Abstract
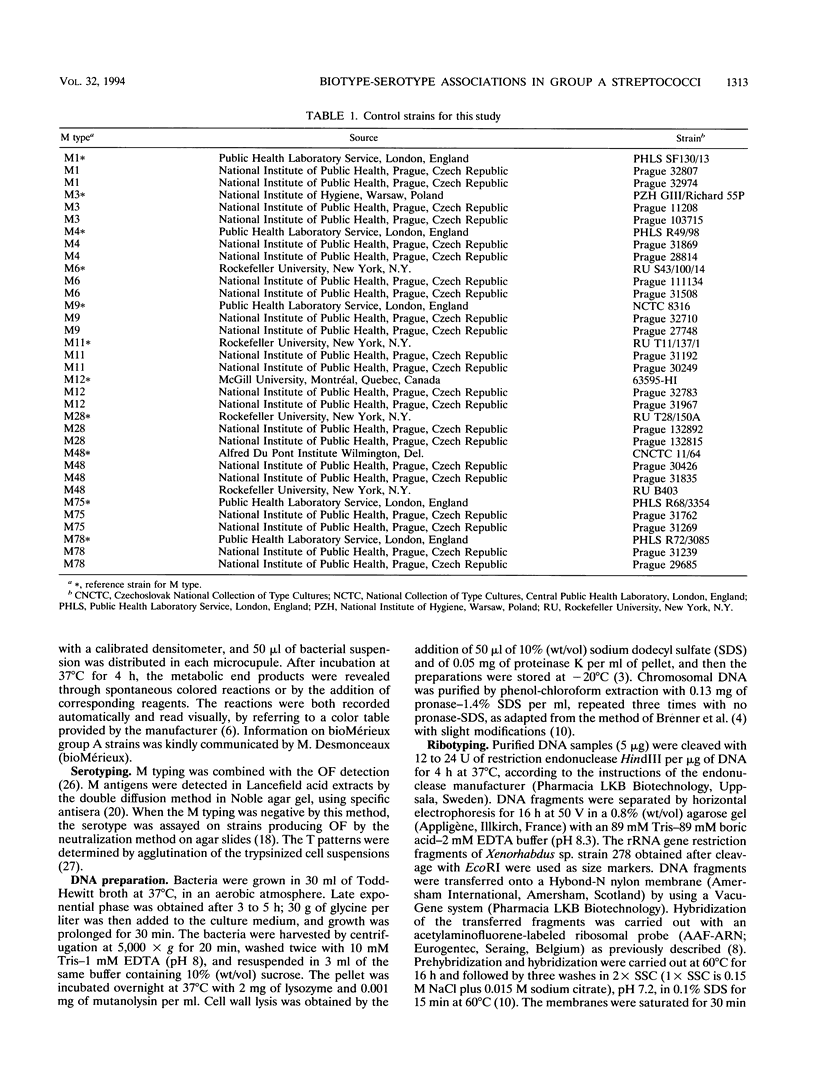
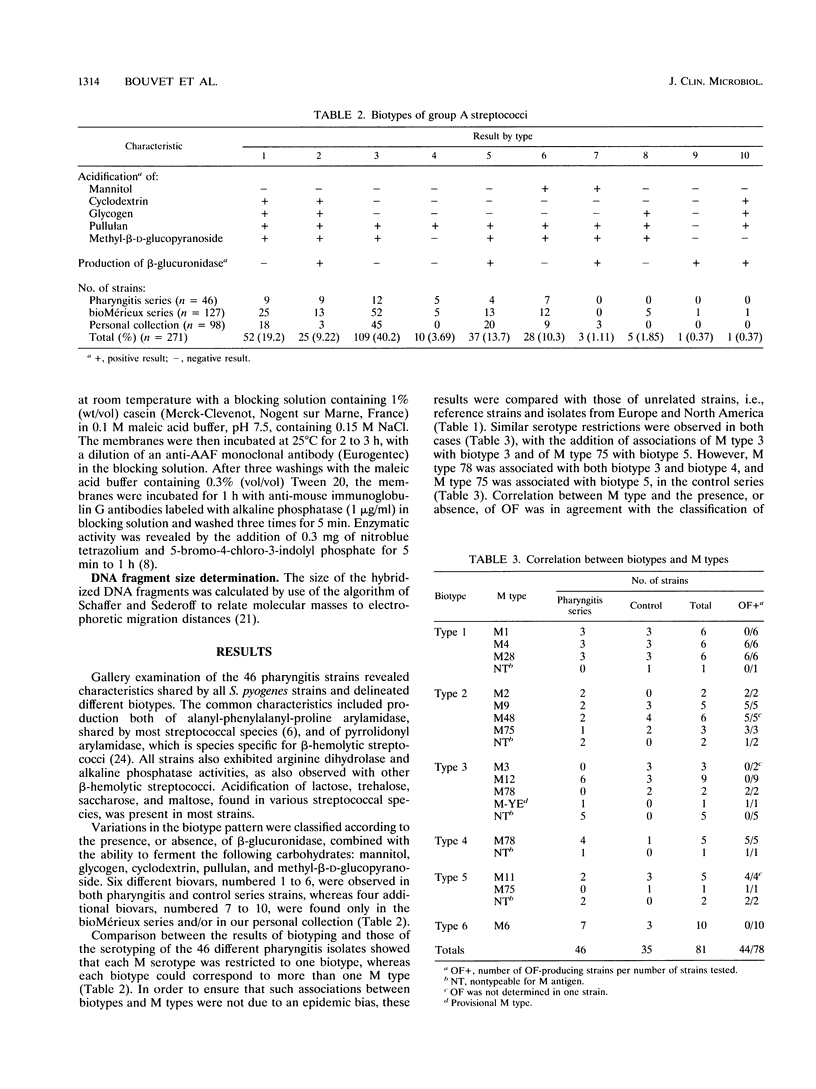
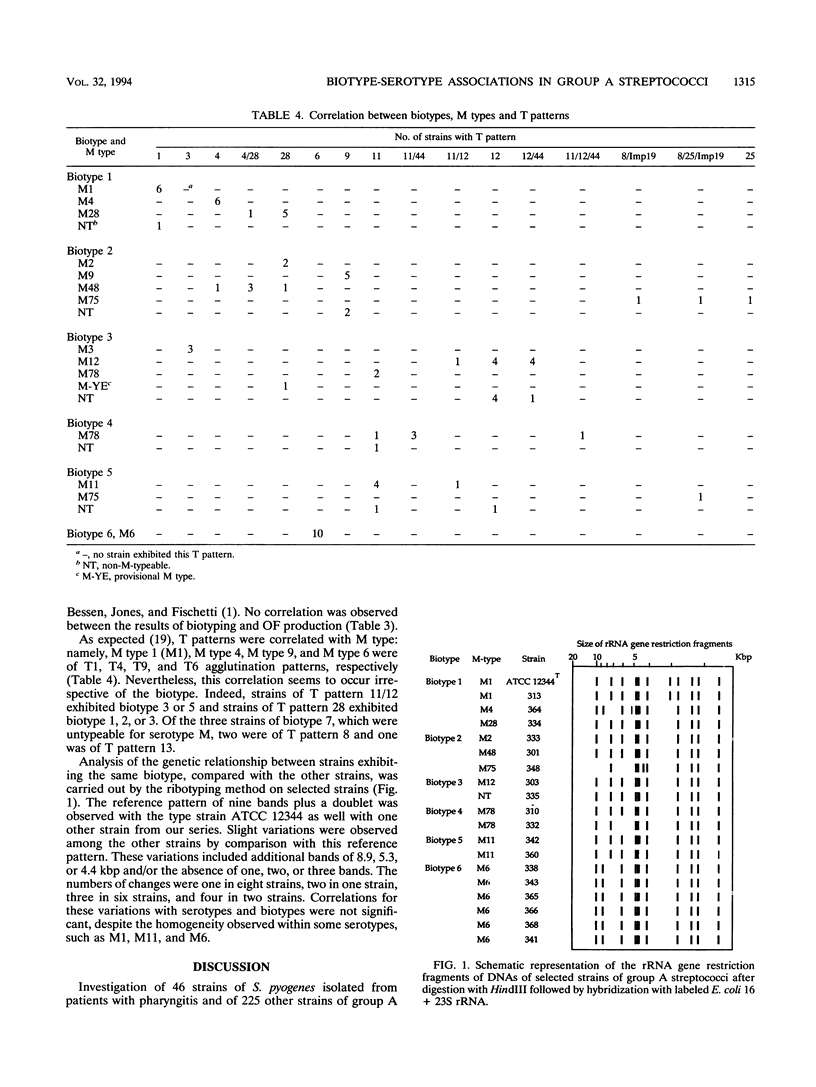
Investigating individual variations between different isolates of group A streptococci, we observed a close correlation between biotypes and serotypes in 46 strains from pharyngitis patients. Biotyping, carried out with a commercially available rapid identification gallery, delineated 10 different associations of characteristics, designated biotypes 1 to 10, observed both in the manufacturer's (127 strains) and our personal (98 strains) collections of group A strains. Only the most frequent biotypes (biotypes 1 to 6) were observed in the pharyngitis cohort, but the overall frequencies of the biotypes did not display striking differences compared with the control collections. Serotyping of the pharyngitis strains showed that each M type was restricted to a sole biotype. For example, M types 1, 4, and 28 were found only in biotype 1 and M type 6 was found only in biotype 6 strains. This association was not due to an epidemiologic bias, since it was also observed in a control series consisting of reference strains and isolates from distant countries (the United States and Czech Republic versus France). An exception was for M type 78, which exhibited biotype 3 or biotype 4. Investigation of the heterogeneity of the strains at the DNA level showed no significant variations of the ribotype patterns between strains of different biotypes, confirming that group A streptococci belong to a unique and homogeneous species. This previously undescribed association between serotypes and biotypes is of interest for a rapid and preliminary characterization of strains isolated in individual patients or during an outbreak. A possible pathogenic association of some biotypic characteristics with specific M proteins is envisaged.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bessen D., Jones K. F., Fischetti V. A. Evidence for two distinct classes of streptococcal M protein and their relationship to rheumatic fever. J Exp Med. 1989 Jan 1;169(1):269–283. doi: 10.1084/jem.169.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisno A. L. Group A streptococcal infections and acute rheumatic fever. N Engl J Med. 1991 Sep 12;325(11):783–793. doi: 10.1056/NEJM199109123251106. [DOI] [PubMed] [Google Scholar]
- Bouvet A., Grimont F., Grimont P. A. Intraspecies variations in nutritionally variant streptococci: rRNA gene restriction patterns of Streptococcus defectivus and Streptococcus adjacens. Int J Syst Bacteriol. 1991 Oct;41(4):483–486. doi: 10.1099/00207713-41-4-483. [DOI] [PubMed] [Google Scholar]
- Brenner D. J., McWhorter A. C., Knutson J. K., Steigerwalt A. G. Escherichia vulneris: a new species of Enterobacteriaceae associated with human wounds. J Clin Microbiol. 1982 Jun;15(6):1133–1140. doi: 10.1128/jcm.15.6.1133-1140.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischetti V. A. Streptococcal M protein: molecular design and biological behavior. Clin Microbiol Rev. 1989 Jul;2(3):285–314. doi: 10.1128/cmr.2.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freney J., Bland S., Etienne J., Desmonceaux M., Boeufgras J. M., Fleurette J. Description and evaluation of the semiautomated 4-hour rapid ID 32 Strep method for identification of streptococci and members of related genera. J Clin Microbiol. 1992 Oct;30(10):2657–2661. doi: 10.1128/jcm.30.10.2657-2661.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimont F., Chevrier D., Grimont P. A., Lefevre M., Guesdon J. L. Acetylaminofluorene-labelled ribosomal RNA for use in molecular epidemiology and taxonomy. Res Microbiol. 1989 Sep;140(7):447–454. doi: 10.1016/0923-2508(89)90065-x. [DOI] [PubMed] [Google Scholar]
- Grimont F., Grimont P. A. Ribosomal ribonucleic acid gene restriction patterns as potential taxonomic tools. Ann Inst Pasteur Microbiol. 1986 Sep-Oct;137B(2):165–175. doi: 10.1016/s0769-2609(86)80105-3. [DOI] [PubMed] [Google Scholar]
- Johnson D. R., Stevens D. L., Kaplan E. L. Epidemiologic analysis of group A streptococcal serotypes associated with severe systemic infections, rheumatic fever, or uncomplicated pharyngitis. J Infect Dis. 1992 Aug;166(2):374–382. doi: 10.1093/infdis/166.2.374. [DOI] [PubMed] [Google Scholar]
- Kaplan E. L., Johnson D. R., Cleary P. P. Group A streptococcal serotypes isolated from patients and sibling contacts during the resurgence of rheumatic fever in the United States in the mid-1980s. J Infect Dis. 1989 Jan;159(1):101–103. doi: 10.1093/infdis/159.1.101. [DOI] [PubMed] [Google Scholar]
- Kaplan E. L. The resurgence of group A streptococcal infections and their sequelae. Eur J Clin Microbiol Infect Dis. 1991 Feb;10(2):55–57. doi: 10.1007/BF01964407. [DOI] [PubMed] [Google Scholar]
- LANCEFIELD R. C. Current knowledge of type-specific M antigens of group A streptococci. J Immunol. 1962 Sep;89:307–313. [PubMed] [Google Scholar]
- MOODY M. D., PADULA J., LIZANA D., HALL C. T. EPIDEMIOLOGIC CHARACTERIZATION OF GROUP A STREPTOCOCCI BY T-AGGLUTINATION AND M-PRECIPITATION TESTS IN THE PUBLIC HEALTH LABORATORY. Health Lab Sci. 1965 Jul;2:149–162. [PubMed] [Google Scholar]
- Manjula B. N., Khandke K. M., Fairwell T., Relf W. A., Sriprakash K. S. Heptad motifs within the distal subdomain of the coiled-coil rod region of M protein from rheumatic fever and nephritis associated serotypes of group A streptococci are distinct from each other: nucleotide sequence of the M57 gene and relation of the deduced amino acid sequence to other M proteins. J Protein Chem. 1991 Aug;10(4):369–384. doi: 10.1007/BF01025251. [DOI] [PubMed] [Google Scholar]
- Martin P. R., Høiby E. A. Streptococcal serogroup A epidemic in Norway 1987-1988. Scand J Infect Dis. 1990;22(4):421–429. doi: 10.3109/00365549009027073. [DOI] [PubMed] [Google Scholar]
- Maxted W. R., Widdowson J. P., Fraser C. A., Ball L. C., Bassett D. C. The use of the serum opacity reaction in the typing of group-A streptococci. J Med Microbiol. 1973 Feb;6(1):83–90. doi: 10.1099/00222615-6-1-83. [DOI] [PubMed] [Google Scholar]
- Schaffer H. E., Sederoff R. R. Improved estimation of DNA fragment lengths from Agarose gels. Anal Biochem. 1981 Jul 15;115(1):113–122. doi: 10.1016/0003-2697(81)90533-9. [DOI] [PubMed] [Google Scholar]
- Schwartz B., Elliott J. A., Butler J. C., Simon P. A., Jameson B. L., Welch G. E., Facklam R. R. Clusters of invasive group A streptococcal infections in family, hospital, and nursing home settings. Clin Infect Dis. 1992 Aug;15(2):277–284. doi: 10.1093/clinids/15.2.277. [DOI] [PubMed] [Google Scholar]
- Severin W. P. Latex agglutination in the diagnosis of meningococcal meningitis. J Clin Pathol. 1972 Dec;25(12):1079–1082. doi: 10.1136/jcp.25.12.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS R. E. Laboratory diagnosis of streptococcal infections. Bull World Health Organ. 1958;19(1):153–176. [PMC free article] [PubMed] [Google Scholar]
- Wellstood S. A. Rapid, cost-effective identification of group A streptococci and enterococci by pyrrolidonyl-beta-naphthylamide hydrolysis. J Clin Microbiol. 1987 Sep;25(9):1805–1806. doi: 10.1128/jcm.25.9.1805-1806.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiley R. A., Fraser H., Hardie J. M., Beighton D. Phenotypic differentiation of Streptococcus intermedius, Streptococcus constellatus, and Streptococcus anginosus strains within the "Streptococcus milleri group". J Clin Microbiol. 1990 Jul;28(7):1497–1501. doi: 10.1128/jcm.28.7.1497-1501.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdowson J. P., Maxted W. R., Grant D. L. The production of opacity in serum by group A streptococci and its relationship withthe presence of M antigen. J Gen Microbiol. 1970 Jun;61(3):343–353. doi: 10.1099/00221287-61-3-343. [DOI] [PubMed] [Google Scholar]


