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pope C, Kim S-K, Marzo A, Williams K, Jiang J, Shen H, Lefrancois L. Organ-Specific Regulation of the CD8 T Cell Response to Listeria monocytogenes Infection. J Immunol. 2001;166:3402–3409. doi: 10.4049/jimmunol.166.5.3402. [DOI] [PubMed] [Google Scholar]
- 29.Altman JD, Moss PAH, Goulder PJR, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Phenotypic Analysis of Antigen-Specific T Lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 30.Badovinac VP, Messingham KA, Jabbari A, Haring JS, Harty JT. Accelerated CD8+ T-cell memory and prime-boost response after dendriticcell vaccination. Nat Med. 2005;11:748–756. doi: 10.1038/nm1257. [DOI] [PubMed] [Google Scholar]
- 31.Ishida TK, Tojo T, Aoki T, Kobayashi N, Ohishi T, Watanabe T, Yamamoto T, Inoue J. TRAF5, a novel tumor necrosis factor receptor-associated factor family protein, mediates CD40 signaling. Proc Natl Acad Sci U S A. 1996;93:9437–9442. doi: 10.1073/pnas.93.18.9437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamilton SE, Harty JT. Quantitation of CD8+ T Cell Expansion, Memory, and Protective Immunity After Immunization with Peptide-Coated Dendritic Cells. J Immunol. 2002;169:4936–4944. doi: 10.4049/jimmunol.169.9.4936. [DOI] [PubMed] [Google Scholar]
- 33.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 34.Slavik JM, Hutchcroft JE, Bierer BE. CD28/CTLA-4 and CD80/CD86 families: signaling and function. Immunol Res. 1999;19:1–24. doi: 10.1007/BF02786473. [DOI] [PubMed] [Google Scholar]
- 35.Idziorek T, Estaquier J, De Bels F, Ameisen J-C. YOPRO-1 permits cytofluorometric analysis of programmed cell death (apoptosis) without interfering with cell viability. Journal of Immunological Methods. 1995;185:249–258. doi: 10.1016/0022-1759(95)00172-7. [DOI] [PubMed] [Google Scholar]
- 36.French RR, Taraban VY, Crowther GR, Rowley TF, Gray JC, Johnson PW, Tutt AL, Al-Shamkhani A, Glennie MJ. Eradication of lymphoma by CD8 T cells following anti-CD40 monoclonal antibody therapy is critically dependent on CD27 costimulation. Blood. 2007;109:4810–4815. doi: 10.1182/blood-2006-11-057216. [DOI] [PubMed] [Google Scholar]
- 37.Hendriks J, Xiao Y, Borst J. CD27 promotes survival of activated T cells and complements CD28 in generation and establishment of the effector T cell pool. J Exp Med. 2003;198:1369–1380. doi: 10.1084/jem.20030916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schildknecht A, Miescher I, Yagita H, van den Broek M. Priming of CD8+ T cell responses by pathogens typically depends on CD70-mediated interactions with dendritic cells. Eur J Immunol. 2007;37:716–728. doi: 10.1002/eji.200636824. [DOI] [PubMed] [Google Scholar]
- 39.Kim M-Y, Bekiaris V, McConnell FM, Gaspal FMC, Raykundalia C, Lane PJL. OX40 Signals during Priming on Dendritic Cells Inhibit CD4 T Cell Proliferation: IL-4 Switches off OX40 Signals Enabling Rapid Proliferation of Th2 Effectors. J Immunol. 2005;174:1433–1437. doi: 10.4049/jimmunol.174.3.1433. [DOI] [PubMed] [Google Scholar]
- 40.Ronchetti S, Zollo O, Bruscoli S, Agostini M, Bianchini R, Nocentini G, Ayroldi E, Riccardi C. GITR, a member of the TNF receptor superfamily, is costimulatory to mouse T lymphocyte subpopulations. Eur J Immunol. 2004;34:613–622. doi: 10.1002/eji.200324804. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.