Abstract
Objective
To characterize the prevalence of amyloid deposition in a clinically unimpaired elderly population, as assessed by Pittsburgh Compound B (PiB) positron emission tomography (PET) imaging, and its relationship to cognitive function, measured with a battery of neuropsychological tests.
Design
Subjects underwent cognitive testing and PiB PET imaging (15 mCi for 90 minutes with an ECAT HR + scanner). Logan graphical analysis was applied to estimate regional PiB retention distribution volume, normalized to a cerebellar reference region volume, to yield distribution volume ratios (DVRs).
Setting
University medical center.
Participants
From a community-based sample of volunteers, 43 participants aged 65 to 88 years who did not meet diagnostic criteria for Alzheimer disease or mild cognitive impairment were included.
Main Outcome Measures
Regional PiB retention and cognitive test performance.
Results
Of 43 clinically unimpaired elderly persons imaged, 9 (21%) showed evidence of early amyloid deposition in at least 1 brain area using an objectively determined DVR cutoff. Demographic characteristics did not differ significantly between amyloid-positive and amyloid-negative participants, and neurocognitive performance was not significantly worse among amyloid-positive compared with amyloid-negative participants.
Conclusions
Amyloid deposition can be identified among cognitively normal elderly persons during life, and the prevalence of asymptomatic amyloid deposition may be similar to that of symptomatic amyloid deposition. In this group of participants without clinically significant impairment, amyloid deposition was not associated with worse cognitive function, suggesting that an elderly person with a significant amyloid burden can remain cognitively normal. However, this finding is based on relatively small numbers and needs to be replicated in larger cohorts. Longitudinal follow-up of these subjects will be required to support the potential of PiB imaging to identify preclinical Alzheimer disease, or, alternatively, to show that amyloid deposition is not sufficient to cause Alzheimer disease within some specified period.
Despite the strong association of amyloid burden and Alzheimer disease (AD), it has also long been recognized that a significant number of individuals without clinical evidence of AD have amyloid deposition at death.1 The ability to identify the proportion of cognitively normal elderly persons with amyloid in their brains, as well as the ability to characterize the relationship of amyloid deposition to cognition function, has historically been limited by the fact that amyloid load was typically determined at autopsy. Nevertheless, through autopsy studies, the prevalence of amyloid burden among cognitively normal elderly persons has been estimated to be more than 25%.2–4 This number rivals the combined prevalence of mild cognitive impairment (MCI) (approximately 19%5) and AD (approximately13%6). If true, this implies that approximately 50% of people older than 65 years have amyloid deposition in their brains and that about half of these people are asymptomatic. These possibilities have important implications when considering strategies for future prevention trials.
Recently, the development of amyloid imaging has made it possible to identify amyloid deposition in vivo.7 Recent studies have suggested that Pittsburgh Compound B (PiB) binds to fibrillar amyloid-β (Aβ) deposits in diffuse as well as dense core plaques and to amyloid angiopathy but not to amorphous Aβ deposits, such as those commonly seen in the cerebellum.8,9 The aim of the present study was to characterize the relationship of amyloid deposition with cognition among elderly persons using PiB positron emission tomography (PET) imaging. Specifically, we were interested in estimating the prevalence of significant amyloid deposition among clinically unimpaired elderly persons, then relating the degree of amyloid deposition to the variability of cognition often seen among older subjects. That is, even though participants were selected for the absence of either clinical MCI or AD, does the presence of amyloid deposition relate to the variability of cognition previously reported among elderly persons?10
If the presence of the Aβ protein is toxic,11 one might expect that the areas in the brain with the greatest amyloid burden would have the greatest change in neural function, and thus cognitive functions related to those regions would be selectively affected. Some neuropathological studies have found that amyloid burden is related to the degree of cognitive impairment in AD and that regional distribution of amyloid burden correlated with changes in the functions related to those areas.12,13 However, several other studies have failed to find correlations between amyloid plaque load and cognition.14,15 Postmortem studies are typically limited to relatively late-stage cases, and the cognitive measures available are often suboptimal owing to the timing of the tests or complications of the premorbid physical state. It is, therefore, possible that postmortem studies could miss correlations between amyloid deposition and cognitive functioning. With PiB PET amyloid imaging, it may be possible to more precisely determine the relationship between early regional amyloid deposition and cognition.
Several previous studies have reported elevated PiB retention among clinically unimpaired elderly persons. In the original report by Klunk et al,7 1 of 6 older controls had PiB retention in the AD range. Lopresti et al16 found 2 of their 8 controls to have elevated PiB retention. Mintun et al17 observed that 2 of 20 nondemented elderly persons had high PiB retention in the range of AD subjects, and an additional 2 had moderate PiB retention. Mintun et al did not find any difference in cognitive testing among amyloid-positive nondemented elderly participants compared with amyloid-negative nondemented elderly participants. Similarly, Rowe et al18 found that 6 of 27 elderly cognitively normal controls (22%) showed high cortical PiB retention despite neuropsychological scores within the normal range. A primary aim of the present study was to replicate and extend these observations. Specifically, our goals were to estimate what level of PiB retention should be considered amyloid-positive among cognitively unimpaired elderly persons, to assess the prevalence of amyloid deposition among clinically unimpaired elderly persons, and to assess the relationship of PiB retention with cognitive abilities.
METHODS
SUBJECTS
For this study, 69 elderly persons were recruited from the community. Participants were recruited primarily from an ad in a local seniors’ newspaper, and others were recruited from previous studies on the effect of normal aging on cognitive performance.19 They were not recruited from an Alzheimer research center and, therefore, had not sought treatment for dementia, nor were they recruited as the spouses or family members of patients with dementia. However, a family history of dementia was not an exclusion criterion for this study. After providing informed consent, subjects underwent eligibility screening, including health screening and neuropsychological testing. Exclusion criteria included the presence of dementia or MCI, either amnestic or amnestic plus other areas of impairment,20 as well as the presence or history of other major neurological or psychiatric diseases or the use of psychoactive medications. The presence of risk factors for AD, such as being an apolipoprotein E ε4 (APOE ε4) allele carrier, was not an exclusion criterion. Of 69 participants, 43 completed the entire study, and 26 did not: 16 did not pass the cognitive screening, 4 could not complete magnetic resonance imaging, 1 could not complete PET scanning, 3 had medical or psychiatric exclusions, and 2 decided not to participate.
In addition, 19 cognitively normal subjects were enrolled through the University of Pittsburgh Alzheimer Disease Research Center and received only the diagnostic neuropsychological screening, PiB PET scanning, and magnetic resonance imaging. These participants were only used to define the cutoff value for amyloid positivity.
DIAGNOSTIC NEUROPSYCHOLOGICAL SCREENING
The neuropsychological screening battery was the same as the assessment battery used at the Pittsburgh Alzheimer Disease Research Center, and thus enabled us to use the Alzheimer Disease Research Center (ADRC) consensus diagnostic criteria20 to identify individuals who would meet NINDS-ADRDA (National Institute for Neurological and Communicative Disorders and Stroke–Alzheimer’s Disease and Related Disorders Association) criteria for probable AD and Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, criteria for dementia of the Alzheimer type21,22 or meet criteria for MCI initially described by Petersen et al.23 The details of our implementation of these criteria have previously been described in detail by Lopez et al5 and include nonamnestic as well as amnestic subtypes. The battery includes the Mini–Mental State Examination24 and Consortium to Establish a Registry for Alzheimer’s Disease Word List Learning25; immediate and delayed recall as well as copy score for the Rey/Osterrieth Complex Figure,26 Digit Span forward and backward; Trail-making Tests, parts A and B27; clock drawing28,29; the 30-item Boston Naming Test30; letter fluency (FAS)31; and category fluency.32
COGNITIVE ASSESSMENTS
A total of 43 participants who were determined to be clinically unimpaired (not meeting criteria for dementia or MCI) and were recruited for the full study protocol underwent further cognitive assessments.
Cognitive Reserve
The recently developed Wechsler Test of Adult Reading33 was used to estimate IQ. Combining an individual’s Wechsler Test of Adult Reading score with his or her educational level accurately predicts an individual’s premorbid full-scale IQ performance on the Wechsler Adult Intelligence Scale,33 which can be used as a measure of cognitive reserve.
Information Processing Speed
Subjects were given 2 different tasks. In the first, perceptual comparison, they were shown 2 shapes side-by-side on a computer screen and pressed a button in their dominant hand if the shapes were physically identical or in their nondominant hand if they were not. In the conceptual comparison task, the subject was shown 2 stimuli (single letters and digits) and had to decide whether they were from the same category (ie, either both letters or both digits, eg, L X or 5 9) or whether they were from different categories (eg, L 5).
Working Memory
Two different working memory tasks were used: the N-back task34 and the letter-number sequencing task from the Wechsler Adult Intelligence Scale III.35
Inhibitory Efficiency
Two different techniques were used to measure efficiency of inhibitory processes. The first was a reaction-time version of the color-word Stroop test, in which naming time was determined on an individual trial basis,36 and the second was the Hayling test,37 which examines a subject’s ability to generate an ending to a sentence that is inconsistent with the overall meaning of the sentence, that is, to inhibit the most salient ending in order to generate an ending that does not make sense in the context of the sentence.
PET IMAGING
The PiB PET scanning was performed within 16 weeks of the clinical screening and cognitive testing. The PiB PET data were acquired as recently described.16 The PET imaging was conducted using an ECAT HR+ scanner (Siemens/CTI Molecular Imaging, Malvern, Pennsylvania) (3-dimensional mode; 15.2-cm field-of-view; 63 planes; reconstructed image resolution of approximately 6 mm). The scanner was equipped with a Neuroinsert (CTI PET Systems, Knoxville, Tennessee) to reduce the contribution of scattered photons.38 Data were reconstructed using filtered back-projection (Fourier rebinning/2-dimensional back projection; 3-mm Hann filter) and corrected for photon attenuation (68Ge-68Ga [germanium 68–gallium 68] rods), scatter,39 and radioactive decay. The participant’s head was immobilized to minimize motion during the scan. The PiB was injected intravenously (mean [SD], 14.3 [2.2] mCi for 20 seconds; mean [SD] specific activity,1.4[0.8] Ci/μmol), and dynamic PET scanning was performed for 90 minutes (34 time frames).
Regions of interest (ROIs) were defined on the coregistered magnetic resonance image as described previously40 and included the following: frontal lobe; anterior cingulate gyrus (ACG); lateral temporal, mesial temporal, occipital, parietal, precuneus cortex (PRC)/posterior cingulate gyrus (PCG), and sensorimotor cortices; and anterior-ventral striatum. A cerebellar ROI was used as the reference region and a pons ROI was included as an example of nonspecific retention in white matter. A global mean of 6 regions (GBL6) was calculated as the arithmetic mean of the PiB binding index of the frontal lobe, ACG, PRC, lateral temporal, and parietal cortices and striatum.
DATA ANALYSIS
The PiB data were analyzed using a Logan graphical analysis with the cerebellar ROI as input and 90 minutes of data.16 This generated a distribution volume ratio (CER90 DVR) for each ROI, which is a reflection of the concentration of binding sites in that volume relative to the concentration in the cerebellum.
In an effort to standardize the definition of amyloid positivity, we used an iterative, objective procedure to identify high outliers in the control group. A “mild” or “suspect” outlier was defined in the standard manner as any observation more than 1.5× the interquartile range higher than the third quartile,41 that is, any observation higher than the upper-inner fence of the box-and-whisker plot of the data. This calculation was performed using 62 clinically unimpaired control subjects (43 from this study and 19 from other related studies). The brain areas used for this calculation were those that most commonly show amyloid deposition in AD patients (ACG; frontal, PRC/PCG, parietal, lateral temporal, and lateral occipital cortices; and occipital pole). After determining which subjects were outliers for any of these neocortical brain areas, we removed those subjects from the analysis and repeated the calculation until there were no outliers remaining. The process required 2 iterations: 12 outliers were removed on the first iteration and 4 on the second. We were left with a core group of 46 controls (74%) who were considered very unlikely to be amyloid-positive. The cutoff value for amyloid positivity was defined as the upper-inner fence of the 46 control subjects for each of the 7 key brain areas listed previously. Any subject who had a PiB DVR value that exceeded this cutoff point in any of these 7 brain areas was defined as amyloid-positive. We then applied this criterion to the 43 subjects who received the full cognitive battery of assessments during this study. Because we wanted to compare cognition among amyloid-positive vs amyloid-negative participants, we eliminated the effect of doubtful cases near this cutoff point by excluding the 5 subjects (12%) with PiB DVR values within 2.5% above or below the cutoff value and compared the 9 subjects clearly above the cutoff point (21%) with the 29 subjects clearly below (67%).
For voxelwise group comparisons, full-resolution 1.5T magnetic resonance images were aligned in the anterior commissure–posterior commissure plane. These images were normalized to the Montreal Neurological Institute template using the unified segmentation technique in SPM5 statistical software (http://www.fil.ion.ucl.ac.uk/spm/software/spm5/). The PiB DVR images were prepared by applying a positive mask, coregistering to the corresponding magnetic resonance image, and transforming them to template space using the factors created during segmentation. Mean PiB DVR images were determined using the arithmetic mean of each voxel. Mean images were then subtracted and rescaled. Voxelwise statistical comparisons were also performed with SPM5 statistical software.
After the regional DVRs were calculated and participants were categorized as amyloid-positive and amyloid-negative, additional statistical tests were done to test our hypotheses about the association of PiB retention with demographic and cognitive variables. Nonparametric Monte Carlo method–based exact tests with a 2-tailed α of .05 were used to compare amyloid-positive and amyloid-negative groups on the key continuous demographic and cognitive variables. Fischer exact tests with a 2-tailed α of.05 were used for the categorical variables (ie, sex, race, and presence of APOE ε4 allele).
RESULTS
SUBJECTS
The 43 participants had a mean (SD) age of 74.4 (6.1) years; 37% were men, 91% were white, and the mean (SD) years of education was 15.1 (2.8). Participants were screened for cognitive impairment. Therefore, as was expected, the mean (SD) Mini–Mental State Examination score of 28.4 (1.7) was within the reference range (minimum score, 24).
PiB RESULTS
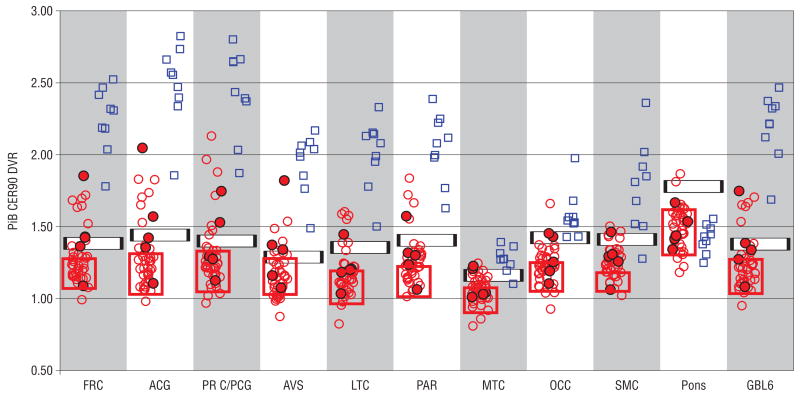
The PiB DVRs for each of the 10 ROIs in addition to the GBL6 are shown as a scatterplot in Figure 1. For comparison, Figure 1 also shows the DVRs for a group of 9 individuals diagnosed with AD. For comparison, the first 9 participants with AD scanned at the University of Pittsburgh Alzheimer Disease Research Center were selected to match the number of amyloid-positive elderly persons. Data from all 9 AD participants have been previously reported.16,42 Visual inspection of the scatterplot illustrates the marked separation of AD participants from the controls, especially in the ACG and PRC/PCG. However, it can also be seen that there is variability in PiB retention among elderly control subjects, with a skew toward higher retention in several brain areas.
Figure 1.
Pittsburgh Compound B (PiB) retention among clinically unimpaired controls (red circles) and patients with Alzheimer disease (blue squares). Filled circles indicate apolipoprotein E ε4 carriers in the control group. Red boxes indicate the range of PiB retention among controls aged 55 years and younger. The black and white horizontal bars indicate the amyloid-positive cutoff points in each brain area (see the “Methods” section for explanation). The width of the bar indicates the “intermediate” zone 2.5% above and below the cutoff value that was used to more conservatively categorize amyloid-positive and amyloid-negative participants. ACG indicates anterior cingulate gyrus; AVS, anterior ventral striatum; DVR, distribution volume ratio; FRC, frontal cortex; GBL6, mean of values for FRC, ACG, PRC, AVS, LTC, and PAR regions; LTC, lateral temporal cortex; MTC, mesial temporal cortex; OCC, occipital cortex (includes primary visual cortex); PAR, parietal cortex; PRC/PCG, precuneus cortex/posterior cingulate gyrus; SMC, sensorimotor cortex.
DEFINING AMYLOID-POSITIVE AND AMYLOID-NEGATIVE
The iterative mild-outlier method objectively identified a cutoff point for each region. To more conservatively define the amyloid-positive and amyloid-negative groups for this study, a lower-bound (97.5% of the cutoff value) was defined such that PiB retention below this cutoff point was in the amyloid-negative (or “normal”) range and an upper-bound (102.5% of the cutoff value) was defined such that PiB retention above this cutoff point would be considered amyloid-positive for that region. Having PiB retention within ±2.5% of the cutoff value was considered “intermediate.” The cutoff boundaries are illustrated in Figure 1. A participant who fell into the amyloid-positive region in any ROI was considered amyloid-positive.
Applying the upper- and lower-bound thresholds categorized 9 of 43 elderly control subjects (21%) as amyloid-positive, 29 (67%) as amyloid-negative, and the remaining 5 (12%) as intermediate. As an independent check of the face validity of these cutoff values, all 19 AD patients assessed at the University of Pittsburgh were classified as amyloid-positive using this approach (although not in every region in every AD patient). As a second check, 8 cognitively normal control subjects aged 55 years or younger (8 from studies separate from this) were recruited through the University of Pittsburgh Alzheimer’s Disease Research Center. These subjects were used for the cutoff definitions, but they were not used for the cognitive analyses in the older subjects described in the “Amyloid-Positive vs Amyloid-Negative Participants” subsection of the “Methods” section. The probability of finding amyloid deposition in these subjects is very low.3 All 8 of these younger subjects were classified as amyloid-negative by using these cutoff values (Figure 1). All amyloid-positive clinically unimpaired subjects had moderately increased PiB retention or higher in the prefrontal and/or PRC/PCG cortex, the areas in which retention is typically increased among AD patients. All 9 subjects classified as amyloid-positive using a combination of all 7 key brain areas also were classified as amyloid-positive according to the PRC/PCG cortex alone. The ACG and frontal cortex both identified 8 of 9 amyloid-positive subjects, reinforcing the observation that the ACG as well as the frontal and PRC/PCG cortex are the most representative regions for overall PiB retention. Six subjects were globally amyloid-positive.
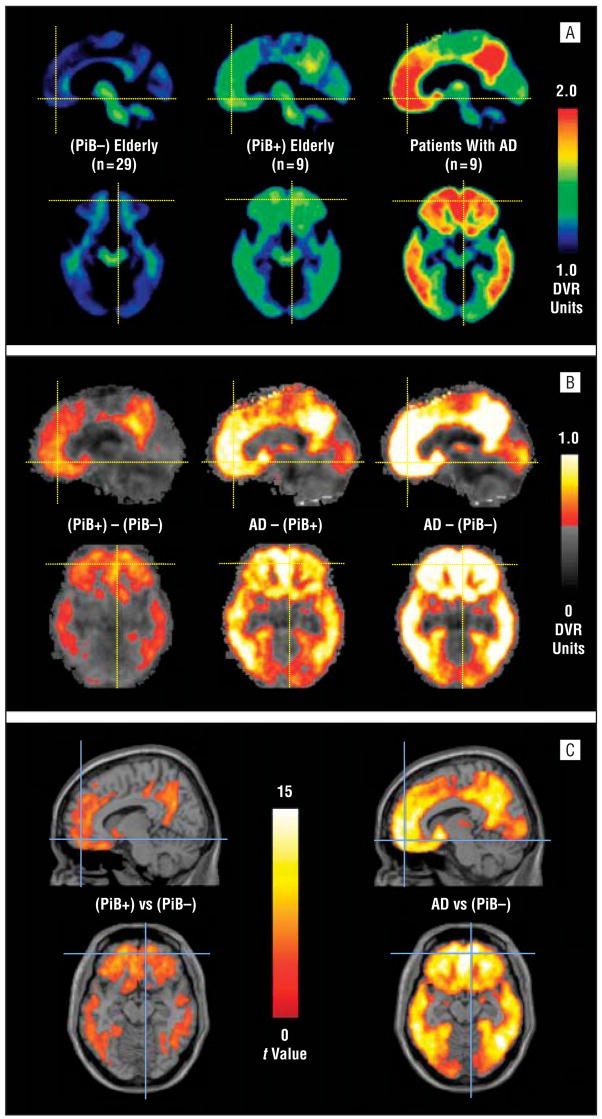
Figure 2 A shows mean DVR images from 29 amyloid-negative elderly participants, 9 amyloid-positive elderly participants, and the 9 AD patients scanned as a comparison. On average, amyloid-positive clinically unimpaired elderly participants had considerably less PiB retention than AD participants, but the distribution was very similar. That is, rather than showing nonspecific white matter accumulation, there appeared to be a pattern of frontal, ACG, PRC, and temporoparietal retention. Figure 2B shows difference images obtained by subtracting the 3 groups from each other (ie, PiB±–PiB−, AD–PiB+, AD–PiB−). Amyloid-positive clinically unimpaired subjects differ from the amyloid-negative group because of increased PiB retention in the frontal cortex, ACG, PRC/PCG, and temporoparietal cortex and striatum. These are the same areas in which PiB retention among AD participants exceeded that of controls, albeit to a much greater degree (Figure 2B). In fact, AD participants exceeded amyloid-positive elderly participants (Figure 2B) by more than amyloid-positive elderly participants exceeded the amyloid-negative group (Figure 2B). Although, on average, an AD-like distribution was seen among amyloid-positive elderly participants, some individuals exhibited asymmetrical PiB retention that was concentrated in the frontal or PRC/PCG cortex.
Figure 2.
A, Mean distribution volume ratio (DVR) images for 29 amyloid-negative clinically unimpaired participants (left), 9 amyloid-positive clinically unimpaired participants (center), and 9 patients with Alzheimer disease (AD) (right). B, Images obtained by subtracting the amyloid-negative mean image from either the mean of amyloid-positive elderly participants (left) or the mean of patients with AD (right) or by subtracting the amyloid-positive mean image from the mean image of patients with AD (center). The gray background is not a magnetic resonance image but represents Pittsburgh Compound B (PiB) retention differences of less than 0.5 DVR units and is shown for orientation. C, Statistical parametric mapping (SPM) software image of t values was determined from the comparison of the amyloid-negative group with the amyloid-positive clinically unimpaired group (left) or the patients with AD (right).
In addition to these simple comparisons of mean images, a groupwise analysis also was performed. Figure 2C shows that the regional distribution of PiB retention among amyloid-positive elderly participants was significantly higher than among amyloid-negative elderly participants in most cortical areas that showed increased PiB retention (Figure 2C), but that the level of the significance is much less than that obtained when comparing AD participants with the amyloid-negative group (Figure 2C). These statistical parametric mapping (SPM) images can be directly compared because the group sizes are 29 and 9 in both comparisons. An AD vs amyloid-positive elderly SPM comparison is not shown because there are only 9 subjects in each group so it could not be directly compared with the other analyses.
AMYLOID-POSITIVE VS AMYLOID-NEGATIVE PARTICIPANTS
We compared the demographic and cognitive variables of amyloid-positive and amyloid-negative participants (Table). We expected that the amyloid-positive group would have a higher cognitive reserve score (premorbid IQ), which may have allowed subjects to have AD pathological characteristics without manifesting cognitive impairment. In addition, we expected greater age and lower cognitive testing scores in the amyloid-positive group because these are known predictors of AD. However, none of these relationships was significant in this sample. In fact, contrary to our prediction, there was a trend toward higher educational level in the amyloid-negative group, and the only significant finding was for lower scores on the delayed recall test in the amyloid-negative group, which was opposite of the expected outcome.
Table.
Data by Amyloid Positivity Status
| Characteristic | Amyloid-Positive (n=9) | Amyloid-Negative (n=29) | P Valuea |
|---|---|---|---|
| Age, mean (SD), y | 74.2 (5.4) | 73.4 (6.0) | .65 |
| Men, No. (%) of participants | 4 (44) | 9 (31) | .23 |
| African American, No. (%) of participants | 0 | 3 (10) | .87 |
| Educational level, mean (SD) | 14.3 (2.8) | 14.9 (2.6) | .57 |
| APOE ε4 allele positive, No. (%) | 2 (22) | 1 (4) | .13 |
| GDS, mean (SD) | 1.56 (2.3) | 1.45 (1.6) | .77 |
| IQ score (WTAR predicted), mean (SD) | 108.2 (8.3) | 108.1 (10.0) | .89 |
| MMSE score, mean (SD) | 28.9 (0.9) | 28.5 (1.5) | .65 |
| Clock drawing, mean (SD) | 14.6 (0.5) | 13.9 (2.0) | .57 |
| Boston 12 score, mean (SD) | 29.1 (1.2) | 28.2 (1.9) | .22 |
| Rey/Osterrieth Complex Figure score,b mean (SD) | |||
| Copy | 20.6 (2.4) | 20.5 (1.5) | .78 |
| Immediate | 17.4 (3.1) | 16.8 (3.6) | .92 |
| Delayed | 15.8 (3.7) | 15.9 (4.0) | .66 |
| Category fluency score, mean (SD) | 20.2 (5.9) | 20.1 (3.9) | .97 |
| Letter fluency score, mean (SD) | 40.7 (14.1) | 43.2 (14.5) | .74 |
| Word list delayed recall score,a mean (SD) | 8.8 (1.1) | 6.9 (1.6) | .02 |
| Digit span forward score, mean (SD) | 6.9 (0.9) | 6.7 (1.3) | .65 |
| Digit span backward score, mean (SD) | 4.6 (1.5) | 5.0 (1.3) | .57 |
| Trail-making Test score, parts A and B, mean (SD) | 3.1 (2.1) | 2.3 (0.8) | .19 |
| WAIS III Letter-number sequencing score, mean (SD) | 8.9 (3.1) | 10.0 (2.6) | .15 |
| N-back task score, mean (SD) | 37.0 (16.5) | 31.6 (11.8) | .26 |
| Stroop incongruent-neutral conditions, ms, mean (SD) | 119.2 (47.2) | 95.2 (64.0) | .13 |
| Sensorimotor speed, ms, mean (SD) | 257.0 (29.0) | 260.3 (44.3) | .87 |
| Conceptual comparison, ms, mean (SD) | 785.3 (183.2) | 745.1 (166.7) | .52 |
| Perceptual comparison, ms, mean (SD) | 781.5 (222.4) | 749.3 (207.9) | .75 |
| Hayling incongruent-congruent conditions, ms, mean (SD) | 2043.3 (2695) | 1681 (1740) med=1260 |
.89 |
| Median | 1010 | 1260 | .89 |
Abbreviations: APOE ε4, apolipoprotein E ε4; GDS, Geriatric Depression Scale; MMSE, Mini-Mental State Examination; WAIS III, Wechsler Adult Intelligence Scale III; WTAR, Wechsler Test of Adult Reading.
Nonparametric tests were used for continuous variables, and the Fisher exact test was used for categorical variables.
These tests were not administered to early participants, so the sample size is smaller (5 amyloid-positive and 23 amyloid-negative participants).
APOE ε4 ALLELE
There were 5 clinically unimpaired participants with an APOE 3/4 genotype and none with 4/4. Of the 5 APOE ε4 allele carriers, 2 were amyloid-positive, 2 had intermediate amyloid status, and 1 was amyloid-negative (Figure 1). One APOE ε4 carrier was globally positive. As a group, PiB retention among the 5 APOE ε4 carriers was not significantly different from that of the 38 participants who were not carriers of an APOE ε4 allele. However, the frequency of the APOE ε4 allele was significantly different among amyloid-negative participants (1 of 29) than it was in the combined amyloid-positive and intermediate group (4 of 14; χ2= 5.80; P=.02).
COMMENT
The present study examined in vivo brain PiB retention in a group of clinically unimpaired elderly persons. Our primary aims were to estimate the prevalence of significant amyloid deposition among clinically unimpaired elderly persons during life, and to characterize the association of amyloid deposition with demographic characteristics and cognitive performance.
Amyloid load and, therefore, PiB retention, is a continuous variable. Even clinically unimpaired elderly persons often have measurable amounts of insoluble Aβ after death. Näslund et al43 have shown that participants who had Clinical Dementia Rating (CDR) scale scores of 0 before death had measurable levels of insoluble Aβ, although these levels were only 4% to 20% of the levels observed among participants with severe AD. That having been said, a dichotomous definition of an amyloid-positive and amyloid-negative state is often useful for group designations in studies such as ours, in which the effect of detectable PiB retention on cognition is being evaluated. Ultimately, the definition of amyloid-positivity will need to be validated by the comparison of in vivo PiB retention with postmortem verification of the presence or absence of significant numbers of Aβ plaques at autopsy. Until then, objective criteria for the definition of amyloid-positivity are needed. Few clear criteria have been posed to define amyloid-positivity. Most studies have defined amyloid-positivity among clinically unimpaired persons or those with MCI either from visual reads of the images (ie, as “AD-like”) or from rough quantitative comparisons of PiB retention among controls with the range of values observed among AD participants (ie, PiB retention above that of the lowest PiB retention value in an AD participant is considered amyloid-positive).7,17,18 Klunk et al7 attempted to provide an objective definition of amyloid-positivity and make it independent of any AD cohort by defining the cutoff point as PiB retention values 1 SD or more above the mean of the entire control group. Pike et al44 have taken a more sophisticated objective approach by using a receiver operating characteristic curve to generate a cutoff point between clinically unimpaired controls and AD participants, although this approach remains dependent on the composition of the AD cohort. Although these 2 objective approaches are an improvement over subjective assessments, they can be disproportionately affected by outliers in either the AD or control groups or by large gaps between the groups.
To remove the contribution of the AD cohort and minimize the impact of controls with high PiB retention on the definition of amyloid-positive cutoff values, we used an iterative outlier removal method and generated an upper- and lower-bound of DVRs for each ROI. Individuals with DVRs above the upper-bound in the ACG or PRC/PCG cortex were considered amyloid-positive. These subjects typically showed a visual pattern of PiB retention that was qualitatively similar to, but quantitatively less than, most AD participants. Individuals with PiB retention below the lower-bound were considered amyloid-negative. Individuals with PiB retention in the ACG or PRC/PCG cortex that was between the lower- and upper-bounds were called intermediate for the purpose of this study. Of 43 subjects, 9 (21%) were amyloid-positive, 29 (67%) amyloid-negative, and 5 (12%) intermediate. Our observation of significant amyloid deposition in a subset of cognitively unimpaired elderly persons is consistent with previous observations from neuropathological studies45–47 and recent amyloid imaging studies.17,18 Our estimate of 21% is similar to that reported by Mintun et al,17 who used a higher threshold for classifying subjects as amyloid-positive, and Rowe et al18 and matches closely the postmortem observations from Knopman et al,47 who reported that 18% of participants met Consortium to Establish a Registry for Alzheimer’s Disease criteria for possible AD.
It should be stressed that the designation of amyloid-positive in the context of clinically unimpaired elderly persons does not imply that these subjects have AD-like results on a PiB PET scan. On the contrary, the scans of most subjects could be easily distinguished from the PiB PET scans of AD patients. This is quantitatively apparent in the global measure (GBL6) in Figure 1 and visually apparent in Figure 2. Only 6 of 9 amyloid-positive subjects are globally positive, and even these 6 are clearly distinguishable from all but 1 AD case with unusually low PiB retention. Although data from only our first 9 consecutive AD patients are shown in Figure 1, we have now studied PiB retention in more than 20 AD participants, and the case shown in Figure 1 is the only one with GBL6 retention that overlaps with that of any amyloid-positive clinically unimpaired participant. This has important implications for the use of amyloid imaging as a diagnostic tool. That is, although subtle amyloid-deposition can be identified by low levels of PiB retention (often focal), it is relatively easy to visually and quantitatively distinguish this type of amyloid deposition from that seen among symptomatic patients with AD.
The degree of PiB retention among amyloid-positive cognitively unimpaired elderly persons also differs from the pattern of PiB retention observed among most participants with MCI. Participants with MCI who also had significant PiB retention typically show a much higher amount of PiB retention than cognitively unimpaired elderly persons, in the range of AD participants.16,40 Most participants with MCI show a dichotomous pattern: 30% to 40% are clearly amyloid-negative, and 50% to 60% have AD-like PiB retention. In the literature, only10% to 15% of participants with MCI fall into the range occupied by the amyloid-positive but clinically unimpaired elderly participants in this study. This has important implications for antiamyloid therapies currently in development, such as that the active phase of Aβ plaque accumulation occurs when a person is asymptomatic. It is likely that antiamyloid therapies will have the highest chance of success during this period. Therefore, if a safe and effective antiamyloid therapy can be developed, it may become critical to identify amyloid-positive individuals at a presymptomatic stage.
Our results strongly suggest that amyloid deposition can be detected at an asymptomatic stage. Similar to the results of several recent neuropathological studies,48,49 we did not find significantly worse cognitive performance among amyloid-positive subjects. In fact, the only significant difference we found between the amyloid-positive and amyloid-negative groups was in a direction opposite to that expected. The amyloid-positive group performed better on the Delayed Word Recall test. It is unclear why this group would show improved performance on a declarative memory task. One speculation is that perhaps subjects with preclinical amyloid deposition who are still clinically unimpaired have particularly high cognitive reserve in areas relatively unaffected by amyloid deposition (ie, medial temporal lobe).
The prevalence estimate of amyloid deposition reported here is based on a very small sample of community volunteers and, therefore, may not reflect the true prevalence in a random sampling of the community. In particular, the sample may be biased by an overrepresentation of more highly educated and motivated volunteers. However, educational level did not differ significantly between amyloid-positive and amyloid-negative groups, so it is unlikely that it would significantly alter the prevalence estimate. In addition, the estimate of 20% to 25% prevalence of amyloid-positivity in this clinically unimpaired cohort with a mean (SD) age of 75(6) years is in keeping with an expected AD prevalence of approximately 25% among people older than 85 years if asymptomatic amyloid deposition invariably leads to AD and if the clinically silent period of amyloid deposition is on the order of 10 years.
Although our primary finding suggests that there is no significant cognitive deficit in the amyloid-positive group compared with the amyloid-negative group, we recognize that this conclusion is based on a relatively small sample of amyloid-positive individuals (n=9) and an even smaller number with complete cognitive data (5 amyloid-positive participants with delayed recall and Rey figure data). Further studies with larger samples are necessary to identify whether subtle differences in cognition may exist among amyloid-positive individuals. Another limitation of this study is that the cognitive testing protocol had only limited executive function assessment and, therefore, it is possible that future studies with more detailed executive function tests may find differences between amyloid-positive and amyloid-negative groups.
In conclusion, to our knowledge, this is one of the first and the largest study to examine in vivo amyloid deposition among clinically unimpaired elderly persons with extensively documented cognitive function. We found that a significant number (21%) of clinically unimpaired elderly persons had amyloid deposition, and the deposition was primarily in regions that ultimately develop heavy amyloid loads in AD patients, especially the ACG and the PRC/PCG cortex. We found mixed results in relating amyloid deposition to demographic and cognitive variables. In particular, we did not find amyloid-positive subjects to have worse cognitive performance than that of the amyloid-negative group, and we did not find any demographic differences between the groups, although there was a suggestion of more frequent amyloid-positivity among those who carry an APOE ε4 allele. Thus, it appears that, in a significant number of elderly, cognitively normal persons, amyloid accumulation does not impair cognitive function.
This study suggests that, among people older than 65 years, amyloid deposition without cognitive sequelae and amyloid deposition accompanied by cognitive impairment in the form of dementia (ie, AD) or MCI have a similar prevalence (approximately 20%). It is possible, but yet unproven, that elderly persons with significant PiB retention may progress to AD and that the preserved cognition observed in this study is owing to compensation. This finding could have profound implications for future prevention strategies, such that it may be possible to predict fairly accurately who will develop AD 5 to 10 years before the onset of symptoms. If compensatory mechanisms are involved in delaying the onset of symptoms, identification of these mechanisms could lead to novel and effective preventative treatment strategies.
Acknowledgments
Funding/Support: This study was supported by grants R01 AG018402, P50 AG005133, K02 AG001039, R01 AG020226, R01 MH070729, K01MH001976, R37 AG025516, and P01 AG025204 from the National Institutes of Health, grant TLL-01-338 from the Alzheimer’s Association, and grant DE-FD02-03 ER63590 from the US Department of Energy.
Footnotes
Author Contributions: Drs Klunk, Mathis, Aizenstein, Nebes, DeKosky, and Price had full access to all the data in this study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Nebes, Saxton, Snitz, DeKosky, and Klunk. Acquisition of data: Nebes, Saxton, Tsopelas, Halligan, and Klunk. Analysis and interpretation of data: Aizenstein, Nebes, Saxton, Price, Mathis, Ziolko, James, Snitz, Houck, Bi, Cohen, Lopresti, DeKosky, and Klunk. Drafting of the manuscript: Aizenstein, Houck, James, Bi, Halligan, and Klunk. Critical revision of the manuscript for important intellectual content: Aizenstein, Nebes, Saxton, Price, Mathis, Tsopelas, Ziolko, Snitz, Cohen, Lopresti, DeKosky, and Klunk. Statistical analysis: Aizenstein, Ziolko, James, Houck, and Klunk. Obtained funding: Nebes, Saxton, DeKosky, and Klunk. Administrative, technical, and material support: Aizenstein, Nebes, Saxton, Price, Mathis, Tsopelas, Bi, Lopresti, DeKosky, and Halligan. Study supervision: Nebes.
Financial Disclosure: GE Healthcare holds a license agreement with the University of Pittsburgh based on the technology described in this manuscript. Drs Klunk and Mathis are coinventors of PiB and, as such, have a financial interest in this license agreement. GE Healthcare provided no grant support for this study and had no role in the design or interpretation of results or preparation of the manuscript. All other authors have no conflicts of interest with this work.
References
- 1.Dickson DW, Crystal HA, Mattiace LA, et al. Identification of normal and pathological aging in prospectively studied nondemented elderly humans. Neurobiol Aging. 1992;13(1):179–189. doi: 10.1016/0197-4580(92)90027-u. [DOI] [PubMed] [Google Scholar]
- 2.Haroutunian V, Perl D, Purohit D, et al. Regional distribution of neuritic plaques in the nondemented elderly and subjects with very mild Alzheimer’s disease. Arch Neurol. 1998;55(9):1185–1191. doi: 10.1001/archneur.55.9.1185. [DOI] [PubMed] [Google Scholar]
- 3.Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol. 1999;45(3):358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 4.Wolf DS, Gearing M, Snowdon DA, Mori H, Markesbery WR, Mirra SS. Progression of regional neuropathology in Alzheimer disease and normal elderly: findings from the Nun Study. Alzheimer Dis Assoc Disord. 1999;13(4):226–231. doi: 10.1097/00002093-199910000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Lopez OL, Jagust WJ, DeKosky ST, et al. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 1. Arch Neurol. 2003;60(10):1385–1389. doi: 10.1001/archneur.60.10.1385. [DOI] [PubMed] [Google Scholar]
- 6.Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60(8):1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 7.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55(3):306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 8.Ikonomovic MD, Klunk WE, Abrahamson EE, et al. Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer’s disease. Brain. 2008;131(pt 6):1630–1645. doi: 10.1093/brain/awn016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lockhart A, Lamb JR, Osredkar T, et al. PIB is a non-specific imaging marker of amyloid-β (Aβ) peptide-related cerebral amyloidosis. Brain. 2007;130(pt 10):2607–2615. doi: 10.1093/brain/awm191. [DOI] [PubMed] [Google Scholar]
- 10.Rabbitt P. Does it all go together when it goes? the 19th Bartlett Memorial Lecture. Q J Exp Psychol A. 1993;46(3):385–434. doi: 10.1080/14640749308401055. [DOI] [PubMed] [Google Scholar]
- 11.Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256(5054):184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 12.Cummings BJ, Cotman CW. Image analysis of β-amyloid load in Alzheimer’s disease and relation to dementia severity. Lancet. 1995;346(8989):1524–1528. doi: 10.1016/s0140-6736(95)92053-6. [DOI] [PubMed] [Google Scholar]
- 13.Nielson KA, Cummings BJ, Cotman CW. Constructional apraxia in Alzheimer’s disease correlates with neuritic neuropathology in occipital cortex. Brain Res. 1996;96(1–2):284–293. doi: 10.1016/s0006-8993(96)00983-3. [DOI] [PubMed] [Google Scholar]
- 14.Braak H, Braak E. Evolution of neuronal changes in the course of Alzheimer’s disease. J Neural Transm Suppl. 1998;53:127–140. doi: 10.1007/978-3-7091-6467-9_11. [DOI] [PubMed] [Google Scholar]
- 15.Terry RD, Masliah E, Salmon DP, et al. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30(4):572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 16.Lopresti BJ, Klunk WE, Mathis CA, et al. Simplified quantification of Pittsburgh Compound-B amyloid imaging PET studies: a comparative analysis. J Nucl Med. 2005;46(12):1959–1972. [PubMed] [Google Scholar]
- 17.Mintun MA, Larossa GN, Sheline YI, et al. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006;67(3):446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- 18.Rowe CC, Ng S, Ackermann U, et al. Imaging β-amyloid burden in aging and dementia. Neurology. 2007;68(20):1718–1725. doi: 10.1212/01.wnl.0000261919.22630.ea. [DOI] [PubMed] [Google Scholar]
- 19.Nebes RD, Meltzer CC, Whyte EM, et al. The relation of white matter hyperintensities to cognitive performance in the normal old: education matters. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2006;13(3–4):326–340. doi: 10.1080/138255890969294. [DOI] [PubMed] [Google Scholar]
- 20.Lopez OL, Becker JT, Klunk W, et al. Research evaluation and diagnosis of probable Alzheimer’s disease over the last two decades: I. Neurology. 2000;55(12):1854–1862. doi: 10.1212/wnl.55.12.1854. [DOI] [PubMed] [Google Scholar]
- 21.The Diagnostic and Statistical Manual of Mental Disorders. 4. Arlington, VA: American Psychiatric Association; 1994. [Google Scholar]
- 22.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 23.Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review); Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56(9):1133–1142. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- 24.Folstein MF, Folstein SE, McHugh PR. Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 25.Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD), part I: clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39(9):1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 26.Rey A. L’examen psychologique dans les cas d’encephalopathie traumatique. Arch Psychol. 1941;28:286–340. [Google Scholar]
- 27.Reitan RM. Validity of the Trail-making Tests as an indication of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- 28.Lopez OL, Becker JT, Klunk W, et al. Research evaluation and diagnosis of possible Alzheimer’s disease over the last two decades: II. Neurology. 2000;55(12):1863–1869. doi: 10.1212/wnl.55.12.1863. [DOI] [PubMed] [Google Scholar]
- 29.Freedman M. Clock Drawing: A Neuropsychological Analysis. Oxford, England: Oxford University Press; 1994. [Google Scholar]
- 30.Saxton J, Ratcliff G, Newman A, et al. Cognitive test performance and presence of subclinical cardiovascular disease in the Cardiovascular Health Study. Neuroepidemiology. 2000;19(6):312–319. doi: 10.1159/000026270. [DOI] [PubMed] [Google Scholar]
- 31.Benton AL. Differential behavioral effects in frontal lobe disease. Neuropsychologia. 1968;6:53–60. [Google Scholar]
- 32.Borkowski JG, Benton AL, Spreen O. Word fluency and brain damage. Neuropsychologia. 1967;5:135–140. [Google Scholar]
- 33.Wechsler D. Wechsler Test of Adult Reading. New York, NY: Psychological Corporation; 2001. [Google Scholar]
- 34.Dobbs AR, Rule BG. Adult age differences in working memory. Psychol Aging. 1989;4(4):500–503. doi: 10.1037//0882-7974.4.4.500. [DOI] [PubMed] [Google Scholar]
- 35.Wechsler D. Wechsler Adult Intelligence Scale. 3. New York, NY: Psychological Corporation; 1997. [Google Scholar]
- 36.Spieler DH, Balota DA, Faust ME. Stroop performance in healthy younger and older adults and in individuals with dementia of the Alzheimer’s type. J Exp Psychol Hum Percept Perform. 1996;22(2):461–479. doi: 10.1037//0096-1523.22.2.461. [DOI] [PubMed] [Google Scholar]
- 37.Burgess PW, Shallice T. Response suppression, initiation, and strategy use following frontal lobe lesions. Neuropsychologia. 1996;34(4):263–272. doi: 10.1016/0028-3932(95)00104-2. [DOI] [PubMed] [Google Scholar]
- 38.Weinhard K. Applications of 3D PET. In: Bendriem B, Townsend D, editors. The Theory and Practice of 3D PET. Boston, MA: Kluwer Academic Publishers; 1998. pp. 133–167. [Google Scholar]
- 39.Watson CC. New, faster, image-based scatter correction for 3D PET. IEEE Trans Nucl Sci. 2000;47(4):1587–1594. [Google Scholar]
- 40.Price JC, Klunk WE, Lopresti BJ, et al. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J Cereb Blood Flow Metab. 2005;25(11):1528–1547. doi: 10.1038/sj.jcbfm.9600146. [DOI] [PubMed] [Google Scholar]
- 41.Schwertman NC, de Silva R. Identifying outliers with sequential fences. Comput Stat Data Anal. 2007;51(8):3800–3810. [Google Scholar]
- 42.Ziolko SK, Weissfeld LA, Klunk WE, et al. Evaluation of voxel-based methods for the statistical analysis of PiB PET amyloid imaging studies in Alzheimer’s disease. Neuroimage. 2006;33(1):94–102. doi: 10.1016/j.neuroimage.2006.05.063. [DOI] [PubMed] [Google Scholar]
- 43.Näslund J, Haroutunian V, Mohs R, et al. Correlation between elevated levels of amyloid β peptide in the brain and cognitive decline. JAMA. 2000;283(12):1571–1577. doi: 10.1001/jama.283.12.1571. [DOI] [PubMed] [Google Scholar]
- 44.Pike KE, Savage G, Villemagne VL, et al. β-Amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer’s disease. Brain. 2007;130(pt 11):2837–2844. doi: 10.1093/brain/awm238. [DOI] [PubMed] [Google Scholar]
- 45.Braak H, Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging. 1997;18(4):351–357. doi: 10.1016/s0197-4580(97)00056-0. [DOI] [PubMed] [Google Scholar]
- 46.Davis DG, Schmitt FA, Wekstein DR, Markesbery WR. Alzheimer neuropathologic alterations in aged cognitively normal subjects. J Neuropathol Exp Neurol. 1999;58(4):376–388. doi: 10.1097/00005072-199904000-00008. [DOI] [PubMed] [Google Scholar]
- 47.Knopman DS, Parisi JE, Salviati A, et al. Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol. 2003;62(11):1087–1095. doi: 10.1093/jnen/62.11.1087. [DOI] [PubMed] [Google Scholar]
- 48.Driscoll I, Resnick SM, Troncoso JC, An Y, O’Brien R, Zonderman AB. Impact of Alzheimer’s pathology on cognitive trajectories in nondemented elderly. Ann Neurol. 2006;60(6):688–695. doi: 10.1002/ana.21031. [DOI] [PubMed] [Google Scholar]
- 49.Goldman WP, Price JL, Storandt M, et al. Absence of cognitive impairment or decline in preclinical Alzheimer’s disease. Neurology. 2001;56(3):361–367. doi: 10.1212/wnl.56.3.361. [DOI] [PubMed] [Google Scholar]