Abstract
Background
The major facilitator superfamily (MFS) is one of the two largest superfamilies of membrane transporters present ubiquitously in bacteria, archaea, and eukarya and includes members that function as uniporters, symporters or antiporters. We report here the complete transportome of MFS proteins of a human pathogenic yeast Candida albicans.
Results
Computational analysis of C. albicans genome enabled us to identify 95 potential MFS proteins which clustered into 17 families using Saier's Transport Commission (TC) system. Among these SP, DHA1, DHA2 and ACS represented major families consisting of 22, 22, 9 and 16 members, respectively. Family designations in C. albicans were validated by subjecting Saccharomyces cerevisiae genome to TC system. Based on the published available genomics/proteomics data, 87 of the putative MFS genes of C. albicans were found to express either at mRNA or protein levels. We checked the expression of the remaining 8 genes by using RT-PCR and observed that they are not expressed under basal growth conditions implying that either these 8 genes are expressed under specific growth conditions or they may be candidates for pseudogenes.
Conclusion
The in silico characterisation of MFS transporters in Candida albicans genome revealed a large complement of MFS transporters with most of them showing expression. Considering the clinical relevance of C. albicans and role of MFS members in antifungal resistance and nutrient transport, this analysis would pave way for identifying their physiological relevance.
Background
Current evidence suggests that Candida albicans acquires azole resistance by employing multiple mechanisms that include (a) alterations in the azole-target protein Erg11p (b) upregulation of the ERG11 gene [1-4] as well as (c) failure of drug accumulation mediated by efflux pumps. Most commonly, genes encoding drug efflux pumps belonging to ATP binding cassette (ABC) and Major facilitator (MFS) superfamilies of proteins are overexpressed in azole resistant Candida isolates [5-9]. ABC family permeases are in general multicomponent primary active transporters, capable of transporting both small molecules and macromolecules which is coupled to ATP hydrolysis while the MFS transporters are single-polypeptide secondary carriers capable only of transporting small solutes in response to chemiosmotic ion gradients. We have earlier annotated and classified ABC transporters in C. albicans [10], however, clinically relevant MFS superfamily in C. albicans largely remains uncharacterized.
MFS superfamily is ubiquitously present in all kingdoms of life and includes members of direct medical and pharmaceutical significance. They are involved in the symport, antiport or uniport of various substrates [11,12] and are known to exhibit specificity for sugars, polyols, drugs, neurotransmitters, Krebs cycle metabolites, phosphorylated glycolytic intermediates, amino acids, peptides, osmolites, siderophores (efflux), iron-siderophores (uptake), nucleosides, organic and inorganic anions, etc [11,12]. Most MFS proteins vary between 400 and 600 amino acid residues in length and possess either 12 or 14 putative transmembrane segments (TMS). The MFS superfamily consists of 61 families according to the Transport Commission (TC) system given by Saier and group http://www.tcdb.org/. TC is a comprehensive classification system for membrane transport proteins and is analogous to the Enzyme Commission (EC) system, except that it incorporates both functional and phylogenetic information [13-15]. This system allocates five digits to each phylogenetic cluster of transporters. The first two digits ("class" and "subclass") identify the transport mode and energy-coupling mechanism. The third digit characterizes phylogenetic "families" or "superfamilies." The fourth digit identifies phylogenetic "subfamilies." The fifth digit ("clusters") corresponds to the substrate specificity, as presumed by experimental data or stringent sequence identity [13]. In TC, the designation 2.A.1 represents MFS and the next digit denotes the family, for instance, 2.A.1.1 represents sugar transporters and so on. Any two transport systems in the same subfamily of a transporter family that transport the same substrate(s) are given the same TC number, regardless of whether they are orthologues or paralogues.
Till date only a few MFS transporters namely MDR1, FLU1, NAG3, NAG4, JEN1, ARN1 and NGT1 [16-22], have been identified and characterized in C. albicans. Additionally, over 20 hexose transporters and glucose sensors are known to exist in C. albicans that reflect the varied niches in which this pathogen thrives [23]. However, very limited knowledge about other MFS transporters is available in C. albicans. Out of all the known MFS in C. albicans MDR1, its alleles and FLU1 are shown to be the only drug efflux pumps transporters. MDR1 was initially identified as a gene, which conferred resistance to the tubulin binding agent benomyl and tetrahydrofolate reductase inhibitor methotrexate [24,25]. MDR1 expression in S. cerevisiae confers resistance to several unrelated drugs and its overexpression has been linked to azole resistance in C. albicans. The expression of MDR1 in C. albicans cells is enhanced by benomyl, methotrexate and several other unrelated drugs, and is found to be more pronounced in some of the azole resistant clinical isolates [26,27]. Keeping in view, the relevance of the MFS multidrug transporters in general and in multidrug resistance (MDR) in particular, in the present study, we have examined MFS superfamily of proteins in C. albicans. Although, earlier annotation of the previous Candida genome assembly (version 19) predicted 71 MFS genes, no systematic classification was given [28]. To address this question, we have applied a comprehensive bioinformatics approach to identify and annotate all MFS transporter genes in the Candida genome from assembly version 21 and systematically searched for evidence of their expression. It is hoped that these findings will provide the scientific community with the necessary framework needed for the functional characterization of the MFS proteins and a better understanding of this medically and pharmaceutically significant superfamily.
Results
Identification and sequence-based functional grouping of C. albicans putative MFS genes
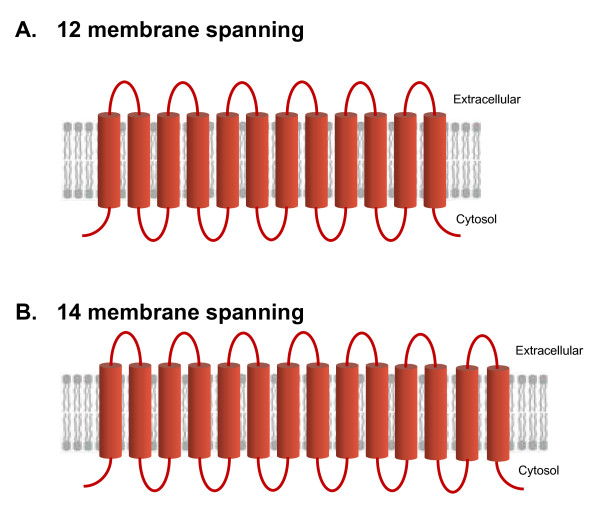
To identify gene loci encoding MFS proteins, multiple TBLASTN searches were performed on assembly (version 21) of the C. albicans genome http://www.candidagenome.org using well known MFS proteins as queries. The ORFs thus identified were subjected to domain analysis followed by sequence-based functional grouping, resulting in clustering of putative Candida proteins into various MFS families as described by Saier's TC system. Using this strategy, we identified a total of 95 loci of putative MFS genes in the Candida genome containing the domains characteristic of MFS proteins and were found to be either 12 or 14 transmembrane spanners (Figure 1). By using TC system, all the putative MFS identified were assigned to 17 TC families (Additional file 1). The family assignment obtained by this approach was validated by applying the same strategy to S. cerevisiae MFS transportome (data not shown). S. cerevisiae homologues of C. albicans MFS proteins were found to take up the same TC cluster thereby addressing the evolutionary relationship between the two yeasts. A summary of the previously known Candida genes together with the new genes is presented in Additional file 1, where, TC family, CGD ORF, gene, alias, TCDB homolog and expression confirmation are listed. In addition, the closest S. cerevisiae member within the family is also presented in Additional file 1.
Figure 1.
Predicted topology of putative MFS proteins of C. albicans. The topology of the putative MFS proteins was predicted using TMHMM program http://www.cbs.dtu.dk/services/TMHMM/. The transmembrane domains were found to be either 12 or 14 transmembrane spanners.
The Sugar Porter (SP) Family (TC # 2.A.1.1)
The SP family is widespread and have members from all of the major groups of living organisms: bacteria, archaea, eukaryotic protists, fungi, animals and plants [11]. It forms one of the largest families with 22 members in C. albicans. We have previously characterized HGT1 [29] and many studies since then have also reported other SP members namely HGT2-HGT20 [23,30]. Apart from identifying HGT1-HGT20, in the present study, we have also identified two previously unidentified members, namely MAL31 (orf19.3981) and orf19.4923 (Additional file 1) both having homologues in S. cerevisiae, MAL31 and YFL040W, respectively. Further, based on the homology, the 22 SP members show significant similarity with various sugars, namely arabinose, quinate, myoinositol, maltose, fructose, glycerol, monosaccharide, glucose, hexoseand xylose (Additional file 1).
The Drug: H+ Antiporter-1 (DHA1) Family (TC # 2.A.1.2)
DHA1 family like the SP is widely distributed and the members include both drug-specific and MDR efflux pumps. Like SP it also forms one of the largest families having 22 members in Candida (Additional file 1). MDR1 gene in C. albicans is one of the best characterized members, originally known to confer resistance to benomyl and methotrexate [19,20,24,25]. Subsequent studies indicated that MDR1 also encodes resistance to cycloheximide, benztriazoles, 4-nitroquinolone-N-oxide and sulfometuron methyl [24,31]. Disruption of the MDR1 gene reduced the virulence of C. albicans [26]. Other characterized members of this family include FLU1, NAG3 and NAG4. Disruption of FLU1 in C. albicans hyper-susceptibility to mycophenolic acid thus suggesting that it could be a preferred substrate for the transporter [17]. On the other hand, NAG3 (TMP1) and NAG4 (TMP2) show susceptibility to a number of unrelated compounds such as cycloheximide, 4-nitroquinoline-N-oxide and 1,10-phenanthroline and are upregulated in response to these drugs, suggesting that they function as multiple drug efflux pumps [21]. Apart from MDR1, which is known as a clinically relevant efflux pump protein, none of the other characterized members have been directly linked to MDR of C. albicans.
The Drug: H+ Antiporter-2 (DHA2) Family (TC # 2.A.1.3)
The DHA2 family of drug:H+ antiporters with 14 predicted transmembrane-spanning segments, consists of nine members in C. albicans which show significant similarity to transporters, namely aminotriazole, 4-nitroquinoline-N-oxide, Me2+-tetracycline antiporter, vacuolar basic amino acid (Arg, Lys, His) transporter and metal:tetracycline/oxytetracycline efflux pump (Additional file 1). In C. albicans no member of this family has yet been characterized whereas in S. cerevisiae two DHA2 proteins, SGE1 and ATR1 are well studied. ATR1 has been shown to confer resistance to the structurally unrelated compounds aminotriazole and 4-nitroquinolone-N-oxide and expression of ATR1 is inducible by the former but not the latter [32,33]. SGE1 appears to confer resistance to crystal violet [34] and ethidium bromide [35,36].
The Fucose: H+ Symporter (FHS) Family (TC # 2.A.1.7)
FHS is a small family with two ORFs identified in Candida namely: orf19.4090 and orf19.7490 (Additional file 1). They are homologous to S. cerevisiae BSC6, which encodes a protein of unknown function exhibiting genomic organization compatible with a translational read through-dependent mode of expression [37].
The Phosphate: H+ Symporter (PHS) Family (TC # 2.A.1.9)
PHS family is unusual in that it has representatives only in yeast, fungi and plants but none in bacteria, animals and other eukaryotes [11]. The occurrence of distant homologues in both the plant and fungal kingdoms suggests that they possess isoforms that diverged from each other well before plants diverged from fungi [11]. Two well characterized members of the PHS family are the Pho84 inorganic phosphate transporter of S. cerevisiae [38] and the GvPT phosphate transporter of Glomus versiforme [39]. In this study, we have identified five ORFs belonging to PHS family in C. albicans with homology to phosphate: H+ symporters (Additional file 1). However, none of the members identified in Candida has yet been characterized.
The Oxalate: Formate Antiporter (OFA) Family (TC # 2.A.1.11)
OFA family members are widely distributed in nature, being present in the bacterial, archaeal and eukaryotic kingdoms [11]. In C. albicans, our searches revealed two members (Additional file 1). OxlT, the oxalate:formate antiporter from Oxalobacter formigenes, is the hallmark protein and provides the basis for naming the OFA family [40,41]. This protein has been purified, reconstituted in an artificial membrane system as well as structurally and functionally characterized [42,43].
The Sialate: H+ Symporter (SHS) Family (TC # 2.A.1.12)
SHS family, like the PHS family, is very small with only two members namely JEN1 and JEN2, identified in the present as well as a previous study [44]. JEN1 has been described as the first monocarboxylate transporter of C. albicans showing loss of all measurable lactate permease activity upon its disruption. Further, lactate uptake by JEN1 was competitively inhibited by pyruvic and propionic acids while acetic acid behaved as a non-competitive substrate [22].
The Monocarboxylate Porter (MCP) Family (TC # 2.A.1.13)
MCP family is exclusively present in yeasts and animals. In mammals, these permeases are known to transport monocarboxylates, namely pyruvate, lactate and mevalonate with inwardly-directed polarity and presumably function as proton symporter [11] while it is reported that the yeast monocarboxylate transporter proteins perform functions other than their mammalian counterparts [45]. This family has six members identified in Candida.
The Anion: Cation Symporter (ACS) Family (TC # 2.A.1.14)
ACS is a relatively large family having representation in bacteria, yeasts and animals comprising mainly of symporters that are known to accumulate their substrates in symport with either Na+ or H+, depending on the system. They may transport either inorganic (e.g. phosphate) or organic anions (e.g. glucarate, hexuronate, tartrate, allantoate or 4-hydroxylphenyl acetate) [11]. In Candida, we have identified 16 members showing significant similarity to transporters having prefered substrates, namely tartrate, allantoate, nicotinate, biotin and pantothenate (Additional file 1).
The Aromatic Acid: H+ Symporter (AAHS) Family (TC # 2.A.1.15)
The members of AAHS family occur exclusively in gram-negative bacteria where they are known to transport a variety of aromatic acids like benzoate, 4-hydroxybenzoate, 3-hydroxyphenylpropionate, 2,4-dichlorophenoxyacetate as well as niacin and cis, cis-muconate [11]. In Candida, a single member of AAHS family has been identified, namely orf19.6952 showing significant similarity to putative niacin uptake porter (Additional file 1). This family has no representation in S. cerevisiae and thus unique to Candida.
The Siderophore-Iron Transporter Family (TC # 2.A.1.16)
All the known members of this family are from yeast species. In C. albicans this family is represented by a single protein known as siderophore transporter, SIT1/ARN1 (orf19.2179) which is required in ferrichrome-iron uptake. Previous reports suggest that deletion of ARN1 leads to reduced ability of C. albicans to use iron bound to the hydroxamate-type siderophore ferrichrome and upon deletion of the two high-affinity iron permease C. albicans genes (FTR1 and FTR2), the activity was completely abolished [18,46]. According to another study, siderophore uptake by Sit1p/Arn1p is required in a specific process of C. albicans infection, namely epithelial invasion and penetration, while in the blood or within organs other sources of iron, including heme, may be used [47].
The Organic Cation Transporter (OCT) Family (TC # 2.A.1.19)
In C. albicans this family is represented by a single uncharacterized member FGR2 (orf19.7071) showing similarity to organic anion: dicarboxylate transporter. These proteins are known to transport organic cations and/or anions and catalyze uptake of cationic drugs such as tetramethyl ammonium, cimetidine, procainamide, quinidine and some endogenous metabolites such as N-methyl-nicotinamide [48-51].
The Vesicular Neurotransmitter Transporter (VNT) Family (TC # 2.A.1.22)
These proteins are more closely related to SP family than to other MFS families. The better characterized members of the VNT family are synaptic vesicle proteins from mammals, the electric eel and insects [52-55]. In C. albicans this family is represented by a single member orf19.6578 with significant similarity to dopamine transporter.
The Peptide-Acetyl-Coenzyme A (PAT) Transporter Family (TC # 2.A.1.25)
Members of the PAT family are present across bacteria, yeast and animals [11]. Amongst the well characterized proteins of this family include acetyl-CoA transporter localized in the endoplasmic reticulum and Golgi membranes of humans [56]. AmpG protein of E. coli belonging to PAT family, brings into the cell peptides, including cell wall degradative peptides and glycopeptides, to act as inducers of β-lactamase synthesis [57]. The acetyl-CoA transporter is expected to function by acetyl-CoA:CoA antiport while the AmpG protein is most likely energized by substrate:H+ symport. In C. albicans this family is represented by a single member orf19.3782 with significant homology to acetyl-CoA:CoA antiporter.
The L-Amino Acid Transporter-3 (LAT3) Family (TC # 2.A.1.44) (also called the SLC43 family)
LAT3 transports neutral amino acids such as L-leucine, L-isoleucine, L-valine and L-phenylalanine by a Na+-independent, electroneutral, facilitated diffusion process and also transports amino acid alcohols. In C. albicans, this family is represented by two ORFs: orf19.6654 and orf19.6316.
The Proton Coupled Folate Transporter/Heme Carrier Protein (PCFT/HCP) Family (TC # 2.A.1.50)
In C. albicans, this family is represented by a single member orf19.6976 showing homology to high-affinity folate transporter. PCFT from human has been shown to act both as an intestinal proton-coupled high-affinity folate transporter and as an intestinal heme transporter which mediates heme uptake from the gut lumen into duodenal epithelial cells. The iron is then released from heme and may be transported into the bloodstream [58,59].
The N-Acetylglucosamine Transporter Family (TC # 2.A.1.58)
NGT1 from C. albicans represents the first eukaryotic N-acetylglucosamine (GlcNAc) transporter and is the only known member of this family. It is required for efficient GlcNAc uptake and for inducing hyphae development at low GlcNAc concentrations [16]. High concentrations of GlcNAc could bypass the need for NGT1 to induce hyphae, indicating that elevated intracellular levels of GlcNAc induce hyphal formation. Expression of NGT1 in S. cerevisiae promoted GlcNAc uptake, indicating that NGT1 acts directly as a GlcNAc transporter [16]. No homologue of NGT1 was detected in S.cerevisiae.
Most of the identified members of MFS superfamily are expressed
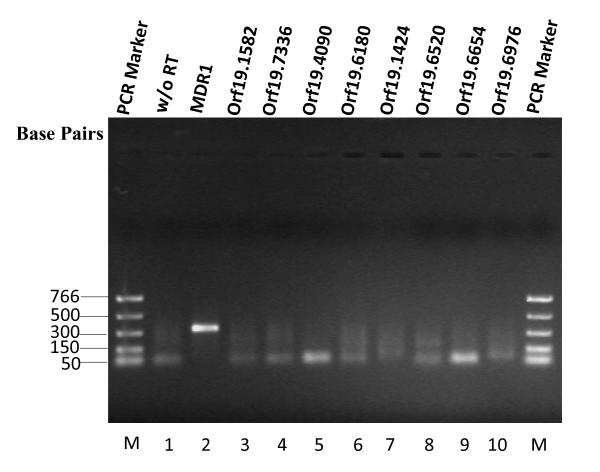
To assess which of the identified Candida MFS transporter genes are transcribed or translated, we analyzed all 95 MFS transporter loci by extensive mining of the data available from the genome or proteome-wide studies in C. albicans. Using this approach, we found that out of the 95 ORFs, 87 were shown to express either at mRNA or protein level under different experimental conditions (see Additional file 1). To validate the expression of the remaining 8 putative MFS genes (orf19.1582, orf19.7336, orf19.4090, orf19.6180, orf19.1424, orf19.6520, orf19.6654 and orf19.6976), we employed reverse transcriptase PCR (RT-PCR) approach taking a well characterized C. albicans MFS transporter, MDR1 as a positive control. The primers utilized for expression analysis are shown in Table 1. Interestingly, no expression was detected in any of the 8 putative genes tested under the basal condition (Figure 2).
Table 1.
Oligomers used for RT-PCR
| Oligomer | Sequence (5'-3') | Expected amplicon length (bp) |
| MDR1-F | CACCGTTATGGAACCAGTTG | 330 |
| MDR1-R | CAGCACCAAACAATGGACCAACCCAATGAG | |
| orf19.1582-F | GAAACTTTGGTATCCTGGAAC | 380 |
| orf19.1582-R | CAACAAAATGGCAAAACCACC | |
| orf19.7336-F | CGCTTTCCAACCATCAATGG | 464 |
| orf19.7336-R | CAGTCATTGAAGAAGCAGAAG | |
| orf19.4090-F | GAGAAGGGGCGTTTTTATTG | 301 |
| orf19.4090-R | CACAATGAAAACCGGTAACAC | |
| orf19.6180-F | GGTTGTTGTTAGGTGTGTTG | 394 |
| orf19.6180-R | CAAAATCTCGTAAACCCACG | |
| orf19.1424-F | CAGTACAAACATTACAAGCCC | 476 |
| orf19.1424-R | CACCACAAATGTCATACCAC | |
| orf19.6520-F | GCCTTACATCCACGCAATTTG | 339 |
| orf19.6520-R | CTAAAATCTAACCTCTTGGCGC | |
| orf19.6654-F | CTATTGGGTTGTTGGGTTTG | 286 |
| orf19.6654-R | GTCGAGCCTCCAATAATACCTG | |
| orf19.6976-F | CTCCCCCTTGGTTATATTAAC | 603 |
| orf19.6976-R | CCAGGCCAACCATTTTTCAAAG |
Figure 2.
Expression analysis of putative MFS genes by RT-PCR. The expression of 8 putative MFS genes, which were not validated by the mRNA/protein profiling data mining, was checked by RT-PCR. Purified poly(A)+ enriched mRNA fractionated from C. albicans isolate SC5314 were amplified by RT-PCR, as described in the Methods. Following electrophoresis through 1.2% agarose gel, the amplified PCR products were visualized by staining with ethidium bromide. Lane M, nucleotide size marker (PCR Marker); lane 1, without RT (negative control); lane 2, MDR1 (positive control, 330 base pairs); lane 3, orf19.1582; lane 4, orf19.7336; lane 5, orf19.4090; lane 6, orf19.6180; lane 7, orf19.1424; lane 8, orf19.6520; lane 9, orf19.6654 and lane 10, orf19.6976.
Discussion and conclusion
In this study, we report the complete transportome of MFS superfamily of C. albicans. Computational analysis of the C. albicans genome assembly (version 21) from CGD enabled us to identify 95 potential MFS permeases. The latter were classified according to both phylogeny and function based TC system earlier developed by Saier [13,15]. This approach enabled us to cluster these 95 MFS proteins into 17 distinct families. Indeed each of the predictions must be tested experimentally before final conclusions are reached with reference to the expression and function of the proteins analyzed.
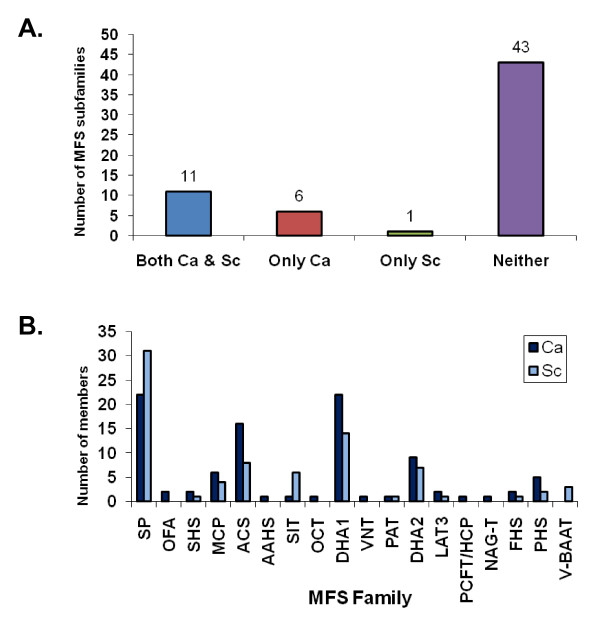
The comparison between C. albicans and S. cerevisiae MFS genes revealed that predominantly most of the families are present in both the organisms (Figure 3A). Notably, there were few families that were present only in C. albicans: OFA, AAHS, OCT, VNT, PCFT/HCP and NAG-T (Figure 3B). Interestingly, in OFA family, the S. cerevisiae MFS genes, MHC1 and YMR155W, although orthologues of C. albicans orf19.6180 and orf19.1424, respectively, yet do not conform to the same TC family designation as Candida, rather these genes were found to be more similar to OxlT of Oxalobacter formigenes, a bacterial oxalate: formate antiporter (Additional file 1). Similar was the case with C. albicans orf19.6976, where the S. cerevisiae orthologue, YJL163C, belong to DHA1 family instead of PCFT/HCP family (Additional file 1). It was also observed in the AAHS family, Candida genes are more closely related to gram-negative bacteria (Additional file 1) as compared to S. cerevisiae which probably explains the absence of homologues of this family in S. cerevisiae. On a similar pattern, the MFS genes of C. albicans belonging to OCT and VNT families are closely related to animals and no significant homologies were detected in S. cerevisiae genome. The NAG-T family also did not find any representation in S. cerevisiae genome. Taken together, these findings point out that probably these genes had diverged during the course of evolution. Interestingly, vacuolar basic amino acid (V-BAAT) family present in S. cerevisiae has no representation in C. albicans (Figure 3B). Although the C. albicans ORFs, orf19.1308 and orf19.7554 show significant homology to S. cerevisiae V-BAAT family members, VBA1 and VBA2, respectively, they belong to DHA2 family supporting the fact that V-BAAT family is most similar to the DHA2 family.
Figure 3.
Distribution of MFS families in C. albicans as per transport commission (TC) system and comparison with S. cerevisiae. (A) Family designations were according to TC system as mentioned in the Methods. Ca and Sc stand for C. albicans and S. cerevisiae, respectively. Out of 61 reported MFS families in TC database http://www.tcdb.org/, 17 were identified in C. albicans as compared to 12 known in S. cerevisiae. (B) A comparison of MFS families between C. albicans and S. cerevisiae revealed that the members of the same family were almost equal in number in both the yeasts. Interestingly, 6 families that were present in C. albicans had no representation in S. cerevisiae whereas there was only one such family in S. cerevisiae which had no counterpart in C. albicans.
Our expression analysis of the published work revealed that out of 95 MFS genes, 87 are expressed under either basal (uninduced) or in different specific experimental conditions (Additional file 1). Most of the genes identified in the present investigation are expressed either at mRNA or protein level thus validating our analysis. The expression of the remaining 8 genes was not detected. This would imply that either these genes are expressed under specific growth condition or that they may be candidate pseudogenes. To dissect the role of each putative member of MFS superfamily, an essential part of the process will now involve construction of multiple knockout mutants, which will enable to unravel their role in drug or nutrient transport.
Methods
Media chemicals were obtained from HiMedia (Mumbai, India). Luria Bertani broth and agar media was purchased from Difco, BD Biosciences, NJ, USA. Taq DNA polymerase, ultra pure deoxyribonucleotides (dATP, dGTP, dCTP and dTTP) were obtained from New England Biolabs (NEB Inc.), USA. Moloney murine leukemia virus (M-MuLV) reverse transcriptases (RT) and RNase inhibitor were obtained from MBI Fermentas. Oligotex mRNA Mini Kit was purchased from Qiagen. Oligonucleotides used were commercially synthesized from Sigma-Aldrich. All Molecular Biology (MB) grade chemicals used in this study were obtained from Sigma Chemical Co. (St. Louis, USA).
Identification of C. albicans MFS transporter genes
C. albicans genome assembly version 21 http://www.candidagenome.org was searched for MFS genes using well known MFS proteins from Swiss-Prot database http://www.expasy.ch/sprot as queries in TBLASTN searches [60]. Our initial query dataset had 230 MFS proteins which were used individually to BLAST Candida genome. Out of 230 sequences, 38 were chosen which gave significant E-values and were maximally dissimilar among themselves covering diversity of MFS from plants, fungi and mammals. It should also be noted that although a rather relaxed E-value (0.0001) cut-off was used, the observed E-values between the test sequence and the closest query sequence were much below this threshold indicating that the hits obtained were highly significant. The high-scoring segment pairs (HSPs) returned from TBLASTN searches were checked for duplications using an in-house written Perl script and only those that gave the lowest E-value with one or the other sequences from the query set were kept for further analysis. The overlapping HSPs were merged so as to obtain the largest contiguous stretch of nucleotides in the C. albicans genome, which had strong sequence homology with the MFS proteins in the query dataset. Since the test sequences always gave significant hits with a number of query sequences the best individual alignments were merged using overlaps. The availability of multiple TBLASTN matches (on account of the large number of query sequences used) made the merging step relatively easy and unambiguous. It also greatly increased the reliability of identifying a true hit and distinguishing it from false positives. The protein sequences were obtained by a six frame translation of the HSPs, using the tool "transeq" from the EMBOSS package http://www.ebi.ac.uk/emboss/transeq and taking the largest open reading frame (ORF). 95 ORFs identified from C. albicans after the initial TBLASTN searches were then pooled with the query dataset of 38 sequences to form a new query dataset and used iteratively for subsequent searches until no new ORFs were obtained. Subsequently, all potential genes were analyzed for MFS domains using the programs ExPASY PROSITE [61], InterPro [62] and Conserved Domain Database at NCBI [63]. Transmembrane domains were predicted using TMHMM http://www.cbs.dtu.dk/services/TMHMM/.
Sequence-based functional grouping of C. albicans MFS genes
C. albicans MFS genes, as identified above, were further subjected to sequence-based classification according to TC system which is based on both functional and phylogenetic information [13,14,64]. Each putative MFS was individually searched against the TCDB. For this purpose the BLAST server at the transporter database http://www.tcdb.org was used with the default settings and E-value cut-off of 1.0 from the given choices of E-values (1000 to 0.0001). It should be noted that here also we chose a rather relaxed E-value cut-off and the potential MFS identified in Candida returned much lower E-values with the MFS sequences in the transporter database. To validate the family designations obtained for C. albicans using TC system all the known S. cerevisiae MFS proteins were also searched against the TC database using the same method as described for C. albicans.
Phylogenetic relationship with S. cerevisiae
A systematic search for S. cerevisiae homologues of the proteins was done with each C. albicans MFS gene by using SGD BLASTP tool http://www.yeastgenome.org/.
Expression analysis of the putative MFS genes
In order to validate the existence of the putative MFS genes in C. albicans, expression analysis was done by extensive mining of the data available from the previous genome and proteome-wide studies (Additional file 1) as well as experimentally by RT-PCR.
Total RNA isolation
Total RNA from C. albicans isolate SC5314 was prepared from mid-logarithmically grown phase cells. In a standard preparation, 10 ml of cells, optical density at 600 nm (OD600) of 1.0, were pelleted and washed with 10 ml of ice-cold H2O and spun at 5000 rpm. The pellet was resuspended in 1.0 ml of TRI® Reagent (Sigma) and 0.3 ml of ice-cold, acid-washed 0.4–0.6 mm diameter glass beads (Sigma, St. Louis, MO, USA) were added and vortexed for 5 min. Chloroform (0.2 ml) without isoamyl alcohol was added and the tubes were shaken vigorously for 15 s. The samples were incubated at room temperature for 15 min, centrifuged at 12,000 × g for 15 min at 4°C. The upper colourless aqueous phase was transferred to a new tube and 0.5 ml of isopropanol was added. The tubes were incubated at room temperature for 10 min, centrifuged at 12,000 × g for 10 min and the pellet washed with 75% ethanol and recentrifuged. The pellet was air dried and resuspended in 100 μl of H2O. All the experiments were done with diethyl pyrocarbonate (DEPC) treated H2O. DNA free RNA was prepared by treating total RNA with DNase RQ1 (Promega). The OD260 and OD280 were measured and the integrity of the total RNA was visualized by subjecting 2–5 μl of the sample to electrophoresis through a denaturing 1% agarose/2.2 M formaldehyde gel. The total RNA preparation isolated was stored at -80°C till further use.
Reverse transcription PCR (RT-PCR)
The nucleotide sequence of the oligonucleotide primers used for the RT-PCR was taken from CGD http://www.candidagenome.org. Total RNA isolated from SC5314 (as described above) was enriched with poly(A)+ (polyadenylated) mRNA using the Oligotex mRNA Mini Kit protocol (Qiagen) and used subsequently for performing the reverse transcription reaction as described elsewhere [65]. To synthesize cDNA, ca. 0.1 μg of poly(A)+ RNA was placed in a 0.5 ml reaction tube with 1 μM of oligo(dT)18 anchor primer stock and the volume was adjusted to 11 μl with DEPC treated water. The mixture was incubated for 10 min at 70°C and chilled on ice for 1 min, after which the remainder of the reaction mixture was added from a master mix to the reaction tube in order for each reaction to contain a 1 mM concentration each of dATP, dCTP, dGTP and dTTP; 40 U of RNase inhibitor in a buffer consisting of 50 mM Tris-HCl (pH 8.3), 50 mM KCl, 4 mM MgCl2 and 10 mM DTT. After brief mixing, the reaction was incubated for 10 min at 37°C followed by addition of 40U of M-MuLV reverse transcriptase. Finally, the reaction was incubated at 37°C for 60 min and then stopped by heating at 70°C for 10 min followed by chilling it on ice for 1 min. The synthesized cDNA was purified from unincorporated dNTPs, oligo(dT)18 anchor primer and proteins by using Oligotex mRNA Mini Kit. Amplification of specific mRNA of each gene was performed using corresponding appropriate dilution of cDNA as template (generally 1:4) and 1 μM of each specific forward and reverse PCR primer as mentioned in Table 1. (parameters: initial denaturation of 95°C for 5 min followed by 35 cycles denaturation at 95°C for 15 s, annealing at 55°C for 30 s, elongation at 72°C for 30 s and final extension at 72°C for 10 min). As a positive control, MDR1 specific forward MDR1-F and reverse MDR1-R primer (corresponding to positions 1038–1396 in the MDR1 genomic sequence) was also used. The negative control (without RT) established that the PCR products generated in the RT-PCR were not due to genomic DNA contamination (data not shown). Resulting RT-PCR products were electrophoresed on a 1.2% agarose gel in 1× TAE.
Authors' contributions
MG identified and annotated MFS proteins in C. albicans and wrote the manuscript. NP and VR facilitated the bioinformatics analyses, particularly with respect to accessing and extracting database information and domain identification. RM was responsible for the experimental part. GM took part in the revision of this article. DC contributed expert knowledge and participated in the design and coordination of the study. RP conceived the study and helped to draft the manuscript. All authors read and approved the final manuscript.
Supplementary Material
A summary of C. albicans potential MFS genes listing TC family designation, CGD ORF, gene, alias, TCDB homolog and expression confirmation along with the closest S. cerevisiae member within the TC family. a Saier's Transport Commission (TC). b CGD ORF number http://www.candidagenome.org/. c TCDB homolog obtained from BLAST searches in the transporter database http://www.tcdb.org. d Systematic search for S. cerevisiae homologues of the proteins was done with each gene by using SGD BLASTP tool http://www.yeastgenome.org/. e Expression Confirmation – M: Microarray, N: Northern Analysis, R: RT-PCR, MS: Mass Spectroscopy. The data has been analysed from the genome/proteome wide studies on C. albicans [16,23,66-71]. Bold, Italics ORFs indicate genes which were experimentally tested by RT-PCR in the present study. * Genes with a strong homolog in fungi but absent from human and murine genomes [28].
Acknowledgments
Acknowledgements
The work presented in this paper has been supported in parts to RP by grants from Department of Biotechnology, (DBT/PR4862/BRB/10/360/2004), Council of Scientific and Industrial Research (38(1122)/06/EMR-II, 22/3/2006), Department of Science and Technology (SR/SO/BB-12/2004, 5/9/2005) and Indo-French Centre for the Promotion of Advanced Research (IFC/A/3403-2/2006). MG and NP acknowledge the University Grants Commission, India and Indian Council for Medical Research, respectively, for the support in the form of junior and senior research fellowships.
Contributor Information
Manisha Gaur, Email: manisha1310@rediffmail.com.
Nidhi Puri, Email: nidhi_puri@rediffmail.com.
Raman Manoharlal, Email: ramanbiotech@gmail.com.
Versha Rai, Email: versharai@yahoo.com.
Gauranga Mukhopadhayay, Email: gm2300@mail.jnu.ac.in.
Devapriya Choudhury, Email: devach@mail.jnu.ac.in.
Rajendra Prasad, Email: rp47@mail.jnu.ac.in.
References
- Mukhopadhyay K, Prasad T, Saini P, Pucadyil TJ, Chattopadhyay A, Prasad R. Membrane sphingolipid-ergosterol interactions are important determinants of multidrug resistance in Candida albicans. Antimicrob Agents Chemother. 2004;48:1778–1787. doi: 10.1128/AAC.48.5.1778-1787.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasrija R, Krishnamurthy S, Prasad T, Ernst JF, Prasad R. Squalene epoxidase encoded by ERG1 affects morphogenesis and drug susceptibilities of Candida albicans. J Antimicrob Chemother. 2005;55:905–913. doi: 10.1093/jac/dki112. [DOI] [PubMed] [Google Scholar]
- Prasad T, Saini P, Gaur NA, Vishwakarma RA, Khan LA, Haq QM, Prasad R. Functional analysis of CaIPT1, a sphingolipid biosynthetic gene involved in multidrug resistance and morphogenesis of Candida albicans. Antimicrob Agents Chemother. 2005;49:3442–3452. doi: 10.1128/AAC.49.8.3442-3452.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad R, Gaur NA, Gaur M, Komath SS. Efflux pumps in drug resistance of Candida. Infect Disord Drug Targets. 2006;6:69–83. doi: 10.2174/187152606784112164. [DOI] [PubMed] [Google Scholar]
- Lopez-Ribot JL, McAtee RK, Lee LN, Kirkpatrick WR, White TC, Sanglard D, Patterson TF. Distinct patterns of gene expression associated with development of fluconazole resistance in serial Candida albicans isolates from human immunodeficiency virus-infected patients with oropharyngeal candidiasis. Antimicrob Agents Chemother. 1998;42:2932–2937. doi: 10.1128/aac.42.11.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harry JB, Song JL, Lyons CN, White TC. Transcription initiation of genes associated with azole resistance in Candida albicans. Med Mycol. 2002;40:73–81. doi: 10.1080/mmy.40.1.73.81. [DOI] [PubMed] [Google Scholar]
- Prasad R, De WP, Goffeau A, Balzi E. Molecular cloning and characterization of a novel gene of Candida albicans, CDR1, conferring multiple resistance to drugs and antifungals. Curr Genet. 1995;27:320–329. doi: 10.1007/BF00352101. [DOI] [PubMed] [Google Scholar]
- Sanglard D, Kuchler K, Ischer F, Pagani JL, Monod M, Bille J. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob Agents Chemother. 1995;39:2378–2386. doi: 10.1128/aac.39.11.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TC. Increased mRNA levels of ERG16, CDR, and MDR1 correlate with increases in azole resistance in Candida albicans isolates from a patient infected with human immunodeficiency virus. Antimicrob Agents Chemother. 1997;41:1482–1487. doi: 10.1128/aac.41.7.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaur M, Choudhury D, Prasad R. Complete inventory of ABC proteins in human pathogenic yeast, Candida albicans. J Mol Microbiol Biotechnol. 2005;9:3–15. doi: 10.1159/000088141. [DOI] [PubMed] [Google Scholar]
- Pao SS, Paulsen IT, Saier MH., Jr Major facilitator superfamily. Microbiol Mol Biol Rev. 1998;62:1–34. doi: 10.1128/mmbr.62.1.1-34.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen IT, Sliwinski MK, Nelissen B, Goffeau A, Saier MH., Jr Unified inventory of established and putative transporters encoded within the complete genome of Saccharomyces cerevisiae. FEBS Lett. 1998;430:116–125. doi: 10.1016/s0014-5793(98)00629-2. [DOI] [PubMed] [Google Scholar]
- Saier MH., Jr A functional-phylogenetic system for the classification of transport proteins. J Cell Biochem. 1999:84–94. doi: 10.1002/(sici)1097-4644(1999)75:32+<84::aid-jcb11>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Saier MH., Jr Molecular phylogeny as a basis for the classification of transport proteins from bacteria, archaea and eukarya. Adv Microb Physiol. 1998;40:81–136. doi: 10.1016/s0065-2911(08)60130-7. [DOI] [PubMed] [Google Scholar]
- Busch W, Saier MH., Jr The transporter classification (TC) system, 2002. Crit Rev Biochem Mol Biol. 2002;37:287–337. doi: 10.1080/10409230290771528. [DOI] [PubMed] [Google Scholar]
- Alvarez FJ, Konopka JB. Identification of an N-acetylglucosamine transporter that mediates hyphal induction in Candida albicans. Mol Biol Cell. 2007;18:965–975. doi: 10.1091/mbc.E06-10-0931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese D, Bille J, Sanglard D. A novel multidrug efflux transporter gene of the major facilitator superfamily from Candida albicans (FLU1) conferring resistance to fluconazole. Microbiology. 2000;146:2743–2754. doi: 10.1099/00221287-146-11-2743. [DOI] [PubMed] [Google Scholar]
- Hu CJ, Bai C, Zheng XD, Wang YM, Wang Y. Characterization and functional analysis of the siderophore-iron transporter CaArn1p in Candida albicans. J Biol Chem. 2002;277:30598–30605. doi: 10.1074/jbc.M204545200. [DOI] [PubMed] [Google Scholar]
- Pasrija R, Banerjee D, Prasad R. Structure and function analysis of CaMdr1p, a major facilitator superfamily antifungal efflux transporter protein of Candida albicans: identification of amino acid residues critical for drug/H+ transport. Eukaryot Cell. 2007;6:443–453. doi: 10.1128/EC.00315-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli A, Gupta V, Krishnamurthy S, Hasnain SE, Prasad R. Specificity of drug transport mediated by CaMDR1: a major facilitator of Candida albicans. J Biosci. 2001;26:333–339. doi: 10.1007/BF02703742. [DOI] [PubMed] [Google Scholar]
- Sengupta M, Datta A. Two membrane proteins located in the Nag regulon of Candida albicans confer multidrug resistance. Biochem Biophys Res Commun. 2003;301:1099–1108. doi: 10.1016/s0006-291x(03)00094-9. [DOI] [PubMed] [Google Scholar]
- Soares-Silva I, Paiva S, Kotter P, Entian KD, Casal M. The disruption of JEN1 from Candida albicans impairs the transport of lactate. Mol Membr Biol. 2004;21:403–411. doi: 10.1080/09687860400011373. [DOI] [PubMed] [Google Scholar]
- Fan J, Chaturvedi V, Shen SH. Identification and phylogenetic analysis of a glucose transporter gene family from the human pathogenic yeast Candida albicans. J Mol Evol. 2002;55:336–346. doi: 10.1007/s00239-002-2330-4. [DOI] [PubMed] [Google Scholar]
- Ben-Yaacov R, Knoller S, Caldwell GA, Becker JM, Koltin Y. Candida albicans gene encoding resistance to benomyl and methotrexate is a multidrug resistance gene. Antimicrob Agents Chemother. 1994;38:648–652. doi: 10.1128/aac.38.4.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fling ME, Kopf J, Tamarkin A, Gorman JA, Smith HA, Koltin Y. Analysis of a Candida albicans gene that encodes a novel mechanism for resistance to benomyl and methotrexate. Mol Gen Genet. 1991;227:318–329. doi: 10.1007/BF00259685. [DOI] [PubMed] [Google Scholar]
- Becker JM, Henry LK, Jiang W, Koltin Y. Reduced virulence of Candida albicans mutants affected in multidrug resistance. Infect Immun. 1995;63:4515–4518. doi: 10.1128/iai.63.11.4515-4518.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta V, Kohli A, Krishnamurthy S, Puri N, Aalamgeer SA, Panwar S, Prasad R. Identification of polymorphic mutant alleles of CaMDR1, a major facilitator of Candida albicans which confers multidrug resistance, and its in vitro transcriptional activation. Curr Genet. 1998;34:192–199. doi: 10.1007/s002940050385. [DOI] [PubMed] [Google Scholar]
- Braun BR, van Het Hoog M, d'Enfert C, Martchenko M, Dungan J, Kuo A, Inglis DO, Uhl MA, Hogues H, Berriman M, Lorenz M, Levitin A, Oberholzer U, Bachewich C, Harcus D, Marcil A, Dignard D, Iouk T, Zito R, Frangeul L, Tekaia F, Rutherford K, Wang E, Munro CA, Bates S, Gow NA, Hoyer LL, Kohler G, Morschhauser J, Newport G, Znaidi S, Raymond M, Turcotte B, Sherlock G, Costanzo M, Ihmels J, Berman J, Sanglard D, Agabian N, Mitchell AP, Johnson AD, Whiteway M, Nantel A. A human-curated annotation of the Candida albicans genome. PLoS Genet. 2005;1:36–57. doi: 10.1371/journal.pgen.0010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma A, Singh BB, Karnani N, Lichtenberg-Frate H, Hofer M, Magee BB, Prasad R. Molecular cloning and functional characterisation of a glucose transporter, CaHGT1, of Candida albicans. FEMS Microbiol Lett. 2000;182:15–21. doi: 10.1111/j.1574-6968.2000.tb08866.x. [DOI] [PubMed] [Google Scholar]
- Palma M, Goffeau A, Spencer-Martins I, Baret PV. A phylogenetic analysis of the sugar porters in hemiascomycetous yeasts. J Mol Microbiol Biotechnol. 2007;12:241–248. doi: 10.1159/000099645. [DOI] [PubMed] [Google Scholar]
- Goldway M, Teff D, Schmidt R, Oppenheim AB, Koltin Y. Multidrug resistance in Candida albicans: disruption of the BENr gene. Antimicrob Agents Chemother. 1995;39:422–426. doi: 10.1128/aac.39.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gompel-Klein P, Brendel M. Allelism of SNQ1 and ATR1, genes of the yeast Saccharomyces cerevisiae required for controlling sensitivity to 4-nitroquinoline-N-oxide and aminotriazole. Curr Genet. 1990;18:93–96. doi: 10.1007/BF00321122. [DOI] [PubMed] [Google Scholar]
- Kanazawa S, Driscoll M, Struhl K. ATR1, a Saccharomyces cerevisiae gene encoding a transmembrane protein required for aminotriazole resistance. Mol Cell Biol. 1988;8:664–673. doi: 10.1128/mcb.8.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenhofer-Murray AE, Wurgler FE, Sengstag C. The Saccharomyces cerevisiae SGE1 gene product: a novel drug-resistance protein within the major facilitator superfamily. Mol Gen Genet. 1994;244:287–294. doi: 10.1007/BF00285456. [DOI] [PubMed] [Google Scholar]
- Amakasu H, Suzuki Y, Nishizawa M, Fukasawa T. Isolation and characterization of SGE1: a yeast gene that partially suppresses the gal11 mutation in multiple copies. Genetics. 1993;134:675–683. doi: 10.1093/genetics/134.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffeau A, Park J, Paulsen IT, Jonniaux JL, Dinh T, Mordant P, Saier MH., Jr Multidrug-resistant transport proteins in yeast: complete inventory and phylogenetic characterization of yeast open reading frames with the major facilitator superfamily. Yeast. 1997;13:43–54. doi: 10.1002/(SICI)1097-0061(199701)13:1<43::AID-YEA56>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Namy O, Duchateau-Nguyen G, Hatin I, Hermann-Le DS, Termier M, Rousset JP. Identification of stop codon readthrough genes in Saccharomyces cerevisiae. Nucleic Acids Res. 2003;31:2289–2296. doi: 10.1093/nar/gkg330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bun-Ya M, Nishimura M, Harashima S, Oshima Y. The PHO84 gene of Saccharomyces cerevisiae encodes an inorganic phosphate transporter. Mol Cell Biol. 1991;11:3229–3238. doi: 10.1128/mcb.11.6.3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison MJ, van Buuren ML. A phosphate transporter from the mycorrhizal fungus Glomus versiforme. Nature. 1995;378:626–629. doi: 10.1038/378626a0. [DOI] [PubMed] [Google Scholar]
- Abe K, Ruan ZS, Maloney PC. Cloning, sequencing, and expression in Escherichia coli of OxlT, the oxalate:formate exchange protein of Oxalobacter formigenes. J Biol Chem. 1996;271:6789–6793. doi: 10.1074/jbc.271.12.6789. [DOI] [PubMed] [Google Scholar]
- Anantharam V, Allison MJ, Maloney PC. Oxalate:formate exchange. The basis for energy coupling in Oxalobacter. J Biol Chem. 1989;264:7244–7250. [PubMed] [Google Scholar]
- Fu D, Maloney PC. Evaluation of secondary structure of OxlT, the oxalate transporter of Oxalobacter formigenes, by circular dichroism spectroscopy. J Biol Chem. 1997;272:2129–2135. doi: 10.1074/jbc.272.4.2129. [DOI] [PubMed] [Google Scholar]
- Ruan ZS, Anantharam V, Crawford IT, Ambudkar SV, Rhee SY, Allison MJ, Maloney PC. Identification, purification, and reconstitution of OxlT, the oxalate: formate antiport protein of Oxalobacter formigenes. J Biol Chem. 1992;267:10537–10543. [PubMed] [Google Scholar]
- Lodi T, Diffels J, Goffeau A, Baret PV. Evolution of the carboxylate Jen transporters in fungi. FEMS Yeast Res. 2007;7:646–656. doi: 10.1111/j.1567-1364.2007.00245.x. [DOI] [PubMed] [Google Scholar]
- Makuc J, Paiva S, Schauen M, Kramer R, Andre B, Casal M, Leao C, Boles E. The putative monocarboxylate permeases of the yeast Saccharomyces cerevisiae do not transport monocarboxylic acids across the plasma membrane. Yeast. 2001;18:1131–1143. doi: 10.1002/yea.763. [DOI] [PubMed] [Google Scholar]
- Yun CW, Ferea T, Rashford J, Ardon O, Brown PO, Botstein D, Kaplan J, Philpott CC. Desferrioxamine-mediated iron uptake in Saccharomyces cerevisiae. Evidence for two pathways of iron uptake. J Biol Chem. 2000;275:10709–10715. doi: 10.1074/jbc.275.14.10709. [DOI] [PubMed] [Google Scholar]
- Heymann P, Gerads M, Schaller M, Dromer F, Winkelmann G, Ernst JF. The siderophore iron transporter of Candida albicans (Sit1p/Arn1p) mediates uptake of ferrichrome-type siderophores and is required for epithelial invasion. Infect Immun. 2002;70:5246–5255. doi: 10.1128/IAI.70.9.5246-5255.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundemann D, Gorboulev V, Gambaryan S, Veyhl M, Koepsell H. Drug excretion mediated by a new prototype of polyspecific transporter. Nature. 1994;372:549–552. doi: 10.1038/372549a0. [DOI] [PubMed] [Google Scholar]
- Urakami Y, Okuda M, Masuda S, Saito H, Inui KI. Functional characteristics and membrane localization of rat multispecific organic cation transporters, OCT1 and OCT2, mediating tubular secretion of cationic drugs. J Pharmacol Exp Ther. 1998;287:800–805. [PubMed] [Google Scholar]
- Kekuda R, Prasad PD, Wu X, Wang H, Fei YJ, Leibach FH, Ganapathy V. Cloning and functional characterization of a potential-sensitive, polyspecific organic cation transporter (OCT3) most abundantly expressed in placenta. J Biol Chem. 1998;273:15971–15979. doi: 10.1074/jbc.273.26.15971. [DOI] [PubMed] [Google Scholar]
- Koepsell H, Busch A, Gorboulev V, Arndt P. Structure and Function of Renal Organic Cation Transporters. News Physiol Sci. 1998;13:11–16. doi: 10.1152/physiologyonline.1998.13.1.11. [DOI] [PubMed] [Google Scholar]
- Bindra PS, Knowles R, Buckley KM. Conservation of the amino acid sequence of SV2, a transmembrane transporter in synaptic vesicles and endocrine cells. Gene. 1993;137:299–302. doi: 10.1016/0378-1119(93)90024-w. [DOI] [PubMed] [Google Scholar]
- Bajjalieh SM, Peterson K, Shinghal R, Scheller RH. SV2, a brain synaptic vesicle protein homologous to bacterial transporters. Science. 1992;257:1271–1273. doi: 10.1126/science.1519064. [DOI] [PubMed] [Google Scholar]
- Gingrich JA, Andersen PH, Tiberi M, el MS, Jorgensen PN, Fremeau RT, Jr, Caron MG. Identification, characterization, and molecular cloning of a novel transporter-like protein localized to the central nervous system. FEBS Lett. 1992;312:115–122. doi: 10.1016/0014-5793(92)80917-6. [DOI] [PubMed] [Google Scholar]
- Janz R, Hofmann K, Sudhof TC. SVOP, an evolutionarily conserved synaptic vesicle protein, suggests novel transport functions of synaptic vesicles. J Neurosci. 1998;18:9269–9281. doi: 10.1523/JNEUROSCI.18-22-09269.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamori A, Nakayama J, Fukuda MN, Stallcup WB, Sasaki K, Fukuda M, Hirabayashi Y. Expression cloning and characterization of a cDNA encoding a novel membrane protein required for the formation of O-acetylated ganglioside: a putative acetyl-CoA transporter. Proc Natl Acad Sci USA. 1997;94:2897–2902. doi: 10.1073/pnas.94.7.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S, Weston-Hafer K, Schmidt H, Pul C, Korfmann G, Erickson J, Sanders C, Martin HH, Normark S. AmpG, a signal transducer in chromosomal beta-lactamase induction. Mol Microbiol. 1993;9:703–715. doi: 10.1111/j.1365-2958.1993.tb01731.x. [DOI] [PubMed] [Google Scholar]
- Shayeghi M, Latunde-Dada GO, Oakhill JS, Laftah AH, Takeuchi K, Halliday N, Khan Y, Warley A, McCann FE, Hider RC, Frazer DM, Anderson GJ, Vulpe CD, Simpson RJ, McKie AT. Identification of an intestinal heme transporter. Cell. 2005;122:789–801. doi: 10.1016/j.cell.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Latunde-Dada GO, Takeuchi K, Simpson RJ, McKie AT. Haem carrier protein 1 (HCP1): Expression and functional studies in cultured cells. FEBS Lett. 2006;580:6865–6870. doi: 10.1016/j.febslet.2006.11.048. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulo N, Bairoch A, Bulliard V, Cerutti L, De CE, Langendijk-Genevaux PS, Pagni M, Sigrist CJ. The PROSITE database. Nucleic Acids Res. 2006;34:D227–D230. doi: 10.1093/nar/gkj063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder NJ, Apweiler R. The InterPro database and tools for protein domain analysis. Curr Protoc Bioinformatics. 2008;Chapter 2 doi: 10.1002/0471250953.bi0207s21. [DOI] [PubMed] [Google Scholar]
- Marchler-Bauer A, Anderson JB, Cherukuri PF, Weese-Scott C, Geer LY, Gwadz M, He S, Hurwitz DI, Jackson JD, Ke Z, Lanczycki CJ, Liebert CA, Liu C, Lu F, Marchler GH, Mullokandov M, Shoemaker BA, Simonyan V, Song JS, Thiessen PA, Yamashita RA, Yin JJ, Zhang D, Bryant SH. CDD: a Conserved Domain Database for protein classification. Nucleic Acids Res. 2005;33:D192–D196. doi: 10.1093/nar/gki069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier MH, Jr, Tran CV, Barabote RD. TCDB: the Transporter Classification Database for membrane transport protein analyses and information. Nucleic Acids Res. 2006;34:D181–D186. doi: 10.1093/nar/gkj001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerads M, Ernst JF. Overlapping coding regions and trancriptional units of two essential chromosomal genes (CCT8, TRP1) in the fungal pathogen Candida albicans. Nucleic Acids Res. 1998;26:5061–5066. doi: 10.1093/nar/26.22.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Jiang B, Ketela T, Lemieux S, Veillette K, Martel N, Davison J, Sillaots S, Trosok S, Bachewich C, Bussey H, Youngman P, Roemer T. Genome-wide fitness test and mechanism-of-action studies of inhibitory compounds in Candida albicans. PLoS Pathog. 2007;3:e92. doi: 10.1371/journal.ppat.0030092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepak A, Nett J, Lincoln L, Marchillo K, Andes D. Time course of microbiologic outcome and gene expression in Candida albicans during and following in vitro and in vivo exposure to fluconazole. Antimicrob Agents Chemother. 2006;50:1311–1319. doi: 10.1128/AAC.50.4.1311-1319.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett RJ, Johnson AD. The role of nutrient regulation and the Gpa2 protein in the mating pheromone response of C. albicans. Mol Microbiol. 2006;62:100–119. doi: 10.1111/j.1365-2958.2006.05367.x. [DOI] [PubMed] [Google Scholar]
- Liu TT, Lee RE, Barker KS, Lee RE, Wei L, Homayouni R, Rogers PD. Genome-wide expression profiling of the response to azole, polyene, echinocandin, and pyrimidine antifungal agents in Candida albicans. Antimicrob Agents Chemother. 2005;49:2226–2236. doi: 10.1128/AAC.49.6.2226-2236.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensen ES, Martin SJ, Li M, Berman J, Davis DA. Transcriptional profiling in Candida albicans reveals new adaptive responses to extracellular pH and functions for Rim101p. Mol Microbiol. 2004;54:1335–1351. doi: 10.1111/j.1365-2958.2004.04350.x. [DOI] [PubMed] [Google Scholar]
- Hromatka BS, Noble SM, Johnson AD. Transcriptional response of Candida albicans to nitric oxide and the role of the YHB1 gene in nitrosative stress and virulence. Mol Biol Cell. 2005;16:4814–4826. doi: 10.1091/mbc.E05-05-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A summary of C. albicans potential MFS genes listing TC family designation, CGD ORF, gene, alias, TCDB homolog and expression confirmation along with the closest S. cerevisiae member within the TC family. a Saier's Transport Commission (TC). b CGD ORF number http://www.candidagenome.org/. c TCDB homolog obtained from BLAST searches in the transporter database http://www.tcdb.org. d Systematic search for S. cerevisiae homologues of the proteins was done with each gene by using SGD BLASTP tool http://www.yeastgenome.org/. e Expression Confirmation – M: Microarray, N: Northern Analysis, R: RT-PCR, MS: Mass Spectroscopy. The data has been analysed from the genome/proteome wide studies on C. albicans [16,23,66-71]. Bold, Italics ORFs indicate genes which were experimentally tested by RT-PCR in the present study. * Genes with a strong homolog in fungi but absent from human and murine genomes [28].