Abstract
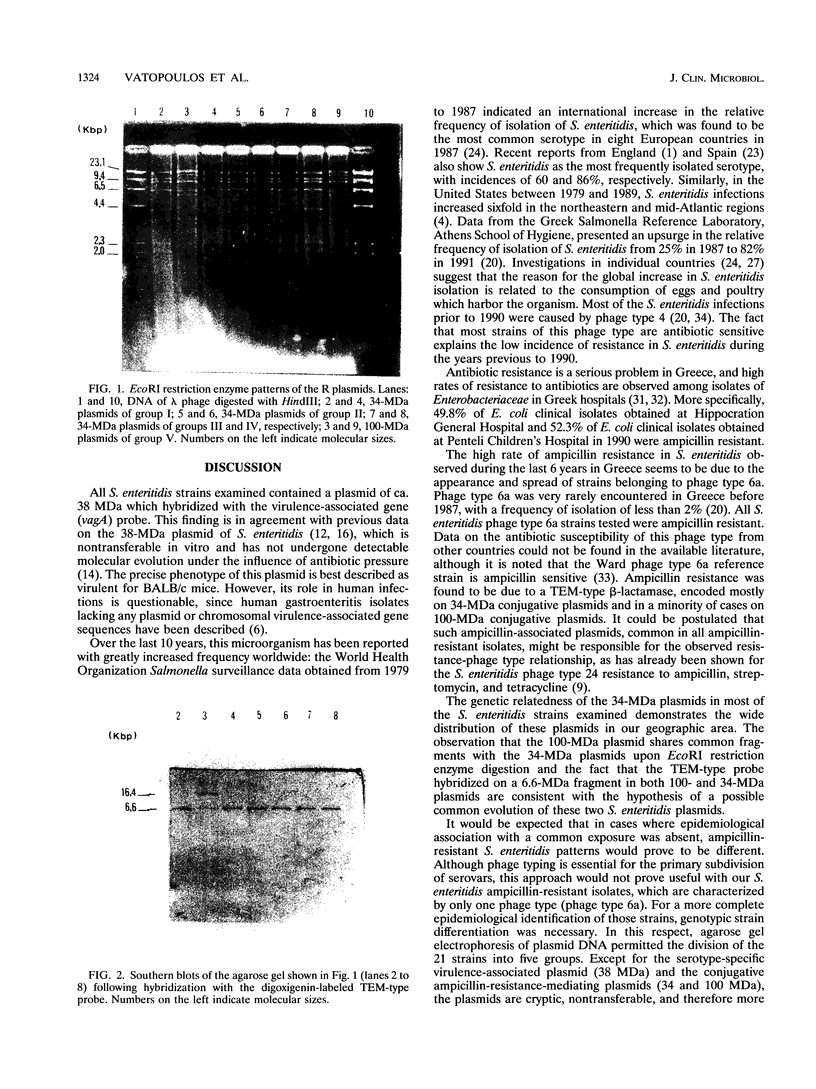
During the last 6 years in Greece, there has been a significant increase in the number of ampicillin-resistant Salmonella clinical isolates reported. In this study 23 ampicillin-resistant Salmonella strains, consecutively isolated from patients with epidemiologically unrelated cases of food poisoning, were investigated. By serotyping and phage typing, 21 of these strains were identified as Salmonella enteritidis phage type 6a, 1 was identified as Salmonella typhimurium, and 1 was identified as Salmonella saintpaul. By plasmid pattern analysis, the 21 S. enteritidis strains were further differentiated into five groups. Group I consisted of 5 strains (carrying two plasmids of ca. 38 and 34 MDa), group II consisted of 10 strains (three plasmids of ca. 38, 34, and 2.5 MDa), group III consisted of 3 strains (four plasmids of ca. 38, 34, 15, and 2.5 MDa), group IV consisted of 1 strain (five plasmids of ca. 100, 38, 34, 24, and 15 MDa), and group V consisted of 2 strains (three plasmids of ca. 100, 38, and 24 MDa). Ampicillin resistance was easily transferred to Escherichia coli and was associated with the transfer of the 34-MDa plasmid, classified in the N incompatibility group for all strains of groups I to IV, and with the transfer of the 100-MDa plasmid for the group V strains. EcoRI restriction endonuclease digestions showed an extensive homology among the 34-MDa conjugative R plasmids. Hybridizations of the EcoRI restriction fragments of the 34-MDa plasmids with a TEM-type probe revealed the locus of the beta-lactamase gene to be situated on a ca. 6.6-MDa fragment, common in all plasmids. These results indicate that ampicillin resistance in Greece is due to the spread of a limited number of clones of S. enteritidis phage type 6A, carrying related 34-MDa R plasmids. Work is in progress to obtain a better understanding of ampicillin resistance in S. enteritidis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archer D. L., Young F. E. Contemporary issues: diseases with a food vector. Clin Microbiol Rev. 1988 Oct;1(4):377–398. doi: 10.1128/cmr.1.4.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burney D. P., Fisher R. D., Schaffner W. Salmonella empyema: a review. South Med J. 1977 Mar;70(3):375–377. doi: 10.1097/00007611-197703000-00041. [DOI] [PubMed] [Google Scholar]
- Chalker R. B., Blaser M. J. A review of human salmonellosis: III. Magnitude of Salmonella infection in the United States. Rev Infect Dis. 1988 Jan-Feb;10(1):111–124. doi: 10.1093/clinids/10.1.111. [DOI] [PubMed] [Google Scholar]
- Chart H., Rowe B. Antibodies to lipopolysaccharide and outer membrane proteins of Salmonella enteritidis PT4 are not involved in protection from experimental infection. FEMS Microbiol Lett. 1991 Dec 1;68(3):345–350. doi: 10.1016/0378-1097(91)90380-s. [DOI] [PubMed] [Google Scholar]
- Chusid M. J., Dunigan T. H., Lewis D. S. Salmonella meningitis in infancy. Wis Med J. 1980 Mar;79(3):23–25. [PubMed] [Google Scholar]
- Frost J. A., Ward L. R., Rowe B. Acquisition of a drug resistance plasmid converts Salmonella enteritidis phage type 4 to phage type 24. Epidemiol Infect. 1989 Oct;103(2):243–248. doi: 10.1017/s0950268800030594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill G. V. Endocarditis caused by Salmonella enteritidis. Br Heart J. 1979 Sep;42(3):353–354. doi: 10.1136/hrt.42.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffernan H. M. Antibiotic resistance among Salmonella from human and other sources in New Zealand. Epidemiol Infect. 1991 Feb;106(1):17–23. doi: 10.1017/s0950268800056405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmuth R., Stephan R., Bunge C., Hoog B., Steinbeck A., Bulling E. Epidemiology of virulence-associated plasmids and outer membrane protein patterns within seven common Salmonella serotypes. Infect Immun. 1985 Apr;48(1):175–182. doi: 10.1128/iai.48.1.175-182.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg S. D., Osterholm M. T., Senger K. A., Cohen M. L. Drug-resistant Salmonella from animals fed antimicrobials. N Engl J Med. 1984 Sep 6;311(10):617–622. doi: 10.1056/NEJM198409063111001. [DOI] [PubMed] [Google Scholar]
- Jones C., Stanley J. Salmonella plasmids of the pre-antibiotic era. J Gen Microbiol. 1992 Jan;138(1):189–197. doi: 10.1099/00221287-138-1-189. [DOI] [PubMed] [Google Scholar]
- Nakamura M., Sato S., Ohya T., Suzuki S., Ikeda S. Possible relationship of a 36-megadalton Salmonella enteritidis plasmid to virulence in mice. Infect Immun. 1985 Mar;47(3):831–833. doi: 10.1128/iai.47.3.831-833.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen J. E. An improved method for rapid isolation of plasmid DNA from wild-type gram-negative bacteria for plasmid restriction profile analysis. Lett Appl Microbiol. 1990 May;10(5):209–212. doi: 10.1111/j.1472-765x.1990.tb01335.x. [DOI] [PubMed] [Google Scholar]
- Ortiz-Neu C., Marr J. S., Cherubin C. E., Neu H. C. Bone and joint infections due to Salmonella. J Infect Dis. 1978 Dec;138(6):820–828. doi: 10.1093/infdis/138.6.820. [DOI] [PubMed] [Google Scholar]
- Paul G. C., Gerbaud G., Bure A., Philippon A. M., Pangon B., Courvalin P. TEM-4, a new plasmid-mediated beta-lactamase that hydrolyzes broad-spectrum cephalosporins in a clinical isolate of Escherichia coli. Antimicrob Agents Chemother. 1989 Nov;33(11):1958–1963. doi: 10.1128/aac.33.11.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera M. J., Rivera N., Castillo J., Rubio M. C., Gómez-Lus R. Molecular and epidemiological study of Salmonella clinical isolates. J Clin Microbiol. 1991 May;29(5):927–932. doi: 10.1128/jcm.29.5.927-932.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigue D. C., Tauxe R. V., Rowe B. International increase in Salmonella enteritidis: a new pandemic? Epidemiol Infect. 1990 Aug;105(1):21–27. doi: 10.1017/s0950268800047609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spies U., Müller H., Becht H. Nucleotide sequence of infectious bursal disease virus genome segment A delineates two major open reading frames. Nucleic Acids Res. 1989 Oct 11;17(19):7982–7982. doi: 10.1093/nar/17.19.7982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S., Nagano Y. Rapid procedure for isolation of plasmid DNA and application to epidemiological analysis. J Clin Microbiol. 1984 Oct;20(4):608–613. doi: 10.1128/jcm.20.4.608-613.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. N., Wachsmuth I. K., Shangkuan Y. H., Schmidt E. V., Barrett T. J., Schrader J. S., Scherach C. S., McGee H. B., Feldman R. A., Brenner D. J. Salmonellosis associated with marijuana: a multistate outbreak traced by plasmid fingerprinting. N Engl J Med. 1982 May 27;306(21):1249–1253. doi: 10.1056/NEJM198205273062101. [DOI] [PubMed] [Google Scholar]
- Telzak E. E., Budnick L. D., Greenberg M. S., Blum S., Shayegani M., Benson C. E., Schultz S. A nosocomial outbreak of Salmonella enteritidis infection due to the consumption of raw eggs. N Engl J Med. 1990 Aug 9;323(6):394–397. doi: 10.1056/NEJM199008093230607. [DOI] [PubMed] [Google Scholar]
- Threlfall E. J., Frost J. A. The identification, typing and fingerprinting of Salmonella: laboratory aspects and epidemiological applications. J Appl Bacteriol. 1990 Jan;68(1):5–16. doi: 10.1111/j.1365-2672.1990.tb02542.x. [DOI] [PubMed] [Google Scholar]
- Threlfall E. J., Hall M. L., Rowe B. Salmonella gold-coast from outbreaks of food-poisoning in the British Isles can be differentiated by plasmid profiles. J Hyg (Lond) 1986 Aug;97(1):115–122. doi: 10.1017/s0022172400064408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfall E. J., Rowe B., Ferguson J. L., Ward L. R. Characterization of plasmids conferring resistance to gentamicin and apramycin in strains of Salmonella typhimurium phage type 204c isolated in Britain. J Hyg (Lond) 1986 Dec;97(3):419–426. doi: 10.1017/s0022172400063609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakris A., Vatopoulos A. C., Johnson A. P., Pitt T. L., Legakis N. J., Tzouvelekis L. S. Prevalence of a plasmid-mediated type II dihydrofolate reductase gene among trimethoprim-resistant urinary pathogens in Greek hospitals. J Antimicrob Chemother. 1992 Apr;29(4):405–413. doi: 10.1093/jac/29.4.405. [DOI] [PubMed] [Google Scholar]
- Vatopoulos A. C., Philippon A., Tzouvelekis L. S., Komninou Z., Legakis N. J. Prevalence of a transferable SHV-5 type beta-lactamase in clinical isolates of Klebsiella pneumoniae and Escherichia coli in Greece. J Antimicrob Chemother. 1990 Nov;26(5):635–648. doi: 10.1093/jac/26.5.635. [DOI] [PubMed] [Google Scholar]
- Ward L. R., Threlfall E. J., Rowe B. Multiple drug resistance in salmonellae in England and Wales: a comparison between 1981 and 1988. J Clin Pathol. 1990 Jul;43(7):563–566. doi: 10.1136/jcp.43.7.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward L. R., de Sa J. D., Rowe B. A phage-typing scheme for Salmonella enteritidis. Epidemiol Infect. 1987 Oct;99(2):291–294. doi: 10.1017/s0950268800067765. [DOI] [PMC free article] [PubMed] [Google Scholar]




