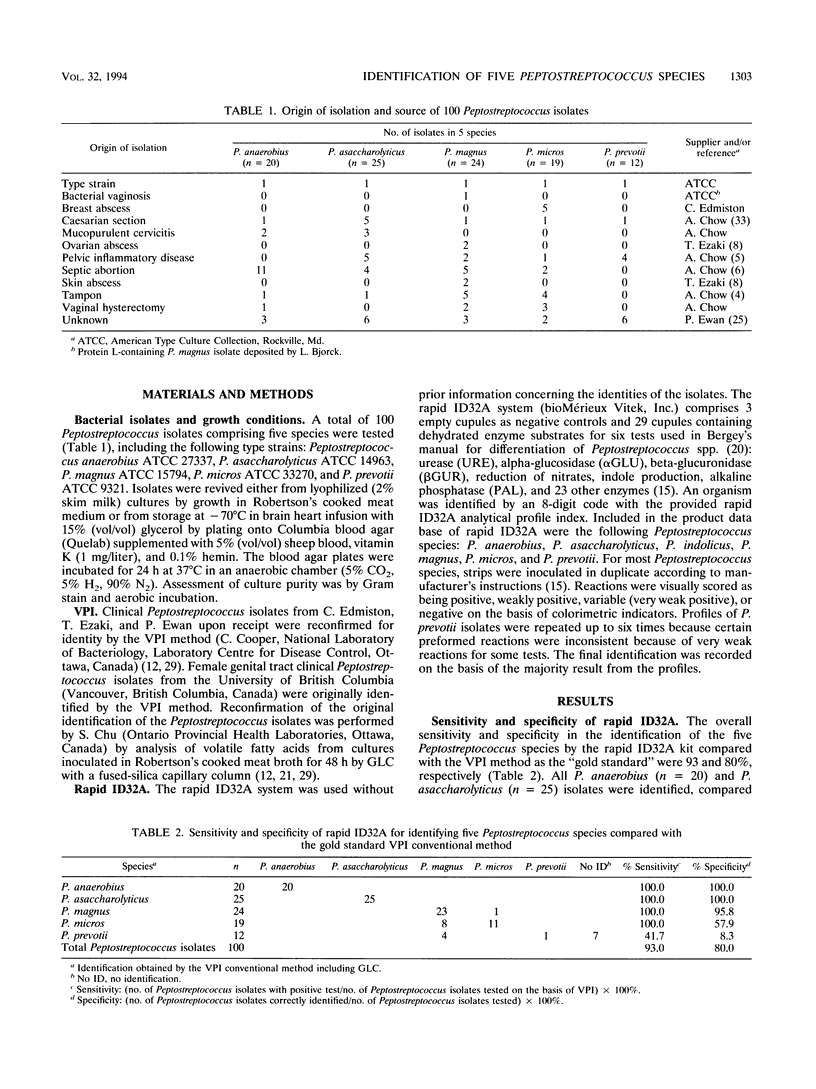
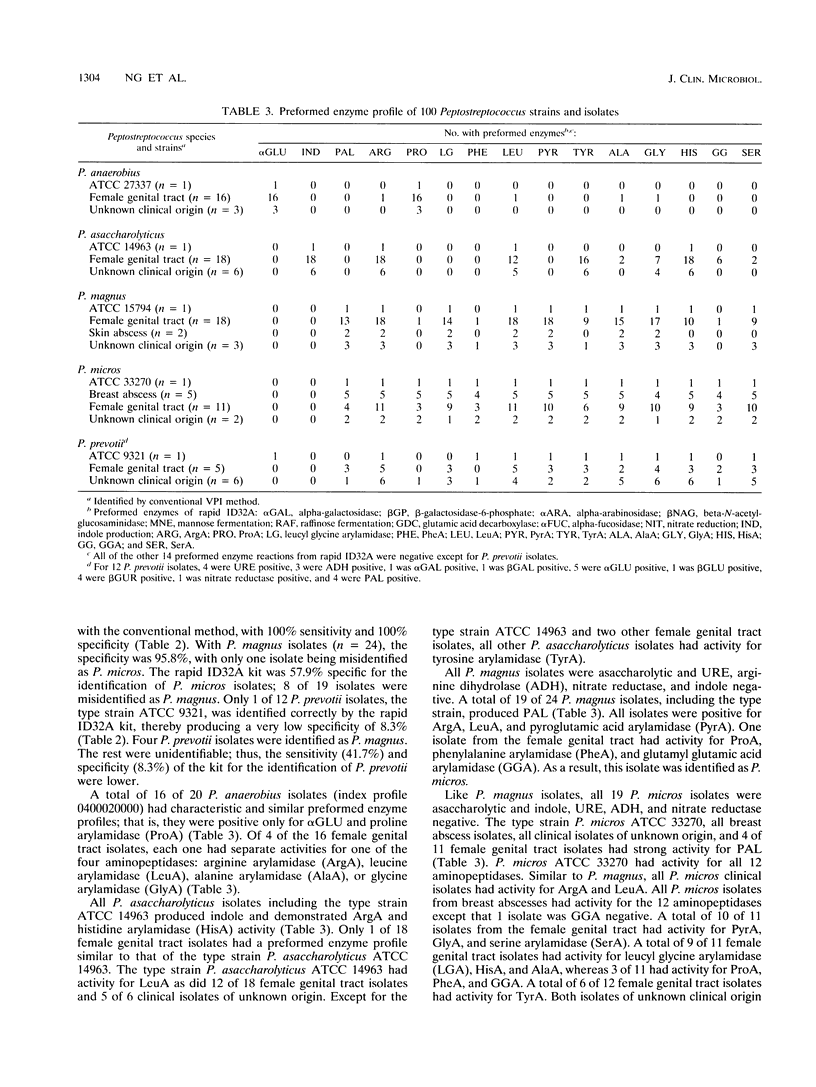
Abstract
The rapid ID32A kit (bioMérieux Vitek, Inc., Hazelwood, Mo.) was evaluated for its ability to identify Peptostreptococcus species compared with conventional biochemical tests and gas-liquid chromatography (Virginia Polytechnic Institute), the current "gold standard" method. A total of 5 Peptostreptococcus American Type Culture Collection strains and 95 clinical isolates comprising Peptostreptococcus anaerobius, P. asaccharolyticus, P. magnus, P. micros, and P. prevotii isolates were included for analysis. Overall, the sensitivity and specificity of the rapid ID32A kit in the identification for five Peptostreptococcus species compared with the Virginia Polytechnic Institute method were 93 and 80%, respectively. All P. anaerobius (n = 20) and P. asaccharolyticus (n = 25) isolates were identified with 100% sensitivity and 100% specificity. For the identification of P. magnus (n = 24) and P. micros (n = 19), the rapid ID32A kit was 100% sensitive for both species; the specificity for P. magnus was 95.8% and that for P. micros was 57.9%. The sensitivity and specificity of the rapid ID32A kit for identification of P. prevotii (n = 12) were poor (41.7 and 8.3%, respectively). The rapid ID32A kit is a useful method for the rapid differentiation of P. anaerobius and P. asaccharolyticus from other Peptostreptococcus spp. Conventional methods should be used to identify to the species level isolates of P. magnus, P. micros, and P. prevotii.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourgault A. M., Rosenblatt J. E., Fitzgerald R. H. Peptococcus magnus: a significant human pathogen. Ann Intern Med. 1980 Aug;93(2):244–248. doi: 10.7326/0003-4819-93-2-244. [DOI] [PubMed] [Google Scholar]
- Brook I. Bacterial synergy in pelvic inflammatory disease. Arch Gynecol Obstet. 1987;241(3):133–143. doi: 10.1007/BF00931309. [DOI] [PubMed] [Google Scholar]
- Brook I., Walker R. I. Pathogenicity of anaerobic gram-positive cocci. Infect Immun. 1984 Aug;45(2):320–324. doi: 10.1128/iai.45.2.320-324.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow A. W., Bartlett K. H. Sequential assessment of vaginal microflora in healthy women randomly assigned to tampon or napkin use. Rev Infect Dis. 1989 Jan-Feb;11 (Suppl 1):S68–S74. doi: 10.1093/clinids/11.supplement_1.s68. [DOI] [PubMed] [Google Scholar]
- Chow A. W., Malkasian K. L., Marshall J. R., Guze L. B. The bacteriology of acute pelvic inflammatory disease. Am J Obstet Gynecol. 1975 Aug 1;122(7):876–879. doi: 10.1016/0002-9378(75)90731-0. [DOI] [PubMed] [Google Scholar]
- Chow A. W., Marshall J. R., Guze L. B. A double-blind comparison of clindamycin with penicillin plus chloramphenicol in treatment of septic abortion. J Infect Dis. 1977 Mar;135 (Suppl):S35–S39. doi: 10.1093/infdis/135.supplement.s35. [DOI] [PubMed] [Google Scholar]
- Harpold D. J., Wasilauskas B. L. Rapid identification of obligately anaerobic gram-positive cocci using high-performance liquid chromatography. J Clin Microbiol. 1987 Jun;25(6):996–1001. doi: 10.1128/jcm.25.6.996-1001.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst E., Wathne B., Hovelius B., Mårdh P. A. Bacterial vaginosis: microbiological and clinical findings. Eur J Clin Microbiol. 1987 Oct;6(5):536–541. doi: 10.1007/BF02014242. [DOI] [PubMed] [Google Scholar]
- Kitch T. T., Appelbaum P. C. Accuracy and reproducibility of the 4-hour ATB 32A method for anaerobe identification. J Clin Microbiol. 1989 Nov;27(11):2509–2513. doi: 10.1128/jcm.27.11.2509-2513.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krepel C. J., Gohr C. M., Walker A. P., Farmer S. G., Edmiston C. E. Enzymatically active Peptostreptococcus magnus: association with site of infection. J Clin Microbiol. 1992 Sep;30(9):2330–2334. doi: 10.1128/jcm.30.9.2330-2334.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert M. A., Armfield A. Y. Differentiation of Peptococcus and Peptostreptococcus by gas-liquid chromatography of cellular fatty acids and metabolic products. J Clin Microbiol. 1979 Oct;10(4):464–476. doi: 10.1128/jcm.10.4.464-476.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N., Hashimoto Y., Adnan S., Miura H., Yamamoto H., Ezaki T. Three new species of the genus Peptostreptococcus isolated from humans: Peptostreptococcus vaginalis sp. nov., Peptostreptococcus lacrimalis sp. nov., and Peptostreptococcus lactolyticus sp. nov. Int J Syst Bacteriol. 1992 Oct;42(4):602–605. doi: 10.1099/00207713-42-4-602. [DOI] [PubMed] [Google Scholar]
- Mardh P. A., Westrom L. Factors that might influence the outcome of studies on the aetiology and epidemiology of acute pelvic inflammatory disease. Scand J Gastroenterol Suppl. 1984;91:73–86. [PubMed] [Google Scholar]
- Moss C. W., Nunez-Montiel O. L. Analysis of short-chain acids from bacteria by gas-liquid chromatography with a fused-silica capillary column. J Clin Microbiol. 1982 Feb;15(2):308–311. doi: 10.1128/jcm.15.2.308-311.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch D. A., Mitchelmore I. J., Nash R. A., Hardie J. M., Tabaqchali S. Preformed enzyme profiles of reference strains of gram-positive anaerobic cocci. J Med Microbiol. 1988 Sep;27(1):65–70. doi: 10.1099/00222615-27-1-65. [DOI] [PubMed] [Google Scholar]
- Murdoch D. A., Mitchelmore I. J., Tabaqchali S. Identification of gram-positive anaerobic cocci by use of systems for detecting pre-formed enzymes. J Med Microbiol. 1988 Apr;25(4):289–293. doi: 10.1099/00222615-25-4-289. [DOI] [PubMed] [Google Scholar]
- Murdoch D. A., Mitchelmore I. J. The laboratory identification of gram-positive anaerobic cocci. J Med Microbiol. 1991 May;34(5):295–308. doi: 10.1099/00222615-34-5-295. [DOI] [PubMed] [Google Scholar]
- Ng L. K., Dillon J. A. Molecular fingerprinting of isolates of the genus Peptostreptococcus using rRNA genes from Escherichia coli and P. anaerobius. J Gen Microbiol. 1991 Jun;137(6):1323–1331. doi: 10.1099/00221287-137-6-1323. [DOI] [PubMed] [Google Scholar]
- Rice P. A., Schachter J. Pathogenesis of pelvic inflammatory disease. What are the questions? JAMA. 1991 Nov 13;266(18):2587–2593. [PubMed] [Google Scholar]
- Smith G. L., Cumming C. G., Ross P. W. Analysis of EDTA-soluble cell surface components of gram-positive anaerobic cocci. J Gen Microbiol. 1986 Jun;132(6):1591–1597. doi: 10.1099/00221287-132-6-1591. [DOI] [PubMed] [Google Scholar]
- Smith G. L., Cumming C. G., Ross P. W. Immunochemical characterization of the cell surface carbohydrate antigens of Peptostreptococcus anaerobius. J Gen Microbiol. 1986 Feb;132(2):525–530. doi: 10.1099/00221287-132-2-525. [DOI] [PubMed] [Google Scholar]
- Taylor E. A., Jackman P. J., Phillips I. The differentiation of asaccharolytic anaerobic gram-positive cocci by protein electrophoresis. J Med Microbiol. 1991 Jun;34(6):339–348. doi: 10.1099/00222615-34-6-339. [DOI] [PubMed] [Google Scholar]
- Williams C. M., Okada D. M., Marshall J. R., Chow A. W. Clinical and microbiologic risk evaluation for post-cesarean section endometritis by multivariate discriminant analysis: role of intraoperative mycoplasma, aerobes, and anaerobes. Am J Obstet Gynecol. 1987 Apr;156(4):967–974. doi: 10.1016/0002-9378(87)90369-3. [DOI] [PubMed] [Google Scholar]
- van Winkelhoff A. J., Clement M., de Graaff J. Rapid characterization of oral and nonoral pigmented Bacteroides species with the ATB Anaerobes ID system. J Clin Microbiol. 1988 May;26(5):1063–1065. doi: 10.1128/jcm.26.5.1063-1065.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]


