Abstract
Kisspeptin, a neuropeptide product of the KiSS-1 gene, has recently been implicated in the regulation of seasonal breeding in a number of species, including Siberian hamsters. In this species, kisspeptin expression is reduced in the anteroventral periventricular nucleus (AVPV) following exposure to inhibitory day lengths, and exogenous kisspeptin activates the reproductive neuroendocrine axis of reproductively quiescent animals. Because sex steroids can impact kisspeptin expression, it is unclear whether changes in kisspeptin occur in direct response to photoperiodic cues or secondarily in response to changes in sex steroid concentrations resulting from the transition to reproductive quiescence. The present study aimed to assess the relative contributions of photoperiod and testosterone in regulating kisspeptin expression in Siberian hamsters. Animals housed in long or short day lengths for 8 weeks were either castrated or received sham surgeries. Half of the hamsters in each photoperiod were given testosterone to mimic long-day sex steroid concentrations. The results obtained indicate that kisspeptin neurones in the AVPV and arcuate nuclei were influenced by both photoperiod and testosterone. In the AVPV, removal of testosterone or exposure to inhibitory day lengths led to a marked reduction in kisspeptin-immunoreactive cells, and testosterone treatment increased cell numbers across conditions. Importantly, long-day castrates exhibited significantly more kisspeptin cells than short-day castrates or intact short-day animals with empty capsules, suggesting the influences of photoperiod, independent of gonadal steroids. In general, the opposite pattern emerged for the arcuate nuclei. Collectively, these data suggest a role for both gonadal-dependent and independent (i.e. photoperiodic) mechanisms regulating seasonal changes in kisspeptin expression in Siberian hamsters.
Keywords: KiSS-1, reproduction, RF amide, seasonal breeding, gonadal steroids
The neuropeptide kisspeptin plays a prominent role in reproductive axis regulation, contributing to the timing of reproductive maturity and adult reproductive neuroendocrine function (1). Both mice and humans lacking functional KiSS-1 and GPR-54 genes, encoding the neuropeptide kisspeptin and its G-protein coupled receptor, GPR-54, respectively, fail to proceed through puberty and remain reproductively immature throughout adulthood (2–5). Exogenous administration of kisspeptin stimulates the gonadotrophin-releasing hormone (GnRH) system (6–18) and accelerates progression into puberty in rats and nonhuman primates (16, 19).
Most animals alter their reproductive status to coordinate off-spring birth and rearing with favourable environmental conditions (20). In temperate zone breeding rodents, changes in photoperiod (i.e. day length) signal time of year and this cue is transduced into changes in the activity of the hypothalamo-pituitary-gonadal (HPG) axis (21, 22). Recent studies suggest a key role for kisspeptin in regulating seasonal changes in reproductive function (11, 23–25). Both Siberian (Phodopus sungorus) and Syrian (Mesocricetus auratus) hamsters held in simulated ‘summer-like’ or ‘winter-like’ photoperiods display marked differences in hypothalamic kisspeptin gene and protein expression (11, 23, 24). In our previous work in Siberian hamsters, we found that animals maintained on long days (16 : 8 h light / dark cycle) have high numbers of kisspeptin-immunoreactive (-ir) neurones in the anteroventral periventricular (AVPV) nucleus that are markedly reduced by exposure to short day lengths (11, 23). Curiously, the arcuate nucleus (Arc) exhibits the opposite pattern of staining; few kisspeptin-ir neurones are found in long-day-housed animals, whereas a large number of kisspeptin-ir cells are present in short-day-housed animals (11, 23). The specific mechanisms mediating these changes remain to be established.
In mice, sex steroids differentially modulate the expression of KiSS-1 in the AVPV and Arc. In the AVPV, castration or ovariectomy reduces KiSS-1 gene expression, whereas sex steroid replacement (testosterone or oestradiol respectively) restores the pattern of KiSS-1 to pre-gonadectomy values; the opposite pattern is observed in the Arc (26, 27). In photoperiodic rodents, inhibitory day lengths lead to gonadal involution and pronounced reductions in circulating gonadal steroids (28). As a result, the specific contribution of photoperiod and / or sex steroids to observed changes in the kisspeptin system need to be assessed independently. Photoperiod-driven changes in kisspeptin could precede the down-regulation of the HPG axis and subsequent decreases in testosterone, indicating a mechanism independent of gonadal steroids. Alternatively, short-day induced decreases in testosterone may drive observed alterations in hypothalamic kisspeptin via feedback mechanisms. Finally, these two processes may interact to precisely regulate kisspeptin. To assess the relative roles of testosterone and photoperiod to changes in kisspeptin expression, adult male Siberian hamsters were housed in long- and short-day photoperiods and circulating levels of testosterone were manipulated by castrations and testosterone replacement.
Materials and methods
Animals and housing
Adult (> 60 days of age) male Siberian hamsters (Phodopus sungorus) (n = 49) were obtained from our breeding colony maintained at Indiana University. The progenitors of these animals were generously provided by Dr Randy Nelson (Ohio State University) and Dr Timothy Bartness (Georgia State University). All animals were group-housed at weaning with same-sex siblings in a long-day photoperiod (16 : 8 h light / dark cycle). Prior to the start of the study, animals were housed individually in polypropylene cages (27.8 × 17.5 × 13.0 cm). Temperature was kept constant at 20 ± 2 °C and relative humidity was maintained at 50 ± 5%. Food (Purina Rat Chow; Ralston Purina Co., St Louis, MO, USA) and tap water were available ad libitum throughout the experiments. All experimental procedures follow NIH guidelines for the Care and Use of Experimental Animals and were approved by the Bloomington Institutional Animal Care and Use Committee.
Experimental procedures
To investigate the relative contribution of photoperiod and gonadal steroids to changes in kisspeptin expression, hamsters were randomly assigned to either long- (16 : 8 h light / dark cycle) (n = 18) or short- (8 : 16 h light / dark cycle) (n = 31) photoperiods (long-day room: lights on 04.00 h; short-day room: lights on 08.00 h). After 8 weeks in photoperiod, hamsters were weighed to the nearest 0.1 g and pelage colouration was noted. Hamsters that did not lose ≥ 10% of their original body mass and did not display the typical progression of pelage colouration from summer grey to winter white were considered to be unresponsive to the short-day photoperiods (called ‘nonresponders’) (29, 30). This assignment was visually confirmed via examination of testis size during surgery. Twelve hamsters were deemed nonresponsive to short-day photoperiods and were removed from the remainder of the study. Animals then received castrations or sham surgeries, followed by Silastic capsules either filled with testosterone or left empty, resulting in one of six treatment groups: (i) long day, sham operated, empty capsule (n = 6), (ii) long day, castrated, empty capsule (n = 6), (iii) long day, castrated, testosterone-filled capsule (n = 6), (iv) short day, sham operated, empty capsule (n = 7), (v) short day, castrated, empty capsule (n = 6) (vi) short day, sham operated, testosterone-filled capsule (n = 6). Two weeks after surgery and capsule implantation, a blood sample was collected from all hamsters and animals were perfused; brain tissue and serum were collected as described below. All sampling was conducted between 09.00 and 12.30 h EST.
Surgical procedures
Surgical procedures have been described previously (31). Briefly, hamsters were anaesthetised with 0.05 ml of a ketamine (20 mg / ml) / xylazine (4 mg / ml) cocktail in 0.9% saline. Lateral abdominal incisions were made and testes were excised, the abdominal cavity was sutured and skin was closed with 9-mm surgical clips (Clay Adams, Parsipany, NJ, USA). All above procedures were followed for the sham operation, except the testes were left in tact. Immediately after surgery, hamsters received either a 10-mm long Silastic capsule implant (inner diameter 1.47 mm, outer diameter 1.95 mm; American Scientific Product, McGraw Park, IL, USA) filled either with testosterone (Sigma, St Louis, MO, USA) or left empty as described previously (31). Implants were applied subcutaneously via a 5-mm incision on the intrascapular surface made perpendicular to the midline. The incision was closed with a 9-mm surgical clip. Both castration and implant sites received an application of nitrofurazone antibacterial ointment (Squire laboratories, Revere, MA, USA) to prevent infection and animals received meloxicam (Boehringer Ingelheim Vetmedica, Inc., St Joseph, MO, USA) orally for analgesia. Animals were returned to their respective photoperiods for an additional 2 weeks.
Blood sampling and perfusion
At the conclusion of the experiment (i.e. 2 weeks after surgery and implantation) a blood sample was collected via the retro-orbital sinus. Clots were removed and the blood spun for 30 min at 5000 g, serum was collected and stored at −80 °C until assayed for testosterone. Hamsters were weighed to the nearest 0.1 g and then deeply anaesthetised with 0.3 ml of ketamine cocktail and perfused transcardially with 50 ml of 0.9% saline, followed by 100–150 ml of 4% paraformaldehyde in 0.1 m phosphate-buffered saline (PBS) (pH 7.3). Brains were post-fixed for 3 h at room temperature in 4% paraformaldehyde, and cryoprotected in 20% sucrose in 0.1 m PBS and stored at 4 °C until processed. Coronal sections (40 µm) were cut on a cryostat and processed as free-floating sections, beginning rostrally at the medial septum / diagonal band of Broca and extending caudally to the brainstem.
Immunohistochemistry, microscopy, cell counts, and optical density
Kisspeptin-immunoreactive cells were labelled using a rabbit anti-human kisspeptin serum (T-4771; Peninsula Laboratories Inc, Bachem, San Carlos, CA, USA) raised against the following amino acids Tyr-Asn-Trp-Asn-Ser-Phe-Gly-Leu-Arg-Phe-NH2, corresponding to amino acids 4–13, diluted at 1 : 7500. Preliminary trial runs with this commercially available antiserum revealed immunoreactivity in the dordomedial hypothalamus (23), an area where the homologue of the avian RFamide gonadotrophin inhibitory hormone (GnIH) has been detected in rodents (32, 33), and an area where KiSS-1 mRNA is not expressed in other rodents. To eliminate potential cross-reactivity of the kisspetpin antibody with this RFamide peptide, kisspeptin antiserum was first preadsorbed with GnIH peptide (diluted 1 : 5000) overnight. This antiserum is polyclonal, and thus contains a host of antibodies targeting specific epitopes of the peptide of interest. Preadsorption with GnIH allows the blocking of antibodies that are promiscuous for GnIH while maintaining those specific to kisspeptin. This procedure has been previously described and shown to specifically block GnIH labelling and maintain kisspeptin expression in this species (23). Sections were then mounted onto gelatin-coated slides, dehydrated in a graded series of ethanol solutions (70, 95 and 100%), and cleared in xylenes (Fisher Scientific Co. Pittsburgh, PA, USA) before the application of cover slips.
Slides were examined under bright field illumination on a Zeiss Z1 microscope (Carl Zeiss, Oberkochen, Germany) by independent observers naïve to the experimental conditions. Kisspeptin-ir cells were located by visually scanning the brains under × 200 magnification. Cell populations were restricted to the AVPV region of the preoptic area and the Arc. All cells were confirmed at a minimum magnification of × 400. Counted cells were photographed with a Zeiss Axiocam Cooled CCD camera at × 400 magnification for cell size and density analyses. All cells in every fourth section were counted through the rostro-caudal extent of the AVPV and Arc. Both cells with a clearly discernable nucleus and cells showing clear soma and processes without a clear, unstained nucleus were counted. Because the inclusion of cells without a clearly-defined nucleus may result in counting overestimates, an Abercrombie correction was applied prior to data analysis.
Soma size and optical density (OD) measurements were performed on images captured at × 400 magnification for all cells on those two sections in the AVPV and Arc containing the highest number of cells. For each animal and brain region, a single mean optical density and cell size was calculated by taking the average value from the two sections examined. All cells examined had optical densities at least two standard deviations above the mean background OD measures for an individual brain. Cell bodies were outlined and the two-dimensional area was calculated using ImageJ, version 1.32 (National Institutes of Health, Bethesda, MD, USA). Each pixel in the greyscale image capture has a measurable specific intensity, with values ranging from 0 (white) to 256 (black). The average value for all pixels in an outlined area is taken as the mean intensity of staining for a given region of the image. OD measures were normalised to minimise differences between replications of immunohistochemistry. First, a background measurement was taken by placing a square outline, four times, on non-overlapping, unstained areas of each section. The mean of these four measures provided the background OD for each section. The OD for each cell body was assessed by outlining the cell body, obtaining a density measure using ImageJ, and subtracting the background OD from the OD of each cell.
Testosterone measurement
To confirm the effectiveness of castrations and testosterone implants, serum testosterone levels were measured via a commercial EIA kit (Correlate-EIA Kit #900–065; Assay Designs, Ann Arbor, MI, USA). Serum samples were run on two separate plates. Samples were diluted 1 : 20 and run in duplicate for each sample. The sensitivity of the assay was 3.82 pg / ml and the intraassay coefficient of variation was less than 5% on both plates and the inter-assay coefficient of variation was 5.5%. This assay has been previously validated for use in Siberian hamsters (34).
Statistical analysis
The effect of experimental treatment on circulating levels of testosterone, body mass, and the number of kisspeptin immuno-reactive cells, cell size and optical density in the AVPV and Arc were analysed using a one-way analysis of variance (ANOVA). When an ANOVA revealed a significant effect of treatment, differences between treatment groups were probed using a Tukey HSD test. In addition, we probed for a relationship between circulating levels of testosterone and the number of kisspeptin cells, cell size and optical density in both the AVPV and Arc, regardless of treatment, using a Pearson’s correlation. Prior to statistical examination, variables not meeting assumptions of parametric statistics were transformed accordingly. All statistics were performed using Minitab 15 (Minitab Inc., State College, PA, USA) for Windows, with α< 0.05.
Results
Treatment effects on circulating testosterone titres
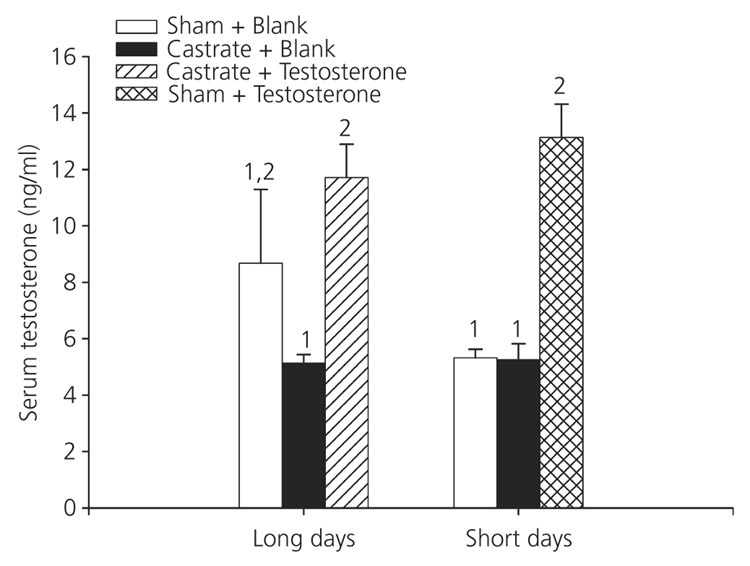
Treatment had a significant effect on serum testosterone titres (F5,28 = 6.73, P < 0.001; Fig. 1). Post-hoc analysis revealed that hamsters receiving testosterone implants, regardless of photoperiod, had the highest titres, and did not differ from each other (P > 0.05). Long-day castrates given testosterone treatment and short-day intact animals receiving testosterone did not differ from long-day sham controls receiving empty implants (P > 0.05). Short-day hamsters that did not receive testosterone, regardless of surgical condition, did not differ from each other, and had significantly lower concentrations of testosterone than both long- and short-day animals receiving testosterone (P < 0.05); neither group differed from long-day castrates or long-day sham groups that received empty implants (P > 0.05).
Fig. 1.
Circulating testosterone titres to experimental treatment: circulating levels of testosterone were significantly altered in response to experimental manipulations (P < 0.05). Animals were held in either long- or short-day lengths, and received either surgical castration or a sham operation, combined with a silastic capsule either filled with testosterone or left blank. Different numbers denote that groups significantly differed from each other (P < 0.05).
Treatment effects on body mass
Treatment had a significant effect on the change in body mass across the 10 weeks of the study (F5,31 = 16.12, P < 0.001) (Table 1). Hamsters in short days lost significantly more body mass than long-day-housed hamsters, regardless of treatment (P < 0.05). Within each photoperiod, treatment did not influence body mass (P > 0.05 in all cases).
Table 1.
Mean ± SEM Body Mass Values for Each Experimental Group.
| Treatment | Initial mass | Week 8 mass | Week 10 mass |
|---|---|---|---|
| LD-sham-empty | 41.73 (2.45) | 42.11 (2.78) | 41.12 (2.61)1 |
| LD-cast-empty | 38.47 (1.74) | 41.63 (2.00) | 37.66 (2.51)1 |
| LD-cast-T | 38.47 (1.79) | 38.05 (2.71) | 35.90 (2.23)1 |
| SD-sham-empty | 41.51 (1.18) | 32.93 (1.59) | 31.62 (1.50)2 |
| SD-cast-empty | 36.70 (2.69) | 28.76 (1.11) | 28.66 (1.02)2 |
| SD-sham-T | 37.57 (1.53) | 29.56 (1.72) | 31.46 (1.20)2 |
Superscript numbers denotes groups differed significantly (P < 0.05) in the change in body mass across the 10-week period of the study. LD, long-day photoperiod; sham, sham operation; empty, empty capsule; cast, castrated; T, testosterone-filled capsule.
Treatment effects on kisspeptin-ir cells in the AVPV
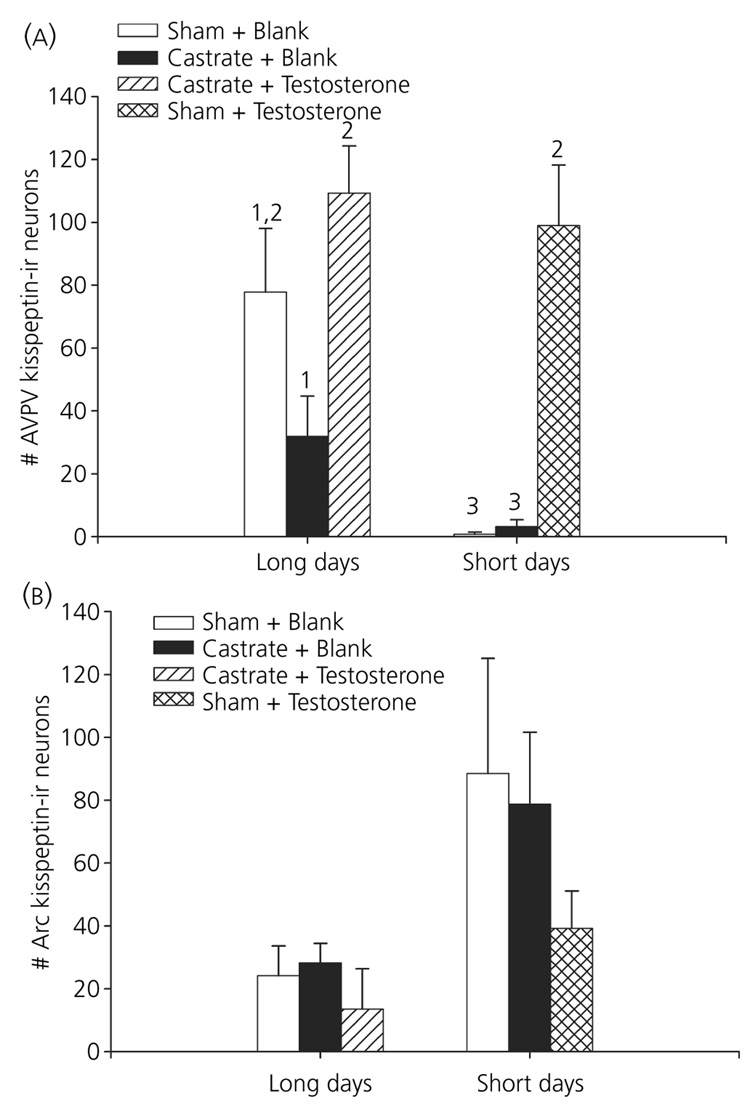
There was a significant effect of treatment on the number of kisspeptin-ir neurones in the AVPV (F5,31 = 27.99, P < 0.001; Fig 2 and Fig 3). Short-day-housed animals receiving sham surgeries or castrations combined with empty implants displayed the fewest kisspeptin-ir neurones in the AVPV; all other groups had significantly more kisspeptin-ir neurones (P < 0.05). Castrated, long-day-housed hamsters with empty implants displayed significantly fewer kisspeptin-ir neurones than long-day hamsters receiving testosterone implants; both castrated long-day and sham-operated short-day-housed hamsters given testosterone had significantly more kisspeptin-ir neurones than long-day-castrated hamsters with empty implants (P < 0.05). Long-day-housed castrated hamsters with empty implants displayed fewer, but not a statistically different numbers of kisspeptin-ir neurones, compared to long-day-housed sham operated hamster-with empty implants (P > 0.05).
Fig. 2.
Effect of treatment on the number of kisspeptin-immunoreactive (-ir) neurones: experimental treatment had a significant effect on the number of kisspeptin-ir neurones in both the anteroventral periventricular nucleus (AVPV) (a) and arcuate nucleus (Arc) (b) of the hypothalamus (P < 0.05). Animals were held in either long or short day lengths, received either surgical castration or a sham operation, combined with a silastic capsule either filled with testosterone or left blank. Different numbers denote that groups significantly differed from each other (P < 0.05).
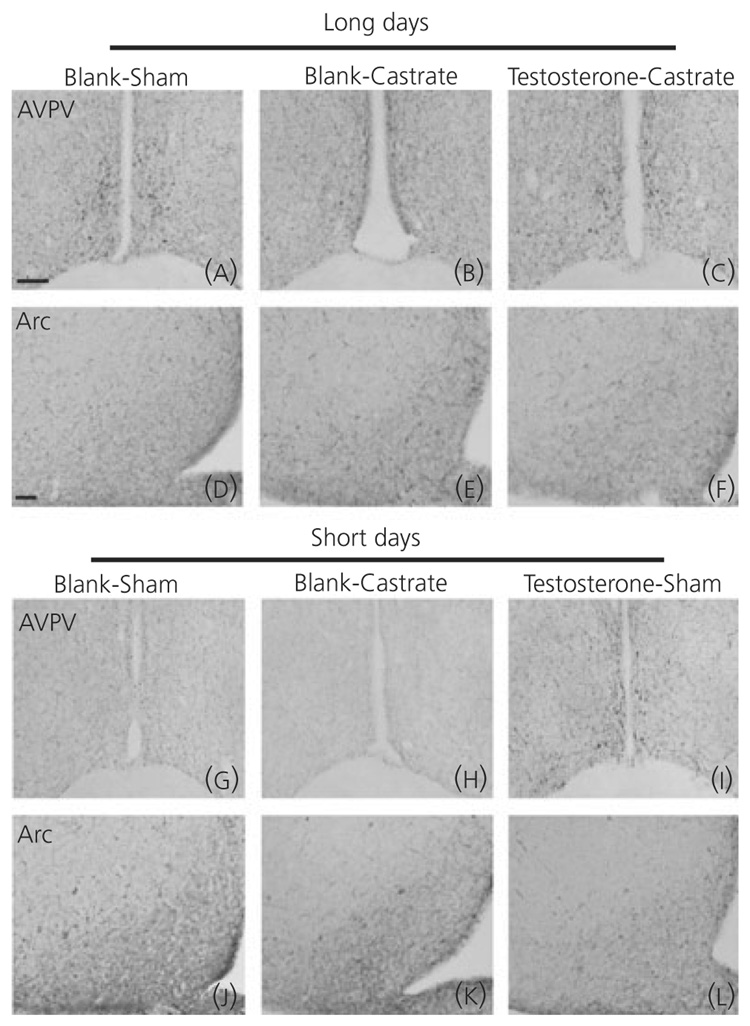
Fig. 3.
Response of kisspeptin-immunoreactive (-ir) neurones to experimental treatment: low-power photomicrographs demonstrating the effect of photoperiod and testosterone on kisspeptin expression in Siberian hamsters. Animals were held in either long- (a–f) or short- (g–l) day lengths and either castrated or left intact. Castrated long-day animals were either treated with testosterone or vehicle. Because intact short-day hamsters exhibit testosterone values equal to those of long-day castrates, one group of intact short-day hamsters (i,l) was treated with testosterone. Scale bars = 50 µm for anteroventral periventricular nucleus and 100 µm for arcuate nucleus. AVPV, anteroventral periventricular nucleus; Arc, arcuate nucleus.
Optical density and cell size in short-days castrated hamsters receiving empty implants was not calculated due to a lack of kisspeptin-ir neurones. When comparing the remaining groups, no effect of treatment was found on cellular optical density (F4,22 = 0.15, P > 0.05). Treatment did have a significant effect on cell size in the remaining groups (F4,22 = 6.00, P = 0.002). Castrated long-day animals with empty implants had significantly smaller cells than short-day sham-operated hamsters receiving testosterone (T = 4.85, P < 0.001), and tended to have smaller cells compared to short-day sham-operated hamsters with empty implants (T = 2.74, P = 0.079). All other treatment groups had kisspeptin-ir cells of similar size (P > 0.05).
Treatment effects on kisspeptin-ir cells in the Arc
There was a significant effect of treatment on the number of kisspeptin-ir neurones in the Arc (F5,22 = 2.80, P < 0.05; Fig 2 and Fig 3). Short-day, sham-operated hamsters with blank implants tended to have more Arc kisspeptin-ir neurones compared to long-day castrated hamsters provided with exogenous testosterone (P < 0.1); however, post-hoc analyses revealed no significant pair-wise differences between groups (P > 0.05 in all cases). Treatment had no effect on either cellular optical density (P > 0.05) or cell size (P > 0.05) (Table 2).
Table 2.
Mean ± SEM of Optical Density (OD) and Cell Size.
| Treatment | AVPV OD | Arc OD | AVPV cell size | Arc cell size |
|---|---|---|---|---|
| LD-sham-empty | 29.75 (3.94) | 25.75 (4.24) | 78.07 (5.00) | 115.85 (14.25) |
| LD-cast-empty | 28.82 (4.59) | 23.39 (2.76) | 65.18 (2.19)* | 104.60 (7.28) |
| LD-cast-T | 31.05 (4.40) | 24.98 (5.82) | 78.07 (3.66) | 116.36 (26.08) |
| SD-sham-empty | 26.03 (6.19) | 24.28 (1.96) | 81.38 (4.32) | 176.65 (62.31) |
| SD-cast-empty | – | 22.54 (2.73) | – | 93.83 (9.16) |
| SD-sham-T | 28.91 (3.43) | 21.15 (2.02) | 90.81 (4.37)* | 98.16 (5.55) |
AVPV, anteroventral periventricular nucleus; Arc, arcuate nucleus; LD, long-day photoperiod; SD, short-day photoperiod; sham, sham operation; cast, castrated; empty, empty capsule; T, testosterone-filled capsule.
Signifcantly differ from each other (P < 0.05).
Relationship between circulating testosterone levels and kisspeptin
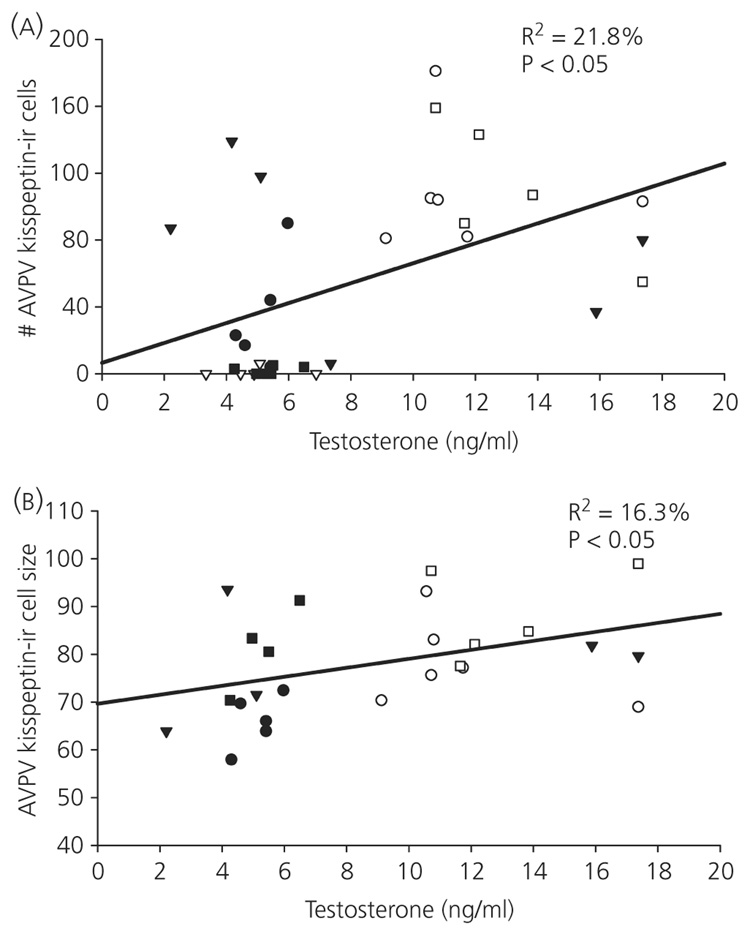
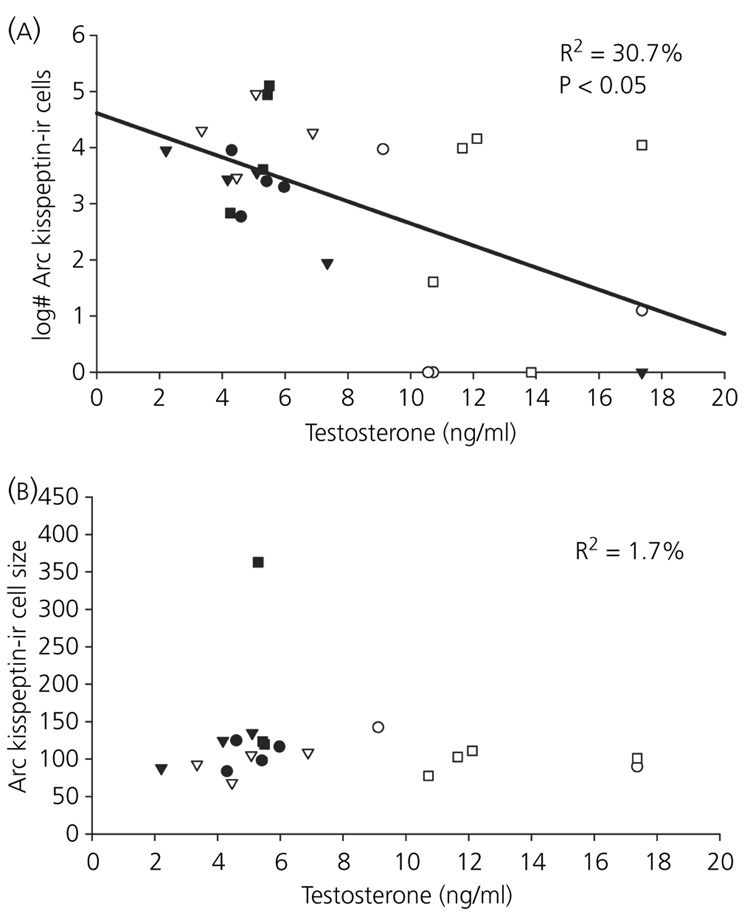
The number of kisspeptin-ir cells in the AVPV was positively correlated with circulating testosterone concentrations (r2 = 0.22, P < 0.05; Fig. 4). Additionally, in detectable cells in the AVPV, the size of kisspeptin-ir cells was positively correlated with serum testosterone concentrations (r2 = 0.16, P < 0.05; Fig. 4). Serum testosterone and optical density did not covary in AVPV kisspeptin-ir cells (P > 0.05). Additionally, a significant negative relationship between testosterone and the number of Arc kisspeptin-ir neurones was observed (r2 = 0.31, P < 0.01; Fig. 5). Circulating concentrations of testosterone did not correlate with either optical density (P > 0.05) or cell size (P > 0.05; Fig. 5) in the Arc.
Fig. 4.
Relationship between testosterone and kisspeptin in the anteroventral periventricular nucleus (AVPV): across all individuals, testosterone levels were significantly positively related with both the number (a) and size (b) of kisspeptin-immunoreactive (-ir) neurones in the AVPV. ▼, long-day controls (sham + blank capsule); ●, long-day castrated + blank capsule; ○, long-day castrated + testosterone; ■, short-day controls (sham + blank capsule); ▽, short-day castrates + blank capsules; □, short-day sham + testosterone.
Fig. 5.
Relationship between between testosterone and kisspeptin in the arcuate nucleus (Arc): across all individuals, testosterone levels were significantly negatively related with the number of kisspeptin-immunoreactive (-ir) neurones in the Arc (a). There was no relationship between testosterone and the size of Arc kisspeptin-immunoreactive neurones (b).▼, long-day controls (sham + blank capsule); ●, long-day castrated + blank capsule; ○, long-day castrated + testosterone; ■ short-day controls (sham + blank capsule); ▽, short-day castrates + blank capsules; □, short-day sham + testosterone.
Discussion
The present study examined the relative contributions of photoperiod and gonadal steroids to kisspeptin regulation in seasonally breeding animals. Specifically, studies were designed to determine whether photoperiodic signals drive the down-regulation of the HPG axis and decreases in testosterone via kiss-peptin, if short-day induced decreases in testosterone drive observed alterations in kisspeptin, or whether both variables contribute to kisspeptin control. The present findings indicate that both photoperiod and circulating testosterone concentrations affect expression of kisspeptin in the AVPV and Arc, with low circulating sex steroids and short day lengths leading to low AVPV and high Arc expression of kisspeptin. Whereas the effects of short days on kisspeptin-ir were reversed with testosterone treatment, removal of testosterone via castration was either unable to induce short-day-like kisspeptin-ir expression in long-day animals (AVPV), or had no effect (Arc). Taken together, these findings indicate that both photoperiod and testosterone impact the kisspeptin system in Siberian hamsters.
The finding that testosterone influences kisspeptin expression in Siberian hamsters is consistent with observations in mice in which gonadectomised males and females display fewer KiSS-1 neurones in the AVPV and an increase in neurones in the Arc compared to intact animals and individuals provided exogenous sex steroids (26, 27). Although the present study investigated peptide content, future studies will be needed to investigate treatment effects on KiSS-1 gene expression, as changes at the transcriptional level likely contribute to seasonal changes in kisspeptin peptide content.
When combined across all treatment groups, a significant positive relationship between circulating concentrations of testosterone and the number of AVPV kisspeptin-ir neurones and a negative relationship in the Arc was observed. This finding suggests potential positive and negative feedback effects of testosterone on AVPV and Arc kisspeptin expression, respectively, in this species. The Arc plays an important role in negative feedback of sex steroids in mammals (35, 36), whereas, in females, the AVPV is an important locus for positive feedback effects of oestradiol necessary to stimulate the pre-ovulatory luteinising hormone (LH) surge (37, 38). Recently, Arc kisspeptin has been proposed to mediate the negative feedback effects of gonadal hormones (26, 27), possibly via alterations of the GnRH pulse-generator (39). Consistent with the roles of these brain regions, sex differences in expression patterns of kisspeptin have been noted, with females having higher expression in the AVPV compared to males (40, 41), and AVPV kisspeptin neurones playing a prominent role in the pre-ovulatory LH surge (42). The role of steroid positive feedback on AVPV kisspeptin neurones in male rodents remains unclear, but has been suggested to potentially play a role in sexual behaviours (43). The noted differential effects of sex steroids on the AVPV and Arc in hamsters and mice, combined with noted sex differences in kisspeptin expression in these regions, suggest differential regulation between the sexes. It is noteworthy, however, that our present results suggest that testosterone may positively drive kisspeptin expression in the AVPV of males, an exciting opportunity for further exploration.
In addition to the effects of gonadal steroids on kisspeptin labelling, the present studies provide evidence for photoperiodic influences on kisspeptin that are independent of gonadal steroids. Short-day hamsters that display naturally low levels of circulating testosterone had similar testosterone titres to long-day-castrated hamsters. Importantly, however, even though these hamsters had comparable testosterone titres, short-day hamsters (both intact and castrated) displayed significantly fewer kisspeptin-ir neurones in the AVPV, and greater numbers in the Arc, compared to castrated long-day-housed animals; post-hoc analyses, however, did not reveal these differences to be significant in the Arc. When considering this comparison in isolation, this finding suggests that AVPV kisspeptin may be more sensitive to the negative feedback effects of testosterone in short days, rather than representing a gonadal steroid-independent effect of photoperiod. If true, then exogenous testosterone replacement should lead to suppression of kisspeptin in short-day animals. By contrast, testosterone treatment instead leads to a robust increase in kisspeptin in short-day animals. Taken together with all other group differences, these observations suggest that both photoperiod and circulating testosterone concentrations affect expression of kisspeptin.
It is possible that the alterations in kisspeptin staining seen in short days compared with long-day-castrated hamsters can be attributed to short-day animals experiencing a prolonged period of reduced gonadal steroids (i.e. approximately 6 weeks) relative to castrated long-day animals (i.e. 2 weeks). In common with all studies using castration and steroid replacement, these manipulations do not fully mimic natural changes in testosterone titres experienced over short (e.g. circadian changes in testosterone) or long time scales (e.g. gradual declines in testosterone in animals transitioning through gonadal regression). Although these possibilities cannot be ruled out, it is unlikely to fully explain these findings, as testosterone has significant effects on androgen receptor levels in as little as 11 days in this species (44), and castration has noticeable effects on HPG axis activity in as little as 1 week in another seasonal breeder, the prairie vole (Microtus ochrogaster) (45).
Photoperiod, independent of sex steroid hormones, is known to alter a variety of physiological and behavioural traits in seasonal mammals (46–57). For example, photoperiod alters circulating levels of gonadotrophins independent of gonadal steroids in Siberian hamsters (48, 51). This observation, combined with the results of the current study, indicates that these steroid-independent changes in HPG axis activity may be, at least in part, attributable to steroid-independent changes in kisspeptin levels. The mechanisms by which photoperiod impacts the kisspeptin system independent of gonadal steroids represents an exciting area for further empirical investigation, and may help to clarify the observed variation in kisspeptin expression that is not explained by sex steroid concentrations. Future studies aiming to examine the interactive effects of photoperiod and energy status on the kisspeptin system, for example, represent an important area for further inquiry. Siberian hamsters provide an ideal model for such investigations; food intake and body mass is reduced in Siberian hamsters held on short days (58). Similarly, pronounced effects of energetics on kisspeptin have been noted; kisspeptin expression is reduced in laboratory animals held on a restricted diet (59–61) and ob / ob mice exhibit increased kisspeptin following leptin administration (62).
A significant interaction between photoperiod and circulating testosterone titres on androgen receptor levels in the Arc has been observed in Siberian hamsters (44). In the present study, although treatment had a significant effect on the number of kisspeptin-ir neurones in the Arc, no significant relationship was observed between any groups. If Arc kisspeptin-ir neurones are responsive to both photoperiod and sex steroids, then the interaction between photoperiod and testosterone concentrations may modulate Arc kisspeptin expression such that sex steroid feedback has a differential effect on kisspeptin immunoreactivity in long versus short days. The majority of Arc kisspeptin neurones in mice express mRNA for both androgen receptor and oestrogen receptor-α (26), supporting the possibility that Arc kisspeptin neurones are modulated by the interactive effects of photoperiod and testosterone on sex steroid receptors.
In one previous study, 4 weeks of testosterone treatment provided to reproductively quiescent Syrian hamsters did not alter Arc KiSS-1 expression (24). In Syrian hamsters, Arc KiSS-1 mRNA levels are opposite to the pattern observed in Siberian hamsters (23), with higher mRNA levels observed in reproductive than nonreproductive individuals (24). It is important to note that, in the present study, testosterone levels in manipulated hamsters were within the physiological range of values reported for long-day sham controls (i.e., individuals with highest testosterone levels in both long-day sham control and long-day castrate plus testosterone had approximately 17 ng / ml testosterone). Testosterone titres were negatively correlated with the number of kisspeptin-ir cells in the Arc, and, although not significant, testosterone treatment in short-day hamsters reduced the number of kisspeptin-ir cells to long-day like levels, suggesting additional species differences. In female sheep, which also breed seasonally, the majority of kisspeptin neurones are found in the Arc, and, similar to observations in laboratory mice, ovariectomy increases KiSS-1 mRNA, whereas sex steroids (oestradiol, progesterone) return KiSS-1 mRNA levels to values observed in intact animals (25). In Medaka fish, KiSS-1 in the nucleus posterioris periventricularis is not responsive to changes in ovarian steroids, whereas KiSS-1 neurones in the nucleus ventral tuberis are positively regulated by oestrogens (63). Future studies, in a greater variety of animals, will be needed to determine the relative contribution of sex steroids to kisspeptin regulation in discrete nuclei at the transcriptional and post-transcriptional levels of control.
The present observations provide further support for the role of kisspeptin as a key regulator of seasonal changes in reproductive function and as a mechanism for transducing and relaying environmental / internal signals to the GnRH system. Through manipulations of testosterone and photoperiod, the present study provides strong evidence indicating that both photoperiodic signals and gonadal steroid feedback work together to precisely regulate kisspeptin expression in Siberian hamsters. Studies aimed at uncovering the specific mechanisms by which these steroid independent effects are generated (e.g. melatonin regulation versus direct neural input), as well as the relative contributions of other relevant stimuli that also vary seasonally (e.g. temperature or food availability) to the regulation of this system, are required.
Acknowledgements
The authors wish to thank Devin Zysling, Emily Chester and Nick Garcia for technical assistance, and Ellen Ketterson, G. Troy Smith and Jill Lodde for helpful discussion. This work was supported by a SICB Grant-in-Aid and NIH / T32 training grant HD049336-0 (T.J.G.), an Eli Lilly METACyt grant, Indiana University Faculty Research Support Programme and NSF IOB:0543798 (G.E.D), and NIH HD050470 and the UC Berkeley Committee on research grant (L.J.K.).
References
- 1.Navarro VM, Castellano JM, Garcia-Galiano D, Tena-Sempere M. Neuroendocrine factors in the initiation of puberty: the emergent role of kisspeptin. Rev Endocr Metab Disord. 2007;8:11–20. doi: 10.1007/s11154-007-9028-2. [DOI] [PubMed] [Google Scholar]
- 2.Funes S, Hedrick JA, Vassileva G, Markowitz L, Abbondanzo S, Golovko A, Yang SJ, Monsma FJ, Gustafson EL. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem Biophys Res Commun. 2003;312:1357–1363. doi: 10.1016/j.bbrc.2003.11.066. [DOI] [PubMed] [Google Scholar]
- 3.d’Anglemont de Tassigny X, Fagg LA, Dixon JPC, Day K, Leitch HG, Hendrick AG, Zahn D, Franceschini I, Caraty A, Carlton MBL, Aparicio SAJR, Colledge WH. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci USA. 2007;104:10714–10719. doi: 10.1073/pnas.0704114104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, zahn D, Dixon J, Kaiser UB, slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MBL, Crowley WF, Aparicio S, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 6.Castellano JM, Navarro VM, Fernandez-Fernandez R, Castano JP, Malagon MM, Aguilar E, Dieguez C, Magni P, Pinilla L, Tena-Sempere M. Ontogeny and mechanisms of action for the stimulatory effect of kisspeptin on gonadotropin-releasing hormone system of the rat. Mol Cell Endocrinol. 2006;257:875–883. doi: 10.1016/j.mce.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Dhillo WS, Chaudhri OB, Patterson M, Thompson EL, Murphy KG, Badman MK, McGowan BM, Amber V, Patel S, Ghatei MA, Bloom SR. Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. J Clin Endocrinol Metab. 2005;90:6609–6615. doi: 10.1210/jc.2005-1468. [DOI] [PubMed] [Google Scholar]
- 8.Gottsch ML, Cunningham MJ, Smith JT, Popaz SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145:4073–4077. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- 9.Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25:11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Irwig MS, Fraleyb GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80:264–272. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- 11.Mason AO, Greives TJ, Scotti MAL, Levine J, Frommeyer S, Ketterson ED, Demas GE, Kriegsfeld LJ. Suppression of kisspeptin expression and gonadotropic axis sensitivity following exposure to inhibitory day lengths in female Siberian hamsters. Horm Behav. 2007;52:492–498. doi: 10.1016/j.yhbeh.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsui H, Takatsu Y, Kumano S, Matsumoto H, Ohtaki T. Peripheral administration of metastin induces marked gonadotropin release and ovulation in the rat. Biochem Biophys Res Commun. 2004;320:383–388. doi: 10.1016/j.bbrc.2004.05.185. [DOI] [PubMed] [Google Scholar]
- 13.Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MBL, Colledge WH, Caraty A, Aparicio S. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA. 2005;102:1761–1766. doi: 10.1073/pnas.0409330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Navarro VM, Castellano JM, Fernandez-Fernandez R, Tovar S, Roa J, Mayen A, Nogueiras R, Vazquez MJ, Barreiro ML, Magni P, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Characterization of the potent luteinizing hormone-releasing activity of KiSS-1 peptide, the natural ligand of GPR54. Endocrinology. 2005;146:156–163. doi: 10.1210/en.2004-0836. [DOI] [PubMed] [Google Scholar]
- 15.Patterson M, Murphy KG, Thompson EL, Patel S, Ghatei MA, Bloom SR. Administration of kisspeptin-54 into discrete regions of the hypothalamus potently increases plasma luteinising hormone and testosterone in male adult rats. J Neuroendocrinol. 2006;18:349–354. doi: 10.1111/j.1365-2826.2006.01420.x. [DOI] [PubMed] [Google Scholar]
- 16.Plant TM, Ramaswamy S, DiPietro MJ. Repetitive activation of hypothalamic G protein-coupled receptor 54 with intravenous pulses of kisspeptin in the juvenile monkey (Macaca mulatta) elicits a sustained train of gonadotropin-releasing hormone discharges. Endocrinology. 2006;147:1007–1013. doi: 10.1210/en.2005-1261. [DOI] [PubMed] [Google Scholar]
- 17.Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: A potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci USA. 2005;102:2129–2134. doi: 10.1073/pnas.0409822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson EL, Patterson M, Murphy KG, Smith KL, Dhillo WS, Todd JF, Ghatei MA, Bloom SR. Central and peripheral administration of kisspeptin-10 stimulates the hypothalamic-pituitary-gonadal axis. J Neuroendo-crinol. 2004;16:850–858. doi: 10.1111/j.1365-2826.2004.01240.x. [DOI] [PubMed] [Google Scholar]
- 19.Navarro VM, Fernandez-Fernandez R, Castellano JM, Roa J, Mayen A, Barreiro ML, Gaytan F, Aguilar E, Pinilla L, Dieguez C, Tena-Sempere M. Advanced vaginal opening and precocious activation of the reproductive axis by KiSS-1 peptide, the endogenous ligand of GPR54. J Physiol Lond. 2004;561:379–386. doi: 10.1113/jphysiol.2004.072298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bronson FH. Mammalian Reproductive Biology. Chicago, IL: University of Chicago Press; 1989. [Google Scholar]
- 21.Bartness TJ, Powers JB, Hastings MH, Bittman EL, Goldman BD. The timed infusion paradigm for melatonin delivery - what has it taught us about the melatonin signal, its reception, and the photoperiodic control of seasonal responses. J Pineal Res. 1993;15:161–190. doi: 10.1111/j.1600-079x.1993.tb00903.x. [DOI] [PubMed] [Google Scholar]
- 22.Goldman BD. Mammalian photoperiodic system: formal properties and neuroendocrine mechanisms of photoperiodic time measurement. J Biol Rhythms. 2001;16:283–301. doi: 10.1177/074873001129001980. [DOI] [PubMed] [Google Scholar]
- 23.Greives TJ, Mason AO, Scotti MAL, Levine J, Ketterson ED, Kriegsfeld LJ, Demas GE. Environmental control of kisspeptin: implications for seasonal reproduction. Endocrinology. 2007;148:1158–1166. doi: 10.1210/en.2006-1249. [DOI] [PubMed] [Google Scholar]
- 24.Revel FG, Saboureau M, Masson-Pevet M, Pevet P, Mikkelsen JD, Simonneaux V. Kisspeptin mediates the photoperiodic control of reproduction in hamsters. Curr Biol. 2006;16:1730–1735. doi: 10.1016/j.cub.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 25.Smith JT, Clay CM, Caraty A, Clarke IJ. KiSS-1 messenger ribonucleic acid expression in the hypothalamus of the ewe is regulated by sex steroids and season. Endocrinology. 2007;148:1150–1157. doi: 10.1210/en.2006-1435. [DOI] [PubMed] [Google Scholar]
- 26.Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology. 2005;146:2976–2984. doi: 10.1210/en.2005-0323. [DOI] [PubMed] [Google Scholar]
- 27.Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146:3686–3692. doi: 10.1210/en.2005-0488. [DOI] [PubMed] [Google Scholar]
- 28.Goldman BD, Nelson RJ. Melatonin and seasonality in mammals. In: Yu HS, Reiter RJ, editors. Melatonin: Biosynthesis, Physiological Effects, and Clinical Applications. Boca Raton, FL: CRC Press; 1993. [Google Scholar]
- 29.Duncan MJ, Goldman BD. Hormonal regulation of the annual pelage color cycle in the Djungarian hamster, Phodopus sungorus. J Exp Zool. 1984;230:89–95. doi: 10.1002/jez.1402300112. [DOI] [PubMed] [Google Scholar]
- 30.Greives TJ, Kriegsfeld LJ, Demas GE. Exogenous kisspeptin does not alter photoperiod-induced gonadal regression in Siberian hamsters (Phodopus sungorus) Gen Comp Endocrinol. 2008;156:552–558. doi: 10.1016/j.ygcen.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jasnow AM, Huhman KL, Bartness TJ, Demas GE. Short-day increases in aggression are inversely related to circulating testosterone concentrations in male Siberian hamsters (Phodopus sungorus) Horm Behav. 2000;38:102–110. doi: 10.1006/hbeh.2000.1604. [DOI] [PubMed] [Google Scholar]
- 32.Kriegsfeld LJ, Mei DF, Bentley GE, Ubuka T, Mason AO, Inoue K, Ukena K, Tsutsui K, Silver R. Identification and characterization of a gonadotropin-inhibitory system in the brains of mammals. Proc Natl Acad Sci USA. 2006;103:2410–2415. doi: 10.1073/pnas.0511003103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Revel FG, Saboureau M, Pevet P, Simonneaux V, Mikkelsen JD. RFamide-related peptide gene is a melatonin-driven photoperiodic gene. Endocrinology. 2008;149:902. doi: 10.1210/en.2007-0848. [DOI] [PubMed] [Google Scholar]
- 34.Demas GE, Johnson C, Polacek KM. Social interactions differentially affect reproductive and immune responses of Siberian hamsters. Physiol Behav. 2004;83:73–79. doi: 10.1016/j.physbeh.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 35.Ferin M, Carmel PW, Zimmerman EA, Warren M, Perez R, Vande Wiele RL. Location of intrahypothalamic estrogen-responsive sites influencing LH secretion in the female Rhesus monkey. Endocrinology. 1974;95:1059–1068. doi: 10.1210/endo-95-4-1059. [DOI] [PubMed] [Google Scholar]
- 36.Smith ER, Davidson JM. Location of feedback receptors: effects of intracranially implanted steroids on plasma LH and LRF response. Endocrinology. 1974;95:1566–1573. doi: 10.1210/endo-95-6-1566. [DOI] [PubMed] [Google Scholar]
- 37.Herbison AE. Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocr Rev. 1998;19:302–330. doi: 10.1210/edrv.19.3.0332. [DOI] [PubMed] [Google Scholar]
- 38.Wiegand SJ, Terasawa E. Discrete lesions reveal functional heterogeneity of suprachiasmatic structures in regulation of gonadotropin secretion in the female rat. Logo. 1982;34:242–247. doi: 10.1159/000123335. [DOI] [PubMed] [Google Scholar]
- 39.Maeda KI, Adachi S, Inoue K, Ohkura S, Tsukamura H. Metastin / Kisspeptin and control of estrous cycle in rats. Rev Endocr Metab Disord. 2007;8:21–29. doi: 10.1007/s11154-007-9032-6. [DOI] [PubMed] [Google Scholar]
- 40.Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147:5817–5825. doi: 10.1210/en.2006-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, Hoffman GE, Steiner RA, Tena-Sempere M. Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology. 2007;148:1774–1783. doi: 10.1210/en.2006-1540. [DOI] [PubMed] [Google Scholar]
- 42.Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, Takase K, Sugiura H, Ohtaki T, Matsumoto H, Uenoyama Y, Tsukamura H, Inoue K, Maeda KI. Involvement of anteroventral periventricular metastin / kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev. 2007;53:367–378. doi: 10.1262/jrd.18146. [DOI] [PubMed] [Google Scholar]
- 43.Smith JT. Kisspeptin signalling in the brain: steroid regulation in the rodent and ewe. Brain Res Rev. 2008;57:288–298. doi: 10.1016/j.brainresrev.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 44.Bittman EL, Ehrlich DA, Ogdahl JL, Jetton AE. Photoperiod and testosterone regulate androgen receptor immunostaining in the Siberian hamster brain. Biol Reprod. 2003;69:876–884. doi: 10.1095/biolreprod.102.010900. [DOI] [PubMed] [Google Scholar]
- 45.Kriegsfeld LJ, Drazen DL, Nelson RJ. Effects of photoperiod and reproductive responsiveness on pituitary sensitivity to GnRH in male prairie voles (Microtus ochrogaster) Gen Comp Endocrinol. 1999;116:221–228. doi: 10.1006/gcen.1999.7414. [DOI] [PubMed] [Google Scholar]
- 46.Demas GE, Nelson RJ. Short-day enhancement of immune function is independent of steroid hormones in deer mice (Peromyscus maniculatus) J Comp Physiol [B] 1998;168:419–426. doi: 10.1007/s003600050161. [DOI] [PubMed] [Google Scholar]
- 47.Turek FW, Ellis GB. Steroid-dependent and steroid-independent aspects of the photoperiodic control of seasonal reproductive cycles in male hamsters. In: Follett BK, Follett DE, editors. Biological Clocks in Seasonal Reproductive Cycles Wright. Bristol: Bristol; 1981. pp. 251–260. [Google Scholar]
- 48.Bittman EL, Jetton AE, Villalba C, Devries GJ. Effects of photoperiod and androgen on pituitary function and neuropeptide staining in Siberian hamsters. Am J Physiol Regul Integr Comp Physiol. 1996;40:R64–R72. doi: 10.1152/ajpregu.1996.271.1.R64. [DOI] [PubMed] [Google Scholar]
- 49.Krey LC, Ronchi E, Bittman EL. Effects of daylength on androgen metabolism and pulsatile luteinizing-hormone secrection in male golden-hamsters. Neuroendocrinology. 1989;50:533–542. doi: 10.1159/000125277. [DOI] [PubMed] [Google Scholar]
- 50.Goodman RL, Bittman EL, Foster DL, Karsch FJ. Alterations in the control of Luteinizing-hormone pulse frequency underlie the seasonal-variation in estadiol negative feedback in the ewe. Biol Reprod. 1982;27:580–589. doi: 10.1095/biolreprod27.3.580. [DOI] [PubMed] [Google Scholar]
- 51.Simpson SM, Follett BK, Ellis DH. Modulation by photoperiod of gonadotropin secretion in intact and castrated Djungarian hamsters. J Reprod Fertil. 1982;66:243–250. doi: 10.1530/jrf.0.0660243. [DOI] [PubMed] [Google Scholar]
- 52.Tamarkin L, Hutchison JS, Goldman BD. Regulation of serum gonadotropins by photoperiod and testicular hormones in the Syrian-hamster. Endocrinology. 1976;99:1528–1533. doi: 10.1210/endo-99-6-1528. [DOI] [PubMed] [Google Scholar]
- 53.Turek FW, Elliott JA, Alvis JD, Menaker M. Interaction of castration and photoperiod in regulation of hypophyseal and serum gonadotropin levels in male golden-hamsters. Endocrinology. 1975;96:854–860. doi: 10.1210/endo-96-4-854. [DOI] [PubMed] [Google Scholar]
- 54.Miernicki M, Pospichal MW, Powers JB. Short photoperiods affect male hamster sociosexual behaviors in the presence and absence of testosterone. Physiol Behav. 1990;47:95–106. doi: 10.1016/0031-9384(90)90046-7. [DOI] [PubMed] [Google Scholar]
- 55.Urbanski HF. Photoperiodic modulation of luteinizing hormone secretion in orchidectomized Syrian hamsters and the influence of excitatory amino acids. Endocrinology. 1992;131:1665–1669. doi: 10.1210/endo.131.4.1396311. [DOI] [PubMed] [Google Scholar]
- 56.Scotti MAL, Place NJ, Demas GE. Short-day increases in aggression are independent of circulating gonadal steroids in female Siberian hamsters (Phodopus sungorus) Horm Behav. 2007;52:183–190. doi: 10.1016/j.yhbeh.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 57.Prendergast BJ, Baillie SR, Dhabhar FS. Gonadal hormone-dependent and-independent regulation of immune function by photoperiod in Siberian hamsters. Am J Physiol Regul Integr Comp Physiol. 2008;294:R384–R392. doi: 10.1152/ajpregu.00551.2007. [DOI] [PubMed] [Google Scholar]
- 58.Wade GN, Bartness TJ. Effects of photoperiod and gonadectomy on food intake, body weight, and body composition in Siberian hamsters. Am J Physiol Regul Integr Comp Physiol. 1984;246:26–30. doi: 10.1152/ajpregu.1984.246.1.R26. [DOI] [PubMed] [Google Scholar]
- 59.Castellano JM, Navarro VM, Fernandez-Fernandez R, Nogueiras R, Tovar S, Roa J, Vazquez MJ, Vigo E, Casanueva FF, Aguilar E, Pinilla L, Dieguez C, Tena-Sempere M. Changes in hypothalamic KiSS-1 system and restoration of pubertal activation of the reproductive axis by kisspeptin in undernutrition. Endocrinology. 2005;146:3917–3925. doi: 10.1210/en.2005-0337. [DOI] [PubMed] [Google Scholar]
- 60.Castellano JM, Navarro VM, Fernandez-Fernandez R, Roa J, Vigo E, Pineda R, Dieguez C, Aguilar E, Pinilla L, Tena-Sempere M. Expression of hypothalamic KiSS-1 system and rescue of defective gonadotropic responses by kisspeptin in streptozotocin-induced diabetic male rats. Diabetes. 2006;55:2602–2610. doi: 10.2337/db05-1584. [DOI] [PubMed] [Google Scholar]
- 61.Luque RM, Kineman RD, Tena-Sempere M. Regulation of hypothalamic expression of KiSS-1 and GPR54 genes by metabolic factors: analyses using mouse models and a cell line. Endocrinology. 2007;148:4601–4611. doi: 10.1210/en.2007-0500. [DOI] [PubMed] [Google Scholar]
- 62.Smith JT, Acohido BV, Clifton DK, Steiner RA. KiSS-1 neurones are direct targets for leptin in the ob / ob mouse. J Neuroendocrinol. 2006;18:298–303. doi: 10.1111/j.1365-2826.2006.01417.x. [DOI] [PubMed] [Google Scholar]
- 63.Kanda S, Akazome Y, Matsunaga T, Yamamoto N, Yamada S, Tsukamura H, Maeda K-i, Oka Y. Identification of KiSS-1 product kisspeptin and steroid-sensitive sexually dimorphic kisspeptin neurons in medaka (Oryzias latipes) Endocrinology. 2008;149:2467–2476. doi: 10.1210/en.2007-1503. [DOI] [PubMed] [Google Scholar]