Abstract
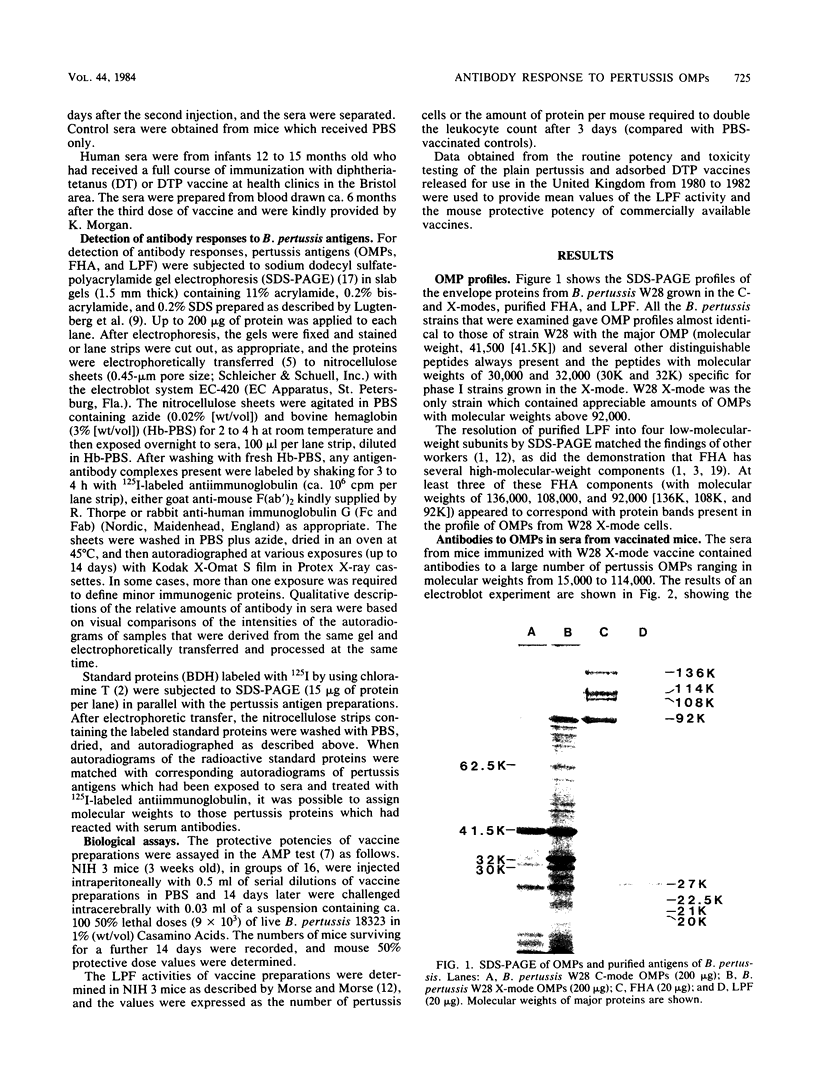
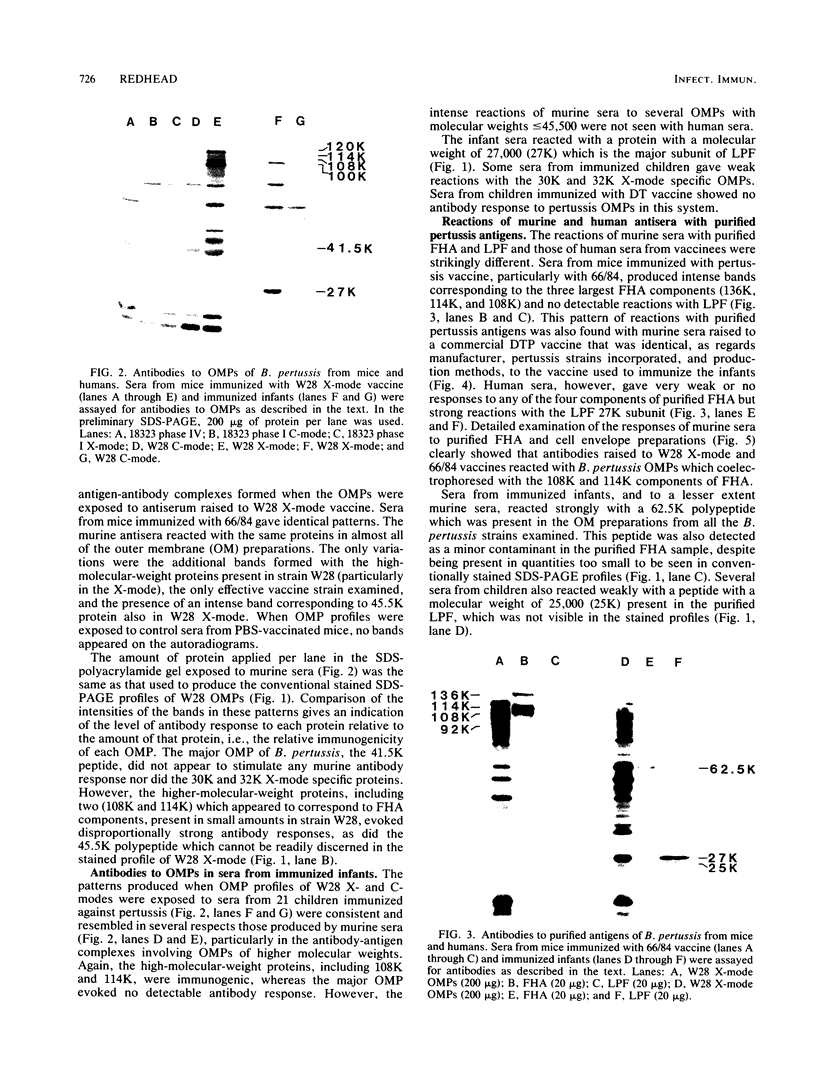
The serum antibody responses to the outer membrane proteins, purified filamentous hemagglutinin, and leukocytosis-promoting factor of Bordetella pertussis were examined in mice and children immunized with pertussis whole-cell vaccine. It was found that, although there were many similarities in the responses of mice and children, there were important differences. Sera from vaccinated mice reacted strongly with purified filamentous hemagglutinin and gave weak or undetectable responses to the components of purified leukocytosis-promoting factor. The converse was found with sera from vaccinated children. Antibodies to leukocytosis-promoting factor may thus be of importance in protecting children against pertussis, although they appear to play no role in the active mouse protection test for vaccine efficacy. These results cast doubt on the value of the mouse as an animal model for the potency testing of extracted acellular pertussis vaccines.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai H., Sato Y. Separation and characterization of two distinct hemagglutinins contained in purified leukocytosis-promoting factor from Bordetella pertussis. Biochim Biophys Acta. 1976 Oct 22;444(3):765–782. doi: 10.1016/0304-4165(76)90323-8. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irons L. I., MacLennan A. P. Isolation of the lymphocytosis promoting factor-haemagglutinin of Bordetella pertussis by affinity chromatography. Biochim Biophys Acta. 1979 Sep 29;580(1):175–185. doi: 10.1016/0005-2795(79)90208-3. [DOI] [PubMed] [Google Scholar]
- Kanbayashi Y., Nakamura T., Hosoda K., Nogimori K., Yajima M., Ui M. Subunit structure of islets-activating protein (IAP), a new protein isolated from the culture media of Bordetella pertussis. J Biochem. 1978 Aug;84(2):443–451. doi: 10.1093/oxfordjournals.jbchem.a132145. [DOI] [PubMed] [Google Scholar]
- Kendrick P. L., Eldering G., Dixon M. K., Misner J. Mouse Protection Tests in the Study of Pertussis Vaccine: A Comparative Series Using the Intracerebral Route for Challenge. Am J Public Health Nations Health. 1947 Jul;37(7):803–810. [PMC free article] [PubMed] [Google Scholar]
- Loeb M. R., Smith D. H. Human antibody response to individual outer membrane proteins of Haemophilus influenzae type b. Infect Immun. 1982 Sep;37(3):1032–1036. doi: 10.1128/iai.37.3.1032-1036.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Mertsola J., Viljanen M. K., Ruuskanen O. Current status of pertussis and pertussis vaccination in Finland. Ann Clin Res. 1982 Dec;14(5-6):253–259. [PubMed] [Google Scholar]
- Morse S. I., Morse J. H. Isolation and properties of the leukocytosis- and lymphocytosis-promoting factor of Bordetella pertussis. J Exp Med. 1976 Jun 1;143(6):1483–1502. doi: 10.1084/jem.143.6.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz J. J., Arai H., Bergman R. K., Sadowski P. L. Biological activities of crystalline pertussigen from Bordetella pertussis. Infect Immun. 1981 Sep;33(3):820–826. doi: 10.1128/iai.33.3.820-826.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz J. J., Arai H., Cole R. L. Mouse-protecting and histamine-sensitizing activities of pertussigen and fimbrial hemagglutinin from Bordetella pertussis. Infect Immun. 1981 Apr;32(1):243–250. doi: 10.1128/iai.32.1.243-250.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman M. Letter: Protective activity of whooping-cough convalescent serum and serum-IgA level in mice infected with Bordetella pertussis. Lancet. 1976 Jul 17;2(7977):156–156. doi: 10.1016/s0140-6736(76)92896-8. [DOI] [PubMed] [Google Scholar]
- Robinson A., Hawkins D. C. Structure and biological properties of solubilized envelope proteins of Bordetella pertussis. Infect Immun. 1983 Feb;39(2):590–598. doi: 10.1128/iai.39.2.590-598.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A., Irons L. I. Synergistic effect of Bordetella pertussis lymphocytosis-promoting factor on protective activities of isolated Bordetella antigens in mice. Infect Immun. 1983 May;40(2):523–528. doi: 10.1128/iai.40.2.523-528.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Izumiya K., Sato H., Cowell J. L., Manclark C. R. Role of antibody to leukocytosis-promoting factor hemagglutinin and to filamentous hemagglutinin in immunity to pertussis. Infect Immun. 1981 Mar;31(3):1223–1231. doi: 10.1128/iai.31.3.1223-1231.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacterle G. R., Pollack R. L. A simplified method for the quantitative assay of small amounts of protein in biologic material. Anal Biochem. 1973 Feb;51(2):654–655. doi: 10.1016/0003-2697(73)90523-x. [DOI] [PubMed] [Google Scholar]
- Schnaitman C. A. Solubilization of the cytoplasmic membrane of Escherichia coli by Triton X-100. J Bacteriol. 1971 Oct;108(1):545–552. doi: 10.1128/jb.108.1.545-552.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stainer D. W., Scholte M. J. A simple chemically defined medium for the production of phase I Bordetella pertussis. J Gen Microbiol. 1970 Oct;63(2):211–220. doi: 10.1099/00221287-63-2-211. [DOI] [PubMed] [Google Scholar]
- Wardlaw A. C., Parton R., Hooker M. J. Loss of protective antigen, histamine-sensitising factor and envelope polypeptides in cultural variants of Bordetella pertussis. J Med Microbiol. 1976 Feb;9(1):89–100. doi: 10.1099/00222615-9-1-89. [DOI] [PubMed] [Google Scholar]