Abstract
A highly efficient kinetic resolution of racemic cis-4-(2-tert-butyldimethylsilyloxy-1,1-dimethyl)ethyl-3-tert-butyldimethylsilyloxy-azetidin-2-one with 7-O-triethylsilylbaccatin III was carried out to furnish 10-O-acetyl-5′-hydroxybutitaxel after removal of the silyl protecting groups. The compound was 50% as active as paclitaxel in a tubulin assembly assay and showed significantly decreased activity against MCF7 cell proliferation compared to paclitaxel.
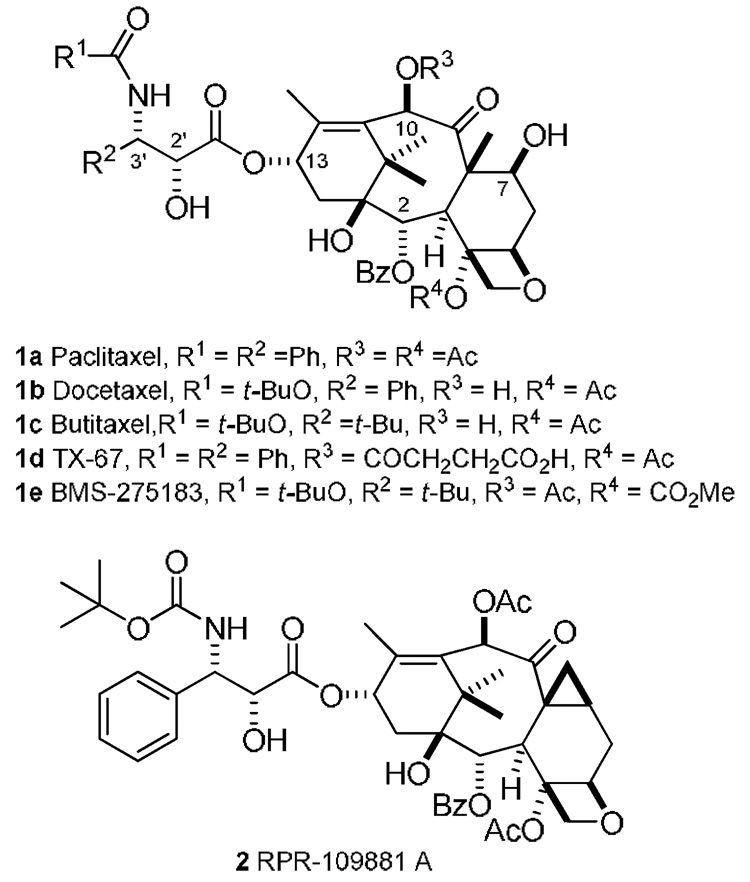
The structurally and biologically unique diterpenoid paclitaxel (1a, Figure 1),1 and its semisynthetic derivative docetaxel (1b, Figure 1),1 are among the most effective anticancer agents known2 for the treatment of metastatic breast cancer, refractory ovarian cancer and Kaposi's sarcoma.3 Paclitaxel suffers from a number of disadvantages, including low aqueous solubility, low oral bioavailability, development of drug resistance, and inability to cross the blood-brain barrier (BBB).4 Therefore, the continued synthesis and evaluation of new analogues has been important and has led to promising new results.5 For example, butitaxel analogue BMS-275183 (1e, Figure 1),6 is orally bioavailable, RPR-109881 A (2, Figure 1)7 is effective against MDR-positive and taxane resistant human tumor xenografts, and TX-67 (1c, Figure 1)8 was shown to permeate the BBB in situ.
Figure 1.
Structures of paclitaxel (1a), docetaxel (1b) butitaxel (1c), Tx-67 (1d), BMS-275183 (1e), and RPR-109881 A (2).
The efficient semisynthesis of paclitaxel analogues can be achieved by reacting baccatin III derivatives with optically pure β-lactams, the precursors for the C13 N-acyl-3′-phenylisoserine side chain. Enantiopure (3R,4S)-β-lactams can be obtained through an asymmetric ester enolate-imine cyclocondensation or via enzyme-catalyzed resolutions.9–13 An alternative semisynthesis, involving the kinetic resolution of racemic β-lactams with 7-O-triethylsilylbaccatin III derivatives has been studied for more than 10 years after being first described by Holton et al.14
This method has not been used widely for the semisynthesis of paclitaxel analogues because of unpredictable diastereoselectivities obtained during the kinetic resolution.6,15,16
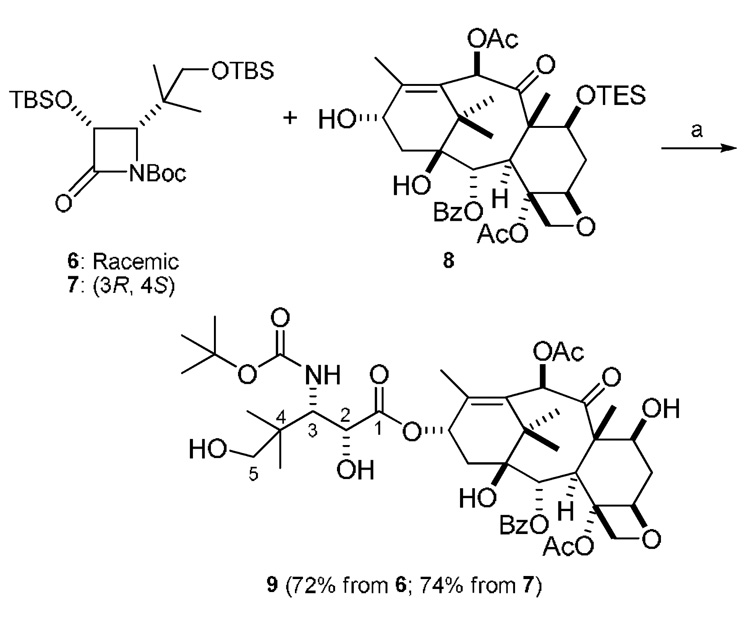
Our recent study of the kinetic resolution of racemic β-lactams with 7-O-triethylsilylbaccatin III17 showed that the structure of the β-lactam played an important role in controlling the diastereoselectivity of the kinetic resolution. High diastereoselectivities were observed when sterically demanding C3-hydroxy protecting groups or sterically demanding C4 substituents at the β-lactam rings were present. In this communication we are reporting the semisynthesis of 10-O-acetyl-5′-hydroxybutitaxel (9) via kinetic resolution with racemic β-lactam 6 that carries two sterically demanding groups: the tert-butylsilyoxy group at C3 and the 1-hydroxy-2-methylpropan-2-yl group at C4 of the β-lactam ring (Scheme 2). The new hydroxylated butitaxel analogue 9 was expected to possess better aqueous solubility (cLogP = 3.7) than paclitaxel (cLogP = 4.2). In addition, analogue 9 is a potential template for structure-activity studies as it allows the facile attachment of a variety of groups at the 5’-hydroxyl group of the C13 side chain.18
Scheme 2.
Synthesis of 10-O-acetyl-5′-hydroxybutitaxel. Reagents and conditions: (a) LiHMDS, THF, −40 °C; then HF-pyridine, pyridine, 0 °C-rt.
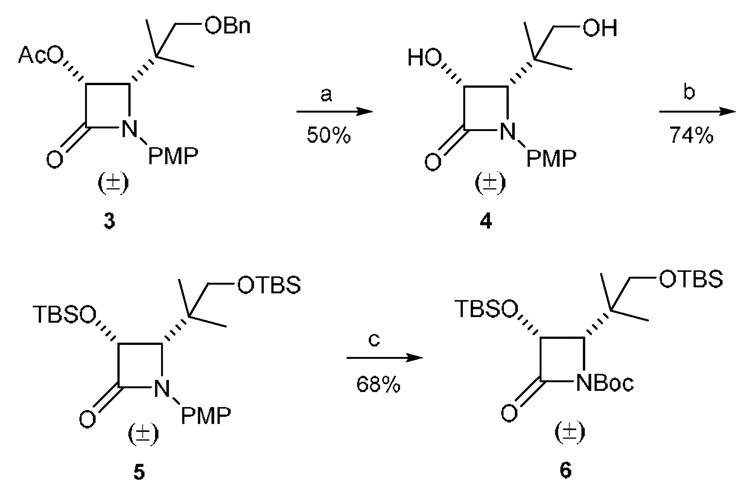
The synthesis of the racemic cis-β-lactam 6 is shown in Scheme 1, starting with the hydrolysis (KOH) of the acetate in β-lactam 3,19 followed by deprotection of the benzyl group to provide diol 4. Protection of the two hydroxyl groups of 4 with tert-butyldimethylsilyl chloride provided intermediate 5. The 4-methoxyphenyl moiety (PMP) of 5 was removed oxidatively with ammonium cerium(IV) nitrate (CAN),20 and the resulting N-unsubstituted β-lactam was then treated with tert-butyl dicarbonate to afford racemic N-Boc-β-lactam 6.
Scheme 1.
Synthesis of racemic β-lactam 6. Reagents and conditions: (a) KOH, THF, 0 °C; then 10% Pd/C, HCO2NH4, MeOH, reflux; (b) TBSCl, imidazole, DMF, 35 °C; (c) CAN, CH3CN, −10 °C; then Boc2O, DMAP, Et3N, CH2Cl2, rt.
We then carried out the kinetic resolution between racemic β-lactam 6 and 7-O-triethylsilylbaccatin III (8)21 using the previously reported reaction conditions (Scheme 2).17 Thus, four equivalents of 6 were used to react with the baccatin derivative 8 in the presence of 1.5 equivalents of LiHMDS in THF at −40 °C. The crude reaction product was subsequently dissolved in pyridine and treated with hydrogen fluoride to furnish derivative 9. The isomeric ratio of 9 was determined by HPLC analysis as described before.22 Excellent diastereoselectivity (216:1) was observed. In our previous study we had observed that diastereoselectivities increased with the size of the C4-substutients of N-Boc-3-OTBS-β-lactams. The 4-phenyl-β-lactam showed a 41:1 diastereomeric ratio and the 4-tert-butyl-β-lactam an 82:1 diastereomeric ratio.17 In the present study, the even more sterically demanding 4-(2-tert-butyldimethylsilyloxy-1,1-dimethyl)ethyl group in β-lactam 6 provided an enhanced diastereomeric ratio (216:1), further supporting the hypothesis that C4-substituents play an important role in controlling the diastereoselectivity of this kinetic resolution.
To verify the efficiency of the kinetic resolution, we coupled 8 with enantiopure β-lactam 7 prepared according to the reported procedure19 under the standard reaction conditions to generate analogue 9 in 74% yield after deprotection of the silyl groups (Scheme 2). Spectral data and HPLC retention time of this analogue are identical to the product obtained in the kinetic resolution. This result demonstrated the highly efficient feature of the kinetic resolution method.
Analogue 9 was tested in a tubulin assembly assay and as an inhibitor of proliferation of the MCF7 and NCI/ADR-RES breast cancer lines (Table 1).23,24 In the tubulin assembly assay analogue 9 was just slightly less active than paclitaxel. However, it showed significantly reduced activity against MCF7 proliferation (267-fold).
Table 1.
ED50 values for tubulin assembly and cytoxicity assays.
In conclusion, a new paclitaxel analogue was synthesized through a highly efficient kinetic resolution of racemic β-lactam 6 with 7-O-triethylsilylbaccatin III. The observed high diastereoselectivity confirms earlier observations that sterically demanding C4-β-lactams substituents are a key factor in the efficiency of this kinetic resolution. Although the new paclitaxel analogue showed excellent activity in the tubulin assembly assay, it had significantly decreased activity against MCF7 cell proliferation. The results suggest that the compound may not enter the cells as well as paclitaxel because it is more hydrophilic or possibly because it is a much better substrate for the multidrug transporter than paclitaxel. A similar result was observed for the phenolic paclitaxel metabolite that carries a 4-hydroxy group at the C3 phenyl group.25
Acknowledgments
The authors wish to thank the National Cancer Institute for financial support of this research (NIH CA82801 and NIH CA105305), NBIF Research Assistantship Initiative, and Tapestry Pharmaceuticals (Boulder, CO) for a generous gift of 10-deacetylbaccatin III. We wish to thank Christopher Schneider for his help with the HPLC analysis. We also thank Jacquelyn K. Huff for her excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Notes
- 1.Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. J. Am. Chem. Soc. 1971;93:2325. doi: 10.1021/ja00738a045. [DOI] [PubMed] [Google Scholar]
- 2.For review: Spencer CM, Faulds D. Drugs. 1994;48:794. doi: 10.2165/00003495-199448050-00009.
- 3.For review: Zhou J, Giannakakou P. Curr. Med. Chem. - Anticancer Agents. 2005;5:65. doi: 10.2174/1568011053352569.
- 4.Ge H, Vasandani V, Huff JK, Audus KL, Himes RH, Seelig A, Georg GI. Bioorg. Med. Chem. Lett. 2006;16:433. doi: 10.1016/j.bmcl.2005.09.043. [DOI] [PubMed] [Google Scholar]
- 5.For review: Kingston DGI, Jagtap PG, Yuan H, Samala L. Prog. Chem. Org. Nat. Prod. 2002;84:53. doi: 10.1007/978-3-7091-6160-9_2.
- 6.Mastalerz H, Cook D, Fairchild CR, Hansel S, Johnson W, Kadow JF, Long BH, Rose WC, Tarrant J, Wu M-J, Xue M-Q, Zhang G-F, Zoeckler M, Vyas DM. Bioorg. Med. Chem. 2003;11:4315. doi: 10.1016/s0968-0896(03)00495-4. [DOI] [PubMed] [Google Scholar]
- 7.Gelmon KA, Latreille J, Tolcher A, Genier L, Fisher B, Forand D, D’Aloisio S, Vernillet L, Daigneault L, Lebecq A, Besenval M, Eisenhauser E. J. Clin. Onco. 2001;18:4098. doi: 10.1200/JCO.2000.18.24.4098. [DOI] [PubMed] [Google Scholar]
- 8.Rice A, Liu Y, Michaelis ML, Himes RH, Georg GI, Audus K. J. Med. Chem. 2005;48:832. doi: 10.1021/jm040114b. [DOI] [PubMed] [Google Scholar]
- 9.For review: Boge TC, Georg GI. The Medicinal Chemistry of β-Amino Acids: Paclitaxel as an Illustrative Example. In: Juaristi E, editor. Enantioselective Synthesis of β-Amino Acids. New York: Wiley-VCH; 1997. p. 1.
- 10.Kayser MM, Mihovilovic MD, Kearns J, Feicht A, Stewart JD. J. Org. Chem. 1999;64:6603. doi: 10.1021/jo9900681. [DOI] [PubMed] [Google Scholar]
- 11.Kayser MM, Yang Y, Mihovilovic MD, Feicht A, Rochon FD. Can. J. Chem. 2002;80:796. [Google Scholar]; Yang Y, Wang F-Y, Rochon FD, Kayser MM. Can. J. Chem. 2005;83:28. [Google Scholar]
- 12.Patel RN, Howell J, Chidambaram R, Benoit S, Kant J. Tetrahedron: Asymmetry. 2003;14:3673. [Google Scholar]
- 13.Carr JC, Al-Azemi TF, Long TE, Shim J-Y, Coates CM, Turos E, Bisht KS. Tetrahedron. 2003;59:9147. [Google Scholar]
- 14.Holton RA, Biedinger RJ, Boatman PD. Semisynthesis of Taxol and Taxotere. In: Suffness M, editor. Taxol® Science and Applications. CRC: Boca Raton, Fl; 1995. p. 97. [Google Scholar]
- 15.Ojima I, Slater JC. Chirality. 1997;9:487. doi: 10.1002/(SICI)1520-636X(1997)9:5/6<487::AID-CHIR15>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 16.Takeda Y, Uoto K, Iwahana M, Jimbo T, Nagata M, Atsumi R, Ono C, Tanaka N, Terasawa H, Soga T. Bioorg. Med. Chem. Lett. 2004;14:3209. doi: 10.1016/j.bmcl.2004.03.109. [DOI] [PubMed] [Google Scholar]
- 17.Ge H, Spletstoser JT, Yang Y, Kayser M, Georg GI. J. Org. Chem. 2007;72:756. doi: 10.1021/jo061339s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.For review: Zefirova ON, Nurieva EV, Ryzhov AN, Zyk NV, Zefirov NS. Russ. J. Org. Chem. 2005;41:315.
- 19.Yang Y, Wang J, Kayser MM. Tetrahedron: Asymmetry. 2007;18:2021. [Google Scholar]
- 20.Georg GI, Kant J, Gill HJ. J. Am. Chem. Soc. 1987;109:1129. [Google Scholar]
- 21.Denis J-N, Greene AE, Guénard D, Guéritte-Voegelein F, Mangatal L, Potier P. J. Am. Chem. Soc. 1988;110:5917. [Google Scholar]
- 22.HPLC conditions. Jupiter 5 µ C4-reverse phase column (10 × 250 mm) from Phenomenex USA, employing a gradient of H2O-CH3CN (0–70%, v/v) with 0.1% TFA as the solvent system with a flow rate of 2.0 mL/min for 100 min. Average retention time for the major isomer (2'R,3'S)-9 = 65.75 min; minor isomer (2'S,3'R)-9 = 64.98 min. All reaction intermediates and the final products showed spectroscopic properties in agreement with their structures.
- 23.Barron DM, Chatterjee SK, Ravindra R, Roof R, Baloglu E, Kingston DG, Bane S. Anal. Biochem. 2003;315:49. doi: 10.1016/s0003-2697(02)00691-7. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Boge TC, Victory S, Ali SM, Zygmunt J, Georg GI, Marquez RT, Himes RH. Combi. Chem. High Through. Screen. 2002;5:39. doi: 10.2174/1386207023330615. [DOI] [PubMed] [Google Scholar]
- 25.Park H, Hepperle M, Boge TC, Himes RH, Georg GI. J. Med. Chem. 1996;39:2705. doi: 10.1021/jm960142x. [DOI] [PubMed] [Google Scholar]