Abstract
At the onset of anaphase, sister-chromatid cohesion is dissolved abruptly and irreversibly, ensuring that all chromosome pairs disjoin almost simultaneously. The regulatory mechanisms that generate this switch-like behavior are unclear. Anaphase is initiated when a ubiquitin ligase, the Anaphase-Promoting Complex (APC), triggers the destruction of securin, thereby allowing the protease, separase, to disrupt sister-chromatid cohesion 1–4. Here we demonstrate that Cdk1-dependent phosphorylation of securin near its destruction-box motif inhibits securin ubiquitination by the APC. The phosphatase Cdc14 reverses securin phosphorylation, thereby increasing the rate of securin ubiquitination. Because separase is known to activate Cdc14 5,6, our results support the existence of a positive feedback loop that increases the abruptness of anaphase. Consistent with this model, we show that mutations that disrupt securin phosphoregulation decrease the synchrony of chromosome segregation. Our results also suggest that coupling securin degradation with changes in Cdk1 and Cdc14 activities helps coordinate the initiation of sister-chromatid separation with changes in spindle dynamics.
Securin is known to integrate multiple signaling pathways to delay anaphase in response to perturbations such as DNA damage 7,8. We hypothesized that securin might also receive regulatory inputs that make separase activation more switch-like. To address this possibility, we used mass spectrometry to analyze the phosphorylation state of securin from mitotic budding yeast cells (Fig. 1a) 9. We identified six phosphorylation sites, including four of the five sites corresponding to the consensus sequence of the cyclin-dependent-kinase, Cdk1 (S/T*-P) (see also Suppl. Fig. 1). Three Cdk1 sites were found in securin’s C-terminal domain, which is known to interact with separase 10; mutations at these sites are known to modulate securin’s ability to bind separase and import it into the nucleus 11. The fourth Cdk1 phosphopeptide, near the N terminus of securin, has not been characterized.
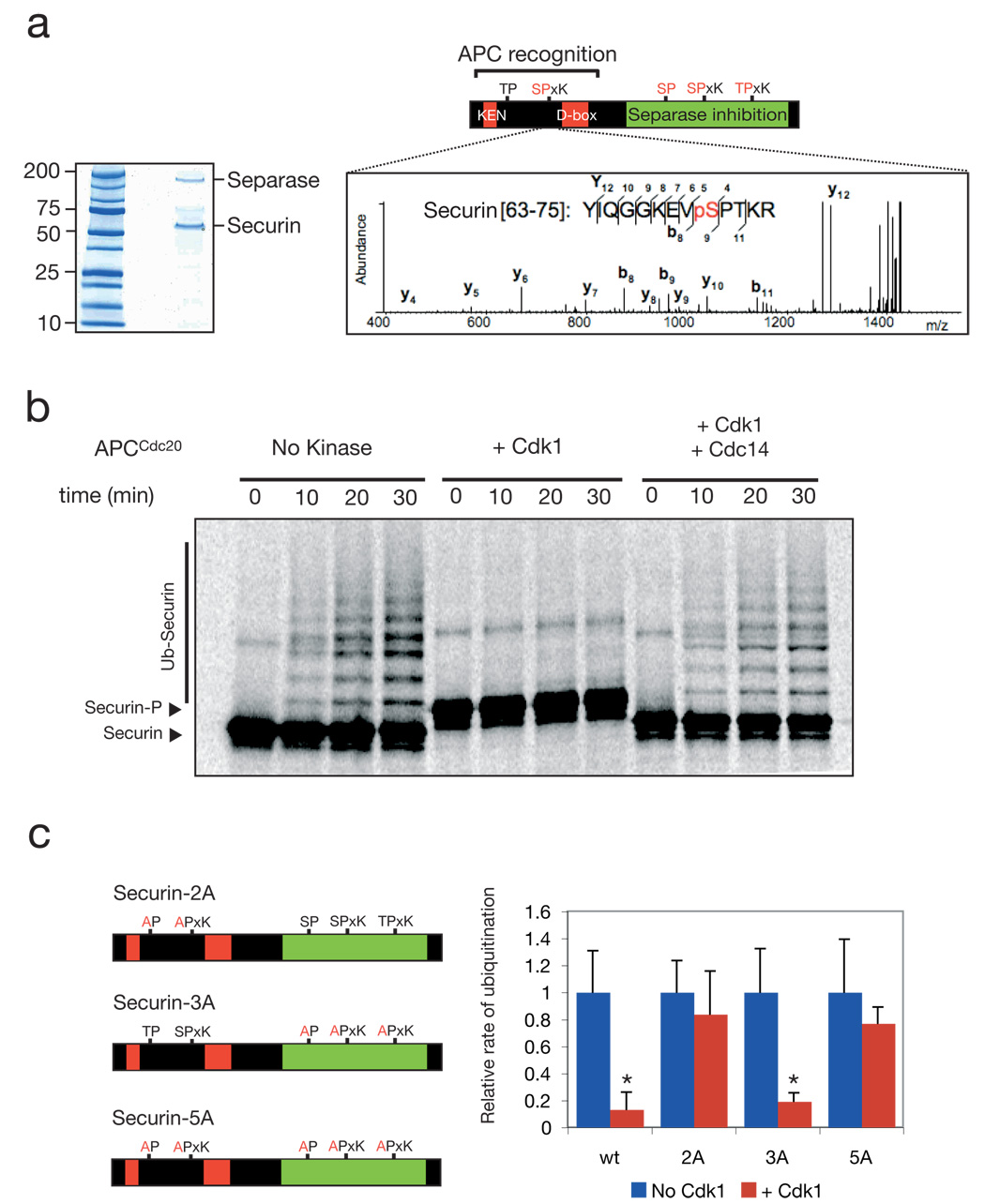
Figure 1. Cdk1 and Cdc14 control the phosphorylation state of securin near its destruction-box and modulate the rate of securin ubiquitination.
a, Securin was purified from a benomyl-arrested strain carrying SECURIN-3FLAG-6HIS (left panel), and MALDI-MS/MS analysis resulted in the detection of ten phosphopeptides (Suppl. Fig. 1), including four peptides phosphorylated at Cdk1 consensus sites (red). One of these sites lies next to the securin destruction-box motif (the MS3 spectrum of the phosphopeptide is shown to the right, with the phosphopeptide sequence most consistent with the data). b, 35S-methionine-labeled securin was produced by translation in vitro and incubated with purified APCCdc20 and other ubiquitination components. Prior to the reactions, as indicated, some samples were incubated with either purified Clb2-Cdk1 or with Clb2-Cdk1 and then Cdc14 sequentially. The substrate was purified away from kinase and phosphatase prior to addition of APCCdc20. c, Quantitation of experiments conducted as in panel b using either wild-type securin, securin-2A (T27A, S71A), securin-3A (S277A, S292A, T304A), or securin-5A as substrate. Error bars indicate standard deviation from 3 experiments. Asterisks indicate a t-test P-value of <0.05 when comparing results with and without kinase.
The N-terminal Cdk1 site is near the destruction-box of securin, a motif important for recognition of substrates by the APC 2–4, 12. To assess whether phosphorylation of this site affects securin ubiquitination by the APC, we tested the ability of purified APC to ubiquitinate purified securin before and after phosphorylation in vitro by Cdk1. We found that if securin was phosphorylated by Cdk1, the rate of ubiquitination by APCCdc20 (the APC-activator complex that controls anaphase onset) was reduced 10-fold (Fig. 1b, c) and the rate of ubiquitination by APCCdh1 was reduced 5-fold (Suppl. Fig. 2).
This regulatory mechanism may be conserved – Drosophila and human homologues of securin have S/T-P motifs near their destruction-boxes, and both were ubiquitinated less efficiently by the APC after phosphorylation by Cdk1 (Suppl. Fig. 3).
Cdc14 is a phosphatase that removes phosphates from Cdk1 substrates during anaphase and late mitosis 4,13–16. We found that Cdc14 removed Cdk1-dependent phosphates from securin, as judged by the loss of a gel-mobility shift that results from phosphorylation (Fig. 1b; Suppl. Fig. 2a). Dephosphorylation reactivated securin as an efficient APC substrate.
To confirm that regulation depends on the canonical Cdk1 sites, we mutagenized securin to remove the N-terminal Cdk1 sites (securin-2A), the three C-terminal sites (securin-3A), or all five sites (securin-5A). Securin-2A and securin-5A were still recognized by the APC after incubation with Cdk1 (Fig. 1c; Suppl. Fig. 2b), while securin-3A ubiquitination was inhibited by Cdk1 (Fig. 1c). We conclude that the ability of Cdk1 and Cdc14 to control securin ubiquitination depends on one or both of the N-terminal Cdk1 sites.
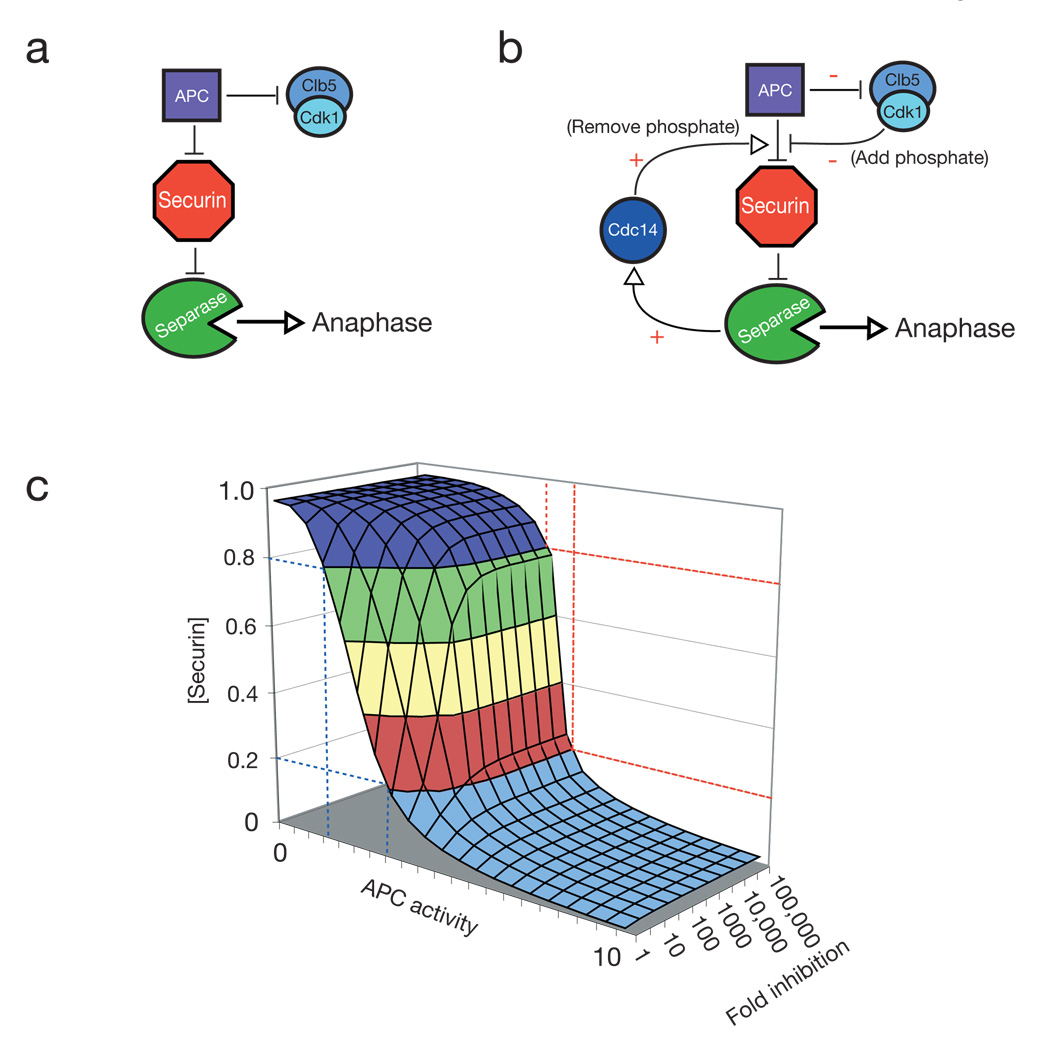
Current models of anaphase regulation do not provide many opportunities for switch-like behavior - in particular there is no known positive feedback in this system (Fig. 2a) 17,18. It is therefore interesting that separase, in addition to cleaving cohesin, initiates the activation of Cdc14 in early anaphase 5,6. Thus, our observation that Cdc14 can dephosphorylate securin, and thereby increase its efficiency as an APC substrate, leads to the potential for a positive feedback loop that is predicted to make anaphase more switch-like (Fig. 2b).
Figure 2. Modulation of securin ubiquitination by Cdk1 and Cdc14 gives rise to a potential positive feedback loop in the anaphase regulatory network.
a, A simplified prior model for anaphase regulation in S. cerevisiae: the APC targets securin and some mitotic cyclins for destruction, which liberates separase to cleave cohesin and initiate anaphase. b, A modified model of anaphase control: Cdk1 phosphorylates securin, reducing the rate at which it is ubiquitinated by the APC. Upon accumulation of high levels of APC activity, some securin is destroyed, releasing a small amount of separase. Separase activates Cdc14, which dephosphorylates securin, increasing the rate at which it is ubiquitinated by the APC. Concomitantly, some mitotic cyclins are destroyed, reducing Cdk1 activity and allowing Cdc14 to more efficiently dephosphorylate securin. c, A set of numerical solutions to a simplified mathematical model of the network in panel b (but excluding cyclin degradation by the APC), showing the steady-state levels of securin remaining (y-axis) as APC activity is varied (x-axis) when securin ubiquitination is inhibited to various degrees by phosphorylation (zaxis; see Suppl. Fig. 3 for details of model).
APCCdc20 activation triggers the destruction of cyclins as well as securin (Fig. 2a, b). Thus, the kinases responsible for securin phosphorylation are partly inactivated at anaphase initiation, providing an additional mechanism by which securin destruction is promoted – and potentially increasing the robustness of this switch.
We explored a simple mathematical version of this model (Fig. 2c – see Suppl. Fig. 4 for details) by numerically determining the steady-state levels of securin as a function of APC activity under a range of parameters. We did not include the effects of the APC on Cdk activity in the model, as it did not significantly affect the outcome of the simulations (Suppl. Fig. 5). To explore how the modulation of securin destruction might affect the abruptness of anaphase, we varied the effect of securin phosphorylation on the rate of its destruction. As we increased the inhibitory effect of phosphorylation from 1- to 100-fold, securin degradation became more switch-like (Fig. 2c).
To investigate the relevance of our model in vivo, we created a yeast strain in which we specifically disrupted the putative positive feedback loop by replacing the securin gene with a mutant gene encoding securin-2A. This strain was viable at both 30°C (the restrictive temperature for a securinΔ strain) and 37°C, suggesting that securin-2A is functional in its ability to bind and fold separase. We measured the timing of securin-2A degradation in cells released from a G1 arrest and found no significant difference between the mutant and wild-type securin (Suppl. Fig. 6). We suspected, however, that asynchrony in the cell population in these experiments prevented the detection of small differences in the timing and abruptness of securin destruction. We therefore attempted to develop microscopic methods to measure securin destruction in single cells. GFP-tagged securin was undetectable when expressed at normal levels, and overexpressed securin-GFP was detectable but delayed cells in late mitosis. It was possible, however, to assess the relative stabilities of securin and securin-2A in late mitosis by simultaneously overexpressing and analyzing the levels of both proteins, each tagged with a different GFP variant. We found that mitotic securin-2A levels were lower than those of wild-type securin (Suppl. Fig. 7).
To analyze the consequences of securin phosphoregulation in more detail, we developed methods for the analysis of spindle dynamics and the abruptness of anaphase onset in single cells. To quantify spindle length, we tagged the spindle pole body protein, Spc42, with dsRed. To visualize the rate and abruptness of chromosome segregation, we integrated LAC and TET operator arrays at two independent loci, TRP1 and URA3, respectively, and expressed GFP-tagged Lac and Tet repressors in the cell to generate fluorescent marks at these loci 19,20. Chromosomes IV (TRP1-LACO) and V (URA3-TETO) were thereby marked at loci that are close to the centromere (12 and 35 kbp, respectively) but far enough away that they are not resolved until cohesin is cleaved by separase. One GFP-labeled locus was consistently brighter than the other, and we found that this dot disappeared upon addition of tetracycline, suggesting that the bright dot labels chromosome V (Suppl. Fig. 8).
We used a spinning-disk confocal microscope to obtain movies of anaphase at 10-second time resolution. These movies allowed us to determine the time lag between the segregation of one chromosome and that of a second, providing a direct measure of the abruptness of anaphase. In addition, we measured the rates and coordination of spindle elongation and chromosome segregation.
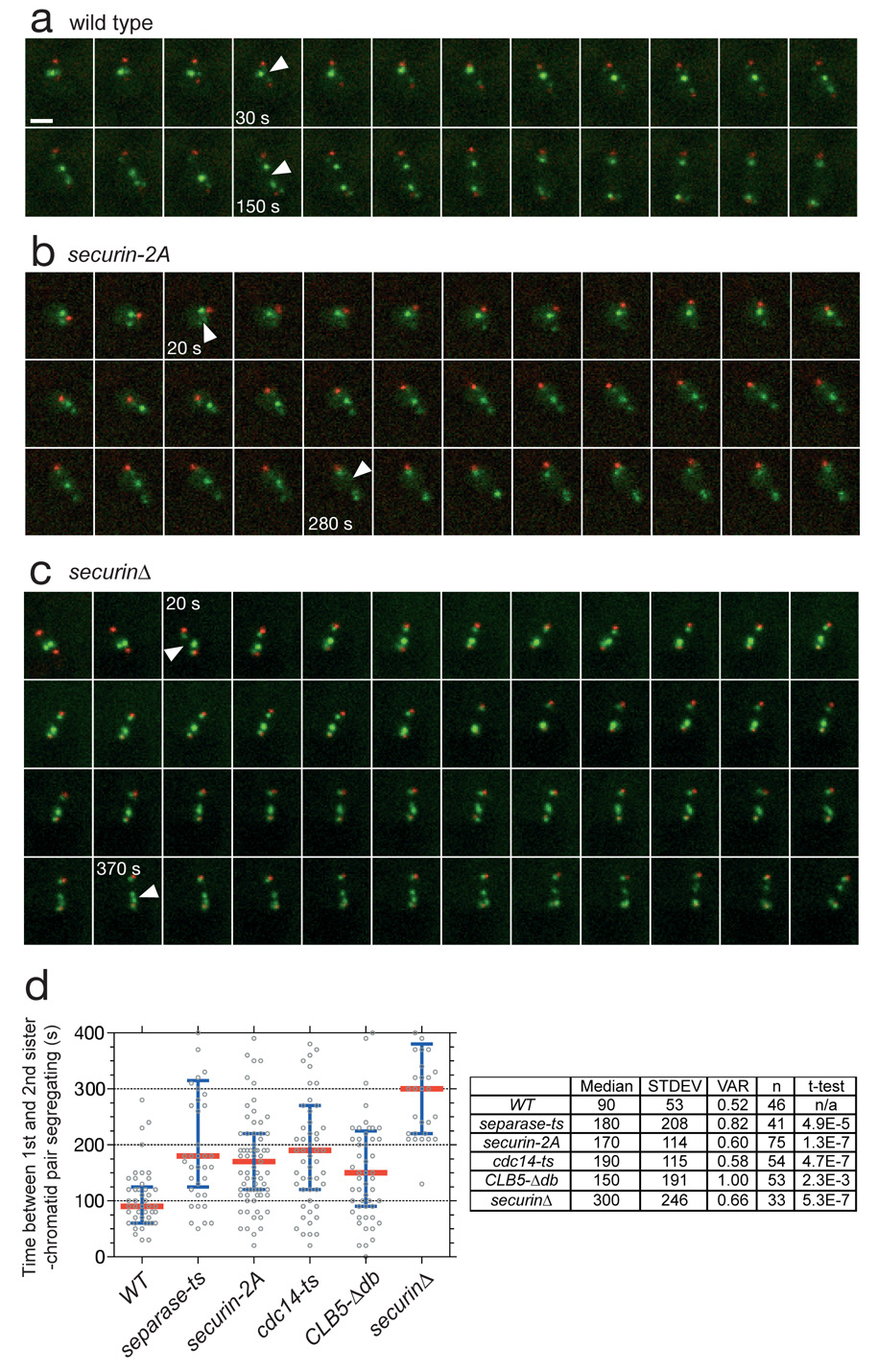
If there is any variation in the inherent sensitivity of each chromosome to separase activity – that is, if different chromatid pairs disjoin at different thresholds of separase activity – then the time lag between segregation of one pair of sister chromatids and another is a measure of the abruptness of separase activation (Suppl. Fig. 9). Indeed, we found that there was a reproducible time lag between the segregation of the two labeled chromosomes in our strain. In addition, the order of chromosome segregation was invariant – in 100% of cells, the bright dot on chromosome V segregated after the dot on chromosome IV. Quantification of 40 wild-type anaphases (Fig. 3a, d) revealed that the median time between the two chromosome segregation events (tseg) was 90 s, with most cells clustered tightly around this value. This abrupt timing of anaphase depends on rapid activation of separase – a mutant with reduced separase function (esp1-1) displayed a less synchronous anaphase (median tseg = 180 s).
Figure 3. Modulation of securin ubiquitination by Cdk1 is required for an abrupt anaphase.
a, A strain containing SPC42-dsRed:NAT; trp1∷256xLACO:TRP1; his3∷CUP1-GFP-LACI:HIS3; ura3∷112xTETO:URA3; leu2∷TETR-GFP:LEU2 has red spindle poles and GFP dots marking chromosomes IV and V. Each panel in this montage represents 10 s in a time-lapse sequence during progression through anaphase. Arrowheads indicate the 1st and 2nd chromosome separation. Each panel is a maximum intensity projection of a 7 µm stack. Note that the daughter spindle pole can be difficult to visualize due to slow maturation of the dsRed fluorophore. Scale bar is 2 µM. b, Anaphase onset in a securin-2A strain. c, Anaphase onset in a securinΔ strain. d, Anaphase synchrony (time between the 1st and 2nd chromosomes segregating) in approx. 40 cells of wild-type, separase mutant (esp1-1), securin-2A, cdc14-1, CLB5-Δdb, and securinΔ strains. The time of sister-chromatid separation was defined as the first time point when chromatids separated and did not reanneal in subsequent frames. The red line is the median and the blue bars indicate the inter-quartile range. The table at right shows the median, standard deviation and variance (standard deviation divided by the mean) for each strain. The movies corresponding to panels a–c are found in Supplementary Movies 1– 3, respectively.
If the positive feedback loop proposed in Fig. 2b is operating in vivo, then disruption of this loop should decrease the abruptness of anaphase. Indeed, the securin-2A mutant strain had a tseg of 170 s, almost double that of wild-type cells (Fig. 3b, d).
Our model (Fig. 2b) predicts that positive feedback should be weakened by reducing Cdc14 activity or increasing Cdk1 activity. Consistent with this possibility, we observed less anaphase synchrony in cdc14-1 mutant cells (tseg = 190 s) and in cells expressing Clb5-Δdb, a partially stabilized cyclin mutant that lacks its destruction-box and thus generates high levels of Cdk1 activity in anaphase (tseg = 150 s) 21. Defects in Cdc14 or Clb5 affect numerous processes in addition to securin degradation. Nevertheless, when taken together with the effects of the securin-2A mutant, these results support the hypothesis that phosphorylation of the N-terminal Cdk1 sites of securin creates a positive feedback loop that makes anaphase more switch-like.
We also analyzed cells carrying a deletion of securin, the loss of which reduces separase activity and partially uncouples anaphase initiation from APC activity. Securin deletion dramatically reduced the synchrony of sister separation (tseg = 300 s; Fig. 3c, d), arguing that a major role for securin is to increase the abruptness of the metaphase-to-anaphase transition.
A second possible function for phosphoregulation of securin is the coordination of separase activation with changes in anaphase spindle behavior. Following APCCdc20 activation, partial cyclin destruction, together with Cdc14 activation by separase, results in dephosphorylation of a subset of Cdk1 substrates that trigger important changes in the behavior of spindle and kinetochore proteins 4,22–25. These changes must be coordinated with the cleavage of cohesin. We propose that this coordination depends in part on phosphoregulation of securin, which we predict delays securin degradation relative to that of cyclins, allowing more robust dephosphorylation of Cdk1 substrates by the time cohesin cleavage begins. We also hypothesize that switch-like activation of separase generates switch-like activation of Cdc14, resulting in robust and coordinated dephosphorylation of the many factors controlling anaphase spindle behavior.
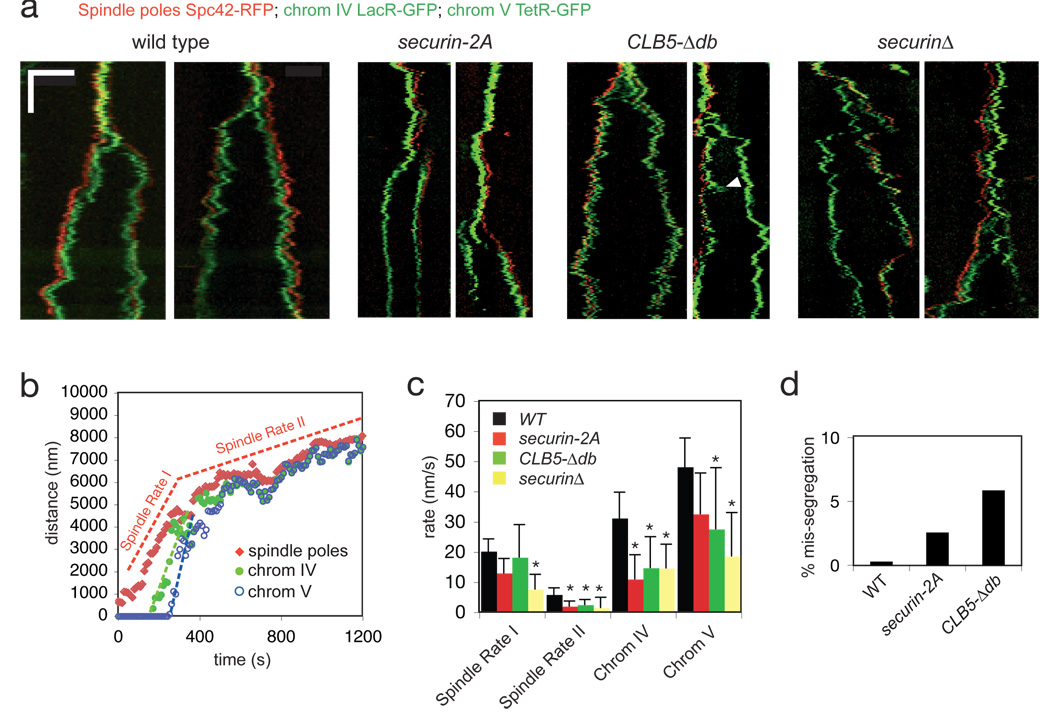
We therefore investigated anaphase dynamics in wild-type cells and cells with perturbed securin regulation. In wild-type cells (Fig. 4a), anaphase was robust and stereotypical, as illustrated by the spindle pole and chromosome tracking data in Fig. 4b. Each pair of sister chromatids segregated rapidly and unidirectionally (quantified in Fig. 4c), and the spindle elongated robustly in two phases: a rapid initial phase (spindle rate I) and a slower second phase (spindle rate II), as described previously 19. Spindle elongation initiated at the same time as chromosome disjunction (Fig. 4a), illustrating the tight temporal coordination of cohesin cleavage with changes in spindle dynamics.
Figure 4. Modulation of securin ubiquitination by Cdk1 helps coordinate anaphase onset with changes in spindle dynamics.
a, Representative kymographs from wild-type and mutant cells. The x-axis is a one dimensional projection along the spindle axis (scale bar is 5 microns) and the y-axis is time (scale bar is 5 min, total time is 30 min). b, Measurements of spindle length (red diamonds) and chromosome segregation (green dots, chromosome IV; blue circles, chromosome V) in a wild-type anaphase, using a spot-tracking algorithm 30. Rates of the two phases of spindle elongation are highlighted by red dashed lines; initial rates of chromatid segregation are highlighted with green and blue dashed lines. When the daughter spindle pole was difficult to detect because of the slow maturation of the dsRed fluorophore, the position of the associated sister chromatid was used as a proxy for spindle pole position after anaphase A. c, Average rates of spindle elongation and sister-chromatid separation in wild-type and mutant cells. Error bars indicate standard deviation (n = 10 cells). Asterisks indicate a t-test P-value of <0.05 when comparing the wild type to the mutant. d, Rate of chromosome mis-segregation in wild-type and mutant cells. Only cells with more than the expected number of GFP dots were scored as a mis-segregation event.
We next analyzed anaphase in the securin-2A strain, in which we predict the normal delay of separase activation is lost, resulting in cohesin cleavage early relative to dephosphorylation of Cdk1 substrates. We also analyzed the effects of the CLB5-Δdb mutation, which is likewise predicted to introduce a delay in the dephosphorylation of Cdk1 substrates relative to separase activation. Finally, we analyzed securinΔ cells, where separase activation is less tightly linked to changes in Cdk1 activity. We observed a wide range of anaphase defects in all mutants (Fig. 4). In a subset of securin-2A cells, the disjunction of chromatids was not coordinated with elongation of the spindle, chromosomes failed to segregate processively, and the rates of chromatid separation at anaphase A were significantly reduced (Fig. 4a, c). Spindle defects were more pronounced in CLB5-Δdb and securinΔ cells, in which the spindle frequently failed to elongate or elongated and then collapsed back to a shorter length (Fig. 4a). Our results are consistent with a general role for securin in the coordination of chromosome disjunction with changes in the phosphorylation state of Cdk1 substrates on the spindle and kinetochores. They also support the specific hypothesis that modulation of securin ubiquitination by phosphorylation helps coordinate the events of anaphase.
We assessed the accuracy of anaphase in our GFP-labeled yeast strain. Each cell in this strain is expected to have two GFP dots before anaphase, and cells containing more than two dots are products of a mis-segregation event. We found that loss of phosphoregulation of securin in the securin-2A mutant led to an elevated rate of chromosome mis-segregation, as did inappropriate Cdk1 activity in the CLB5-Δdb mutant (Fig. 4d).
We conclude that positive feedback in securin regulation promotes accurate chromosome segregation. Positive feedback could generate bistability in anaphase control 17,18, ensuring that the cell is irreversibly committed to complete sister-chromatid separation. Synchronous sister separation may have several benefits: for example, if disjunction of the first chromatid pair is sensed by the spindle assembly checkpoint, then rapid separation of the remaining sisters would reduce the danger of inappropriate checkpoint activation before anaphase is complete. In addition, the initiation of chromosome segregation might cause local perturbations to the spindle, making it important to transition rapidly to the segregation mode of spindle function to avoid mechanical catastrophe.
Our studies support the possibility that each sister chromatid pair separates at a distinct threshold of separase activity (Suppl. Fig. 9). Such differences in separase sensitivity would help explain the observation that sister chromatids separate in a specific order, as we observed in yeast and as previously observed in plant and animal cells 26,27. The ordered segregation of chromatids provides a mechanism to maintain nuclear architecture through successive generations 27. However, the existence of distinct separase thresholds provides a challenge for the rapid execution of anaphase. The most effective mechanism for achieving chromosome segregation that is both ordered and abrupt is to make separase activation rapid and switch-like.
Methods
Yeast Strains
Saccharomyces cerevisiae strains were derivatives of a W303 diploid strain containing: SPC42-dsRed:NAT; trp1∷256xLACO:TRP1/trp1-1; his3∷CUP1-GFP-LACI:HIS3/his3-11; ura3∷112xTETO:URA3/ura3-1; leu2∷TETR-GFP:LEU2/leu2-1. This strain was created by mating strain LH557 (Mat a; trp1∷256xLACO:TRP1; his3∷CUP1-GFP-LACI:HIS3; SPC42-dsRed-NAT) with LH554 (Mat alpha; leu2∷TETR-GFP:LEU2; ura3∷112xTETO:URA3; SPC42-dsRed-NAT).
Protein Purification
Securin-3FLAG-6HIS was purified by the tandem affinity method 9. APC, Ubc4, Uba1 and Cdh1 were purified as described 28. Clb2 was expressed from a 2μ PGAL1-TAP plasmid, and Clb2-Cdk1 complexes were purified using a C-terminal TAP tag on Clb2 29. GST-Cdc14 was purified from bacteria 15. Securin and Cdc20 were expressed in the TNT reticulocyte lysate coupled transcription/translation system (Promega) with a C- and N-terminal TEV-ZZ tag, respectively, purified rapidly on M-270 Epoxy dynabeads (Invitrogen) coupled to IgG, and eluted with TEV protease.
Mass Spectrometry
Identification of phosphorylation sites was performed using a modular mass spectrometric tool as described 9.
Ubiquitination assays
Ubiquitination assays were performed as described 28, except where securin was pre-incubated with Cdk1-Clb2 in a 10 µl reaction volume containing 25 mM Hepes pH 7.4, 150 mM NaCl, 10% glycerol, 0.1 mg ml−1 BSA, 1 mM ATP, and 10 mM MgCl2 for 20 min at room temperature. Kinase reactions were terminated by 5 min incubation with 50 mM EDTA. In some cases, Cdc14 was then added to this mixture for a further 20 min at room temperature prior to ubiquitination assays.
Microscopy
All microscopy was carried out in the UCSF Nikon Imaging Center using a TE2000U Inverted Microscope (Nikon) with Yokogawa CSU22 Spinning Disk Confocal illumination (Solamere Technology Group) and a Cascade II CCD Camera (Photometrics). Stacks of 7 images 1 micron apart were acquired every 10 s for 30 min at 25°C. Images were acquired using micro-manager software (http://micro-manager.org/) and analyzed using ImageJ (http://rsb.info.nih.gov/ij/) with the SpotTracker2D plugin (http://bigwww.epfl.ch/sage/soft/spottracker/gasser.html).
Supplementary Material
Acknowledgements
We thank P. H. O’Farrell, A. D. Johnson and M. J. Sullivan for thoughtful discussions; J. A. Ubersax, G. Goshima and O. Cohen-Fix for reagents; S. Foster, M. C. Rodrigo-Brenni, M. Enquist-Newman and the Morgan lab for help generating strains and reagents; J. M. Pedraza & A. van Oudenaarden for help with the model; K. S. Thorn and the UCSF Nikon Imaging Center for help with microscopy, and M. J. Sullivan and J. L. Feldman for critical reading of the manuscript. This work was supported by funding from the National Institute of General Medical Sciences (GM50684; D.O.M.), a grant from the Sandler Family Foundation (A.N.K.) and a fellowship from the National Science Foundation (L.J.H.).
References
- 1.Nasmyth K. Segregating sister genomes: the molecular biology of chromosome separation. Science. 2002;297:559–565. doi: 10.1126/science.1074757. [DOI] [PubMed] [Google Scholar]
- 2.Peters JM. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat Rev Mol Cell Biol. 2006;7:644–656. doi: 10.1038/nrm1988. [DOI] [PubMed] [Google Scholar]
- 3.Thornton BR, Toczyski DP. Precise destruction: an emerging picture of the APC. Genes Dev. 2006;20:3069–3078. doi: 10.1101/gad.1478306. [DOI] [PubMed] [Google Scholar]
- 4.Sullivan M, Morgan DO. Finishing mitosis, one step at a time. Nat Rev Mol Cell Biol. 2007;8:894–903. doi: 10.1038/nrm2276. [DOI] [PubMed] [Google Scholar]
- 5.Stegmeier F, Visintin R, Amon A. Separase, polo kinase, the kinetochore protein Slk19, and Spo12 function in a network that controls Cdc14 localization during early anaphase. Cell. 2002;108:207–220. doi: 10.1016/s0092-8674(02)00618-9. [DOI] [PubMed] [Google Scholar]
- 6.Queralt E, Lehane C, Novak B, Uhlmann F. Downregulation of PP2A(Cdc55) phosphatase by separase initiates mitotic exit in budding yeast. Cell. 2006;125:719–732. doi: 10.1016/j.cell.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 7.Agarwal R, Tang Z, Yu H, Cohen-Fix O. Two distinct pathways for inhibiting pds1 ubiquitination in response to DNA damage. J Biol Chem. 2003;278:45027–45033. doi: 10.1074/jbc.M306783200. [DOI] [PubMed] [Google Scholar]
- 8.Wang H, et al. Pds1 phosphorylation in response to DNA damage is essential for its DNA damage checkpoint function. Genes Dev. 2001;15:1361–1372. doi: 10.1101/gad.893201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blethrow JD, Tang C, Deng C, Krutchinsky AN. Modular mass spectrometric tool for analysis of composition and phosphorylation of protein complexes. PLoS ONE. 2007;2:e358. doi: 10.1371/journal.pone.0000358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hornig NC, Knowles PP, McDonald NQ, Uhlmann F. The dual mechanism of separase regulation by securin. Curr Biol. 2002;12:973–982. doi: 10.1016/s0960-9822(02)00847-3. [DOI] [PubMed] [Google Scholar]
- 11.Agarwal R, Cohen-Fix O. Phosphorylation of the mitotic regulator Pds1/securin by Cdc28 is required for efficient nuclear localization of Esp1/separase. Genes Dev. 2002;16:1371–1382. doi: 10.1101/gad.971402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.King RW, Glotzer M, Kirschner MW. Mutagenic analysis of the destruction signal of mitotic cyclins and structural characterization of ubiquitinated intermediates. Mol. Biol. Cell. 1996;7:1343–1357. doi: 10.1091/mbc.7.9.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wan J, Xu H, Grunstein M. CDC14 of Saccharomyces cerevisiae. J. Biol. Chem. 1992;267:11274–11280. [PubMed] [Google Scholar]
- 14.Visintin R, et al. The phosphatase Cdc14 triggers mitotic exit by reversal of Cdk-dependent phosphorylation. Mol. Cell. 1998;2:709–718. doi: 10.1016/s1097-2765(00)80286-5. [DOI] [PubMed] [Google Scholar]
- 15.Jaspersen SL, Charles JF, Morgan DO. Inhibitory phosphorylation of the APC regulator Hct1 is controlled by the kinase Cdc28 and the phosphatase Cdc14. Curr. Biol. 1999;9:227–236. doi: 10.1016/s0960-9822(99)80111-0. [DOI] [PubMed] [Google Scholar]
- 16.Stegmeier F, Amon A. Closing mitosis: the functions of the Cdc14 phosphatase and its regulation. Annu Rev Genet. 2004;38:203–232. doi: 10.1146/annurev.genet.38.072902.093051. [DOI] [PubMed] [Google Scholar]
- 17.Ferrell JE., Jr Self-perpetuating states in signal transduction: positive feedback, double-negative feedback and bistability. Curr Opin Cell Biol. 2002;14:140–148. doi: 10.1016/s0955-0674(02)00314-9. [DOI] [PubMed] [Google Scholar]
- 18.Tyson JJ, Chen KC, Novak B. Sniffers, buzzers, toggles and blinkers: dynamics of regulatory and signaling pathways in the cell. Curr Opin Cell Biol. 2003;15:221–231. doi: 10.1016/s0955-0674(03)00017-6. [DOI] [PubMed] [Google Scholar]
- 19.Straight AF, Marshall WF, Sedat JW, Murray AW. Mitosis in living budding yeast: anaphase A but no metaphase plate. Science. 1997;277:574–578. doi: 10.1126/science.277.5325.574. [DOI] [PubMed] [Google Scholar]
- 20.Michaelis C, Ciosk R, Nasmyth K. Cohesins: chromosomal proteins that prevent premature separation of sister chromatids. Cell. 1997;91:35–45. doi: 10.1016/s0092-8674(01)80007-6. [DOI] [PubMed] [Google Scholar]
- 21.Wäsch R, Cross F. APC-dependent proteolysis of the mitotic cyclin Clb2 is essential for mitotic exit. Nature. 2002;418:556–562. doi: 10.1038/nature00856. [DOI] [PubMed] [Google Scholar]
- 22.Higuchi T, Uhlmann F. Stabilization of microtubule dynamics at anaphase onset promotes chromosome segregation. Nature. 2005;433:171–176. doi: 10.1038/nature03240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pereira G, Schiebel E. Separase regulates INCENP-Aurora B anaphase spindle function through Cdc14. Science. 2003;302:2120–2124. doi: 10.1126/science.1091936. [DOI] [PubMed] [Google Scholar]
- 24.Woodbury EL, Morgan DO. Cdk and APC activities limit the spindle-stabilizing function of Fin1 to anaphase. Nat Cell Biol. 2007;9:106–112. doi: 10.1038/ncb1523. [DOI] [PubMed] [Google Scholar]
- 25.Parry DH, Hickson GR, O'Farrell PH. Cyclin B destruction triggers changes in kinetochore behavior essential for successful anaphase. Curr Biol. 2003;13:647–653. doi: 10.1016/s0960-9822(03)00242-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vig BK. Sequence of centromere separation: occurrence, possible significance, and control. Cancer Genet Cytogenet. 1983;8:249–274. doi: 10.1016/0165-4608(83)90142-5. [DOI] [PubMed] [Google Scholar]
- 27.Gerlich D, et al. Global chromosome positions are transmitted through mitosis in mammalian cells. Cell. 2003;112:751–764. doi: 10.1016/s0092-8674(03)00189-2. [DOI] [PubMed] [Google Scholar]
- 28.Carroll CW, Morgan DO. Enzymology of the Anaphase-Promoting Complex. Meth. Enzymol. 2005;398:219–230. doi: 10.1016/S0076-6879(05)98018-X. [DOI] [PubMed] [Google Scholar]
- 29.Puig O, et al. The tandem affinity purification (TAP) method: a general procedure of protein complex purification. Methods. 2001;24:218–229. doi: 10.1006/meth.2001.1183. [DOI] [PubMed] [Google Scholar]
- 30.Sage D, et al. Automatic tracking of individual fluorescence particles: application to the study of chromosome dynamics. IEEE Trans Image Process. 2005;14:1372–1383. doi: 10.1109/tip.2005.852787. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.