Abstract
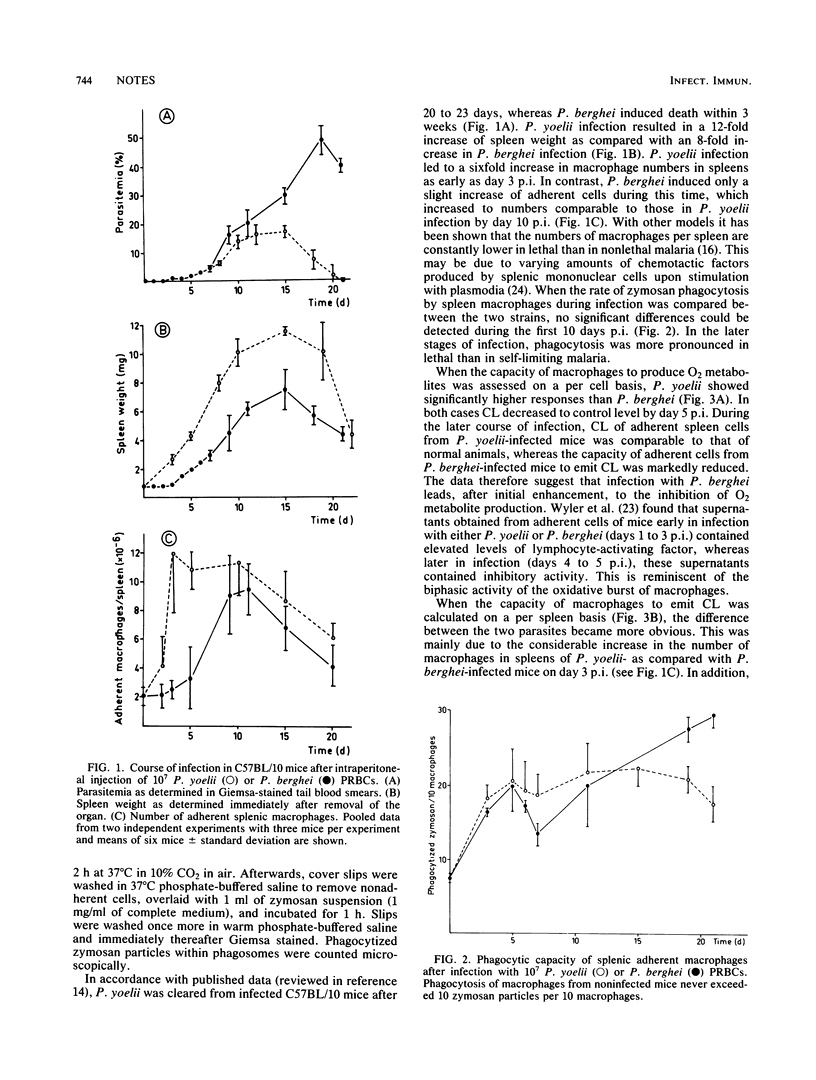
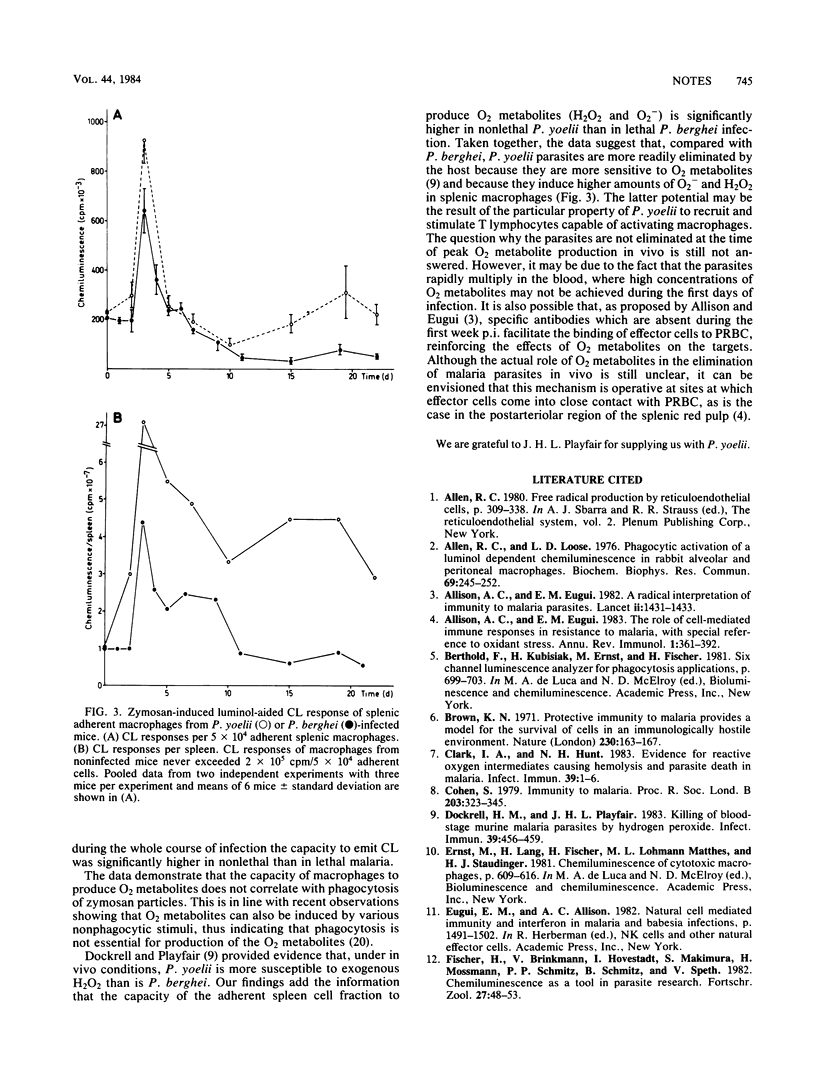
The role of splenic macrophages in resistance to lethal Plasmodium berghei or self-limiting Plasmodium yoelii was studied by testing their rate of phagocytosis and their production of O2 metabolites (H2O2 and O2-) upon nonspecific stimulation with zymosan. It was found that, compared with P. berghei, infection of mice with P. yoelii resulted in an earlier appearance and in higher numbers of adherent cells in the spleen. Furthermore, the capacity of macrophages to generate O2 metabolites was significantly higher in P. yoelii- than in P. berghei-infected mice. This difference in the production of O2 metabolites was more pronounced when calculated on a per spleen basis. On the other hand, phagocytosis by macrophages was similar in both types of infection. The data suggest that lethal and nonlethal malaria species differ in their capacity to induce the production of O2 metabolites by macrophages, thereby influencing the final course of disease.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C., Loose L. D. Phagocytic activation of a luminol-dependent chemiluminescence in rabbit alveolar and peritoneal macrophages. Biochem Biophys Res Commun. 1976 Mar 8;69(1):245–252. doi: 10.1016/s0006-291x(76)80299-9. [DOI] [PubMed] [Google Scholar]
- Allison A. C., Eugui E. M. A radical interpretation of immunity to malaria parasites. Lancet. 1982 Dec 25;2(8313):1431–1433. doi: 10.1016/s0140-6736(82)91330-7. [DOI] [PubMed] [Google Scholar]
- Allison A. C., Eugui E. M. The role of cell-mediated immune responses in resistance to malaria, with special reference to oxidant stress. Annu Rev Immunol. 1983;1:361–392. doi: 10.1146/annurev.iy.01.040183.002045. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Hunt N. H. Evidence for reactive oxygen intermediates causing hemolysis and parasite death in malaria. Infect Immun. 1983 Jan;39(1):1–6. doi: 10.1128/iai.39.1.1-6.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. Immunity to malaria. Proc R Soc Lond B Biol Sci. 1979 Jan 15;203(1153):323–345. doi: 10.1098/rspb.1979.0001. [DOI] [PubMed] [Google Scholar]
- Dockrell H. M., Playfair J. H. Killing of blood-stage murine malaria parasites by hydrogen peroxide. Infect Immun. 1983 Jan;39(1):456–459. doi: 10.1128/iai.39.1.456-459.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingdale M. R., Zavala F., Nussenzweig R. S., Nussenzweig V. Antibodies to the protective antigen of Plasmodium berghei sporozoites prevent entry into cultured cells. J Immunol. 1982 Apr;128(4):1929–1930. [PubMed] [Google Scholar]
- Lelchuk R., Dockrell H. M., Playfair J. H. T-independent macrophage changes in murine malaria. Clin Exp Immunol. 1983 Mar;51(3):487–493. [PMC free article] [PubMed] [Google Scholar]
- Lelchuk R., Taverne J., Agomo P. U., Playfair J. H. Development and suppression of a population of late-adhering macrophages in mouse malaria. Parasite Immunol. 1979 Spring;1(1):61–78. doi: 10.1111/j.1365-3024.1979.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Makimura S., Brinkmann V., Mossmann H., Fischer H. Chemiluminescence response of peritoneal macrophages to parasitized erythrocytes and lysed erythrocytes from Plasmodium berghei-infected mice. Infect Immun. 1982 Aug;37(2):800–804. doi: 10.1128/iai.37.2.800-804.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pick E., Keisari Y. Superoxide anion and hydrogen peroxide production by chemically elicited peritoneal macrophages--induction by multiple nonphagocytic stimuli. Cell Immunol. 1981 Apr;59(2):301–318. doi: 10.1016/0008-8749(81)90411-1. [DOI] [PubMed] [Google Scholar]
- Potocnjak P., Yoshida N., Nussenzweig R. S., Nussenzweig V. Monovalent fragments (Fab) of monoclonal antibodies to a sporozoite surface antigen (Pb44) protect mice against malarial infection. J Exp Med. 1980 Jun 1;151(6):1504–1513. doi: 10.1084/jem.151.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyler D. J., Gallin J. I. Spleen-derived mononuclear cell chemotactic factor in malaria infections: a possible mechanism for splenic macrophage accumulation. J Immunol. 1977 Feb;118(2):478–484. [PubMed] [Google Scholar]
- Wyler D. J., Oppenheim J. J., Koontz L. C. Influence of malaria infection on the elaboration of soluble mediators by adherent mononuclear cells. Infect Immun. 1979 Apr;24(1):151–159. doi: 10.1128/iai.24.1.151-159.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]


