Abstract
The circadian system orchestrates internal physiology on a daily schedule to promote optimal health and maximize disease prevention. Chronic disruptions in circadian function are associated with an increase in a variety of disease states including, heart disease, ulcers, and diabetes. With advanced age, the genes regulating circadian function at the cellar level become disorganized and the ability of the brain clock to entrain to local time diminishes. As a result, aged individuals exhibit a loss of temporal coordination among bodily systems, leading to deficits in homeostasis and sub-optimal functioning. Such disruptions in the circadian system appear to accelerate the aging process and contribute to senescence, with some systems being more vulnerable than others. This review explores aging-associated changes in circadian function and examines evidence linking such alterations to adverse health consequences in late life and promotion of the aging process.
Keywords: Senescence, clock gene, CCG, estrus, ovulation
I. Introduction: The Circadian System in Health, Disease, and Aging
In The Wisdom of the Body, Walter B. Cannon developed the concept of homeostasis to describe the exquisite precision in which countless bodily systems are maintained within finely-tuned operating limits to promote optimal health and avoid disease states. When we consider homeostasis, we often overlook the fact that the optimal limits for a given physiological or biochemical process vary by time of day. The circadian system orchestrates internal events on a daily schedule to ensure that bodily systems are coordinated with environmental time and with each other on a daily schedule (Figure 1). Disruptions in temporal homeostasis have pronounced impact on physiological functioning, overall health, and disease susceptibility. An endogenous, daily time-keeping system is necessary to anticipate environmental change, initiate internal adjustments in advance of the appropriate environmental time, and maintain proper phase relationships among internal systems.
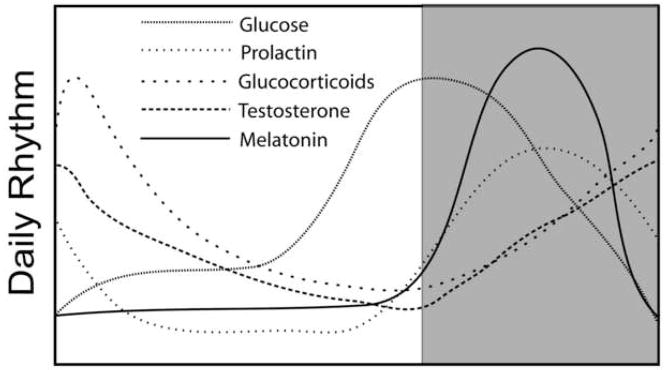
Figure 1.
Examples of circadian rhythms in humans. Shaded area represents times of sleep while the unshaded area represents times of activity. Note that all parameters exhibit unique peaks and troughs relative to each other. This phase relationship among individual rhythms is critical for optimal functioning and homeostasis.
It is not difficult to imagine how physiological functioning would suffer without an internal circadian clock synchronized to the environment. Most of us have experienced this acutely, manifested by general feelings of malaise and other maladies following a long flight across time zones. This loss of synchrony between the circadian clock in the brain and the environment leads to pronounced clinical pathologies. One recent study found that elderly mice subjected to temporal disruptions equivalent to a flight from Washington to Paris, once a week for eight weeks, die as a result of their bodies being out of sync with local time (Davidson et al., 2006). Flight attendants frequently traveling across time zones exhibit cognitive deficits associated with reductions in temporal lobe structures (Cho, 2001; Cho et al., 2000). Numerous studies show that shift workers have a higher incidence of cancer (Conlon et al., 2007), diabetes (Morikawa et al., 2005), ulcers (Koda et al., 2000), hypertension and cardiovascular disease (Kivimaki et al., 2006), psychological disorders (Bildt and Michelsen, 2002), and a host of other clinical issues. These findings, although largely correlational, point to a critical role for internal circadian timing in maintaining normal brain functioning and peripheral physiology.
With advancing age, animals exhibit numerous circadian disruptions (Benloucif et al., 1997; Davidson et al., 2008; Li and Satinoff, 1995; Valentinuzzi et al., 1997; Weinert and Waterhouse, 1999) that contribute to poor health consequences and hastened senescence. In fact, longevity is diminished by circadian perturbations and decelerated by restoration of youthful circadian behavior by transplantation of a fetal clock into the brains of aged animals (Hurd and Ralph, 1998). The following review underscores the significance of two fundamental functions of the circadian system, internal organization and entrainment to the environment, in the aging process.
II. The Circadian System
Whereas a ‘master’ circadian clock has been localized to the suprachiasmatic nucleus (SCN) located in the anterior hypothalamus in mammals (Moore and Eichler, 1972; Stephan and Zucker, 1972), it is now more appropriate to conceptualize the ‘circadian system’ as an assembly comprised not only of a master clock, but also a series of subordinate clocks whose phase and coordinated activity is set by the SCN. Numerous lines of converging evidence from a host of laboratories have confirmed the role of the SCN as a master pacemaker. For example, transplants of donor SCN tissue into the brains of arrhythmic, SCN-lesioned hosts restore circadian rhythmicity in behavior (Lehman et al., 1987; Ralph et al., 1990). Importantly, rhythms are restored with the period of the donor SCN, indicating that the transplanted tissue does not act by restoring host-brain function but that the “clock” is contained in the transplanted tissue. Furthermore, circadian rhythms in neural firing rate persist in isolated SCN tissue maintained in culture (Green and Gillette, 1982), demonstrating that input from extra-SCN brain sites is not necessary for circadian rhythms in this nucleus. As will be described in the following sections, the SCN has direct access to environmental time via retinal projections to the clock. Because subordinate central and peripheral clocks do not have access to such time cues, it is necessary for the SCN to communicate such information throughout the CNS and periphery. In addition to the core clock genes responsible for circadian function at the cellular level, subordinate clock-controlled genes (CCGs) represent important output and local coordination systems. The stability of this hierarchical arrangement is disrupted with advancing age (Davidson et al., 2008).
A. The Molecular Clock
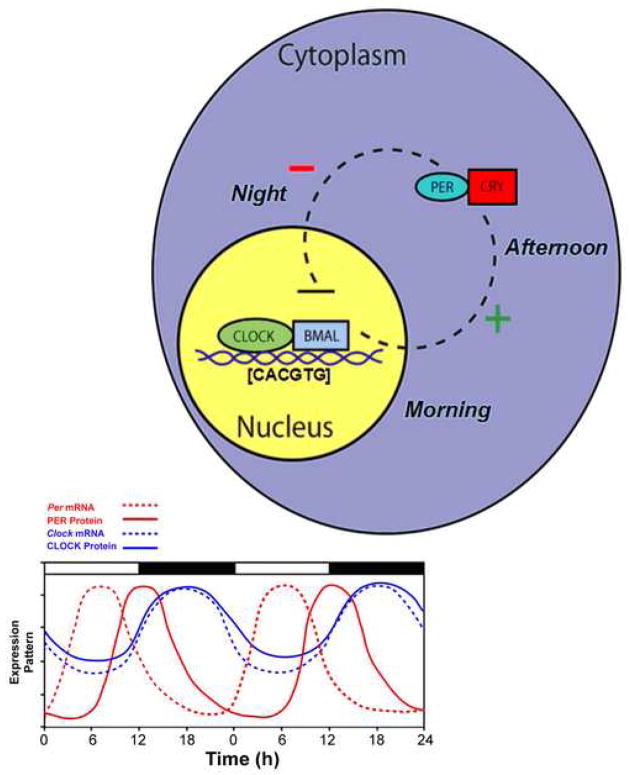
Within a cell, circadian rhythms are produced by an autoregulatory transcriptional/translational negative feedback loop that takes approximately 24 hours. While the general mechanism for circadian oscillations at the cellular level is common among organisms, the components comprising the feedback loop differ. For the purpose of clarity, only the core mammalian feedback loop is described. Earlier work proposed a core feedback loop that begins when two proteins, CLOCK and BMAL1, bind to one another and drive the transcription of messenger RNA (mRNA) of the Period (Per) and Cryptochrome (Cry) genes by binding to the E-box (CACGTG) domain on these gene promoters. Three Period (Per1, Per2, and Per3) and two Cryptochrome (Cry1 and Cry2) genes have been identified. The mRNA for these genes is translated into PER and CRY proteins in the cytoplasm of the cell over the course of the day. Throughout the day, these proteins build up within the cytoplasm, and when they reach high enough levels, they form hetero- and homo-dimers. These newly formed dimers then feed back to the nucleus where they bind to the CLOCK:BMAL1 protein complex to turn off their own transcription (reviewed in (Ko and Takahashi, 2006)) (Figure 2).
Figure 2.
A simplified model of the intracellular mechanisms responsible for mammalian circadian rhythm generation. The process begins when CLOCK and BMAL1 proteins dimerize to drive the transcription of the Per (Per1, Per2, and Per3) and Cry (Cry1 and Cry2) genes. In turn, Per and Cry are translocated to the cytoplasm and translated into their respective proteins. Throughout the day, PER and CRY proteins rise within the cell cytoplasm. When levels of PER and CRY reach a threshold, they form heterodimers, feed back to the cell nucleus and negatively regulate CLOCK:BMAL1 mediated transcription of their own genes. This feedback loop takes approximately 24 h, thereby leading to an intracellular circadian rhythm. (Adapted from Kriegsfeld and Silver, 2006).
More recently, it has become clear that the cellular clockwork is more complex, with a number of integrated feedback loops whose regulators are often controlled by elements of the core clock mechanism. Two other promoter elements have emerged as important for circadian rhythm generation, DBP/E4BP4 binding elements (D boxes) and REV-ERBα/ROR binding elements (RREs) (Ueda et al., 2005). REV-ERBα, an orphan nuclear receptor, negatively regulates the activity of the CLOCK:BMAL1 complex and is also acted upon by PER and CRY. Transcription of REV-ERBα is controlled by the same mechanism controlling Per and Cry transcription. Similarly, the transcription factor DPB is positively regulated by the CLOCK:BMAL1 complex (Ripperger and Schibler, 2006) and acts as an important output mechanism by driving rhythmic transcription of other output genes via a PAR basic leucine zipper (PAR bZIP) (Lavery et al., 1999). Whereas most work to date has focused on transcriptional regulation as the key mechanism driving cellular rhythms, post-transcriptional and post-translational events are also critical for circadian coordination (Baggs and Green, 2003; Kramer et al., 2003; Reddy et al., 2006).
In addition to transcriptional/translational control of cellular clock function, regulatory kinases also play a pronounced role in regulation of circadian period. Over a decade ago, a circadian mutation named tau was identified that resulted in a shortened circadian period in Syrian hamsters (Ralph and Menaker, 1988). It is now known that the tau locus is encoded by casein kinase I epsilon (CKIε) (Lowrey et al., 2000; Wang et al., 2007). In normal rodents, CKIε phosphorylates PER and “tags” it for degradation throughout the day. Eventually, PER acts to overwhelm CKIε, and dimerizes with CRY to feed back to the cell nucleus. The mutant form of CKIε is unable to phosphorylate PER, leading to a short circadian period in tau mutants due to premature nuclear entry of PER:CRY dimers (Lowrey et al., 2000; Vielhaber et al., 2000).
B. Entrainment of the Circadian System
In addition to a direct visual pathway from retinal ganglion cells to the visual cortex, there is also a direct retinohypothalamic tract (RHT) projecting from the optic nerve to the SCN (Klein and Moore, 1979; Moore and Klein, 1974). This second visual pathway is necessary and sufficient to entrain (synchronize) the SCN to the environmental light:dark cycle. Early studies of circadian photoreception investigated the role of traditional rod and cone photoreceptors in entrainment. However, mice lacking both rod and cone photoreceptors (rd/rd) exhibit grossly normal entrainment even though they are visually blind (Foster et al., 1993; Freedman et al., 1999; Lucas et al., 1999; Van Gelder, 2001). These findings led to a search for a novel non-rod, non-cone photoreceptor necessary for photic entrainment. Several years of rapid discovery beginning in this millennium identified a subset of light-responsive ganglion cells containing the photopigment, melanopsin (Berson et al., 2002; Hannibal and Fahrenkrug, 2002). These ganglion cells project directly to the SCN and were initially thought to be the sole photoreceptors necessary for entrainment. However, melanopsin deficient mice exhibit only minor impairments in entrainment (Lucas et al., 2003; Panda et al., 2002; Ruby et al., 2002). This discrepancy was resolved by showing that entrainment is abolished in mice doubly mutant for both melanopsin and traditional rod/cone photoreceptors (Hattar et al., 2003; Panda et al., 2003). These findings suggest that traditional rod/cone photoreceptors project to specialized light-responsive ganglion cells that then transmit this integrated photic information directly to the SCN. Thus, these two types of receptors likely work together to entrain the circadian clock, and either receptor type can support entrainment in the absence of the other.
III. Age-Related Changes in the Circadian System
With advancing age, the circadian system exhibits loss of temporal precision (Benloucif et al., 1997; Davidson et al., 2008; Li and Satinoff, 1995; Valentinuzzi et al., 1997; Weinert and Waterhouse, 1999), contributing to a variety of age-related pathologies. Such age-related changes cannot be explained by cell death or atrophy in the SCN, as aging does not decrease cell size or numbers in the master pacemaker (Madeira et al., 1995). However, the aged SCN shows alterations in peptide expression (vasoactive intestinal polypeptide (VIP) (Kawakami et al., 1997); arginine vasopressin (AVP) (Roozendaal et al., 1987)), and a reduction in the amplitude of circadian rhythms of electrical activity (Satinoff et al., 1993; Watanabe et al., 1995). Transplantation of a ‘young’ SCN into aged animals yields improvements in numerous rhythmic functions, including the diurnal rhythm of corticortropin-releasing hormone (CRH) and behavioral rhythms in locomotion (Cai et al., 1997; Li and Satinoff, 1995). Together, these studies suggest that the SCN is an important locus for age-related changes in the rodent circadian system and treatments targeting this structure may act to ameliorate some of the deterioration seen in aged individuals.
A. Aged-Related Changes in Circadian Entrainment
When the circadian clock is not synchronized to the environment, temporal homeostasis is severely disrupted, comparable to some of the changes seen with aging. Consequently, one potential target of aging in the circadian system is the entrainment apparatus. Aged animals are about 20 times less sensitive to the entraining effects of light relative to young animals, despite the fact that they exhibit unaltered retinal innervation of the SCN (Zhang et al., 1998). This finding suggests that deficits in entrainment result from alterations at the level of the retina or the master clock. As aged mice do not exhibit gross morphological retinal abnormalities (Oster et al., 2003), most research has focused on the SCN as the site of entrainment alterations. Because Per1 transcription is rapidly induced by light and is required for entrainment (Wakamatsu et al., 2001), the induction of Per1 makes a convenient assay for investigating the response of the SCN to a phase-resetting light pulse. In aged animals, Per1 expression following an entraining light stimulus is markedly reduced with a significantly longer delay to resynchronization (Davidson et al., 2008; Kolker et al., 2003). In young animals, disruption of the Period genes leads to aging-like declines in sensitivity to light (Asai et al., 2001; Weinert et al., 2001). These findings suggest that temporal disorganization with advancing age may result, in part, from reductions in the sensitivity of the SCN to retinal stimulation. Future studies using pharmacological approaches to examine the response of the SCN to neurochemical applications simulating retinal stimulation are necessary to rule out abnormal retinal signaling as a mechanism mediating age-related declines in entrainment.
B. Clock Genes and Aging
Given the pronounced decrements in rhythmic function seen in aged animals, several studies have investigated the impact of aging on the expression of the core clock genes. The amplitude of daily Bmal1 expression is reduced in aged hamsters, with lower expression during the subjective night, when Bmal1 expression is normally at its peak, compared to young animals (Kolker et al., 2003). Bmal1 knockout animals are arrhythmic (Bunger et al., 2000), indicating the importance of this gene in normal circadian cycling, and lend support for the possibility that age-related changes in circadian rhythms may result from abnormal Bmal1 expression. Interestingly, reductions in Bmal1 expression are not associated with changes in Per1, Per2 and Cry1 amplitude in the aged SCN, indicating that the downstream targets of Bmal1 responsible for circadian disruption with advanced age may be distinct from the core clock machinery (Asai et al., 2001). Clock expression is also reduced throughout the day in the SCN of aged animals although, importantly, mutation of the Clock gene and resulting arrhythmicity in constant conditions does not impact the aging process (Kolker et al., 2003). As a result, Bmal1 has remained a focus for research exploring the role of clock genes in aging.
Additional evidence for a role of Bmal1 in the aging process comes from studies of knockout mice. Mice deficient in Bmal1 have a reduced lifespan and develop a number of age-related pathologies including sarcopenia, cataracts, and organ shrinkage significantly earlier than their wild-type counterparts (Kondratov et al., 2006). These age-related pathologies indicate that some circadian proteins are important for physiological processes that are not directly linked to circadian function. Since many peripheral tissues express clock genes endogenously (Reppert and Weaver, 2002), understanding the role of peripheral clock gene oscillations in maintaining organ homeostasis will aid in the development of strategies to treat age related pathologies. Future studies aimed at understanding the extent to which endogenous oscillations of peripheral clock genes versus synchronization of these peripheral tissues with the master pacemaker will reveal changes in the maintenance of organ and tissue homeostasis in aging animals.
IV. The Circadian System and Reproductive Aging
Age-related decline in reproductive axis function is common across species, with initiation of this decline typically occurring midway through life. Because females are affected earlier in life and to a greater extent than males, research to date has focused on female reproductive aging and the circadian system. In regularly cycling female rodents, a daily circadian signal from the SCN coinciding with a threshold level of estrogen initiates the gonadotropin-releasing hormone (GnRH) mediated luteinizing hormone (LH) surge required for ovulation (reviewed in (Kriegsfeld and Silver, 2006)). As female rodents age, the ability of the SCN to stimulate the surge weakens.
A delay in the timing, and attenuation of the amplitude, of the LH surge are hallmarks of reproductive aging. In middle-aged hamsters, for example, the peak level of LH is delayed by as many as three hours compared to the onset of the surge in young, reproductively healthy animals (Wise, 1982). The cadence seen in the timing and amplitude of the LH surge in middle-aged animals has been linked to changes in GnRH activation by SCN neuropeptidergic signals. The GnRH system represents the final pathway in control of the reproductive axis in most mammalian species. It would be reasonable to assume that the deficit in LH surge amplitude may be a direct consequence of a decrease in GnRH peptide release. However, concentrations of GnRH peptide do not show an age-related decline. Instead, the decline in reproductive capacity appears to be due to changes in the activation of the GnRH system by the circadian clockwork. In regularly cycling young females, 34–40% of GnRH neurons express immediate early genes on the afternoon of proestrus compared to 9–14% in middle-aged animals; the total number of GnRH-immunoreactive neurons does not differ between the two groups (Lloyd et al., 1994). This finding suggests that the SCN may not provide adequate stimulation to the GnRH system in aged animals. Two SCN peptides, VIP and AVP, have long been implicated in stimulation of the reproductive axis. VIP mRNA but not AVP mRNA becomes arrhythmic in the SCN of middle-aged female hamsters (Krajnak et al., 1998), and suppression of SCN VIP in young female hamsters results in accelerated deficits in GnRH activation and an LH surge that mimic that of an aged population (Gerhold et al., 2005; Harney et al., 1996). Together, these findings suggest that age-related deficits in the neuroendocrine mechanisms mediating ovulation may result from loss of function at the level of the SCN.
V. Aging and Circadian Changes in Energetics
One of the most robust phenotypes associated with aging is change in energy utilization, including body fat stores. Increases in body fat mass and deteriorations in insulin sensitivity are associated with aging in many mammalian species as well as many clinical pathologies, including cardiovascular disease. Data from clock gene mutants demonstrate a strong circadian mechanism regulating adipose stores, as well as the release of insulin, a pancreatic hormone, and leptin, an adipose hormone, all of which activate the anorexigenic pathways of energy homeostasis and reduce food intake. The nocturnal rise in circulating leptin levels of younger animals is attenuated in older animals, including primates (Downs and Urbanski, 2006). The phases and amplitudes of rhythms of hormones associated with metabolic function, such as insulin, corticosterone, and prolactin, are disrupted in obese aged rodents and administration of these hormones at specific times of day mimicking the rhythms of the younger phenotype are able to re-establish metabolic characteristics of younger animals (Cincotta et al., 1993). Age-related alterations in corticosterone secretion are a direct result of the eradication of the diurnal rhythm of its hypothalamic releasing peptide, corticotropin-releasing hormone (CRH) (Cai and Wise, 1996). Fetal SCN grafts implanted into the brains of middle-aged rats restores the diurnal rhythm of CRH (Cai et al., 1997). The reinstatement of CRH rhythms in middle-aged rats by fetal SCN is unique, as other neuroendocrine rhythms are not restored by such grafts (Kriegsfeld and Silver, 2006). Future studies are needed to fully delineate if re-establishing temporal rhythms in aged populations through timed hormonal injections are able to re-set energetic processes to allow for minimization of metabolic deficits.
VI. Conclusions and Perspectives
With increased understanding of the mechanisms driving age-associated changes in the mammalian circadian clockwork, novel insight concerning the nature of age-related pathologies resulting from loss of temporal precision can be gained. Aged individuals exhibit pronounced deficits in the amplitude and timing of core molecular clock genes and entrainment of the master clock to local time. This deterioration in the circadian system manifests as disruptions in the sleep-wake cycle and system-wide physiology. Information on the role circadian abnormalities play in the aging process is limited and enormous opportunity exists for exploring these associations from gene to behavior. Further studies investigating the impact of aging on the master clock in the SCN and the mechanisms coupling the SCN to peripheral clocks will afford great insight into maximizing health with advancing age.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asai M, Yoshinobu Y, Kaneko S, Mori A, Nikaido T, Moriya T, Akiyama M, Shibata S. Circadian profile of Per gene mRNA expression in the suprachiasmatic nucleus, paraventricular nucleus, and pineal body of aged rats. J Neurosci Res. 2001;66:1133–1139. doi: 10.1002/jnr.10010. [DOI] [PubMed] [Google Scholar]
- Baggs JE, Green CB. Nocturnin, a deadenylase in Xenopus laevis retina: a mechanism for posttranscriptional control of circadian-related mRNA. Curr Biol. 2003;13:189–198. doi: 10.1016/s0960-9822(03)00014-9. [DOI] [PubMed] [Google Scholar]
- Benloucif S, Masana MI, Dubocovich ML. Light-induced phase shifts of circadian activity rhythms and immediate early gene expression in the suprachiasmatic nucleus are attenuated in old C3H/HeN mice. Brain Res. 1997;747:34–42. doi: 10.1016/s0006-8993(96)01182-1. [DOI] [PubMed] [Google Scholar]
- Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- Bildt C, Michelsen H. Gender differences in the effects from working conditions on mental health: a 4-year follow-up. Int Arch Occup Environ Health. 2002;75:252–258. doi: 10.1007/s00420-001-0299-8. [DOI] [PubMed] [Google Scholar]
- Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, Simon MC, Takahashi JS, Bradfield CA. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103:1009–1017. doi: 10.1016/s0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai A, Wise PM. Age-related changes in the diurnal rhythm of CRH gene expression in the paraventricular nuclei. Am J Physiol. 1996;270:238–243. doi: 10.1152/ajpendo.1996.270.2.E238. [DOI] [PubMed] [Google Scholar]
- Cai A, Scarbrough K, Hinkle DA, Wise PM. Fetal grafts containing suprachiasmatic nuclei restore the diurnal rhythm of CRH and POMC mRNA in aging rats. Am J Physiol. 1997;273:1764–1770. doi: 10.1152/ajpregu.1997.273.5.R1764. [DOI] [PubMed] [Google Scholar]
- Cho K, Ennaceur A, Cole JC, Suh CK. Chronic jet lag produces cognitive deficits. J Neurosci. 2000;20:66. doi: 10.1523/JNEUROSCI.20-06-j0005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K. Chronic ‘jet lag’ produces temporal lobe atrophy and spatial cognitive deficits. Nat Neurosci. 2001;4:567–568. doi: 10.1038/88384. [DOI] [PubMed] [Google Scholar]
- Cincotta AH, Schiller BC, Landry RJ, Herbert SJ, Miers WR, Meier AH. Circadian neuroendocrine role in age-related changes in body fat stores and insulin sensitivity of the male Sprague-Dawley rat. Chronobiol Int. 1993;10:244–258. doi: 10.1080/07420529309059707. [DOI] [PubMed] [Google Scholar]
- Conlon M, Lightfoot N, Kreiger N. Rotating shift work and risk of prostate cancer. Epidemiology. 2007;18:182–183. doi: 10.1097/01.ede.0000249519.33978.31. [DOI] [PubMed] [Google Scholar]
- Davidson AJ, Sellix MT, Daniel J, Yamazaki S, Menaker M, Block GD. Chronic jet-lag increases mortality in aged mice. Curr Biol. 2006;16:914–916. doi: 10.1016/j.cub.2006.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AJ, Yamazaki S, Arble DM, Menaker M, Block GD. Resetting of central and peripheral circadian oscillators in aged rats. Neurobiol Aging. 2008;29:471–477. doi: 10.1016/j.neurobiolaging.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs JL, Urbanski HF. Aging-related sex-dependent loss of the circulating leptin 24-h rhythm in the rhesus monkey. J Endocrinol. 2006;190:117–127. doi: 10.1677/joe.1.06745. [DOI] [PubMed] [Google Scholar]
- Foster RG, Argamaso S, Coleman S, Colwell CS, Lederman A, Provencio I. Photoreceptors regulating circadian behavior: a mouse model. J Biol Rhythms. 1993;8(Suppl):17–23. [PubMed] [Google Scholar]
- Freedman MS, Lucas RJ, Soni B, von Schantz M, Munoz M, David-Gray Z, Foster R. Regulation of mammalian circadian behavior by non-rod, non-cone, ocular photoreceptors. Science. 1999;284:502–504. doi: 10.1126/science.284.5413.502. [DOI] [PubMed] [Google Scholar]
- Gerhold LM, Rosewell KL, Wise PM. Suppression of vasoactive intestinal polypeptide in the suprachiasmatic nucleus leads to aging-like alterations in cAMP rhythms and activation of gonadotropin-releasing hormone neurons. J Neurosci. 2005;25:62–67. doi: 10.1523/JNEUROSCI.3598-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DJ, Gillette R. Circadian rhythm of firing rate recorded from single cells in the rat suprachiasmatic brain slice. Brain Res. 1982;245:198–200. doi: 10.1016/0006-8993(82)90361-4. [DOI] [PubMed] [Google Scholar]
- Hannibal J, Fahrenkrug J. Melanopsin: a novel photopigment involved in the photoentrainment of the brain’s biological clock? Ann Med. 2002;34:401–407. doi: 10.1080/078538902320772151. [DOI] [PubMed] [Google Scholar]
- Harney JP, Scarbrough K, Rosewell KL, Wise PM. In vivo antisense antagonism of vasoactive intestinal peptide in the suprachiasmatic nuclei causes aging-like changes in the estradiol-induced luteinizing hormone and prolactin surges. Endocrinology. 1996;137:3696–3701. doi: 10.1210/endo.137.9.8756535. [DOI] [PubMed] [Google Scholar]
- Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, Hankins MW, Lem J, Biel M, Hofmann F, Foster RG, Yau KW. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:76–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd MW, Ralph MR. The significance of circadian organization for longevity in the golden hamster. J Biol Rhythms. 1998;13:430–436. doi: 10.1177/074873098129000255. [DOI] [PubMed] [Google Scholar]
- Kawakami F, Okamura H, Tamada Y, Maebayashi Y, Fukui K, Ibata Y. Loss of day-night differences in VIP mRNA levels in the suprachiasmatic nucleus of aged rats. Neurosci Lett. 1997;222:99–102. doi: 10.1016/s0304-3940(97)13355-9. [DOI] [PubMed] [Google Scholar]
- Kivimaki M, Virtanen M, Elovainio M, Vaananen A, Keltikangas-Jarvinen L, Vahtera J. Prevalent cardiovascular disease, risk factors and selection out of shift work. Scand J Work Environ Health. 2006;32:204–208. doi: 10.5271/sjweh.1000. [DOI] [PubMed] [Google Scholar]
- Klein DC, Moore RY. Pineal N-acetyltransferase and hydroxyindole-O-methyltransferase: control by the retinohypothalamic tract and the suprachiasmatic nucleus. Brain Res. 1979;174:245–262. doi: 10.1016/0006-8993(79)90848-5. [DOI] [PubMed] [Google Scholar]
- Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15(Spec No 2):271–277. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- Koda S, Yasuda N, Sugihara Y, Ohara H, Udo H, Otani T, Hisashige A, Ogawa T, Aoyama H. [Analyses of work-relatedness of health problems among truck drivers by questionnaire survey] Sangyo Eiseigaku Zasshi. 2000;42:6–16. doi: 10.1539/sangyoeisei.kj00002552185. [DOI] [PubMed] [Google Scholar]
- Kolker DE, Fukuyama H, Huang DS, Takahashi JS, Horton TH, Turek FW. Aging alters circadian and light-induced expression of clock genes in golden hamsters. J Biol Rhythms. 2003;18:159–169. doi: 10.1177/0748730403251802. [DOI] [PubMed] [Google Scholar]
- Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP. Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes Dev. 2006;20:1868–1873. doi: 10.1101/gad.1432206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajnak K, Kashon ML, Rosewell KL, Wise PM. Aging alters the rhythmic expression of vasoactive intestinal polypeptide mRNA but not arginine vasopressin mRNA in the suprachiasmatic nuclei of female rats. J Neurosci. 1998;18:4767–4774. doi: 10.1523/JNEUROSCI.18-12-04767.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer C, Loros JJ, Dunlap JC, Crosthwaite SK. Role for antisense RNA in regulating circadian clock function in Neurospora crassa. Nature. 2003;421:948–952. doi: 10.1038/nature01427. [DOI] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Silver R. The regulation of neuroendocrine function: Timing is everything. Horm Behav. 2006;49:557–574. doi: 10.1016/j.yhbeh.2005.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavery DJ, Lopez-Molina L, Margueron R, Fleury-Olela F, Conquet F, Schibler U, Bonfils C. Circadian expression of the steroid 15 alpha-hydroxylase (Cyp2a4) and coumarin 7-hydroxylase (Cyp2a5) genes in mouse liver is regulated by the PAR leucine zipper transcription factor DBP. Mol Cell Biol. 1999;19:6488–6499. doi: 10.1128/mcb.19.10.6488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman MN, Silver R, Gladstone WR, Kahn RM, Gibson M, Bittman EL. Circadian rhythmicity restored by neural transplant. Immunocytochemical characterization of the graft and its integration with the host brain. J Neurosci. 1987;7:1626–1638. doi: 10.1523/JNEUROSCI.07-06-01626.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Satinoff E. Changes in circadian rhythms of body temperature and sleep in old rats. Am J Physiol. 1995;269:208–214. doi: 10.1152/ajpregu.1995.269.1.R208. [DOI] [PubMed] [Google Scholar]
- Lloyd JM, Hoffman GE, Wise PM. Decline in immediate early gene expression in gonadotropin-releasing hormone neurons during proestrus in regularly cycling, middle-aged rats. Endocrinology. 1994;134:1800–1805. doi: 10.1210/endo.134.4.8137745. [DOI] [PubMed] [Google Scholar]
- Lowrey PL, Shimomura K, Antoch MP, Yamazaki S, Zemenides PD, Ralph MR, Menaker M, Takahashi JS. Positional syntenic cloning and functional characterization of the mammalian circadian mutation tau. Science. 2000;288:483–492. doi: 10.1126/science.288.5465.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas RJ, Freedman MS, Munoz M, Garcia-Fernandez JM, Foster RG. Regulation of the mammalian pineal by non-rod, non-cone, ocular photoreceptors. Science. 1999;284:505–507. doi: 10.1126/science.284.5413.505. [DOI] [PubMed] [Google Scholar]
- Lucas RJ, Hattar S, Takao M, Berson DM, Foster RG, Yau KW. Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science. 2003;299:245–247. doi: 10.1126/science.1077293. [DOI] [PubMed] [Google Scholar]
- Madeira MD, Sousa N, Santer RM, Paula-Barbosa MM, Gundersen HJ. Age and sex do not affect the volume, cell numbers, or cell size of the suprachiasmatic nucleus of the rat: an unbiased stereological study. J Comp Neurol. 1995;361:585–601. doi: 10.1002/cne.903610404. [DOI] [PubMed] [Google Scholar]
- Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972;42:201–206. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- Moore RY, Klein DC. Visual pathways and the central neural control of a circadian rhythm in pineal serotonin N-acetyltransferase activity. Brain Res. 1974;71:17–33. doi: 10.1016/0006-8993(74)90188-7. [DOI] [PubMed] [Google Scholar]
- Morikawa Y, Nakagawa H, Miura K, Soyama Y, Ishizaki M, Kido T, Naruse Y, Suwazono Y, Nogawa K. Shift work and the risk of diabetes mellitus among Japanese male factory workers. Scand J Work Environ Health. 2005;31:179–183. doi: 10.5271/sjweh.867. [DOI] [PubMed] [Google Scholar]
- Oster H, Baeriswyl S, Van Der Horst GT, Albrecht U. Loss of circadian rhythmicity in aging mPer1-/-mCry2-/- mutant mice. Genes Dev. 2003;17:1366–1379. doi: 10.1101/gad.256103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda S, Sato TK, Castrucci AM, Rollag MD, DeGrip WJ, Hogenesch JB, Provencio I, Kay SA. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science. 2002;298:2213–2216. doi: 10.1126/science.1076848. [DOI] [PubMed] [Google Scholar]
- Panda S, Provencio I, Tu DC, Pires SS, Rollag MD, Castrucci AM, Pletcher MT, Sato TK, Wiltshire T, Andahazy M, Kay SA, Van Gelder RN, Hogenesch JB. Melanopsin is required for non-image-forming photic responses in blind mice. Science. 2003;301:525–527. doi: 10.1126/science.1086179. [DOI] [PubMed] [Google Scholar]
- Ralph MR, Menaker M. A mutation of the circadian system in golden hamsters. Science. 1988;241:1225–1227. doi: 10.1126/science.3413487. [DOI] [PubMed] [Google Scholar]
- Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247:975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- Reddy AB, Karp NA, Maywood ES, Sage EA, Deery M, O’Neill JS, Wong GK, Chesham J, Odell M, Lilley KS, Kyriacou CP, Hastings MH. Circadian orchestration of the hepatic proteome. Curr Biol. 2006;16:1107–1115. doi: 10.1016/j.cub.2006.04.026. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- Ripperger JA, Schibler U. Rhythmic CLOCK-BMAL1 binding to multiple E-box motifs drives circadian Dbp transcription and chromatin transitions. Nat Genet. 2006;38:369–374. doi: 10.1038/ng1738. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, van Gool WA, Swaab DF, Hoogendijk JE, Mirmiran M. Changes in vasopressin cells of the rat suprachiasmatic nucleus with aging. Brain Res. 1987;409:259–264. doi: 10.1016/0006-8993(87)90710-4. [DOI] [PubMed] [Google Scholar]
- Ruby NF, Brennan TJ, Xie X, Cao V, Franken P, Heller HC, O’Hara BF. Role of melanopsin in circadian responses to light. Science. 2002;298:2211–2213. doi: 10.1126/science.1076701. [DOI] [PubMed] [Google Scholar]
- Satinoff E, Li H, Tcheng TK, Liu C, McArthur AJ, Medanic M, Gillette MU. Do the suprachiasmatic nuclei oscillate in old rats as they do in young ones? Am J Physiol. 1993;265:1216–1222. doi: 10.1152/ajpregu.1993.265.5.R1216. [DOI] [PubMed] [Google Scholar]
- Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci U S A. 1972;69:1583–1586. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda HR, Hayashi S, Chen W, Sano M, Machida M, Shigeyoshi Y, Iino M, Hashimoto S. System-level identification of transcriptional circuits underlying mammalian circadian clocks. Nat Genet. 2005;37:187–192. doi: 10.1038/ng1504. [DOI] [PubMed] [Google Scholar]
- Valentinuzzi VS, Scarbrough K, Takahashi JS, Turek FW. Effects of aging on the circadian rhythm of wheel-running activity in C57BL/6 mice. Am J Physiol. 1997;273:1957–1964. doi: 10.1152/ajpregu.1997.273.6.R1957. [DOI] [PubMed] [Google Scholar]
- Van Gelder RN. Non-visual ocular photoreception. Ophthalmic Genet. 2001;22:195–205. doi: 10.1076/opge.22.4.195.2215. [DOI] [PubMed] [Google Scholar]
- Vielhaber E, Eide E, Rivers A, Gao ZH, Virshup DM. Nuclear entry of the circadian regulator mPER1 is controlled by mammalian casein kinase I epsilon. Mol Cell Biol. 2000;20:4888–4899. doi: 10.1128/mcb.20.13.4888-4899.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakamatsu H, Takahashi S, Moriya T, Inouye ST, Okamura H, Akiyama M, Shibata S. Additive effect of mPer1 and mPer2 antisense oligonucleotides on light-induced phase shift. Neuroreport. 2001;12:127–131. doi: 10.1097/00001756-200101220-00033. [DOI] [PubMed] [Google Scholar]
- Wang H, Ko CH, Koletar MM, Ralph MR, Yeomans J. Casein kinase I epsilon gene transfer into the suprachiasmatic nucleus via electroporation lengthens circadian periods of tau mutant hamsters. Eur J Neurosci. 2007;25:3359–3366. doi: 10.1111/j.1460-9568.2007.05545.x. [DOI] [PubMed] [Google Scholar]
- Watanabe A, Shibata S, Watanabe S. Circadian rhythm of spontaneous neuronal activity in the suprachiasmatic nucleus of old hamster in vitro. Brain Res. 1995;695:237–239. doi: 10.1016/0006-8993(95)00713-z. [DOI] [PubMed] [Google Scholar]
- Weinert D, Waterhouse J. Daily activity and body temperature rhythms do not change simultaneously with age in laboratory mice. Physiol Behav. 1999;66:605–612. doi: 10.1016/s0031-9384(98)00342-4. [DOI] [PubMed] [Google Scholar]
- Weinert H, Weinert D, Schurov I, Maywood ES, Hastings MH. Impaired expression of the mPer2 circadian clock gene in the suprachiasmatic nuclei of aging mice. Chronobiol Int. 2001;18:559–565. doi: 10.1081/cbi-100103976. [DOI] [PubMed] [Google Scholar]
- Wise PM. Alterations in the proestrous pattern of median eminence LHRH, serum LH, FSH, estradiol and progesterone concentrations in middle-aged rats. Life Sci. 1982;31:165–173. doi: 10.1016/0024-3205(82)90429-5. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brainard GC, Zee PC, Pinto LH, Takahashi JS, Turek FW. Effects of aging on lens transmittance and retinal input to the suprachiasmatic nucleus in golden hamsters. Neurosci Lett. 1998;258:167–170. doi: 10.1016/s0304-3940(98)00887-8. [DOI] [PubMed] [Google Scholar]