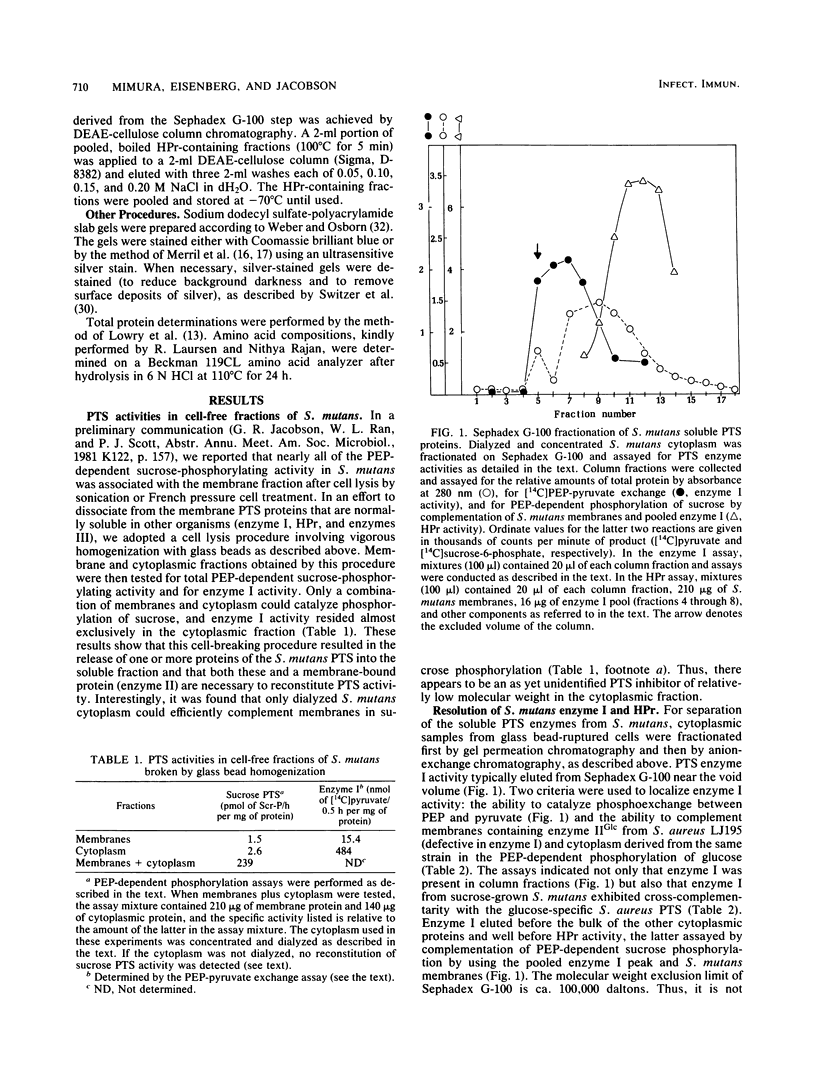
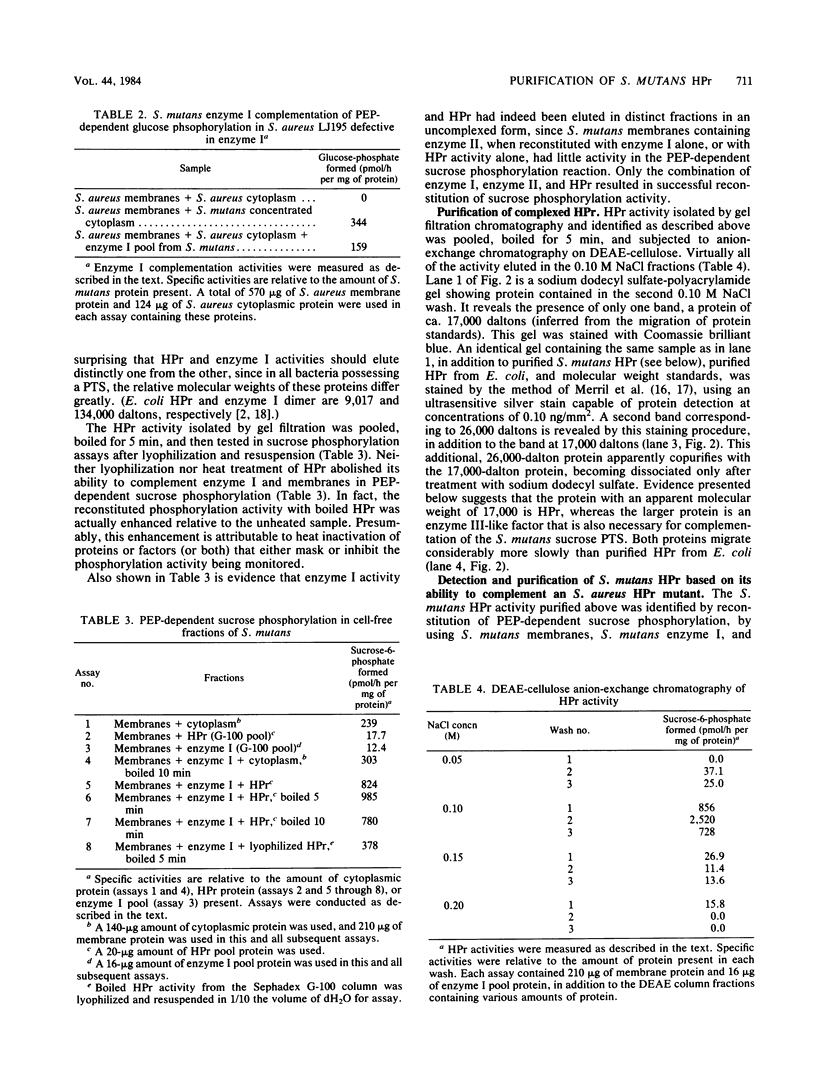
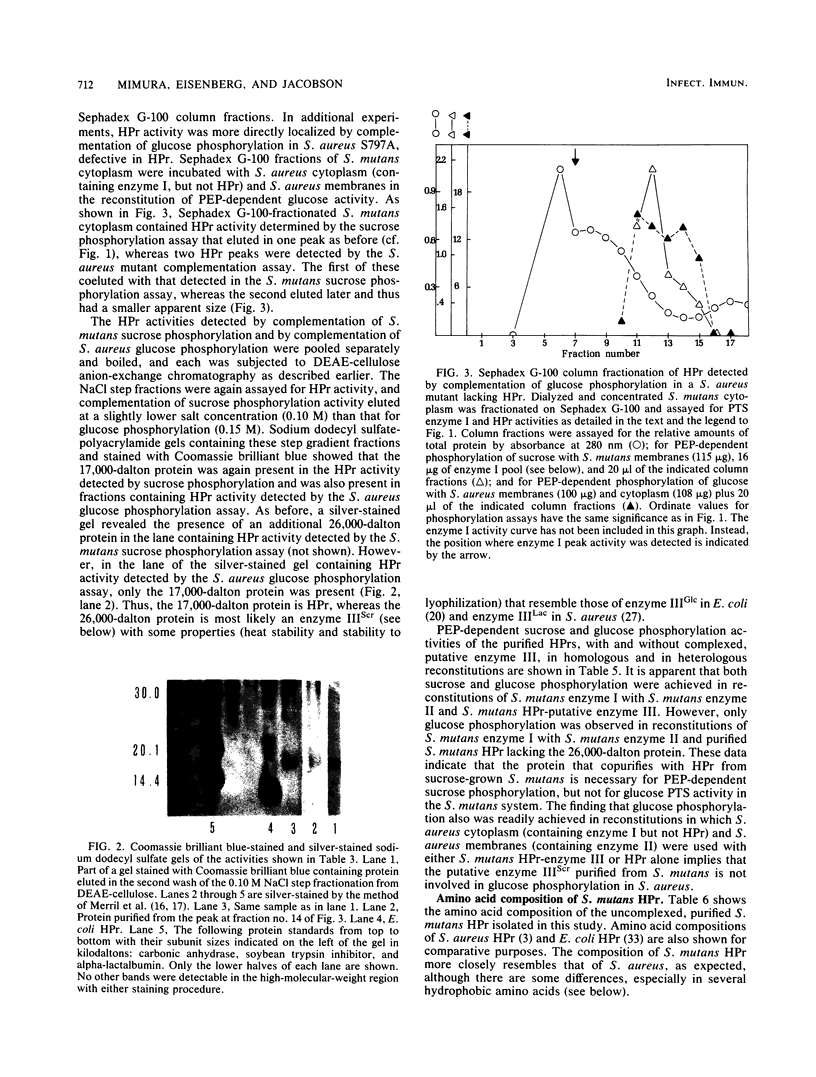
Abstract
The sucrose phosphotransferase system of Streptococcus mutans catalyzes the phosphorylation of sucrose to sucrose-6-phosphate with concomitant translocation of this disaccharide across the cytoplasmic membrane in reactions requiring intracellular phosphoenolpyruvate. Soluble proteins released by vigorous homogenization of cells with glass beads are shown to be necessary for the phosphoenolpyruvate-dependent phosphorylation of sucrose in combination with one or more proteins that remain tightly associated with the membrane fraction. We have partially purified phosphotransferase enzyme I and have purified a heat-stable phosphocarrier protein (HPr) to apparent homogeneity, by gel filtration and ion-exchange chromatography from the soluble fraction. HPr from S. mutans has an apparent molecular weight larger than that of Escherichia coli HPr but has properties similar to those of Staphylococcus aureus HPr. Furthermore, it appears to be partially complexed with a heat-stable enzyme III-like protein in cell-free fractions from S. mutans, and we also report the purification of this complex. Enzyme I from S. mutans is a protein (native Mr greater than 100,000) that cross-complements enzyme I from S. aureus. Preliminary characterizations of homogeneous HPr and its complex with the putative enzyme III are also presented.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson B., Weigel N., Kundig W., Roseman S. Sugar transport. 3. Purification and properties of a phosphocarrier protein (HPr) of the phosphoenolpyruvate-dependent phosphotransferase system of Escherichia coli. J Biol Chem. 1971 Nov 25;246(22):7023–7033. [PubMed] [Google Scholar]
- Beneski D. A., Nakazawa A., Weigel N., Hartman P. E., Roseman S. Sugar transport by the bacterial phosphotransferase system. Isolation and characterization of a phosphocarrier protein HPr from wild type and mutants of Salmonella typhimurium. J Biol Chem. 1982 Dec 10;257(23):14492–14498. [PubMed] [Google Scholar]
- Beyreuther K., Raufuss H., Schrecker O., Hengstenberg W. The phosphoenolpyruvate-dependent phosphotransferase system of Staphylococcus aureus. 1. Amino-acid sequence of the phosphocarrier protein HPr. Eur J Biochem. 1977 May 2;75(1):275–286. doi: 10.1111/j.1432-1033.1977.tb11527.x. [DOI] [PubMed] [Google Scholar]
- Chassy B. M. A gentle method for the lysis of oral streptococci. Biochem Biophys Res Commun. 1976 Jan 26;68(2):603–608. doi: 10.1016/0006-291x(76)91188-8. [DOI] [PubMed] [Google Scholar]
- Dills S. S., Apperson A., Schmidt M. R., Saier M. H., Jr Carbohydrate transport in bacteria. Microbiol Rev. 1980 Sep;44(3):385–418. doi: 10.1128/mr.44.3.385-418.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooijewaard G., Roossien F. F., Robillard G. T. Escherichia coli phosphoenolpyruvate dependent phosphotransferase system. NMR studies of the conformation of HPr and P-HPr and the mechanism of energy coupling. Biochemistry. 1979 Jul 10;18(14):2996–3001. doi: 10.1021/bi00581a014. [DOI] [PubMed] [Google Scholar]
- Hamada S., Slade H. D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980 Jun;44(2):331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson G. R., Kelly D. M., Finlay D. R. The intramembrane topography of the mannitol-specific enzyme II of the Escherichia coli phosphotransferase system. J Biol Chem. 1983 Mar 10;258(5):2955–2959. [PubMed] [Google Scholar]
- Jacobson G. R., Lee C. A., Saier M. H., Jr Purification of the mannitol-specific enzyme II of the Escherichia coli phosphoenolpyruvate:sugar phosphotransferase system. J Biol Chem. 1979 Jan 25;254(2):249–252. [PubMed] [Google Scholar]
- Kalbitzer H. R., Deutscher J., Hengstenberg W., Rösch P. Phosphoenolpyruvate-dependent phosphotransferase system of Staphylococcus aureus: 1H nuclear magnetic resonance studies on phosphorylated and unphosphorylated factor IIIlac and its interaction with the phosphocarrier protein HPr. Biochemistry. 1981 Oct 13;20(21):6178–6185. doi: 10.1021/bi00524a041. [DOI] [PubMed] [Google Scholar]
- Kalbitzer H. R., Hengstenberg W., Rösch P., Muss P., Bernsmann P., Engelmann R., Dörschug M., Deutscher J. HPr proteins of different microorganisms studied by hydrogen-1 high-resolution nuclear magnetic resonance: similarities of structures and mechanisms. Biochemistry. 1982 Jun 8;21(12):2879–2885. doi: 10.1021/bi00541a012. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lengeler J. W., Mayer R. J., Schmid K. Phosphoenolpyruvate-dependent phosphotransferase system enzyme III and plasmid-encoded sucrose transport in Escherichia coli K-12. J Bacteriol. 1982 Jul;151(1):468–471. doi: 10.1128/jb.151.1.468-471.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maryanski J. H., Wittenberger C. L. Mannitol transport in Streptococcus mutans. J Bacteriol. 1975 Dec;124(3):1475–1481. doi: 10.1128/jb.124.3.1475-1481.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer W., Rüterjans H., Schrecker O., Hengstenberg W., Gassner M., Stehlik D. The phosphoenolpyruvate-dependent phosphotransferase system of Staphylococcus aureus. 3. 1H and 31P nuclear-magnetic-resonance studies on the phosphocarrier protein HPr; tyrosine titration and denaturation studies. Eur J Biochem. 1977 May 2;75(1):297–301. doi: 10.1111/j.1432-1033.1977.tb11529.x. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Switzer R. C., Van Keuren M. L. Trace polypeptides in cellular extracts and human body fluids detected by two-dimensional electrophoresis and a highly sensitive silver stain. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4335–4339. doi: 10.1073/pnas.76.9.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robillard G. T. The enzymology of the bacterial phosphoenolpyruvate-dependent sugar transport systems. Mol Cell Biochem. 1982 Jul 7;46(1):3–24. doi: 10.1007/BF00215577. [DOI] [PubMed] [Google Scholar]
- Rösch P., Kalbitzer H. R., Schmidt-Aderjan U., Hengstenberg W. 1H nuclear magnetic resonance studies on the structure and mechanism of the HPr protein of Staphylococcus aureus. Biochemistry. 1981 Mar 17;20(6):1599–1605. doi: 10.1021/bi00509a029. [DOI] [PubMed] [Google Scholar]
- Saier M. H., Jr Bacterial phosphoenolpyruvate: sugar phosphotransferase systems: structural, functional, and evolutionary interrelationships. Bacteriol Rev. 1977 Dec;41(4):856–871. doi: 10.1128/br.41.4.856-871.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr, Cox D. F., Feucht B. U., Novotny M. J. Evidence for the functional association of enzyme I and HPr of the phosphoenolpyruvate-sugar phosphotransferase system with the membrane in sealed vesicles of Escherichia coli. J Cell Biochem. 1982;18(2):231–238. doi: 10.1002/jcb.1982.240180210. [DOI] [PubMed] [Google Scholar]
- Saier M. H., Jr, Schmidt M. R., Lin P. Phosphoryl exchange reaction catalyzed by enzyme I of the bacterial phosphoenolpyruvate: sugar phosphotransferase system. Kinetic characterization. J Biol Chem. 1980 Sep 25;255(18):8579–8584. [PubMed] [Google Scholar]
- Schachtele C. F. Glucose transport in Streptococcus mutans: preparation of cytoplasmic membranes and characteristics of phosphotransferase activity. J Dent Res. 1975 Mar-Apr;54(2):330–338. [PubMed] [Google Scholar]
- Schmid K., Schupfner M., Schmitt R. Plasmid-mediated uptake and metabolism of sucrose by Escherichia coli K-12. J Bacteriol. 1982 Jul;151(1):68–76. doi: 10.1128/jb.151.1.68-76.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Aderjan U., Rösch P., Frank R., Hengstenberg W. The phosphoenolpyruvate-dependent phosphotransferase system of Staphylococcus aureus. Complete tyrosine assignments in the 1H nuclear-magnetic-resonance spectrum of the phosphocarrier protein HPr. Eur J Biochem. 1979 May 2;96(1):43–48. doi: 10.1111/j.1432-1033.1979.tb13011.x. [DOI] [PubMed] [Google Scholar]
- Scholte B. J., Schuitema A. R., Postma P. W. Characterization of factor IIIGLc in catabolite repression-resistant (crr) mutants of Salmonella typhimurium. J Bacteriol. 1982 Feb;149(2):576–586. doi: 10.1128/jb.149.2.576-586.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoni R. D., Nakazawa T., Hays J. B., Roseman S. Sugar transport. IV. Isolation and characterization of the lactose phosphotransferase system in Staphylococcus aureus. J Biol Chem. 1973 Feb 10;248(3):932–940. [PubMed] [Google Scholar]
- Slee A. M., Tanzer J. M. Phosphoenolpyruvate-dependent sucrose phosphotransferase activity in Streptococcus mutans NCTC 10449. Infect Immun. 1979 Jun;24(3):821–828. doi: 10.1128/iai.24.3.821-828.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Martin E. J., Wittenberger C. L. Characterization of a phosphoenolpyruvate-dependent sucrose phosphotransferase system in Streptococcus mutans. Infect Immun. 1979 Jun;24(3):865–868. doi: 10.1128/iai.24.3.865-868.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switzer R. C., 3rd, Merril C. R., Shifrin S. A highly sensitive silver stain for detecting proteins and peptides in polyacrylamide gels. Anal Biochem. 1979 Sep 15;98(1):231–237. doi: 10.1016/0003-2697(79)90732-2. [DOI] [PubMed] [Google Scholar]
- Thompson J., Chassy B. M. Uptake and metabolism of sucrose by Streptococcus lactis. J Bacteriol. 1981 Aug;147(2):543–551. doi: 10.1128/jb.147.2.543-551.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weigel N., Powers D. A., Roseman S. Sugar transport by the bacterial phosphotransferase system. Primary structure and active site of a general phosphocarrier protein (HPr) from Salmonella typhimurium. J Biol Chem. 1982 Dec 10;257(23):14499–14509. [PubMed] [Google Scholar]