Abstract
Relative to other primates, Cebus monkeys display unusually fast postnatal brain growth and motor skill development. The neonatal capuchin brain, at approximately 29–34 g, is a smaller proportion of the adult brain weight (c. 50%) than is the brain of other primates except humans and great apes. Here we describe, from a cross-sectional sample, brain development in 29 brown capuchin monkeys (Cebus apella) using high-resolution structural magnetic resonance images, focusing on growth patterns in total brain volume, cortical gray and white matter volume, frontal lobe gray and white matter volume, and corpus callosum area. Non-linear age-related changes in total brain volume, cortical white matter volume and frontal white matter volume were detected from birth – 5 years. Sex differences in corpus callosum:brain ratio were also found, with males having a 10% smaller corpus callosum:brain ratio than females regardless of age. Female corpus callosum:brain ratio showed significant age-related related changes, whereas males did not display any significant changes across age. Sex differences were also found in cortical gray and frontal lobe gray matter volumes, with males having larger volumes than females. These findings support the conclusion that capuchins undergo rapid neurological change during the first few years of life.
The dynamics of brain growth determine important factors in an organism’s life history, cognitive development, and behavioral maturation. Species that have relatively large adult brains, such as humans, achieve this endpoint by either prolonging the time-course of brain development or by increasing its rate (Leigh, 2004; Vinicius, 2005). In humans, there is a rapid, non-linear increase in total brain volume during early development, from infancy to adolescence (Courchesne et al., 2000; Pfefferbaum et al., 1994; Thompson et al., 2000). Maximum total brain volume is attained around the age of sexual maturity and reflects the summation of separate developmental processes in different neuroanatomical regions (Giedd, 2004; Giedd et al., 1999a). Cortical gray matter volume tends to follow an inverted U – shaped developmental trajectory and shows regional variation (Giedd, 2004; Giedd et al., 1999a). In contrast, beginning at birth and extending through early adulthood, cerebral white matter volume generally increases in a linear fashion (Giedd, 2004; Lenroot and Giedd, 2006; Jernigan and Tallal, 1990; Reiss et al., 1996; Schaefer et al., 1990; Sowell et al., 2002). The increase in the volume occupied by white matter during this period may be due, in part, to the increase in myelination of long axon projections, as layers of the lipid-rich axon insulation are laid down to increase the conduction speed of fibers that interconnect brain regions (van der Knaap and Valk, 1990; Paus et al., 1999). The myelination of the dorsal cortical areas occurs relatively late in development, matching the relatively slow maturation of the cognitive functions they mediate, whereas more ventral areas complete myelination earlier (Toga et al., 2006). The total area of the corpus callosum also shows significant age-related increases, with most of the growth occurring in the posterior half of the corpus callosum, particularly the splenium (Giedd et al., 1999a).
Nonhuman primates share several important characteristics of development with humans, including a prolonged infancy and juvenile period, long lifespan and complex social behavior. Rhesus monkeys parallel human brain development with maximum total brain volume attained around the age of sexual maturity and continuation of white matter development into adulthood (Malkova et al., 2006). One interspecific difference, however, concerns the early rapid and sustained growth in both total brain volume and white matter volume in humans, with this period ending earlier in rhesus monkeys. Unfortunately, there are very limited data on nonhuman primate brain development in comparison to humans. It is important to determine the extent to which the pattern and process of human brain development is distinctive, as such knowledge is crucial for understanding human behavioral evolution. Furthermore, such comparative knowledge of brain development is likely to further our understanding of neurodevelopmental disorders, psychiatric conditions and disease (Dawson et al., 2000).
Capuchin monkeys (Cebus spp.) are New World primates commonly used in biomedical and behavioral research. Capuchins have a large relative brain size as adults (Rilling and Insel, 1999) and are noted for their high degree of skilled motor and extractive foraging habits, complex social behavior, and cognitive abilities (Jack, 2007). However, little is known about brain development in this species, other than that the neonatal capuchin brain is a smaller proportion of the adult brain weight (c. 50%) than is the brain of any other primate except humans and great apes (Elias, 1977; Martin, 1983; Hartwig, 1996; Vinicius, 2005). This indicates that a relatively large amount of brain development in this species occurs postnatally, while in the context of rich environmental and social stimuli. Here we describe, from a cross-sectional sample, brain development in brown capuchin monkeys (Cebus apella) using high-resolution structural magnetic resonance images. We report age and sex effects for the volume of the whole brain, cortical gray and white matter, frontal lobe gray and white matter, and corpus callosum area. Similar to humans and rhesus monkeys, we expected capuchins to show rapid early developmental changes in these regions.
Methods
Subjects
In vivo magnetic resonance images were collected from 29 capuchin monkeys (Cebus apella; male n = 18, female n = 11) ranging in age from 4 days – 20 years. Of the total subjects, 12 were adults (≥ 5 years; male n = 4, female n = 8) and 17 were juveniles (between 4 days – 5 years; male n = 14, female n = 3). Subjects were housed at Hiram College (Hiram, Ohio), Northeastern Ohio Universities College of Medicine (Rootstown, Ohio), the College of Wooster (Wooster, Ohio), or the University of Pittsburgh (Pittsburgh, Pennsylvania). The MRI scanning protocol was approved by the Institutional Animal Care and Use Committee at each institution.
MRI Acquisition Procedure
Capuchins were transported to the Brain Imaging Research Center in Pittsburgh, Pennsylvania for the MR procedure. Once at the facility, subjects were initially immobilized with one of two drug cocktails: a) ketamine (7 mg/kg) injection IM, meditomidine (.06 mg/kg) injection IM and atropine (.05 mg/kg) injection SQ, or b) ketamine (25 mg/kg) injection IM, acetylpromazine (1 mg/kg) injection IM and atropine (.05 mg/kg) injection SQ. For all subjects except the 4 day old infant, an intravenous catheter was then placed in the saphenous vein. Subjects were given a bolus of propofol (2 – 5 mg/kg) intravenously; a constant intravenous drip (250 – 350 µg/kg/min) maintained anaesthesia. Subjects were placed into the scanner chamber and their heads were fitted inside a 16 cm or 12 cm head coil. Subjects remained anaesthetized throughout the MR procedure and respiration rate, heart rate, and oxygen consumption were continually monitored. At the cessation of the scan, subjects receiving drug cocktail “a” received atipamazole (0.06mg/kg) injection IM.
All subjects were scanned on the same Siemens 3T Allegra Scanner. Sagittal T1-weighted 3D MPRAGE MR images were acquired through the entire brain using a TR = 1,500 ms and TE = 3.04 ms with no echo-train. Scan acquisition time was approximately 30 min. For each monkey the number of signals averaged three. Slices were obtained as 0.5-mm thick contiguous sections with a matrix size of 256 × 256 and a field of view of 128 mm × 128 mm, resulting in a final voxel size of 0.5 mm × 0.5 mm × 0.5 mm. Between 100 and 120 contiguous slices were obtained for each subject. Prior to morphometric analysis, data were converted into the ANALYZE 3D volume file format to facilitate reslicing into orthogonal planes. Computer files for individual monkeys were numerically coded prior to measurement to prevent observer bias.
Image Quantification Method
The archived MRI data were transferred to a PC running Analyze 7.0 (Mayo Foundation for Medical Education and Research, Lenexa, Kansas) software and ImageJ 1.26t (http://rsb.info.nih.gov/ij/) for post-image processing. Images were spatially realigned into standard anatomical orientation with the transaxial plane parallel to the anterior commissure – posterior commissure line and perpendicular to the interhemispheric fissure. All volumetric and surface area measures were conducted on the raw image series, with no warping or registration to a template.
We used the Cavalieri method to estimate volumes from a systematic-random series of 10 to 12 MRI slices. For Cavalieri estimates of volume, it has been demonstrated that most within-sample variance in a structure can be captured in 8 to 10 systematic-random sections regardless of the irregularity of the ROI outline (Mouton, 2002). Increasing the number of sections sampled leads to only minor increases in the precision of the estimate. Total brain volume was determined by measuring every 8th slice from the parasagittal series. This yielded, on average, 12.1 slices that were measured per individual to calculate total brain volume. In each slice, voxels representing gray matter, white matter, and ventricular space were identified and separated from the skull using manual segmentation. The entirety of the cerebral hemispheres, cerebellum, midbrain, brainstem, and ventricles were included in the measurement of brain volume, following protocols described in a previous study of nonhuman primate brains (Sherwood et al., 2004). The volume corresponding to these voxels was calculated for each slice and multiplied by the interslice distance to obtain the whole brain volume.
Morphometric measurements of CC area were performed using ImageJ. The CC is readily delineated from a mid-sagittal slice and was easily measured in its entirety. While it could be argued that measuring the midsaggital CC area from a single projection is prone to bias toward increased CC surface area due to oblique slicing, we do not believe that such an error was introduced for two reasons. First, each individual brain was realigned along the AC-PC and interhemispheric fissure to reduce such error. Secondly, the inter-rater measurements of CC involved different individuals realigning the scans and yet there was a high degree of concordance in measures (see below).
Measures of total cortical gray and white matter, and frontal lobe gray and white matter were segmented according to previously described protocols (Semendeferi et al., 1997; Sherwood et al., 2004). Total cortical gray matter volume measurements included the entire cortical mantle, including cortex located mesial to the rhinal sulcus. According to these criteria, our definition of cortical gray matter also includes proisocortex (cingulate gyrus, rostral insula and temporal pole), periallocortex (entorhinal cortex) and allocortex (olfactory cortex and hippocampus). Measurements of the total cortical white matter volume included commissural fibers, association fibers and the internal capsule. Measurements of frontal lobe gray and white matter were performed on coronal sections and were bounded by the Sylvian sulcus and the central sulcus. The position of the central sulcus was outlined as it appeared in sagittal and transverse views and used to guide segmentations in the coronal view. In sections including the central sulcus, only gray and white matter located mesial and superior to the central sulcus was measured. Measurements of the volume of the frontal white matter excluded the internal capsule and external capsule.
Statistical analyses
To statistically adjust CC data for total brain volume, we followed a recommendation by Smith (2005) wherein the square root of the CC area was divided by the cube root of total brain volume for each individual to bring all measures into the same geometric dimensionality. A one-way MANCOVA was conducted to determine the effect of sex on total brain volume, cortical gray and cortical white matter volumes, frontal lobe gray and frontal lobe white matter volumes, and corpus callosum area, while controlling for age. F tests were used to determine whether linear, cubic or quadratic growth models best fit the developmental change in brain regions. Inter-observer reliability (interclass correlation, ICC) of total brain volume and CC area measures were ICC = 0.98 (n = 10), P < 0.01 and ICC = 0.94 (n = 10), P < 0.01, respectively (observers were KAP and CCS). Intra-observer reliability for neocortical gray and white matter, and frontal lobe gray and white volume was 0.91, P < 0.05 (observer was KAP). SPSS 15.0 was used for conducting all analyses.
Results
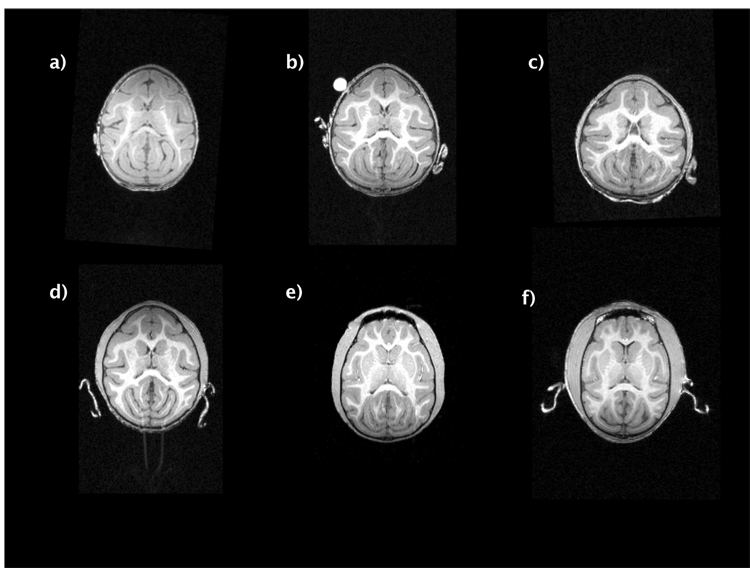
A preliminary MANCOVA using the entire data set violated the assumption of homogeneity of the regression slopes. As there were no juvenile females under the age of 1 year, the four juvenile males that were less than 1 year of age were removed from this analysis. Results revealed a trend toward significant differences between sexes on the combined DV, Wilks’ Λ = .54, F (6, 17) = 2.42, P = .07, multivariate partial η2 = .46. The covariate (age) significantly influenced the combined DV, Wilks’ Λ = .38, F (6, 17) = 4.61, P = .006, multivariate partial η2 = .62. Univariate ANOVA results indicate that CC:brain ratio was significantly influenced by sex, F (1, 22) = 15.01, P = .001, η2 = .29. Male CC:brain ratio was approximately 10% smaller than females (male M = 1.87 ± .09; female M = 2.09 ± .11). Cortical gray matter and frontal lobe gray matter volumes were significantly influenced by sex (cortical gray matter volume, F (1, 22) = 8.38, P = .008, η2 = .25; frontal lobe gray matter volume, F (1, 22) = 5.16, P = .033, η2 =.15), with males having larger volumes of both regions (male cortical gray matter volume M = 40.98 ± 4.75, female cortical gray matter volume M = 34.66 ± 3.99; male frontal lobe gray matter volume M = 7.81 ± 1.02; female frontal lobe gray matter volume M = 6.04 ± 1.66). Cortical white matter volume was significantly effected by the covariate age (F (1, 22) = 6.01, P = .02, η2 = .21). Frontal lobe white matter volume showed a borderline significant effect of the covariate age (F (1, 22) = 3.41, P = .078, η2 = .13). Figure 1 displays a cross-sectional series of horizontal sections from subjects aged 2 months – 5 years, illustrating frontal lobe white matter growth.
Figure 1.
Cross-sectional series illustrating white matter development in capuchins, from early infancy to early adulthood. a) = 2 months, b) = 5 months, c) = 1 year, d) = 1.5 years, e) = 3.5 years, and f) = 5 years. Subject b has a vitamin E capsule marking the left hemisphere.
Growth curves
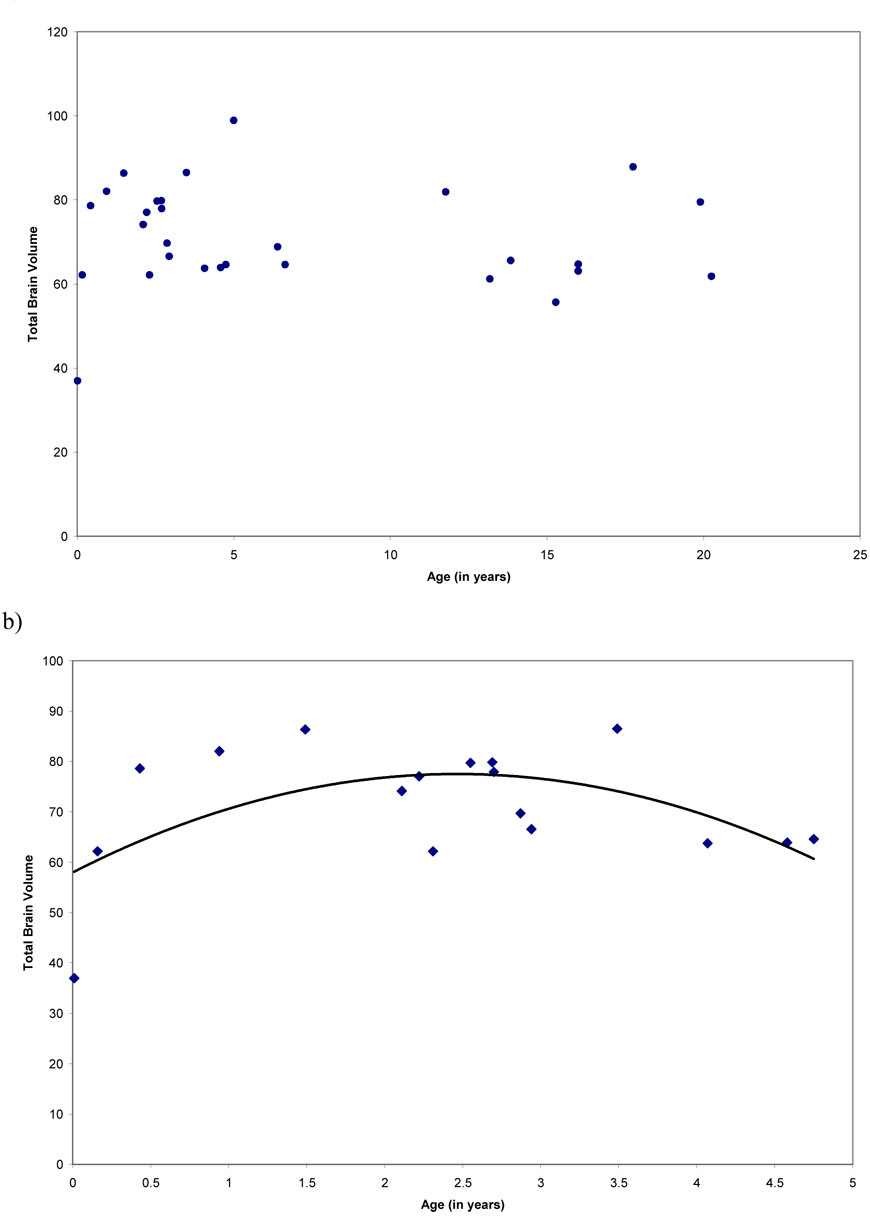
There were no significant age-related changes in total brain volume (linear component, R2 = .01, F (1, 27) = .16, P = .69; cubic component, R2 = .10, F (3, 25) = .88, P =.47; quadratic component, R2 = .01, F (2, 26) = .11, P = .89; Figure 2a). This failure to detect developmental changes in total brain volume is likely due to a combination of the relatively few subjects from the first year of life and the high variability seen in the adult brains in this sample. Therefore, we conducted post hoc analysis to examine age-related changes in total brain volume from birth to sexual maturity (5 years), the developmental stage at which humans attain maximum total brain volume (Courchesne et al., 2000; Giedd, 2004; Giedd et al., 1999a). Using these data (n = 17), significant non-linear age-related changes in total brain volume were found (linear component, R2 = .01, F (1, 15) = .20, P = .66; cubic component, R2 = .49, F (3, 13) = 4.23, P = .027; quadratic component, R2 = .35, F (2, 14) = 3.73, P =.050; Figure 2b). Total brain volume increased approximately 125% between birth and 5 years, with the maximum reached around age 2.5 years.
Figure 2.
Scatterplots representing age effects for a) total brain volume from birth through adulthood, and b) total brain volume from birth through sexual maturity.
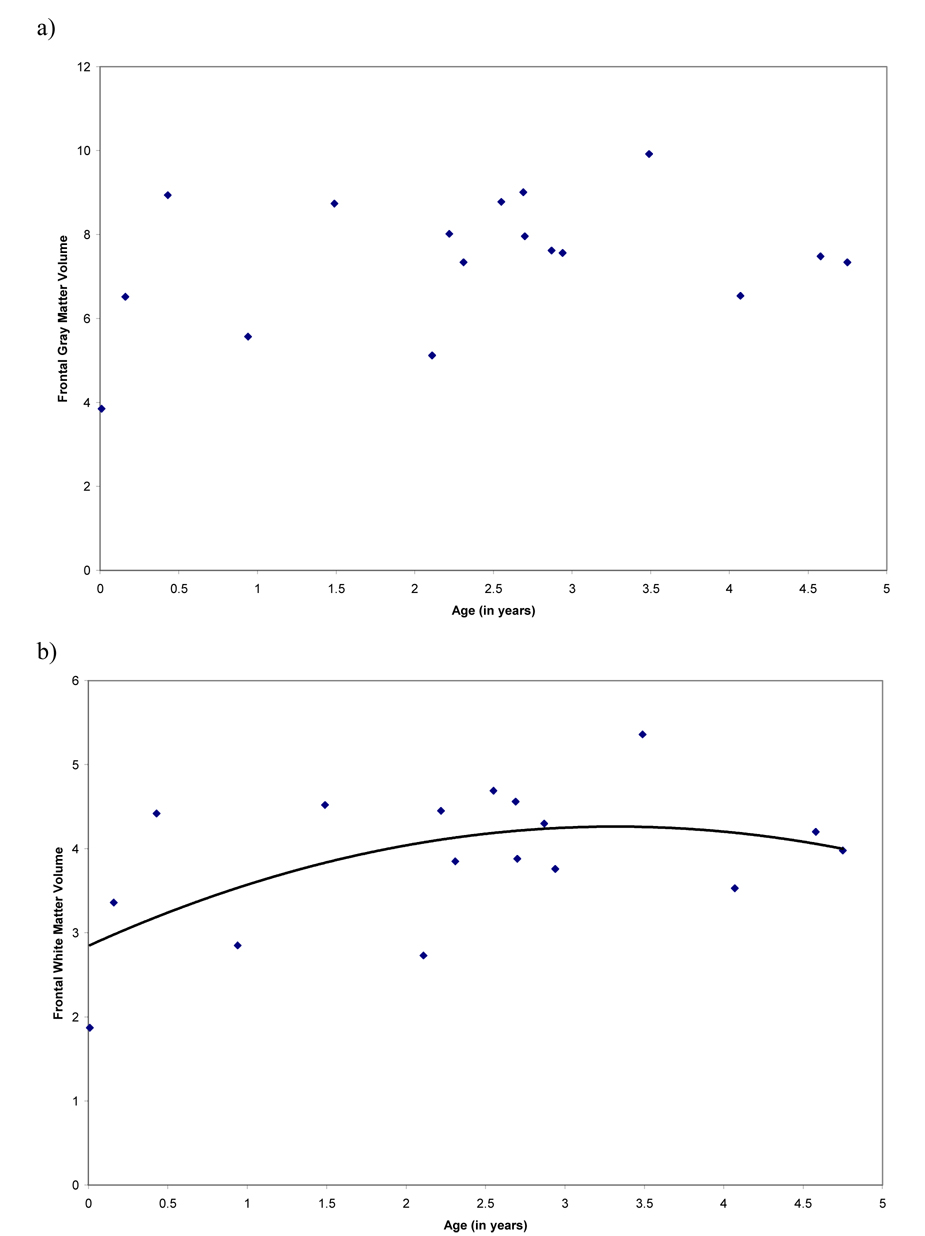
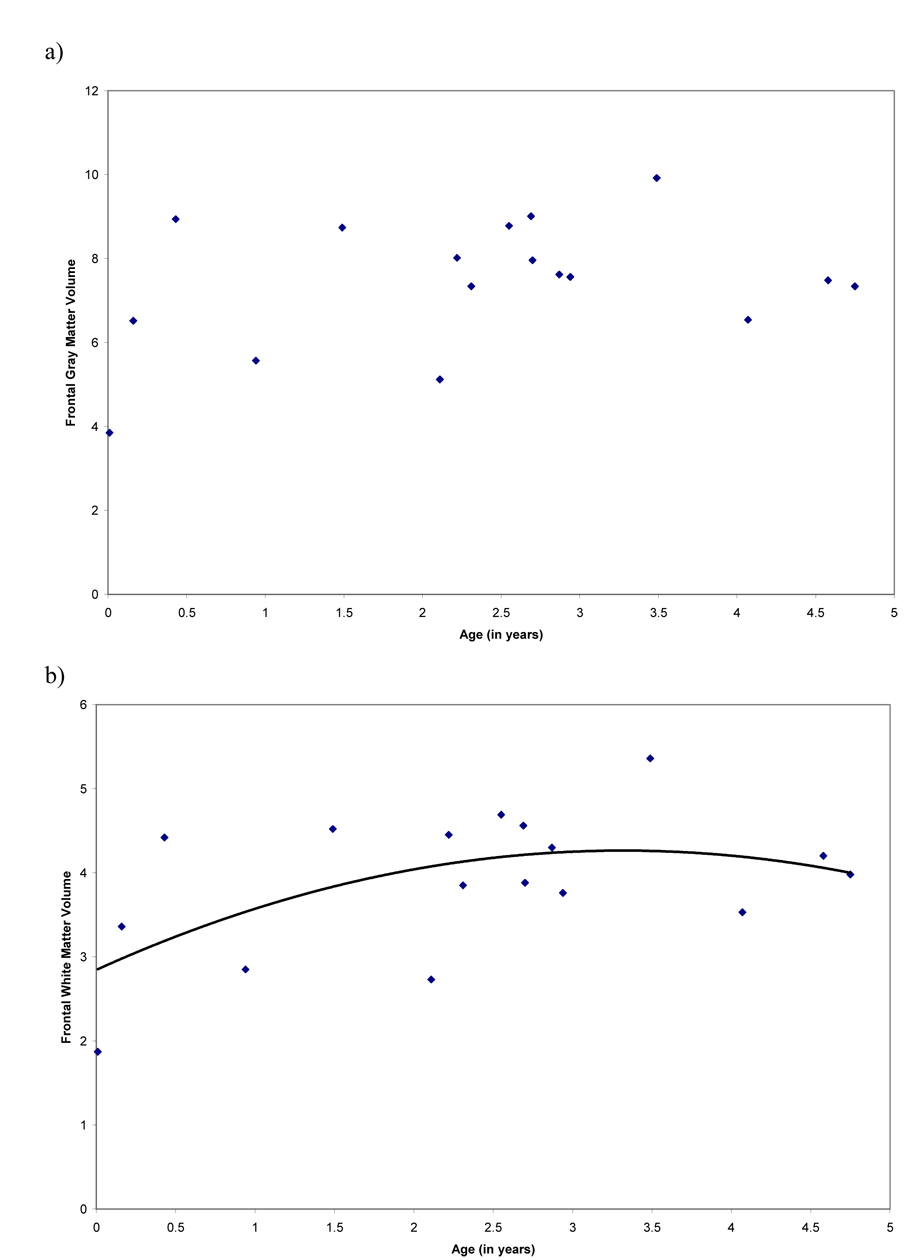
We analyzed age-related changes in cortical gray and white matter volumes, and frontal gray and white matter volumes from birth to sexual maturity. Cortical gray matter volume did not show any age-related changes (linear component, R2 = .04, F (1, 15) = .47, P = .47; cubic component, R2 = .40, F (3, 13) = 2.91, P =.08; quadratic component, R2 = .14, F (2, 14) = 1.11, P = .36; Figure 3a). Cortical white matter volume displayed non-linear change and was similarly explained by quadratic and cubic models (linear component, R2 = .26, F (1, 15) = 5.31, P = .04; cubic component, R2 = .57, F (3, 13) = 5.70, P =.010; quadratic component, R2 = .55, F (2, 14) = 8.48, P = .004; Figure 3b). There were no significant age-related changes in frontal lobe gray matter (linear component, R2 = .05, F (1, 15) = .79, P = .39; cubic component, R2 = .11, F (3, 13) = .55, P =.65; quadratic component, R2 = .11, F (2, 14) = .89, P = .43; Figure 4a). Frontal lobe white matter displayed significant non-linear age-related change and was similarly explained by quadratic and cubic models (linear component, R2 = .42, F (1, 15) = 10.79, P = .005; cubic component, R2 = .63, F (3, 13) = 7.22, P =.004; quadratic component, R2 = .61, F (2, 14) = 10.86, P = .001; Figure 4b). To determine whether there were differences in the development of non-frontal and frontal white matter, we analyzed the growth curve for non-frontal white matter. There were no significant age-related changes in non-frontal white matter from birth through 5 years (linear component, R2 = .01, F (1, 15) = .08, P = .78; cubic component, R2 = .13, F (3, 13) = .64, P = .61; quadratic component, R2 = .03, F (2, 03) = .19, P = .83).
Figure 3.
Scatterplots representing age effects for a) volume of cortical gray matter from birth through sexual maturity and b) volume of cortical white matter from birth through sexual maturity. Cortical white matter displayed significant non-linear change with age.
Figure 4.
Scatterplots representing age effects for a) volume of frontal lobe gray matter and b) volume of frontal lobe white matter. Frontal lobe white matter displayed significant non-linear change with age.
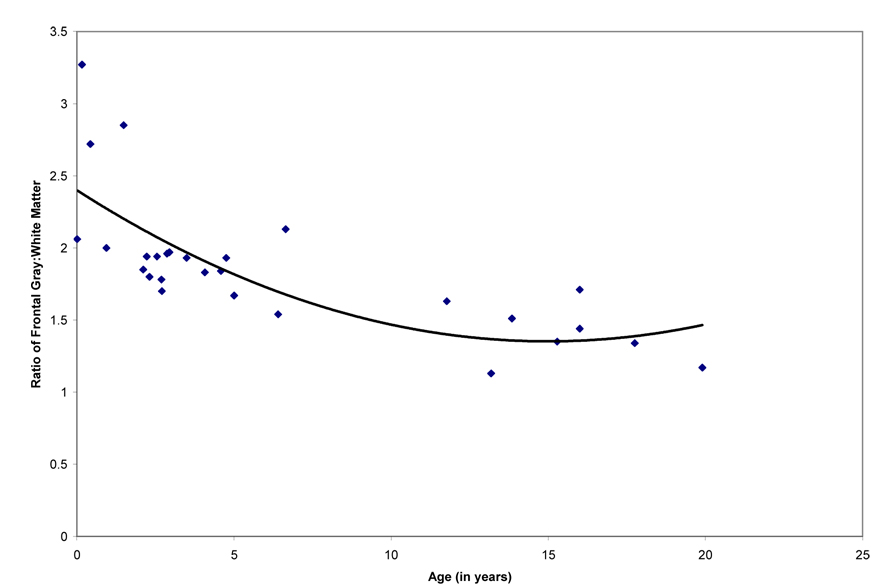
The ratio of non-frontal gray:white matter did not display age-related changes with age (linear component, R2 = .07, F (1, 27) = 2.05, P = .16; cubic component, R2 = .16, F (3, 25) = 1.63, P = .21; quadratic component, R2 = .16, F (2, 26) = 2.51, P = .10). However, the ratio of frontal lobe gray: white matter decreased with age, particularly from birth to 5 years, and was similarly explained by cubic and quadratic models (linear component, R2 = .48, F (1, 27) = 24.68, P < .001; cubic component, R2 = .61, F (3, 25) = 13.05, P < .001; quadratic component, R2 = .57, F (2, 26) = 17.28, P < .001; Figure 5). Combined with the previous results on age effects for raw volumetric changes in these regions, these significant age effects for the ratio of frontal lobe gray: white matter in the absence of age-related changes in the ratio of non-frontal gray:white matter indicate that the rate of white matter increases are significantly greater than changes in gray matter, with particularly rapid relative increases in frontal lobe white matter volume in the first 5 years of life.
Figure 5.
Scatterplot representing age effects for the ratio of frontal lobe gray:white matter.
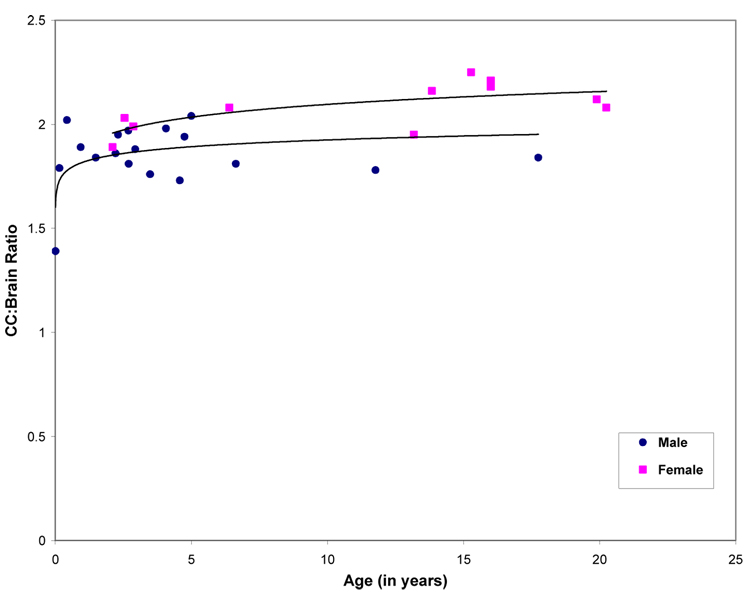
In females, CC:brain ratio showed an age-related increase (linear component, R2 = .41, F (1, 9) = 6.20, P < .05; cubic component, R2 = .52, F (3, 7) = 2.47, P = .15; quadratic component, R2 = .50, F (2, 8) = 4.04, P = .06) . In contrast, males did not display any significant changes in CC:brain ratio across age (linear component, R2 = .01, F (1, 16) = .03, P = .86; cubic component, R2 = .19, F (3, 14) = 1.12, P = .37; quadratic component, R2 = .07, F (2, 15) = .55, P = .58; Figure 6).
Figure 6.
Age-related changes in corpus callosum:brain ratio. Females are indicated as squares, males as diamonds. Throughout all ages, females show a larger cc:brain ratio than males.
Discussion
Our data indicate that both male and female capuchins show significant age-related non-linear changes in the volumes of cortical white matter and frontal lobe white matter. During the first few years of life especially, capuchin brain development is characterized by rapid growth in total brain size, which appears to be primarily related to the expansion of white matter volume. Our data also revealed that sexual dimorphism in CC:brain ratio is persistent across development, indicating that the adult pattern of sex differences in corpus callosum morphology may arise pre- or perinatally. Furthermore, females were shown to have age-related changes in CC:brain ratio. The current results provide comparative data on the pattern of brain growth in a highly encephalized New World monkey species, indicating that capuchins, similar to humans, undergo rapid neurological change during the first few years of life. This rapid white matter growth suggests that experience-dependent plasticity during the early critical period might provide enhanced opportunities for learning to shape neuronal connectivity in species that are born with relatively immature brains, such as humans and capuchins.
Age-related changes in total brain volume were detected from birth – 5 years (sexual maturity), with capuchins showing non-linear growth in total brain volume during this period and the developmental maximum was reached around 2.5 years. Taken together with previous research indicating that only 50% of adult brain size is obtained at birth in capuchins (Elias, 1977; Hartwig, 1996), our results suggest that overall brain growth is relatively rapid during infancy. Compared to rhesus monkeys, whose newborn brain size is approximately 64% of the adult brain (Malkova et al., 2006) and increases 56% between 1 week and 4 years, capuchin brains are less mature at birth. Neonatal capuchin brains are somewhat more mature than neonatal chimpanzee brains, whose postnatal brain growth accounts for approximately 65 – 75% of total brain size (Vinicius, 2005). Neonatal capuchin brains are also more mature than the neonatal human brain, whose size is only one-fourth to one-third of its adult volume. Furthermore, in humans, total brain size increases 100% during the first year (Gilmore et al., 2007), and continues to display exponential growth into adolescence (Giedd, 2004; Giedd et al., 1999a). Thus, human brains are distinctive in having a less mature brain at birth and a longer period of early rapid brain growth.
While capuchins did not display age-related change in the volumes of either cortical gray or frontal lobe gray matter, significant developmental changes were observed in the volumes of cortical white and frontal lobe white matter. Additionally, capuchins displayed a rapid, non-linear change in the ratio of frontal lobe gray:white matter from infancy to approximately 3 years, with sustained but slower change through adulthood. Capuchin brain development can be characterized by a rapid rate of increase in white matter relative to gray matter, and the comparison of growth curves of the ratios of non-frontal gray:white matter and frontal gray:white matter suggest that this change is largely due to increases in frontal lobe white matter. In humans, while both gray and white matter increase rapidly during the first 2 years, gray matter volume peaks early in life (around age 4 years) and then decreases whereas cortical white matter shows continued growth through early adulthood (Giedd, 2004; Lenroot and Giedd, 2006; Jernigan and Tallal, 1990; Matsuzawa et al., 1994; Pfefferbaum et al., 1994; Reiss et al., 1996; Schaefer et al., 1990; Sowell et al., 2002). Additionally, humans display regionally specific differential growth of white matter, with frontal lobe growth more rapid than the temporal lobe (Matsuzawa et al., 1994). Differences in regional growth patterns correspond to changes in the behavioral repertoire. Adenosine-mediated mechanisms have been identified that promote oligodendrocyte proliferation and myelination in response to neuronal activity (Stevens et al. 2002). Early visual deprivation in humans has been shown to cause selective white matter expansion underlying somatosensory and motor cortex in relation to cross-modal compensation (Noppeney et al., 2005). In addition, myelination of language-related areas of the brain from birth to 3 years corresponds to the development of vocabulary (Pujol et al., 2006).
In capuchins, the rapid increase of frontal lobe white matter during the first few years of life corresponds with opportunities for social learning and acquiring technical skills related to skilled foraging behavior. Capuchins are noted for their high degree of manipulative propensities and extractive foraging habits, which are analogous to complex manipulative skills demonstrated by humans and chimpanzees, and require not only manual and oral activity but also fine hand-eye coordination, haptic perception and digit coordination. The development of these skills reflects, in part, a connection to the maturation of brain regions that mediate these behaviors, including the parietal cortex, supplementary motor area, and premotor cortex. By 6 months, capuchins display the major components of the adult foraging repertoire, including bimanual activity and precision grips (Adams-Curtis & Fragaszy, 1994; Costello & Fragaszy, 1989). Around one year of age, capuchins display the manipulative patterns associated with species-typical foraging (Fragaszy & Adams-Curtis, 1997). The developmental progression of capuchin corticospinal projections is unknown – but adults of this species have dense projections to hand motoneurons in the ventral horn, associated with dextrous finger movements (Bortoff & Strick, 1993). In rhesus macaques corticospinal projections, which contribute to manual dexterity, are not adultlike at 11 months (Armand et al., 1997).
Sex differences in CC:brain ratio were found to be persistent across development. At all stages of development, females had a larger CC:brain ratio than males. The finding of sex differences in CC:brain ratio across development is especially noteworthy given that total brain volume and the ratio of frontal lobe gray:white matter did not show sex differences. The finding of sex differences in CC:brain ratio supports and extends the recent evidence of sex influences on corpus callosum morphology in adult capuchins (Phillips et al., 2007). Interestingly, such developmental differences do not appear to be present in humans. A longitudinal study of development of human corpus callosum revealed that, when an adjustment was made for total brain volume, no sex difference existed in midsagittal CC area throughout childhood (Giedd et al., 1999b). Giedd et al. also found no sex difference in the growth patterns of the CC, and suggested that the high variability seen in the size of the CC in humans may be confounding the relationship. Our results suggest sex differences in the organization of the capuchin corpus callosum are present early in life and persist throughout development.
The functional significance of this morphological sex difference in CC:brain ratio in capuchins is unclear, but may be related to behavioral laterality in cognitive and visuospatial tasks. In addition to the sex difference in overall CC:brain ratio reported in this investigation, a previous study reported sex differences in regional subdivisions of the corpus callosum in adult capuchins, including regions involved in motor processing (anterior midbody) and spatial-ability (splenium) (Phillips et al., 2007). Furthermore, these effects were mediated by handedness on a skilled motor task, with right-handed males having significantly smaller anterior midbody than right-handed females. Collectively, these results support the importance of left-hemisphere specialization in skilled motor actions (Serrien et al., 2006). The observed sex difference in corpus callosum morphology may be related to hemispheric specialization for motor integration of visuospatial information in the context of complex foraging. In the wild, capuchins rely heavily on processing complex visuospatial information as they utilize both arboreal and terrestrial substrates in their locomotor repertoire (Jack, 2007). Capuchins are also noted for being very adept at capturing small rapid prey, such as birds, lizards, squirrels, and coatis. Behavioral data investigating sex differences in spatial abilities in the context of foraging, such as prey capture, are needed to understand the functional significance of morphological differences in the corpus callosum.
The limitations of this study include an unbalanced sample with respect to age and sex, as most of the younger animals were male while most of the older animals were female. An additional limitation -is the relatively few samples collected from subjects less than 1 year of age. Nonetheless, this study provides initial insight into the developing capuchin brain, demonstrating that cortical white and frontal lobe white matter development are relatively rapid during the first 3 years and continue more slowly into adulthood. Ongoing research in our laboratory is further investigating capuchin brain development using a longitudinal approach, investigating how brain development is related to the emergence of skilled foraging behavior.
Acknowledgement
This work was supported in part by the NSF BCS-0515484 and BCS-0549117; NIH NS-42867, NS24328 and MH56661; the James S. McDonnell Foundation (22002078); Howard Hughes Medical Institute; and Hiram College. We thank Dr. Peter Strick for providing his MR images of capuchins, Dr. Claudia Thompson for allowing us to acquire MR images from her capuchins, our veterinary staff for their care of the animals during scanning, and the staff of the Brain Imaging Research Center, especially Dr. Kwan-Jin Jung, Debbie Viszlay and Scott Kurdilla.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams-Curtis LE, Fragaszy DM. Development of manipulation in capuchin monkeys during the first 6 months. Developmental Psychobiology. 1994;27:123–136. doi: 10.1002/dev.420270206. [DOI] [PubMed] [Google Scholar]
- Armand J, Olivier E, Edgley SA, Lemon RN. Postnatal development of corticospinal projections from motor cortex to the cervical enlargement in the macaque monkey. The Journal of Neuroscience. 1997;17:251–266. doi: 10.1523/JNEUROSCI.17-01-00251.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortoff GA, Strick PL. Corticospinal terminations in two New-world Primates: Further evidence that corticomotoneuronal connections provide part of the neural substrate for manual dexterity. The Journal of Neuroscience. 1993;13:5105–5118. doi: 10.1523/JNEUROSCI.13-12-05105.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello M, Fragaszy D. Prehension in Saimiri and Cebus: I. Grip type and hand preference. American Journal of Primatology. 1989;15:235–245. doi: 10.1002/ajp.1350150306. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Chisum HJ, Townsend J, Cowles A, Covington J, Egaas B, Harwood M, Hinds S, Press GA. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000;21:672–682. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- Dawson G, Ashman SB, Carver LJ. The role of early experience in shaping behavioral and brain development and its implications for social policy. Developmental Psychopathology. 2000;12:695–712. doi: 10.1017/s0954579400004089. [DOI] [PubMed] [Google Scholar]
- Elias MF. Relative maturity of Cebus and squirrel monkeys at birth and during infancy. Developmental Psychobiology. 1977;10:519–528. doi: 10.1002/dev.420100605. [DOI] [PubMed] [Google Scholar]
- Fragaszy DM, Adams-Curtis LE. Developmental changes in manipulation in tufted capuchins (Cebus apella) from birth through 2 years and their relation to foraging and weaning. Journal of Comparative Psychology. 1997;111:201–211. doi: 10.1037/0735-7036.111.2.201. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999a;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Rajapakse JC, Vaituzis AC, Liu H, Berry YC, Tobin M, Nelson J, Castellanos FX. Development of the human corpus callosum during childhood and adolescence: A longitudinal MRI study. Progress in Neuropsychopharmacology & Biological Psychiatry. 1999b;23:571–588. doi: 10.1016/s0278-5846(99)00017-2. [DOI] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Gilmore JH, Lin W, Prastawa MW, Looney CB, Vetsa SK, Knickmeyer RC, Evans DD, Smith JK, Hamer RM, Lieberman JA, Gerig G. Regional gray matter growth, sexual dimorphism, and cerebral asymmetry in the neonatal brain. Journal of Neuroscience. 2007;27:1255–1260. doi: 10.1523/JNEUROSCI.3339-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig WC. Perinatal life history traits in New World monkeys. American Journal of Primatology. 1996;40:99–130. [Google Scholar]
- Jack KM. The Cebines: Toward an explanation of variable social structure. In: Campbell CJ, Fuentes A, Mackinnon KC, Panger M, Bearder SK, editors. Primates in Perspective. New York: Oxford University Press; 2007. pp. 107–123. [Google Scholar]
- Jernigan TL, Tallal P. Late childhood changes in brain morphology observable with MRI. Developmental Medicine and Child Neurology. 1990;32:379–385. doi: 10.1111/j.1469-8749.1990.tb16956.x. [DOI] [PubMed] [Google Scholar]
- Leigh SR. Brain growth, life history, and cognition in primate and human evolution. American Journal of Primatology. 2004;62:139–164. doi: 10.1002/ajp.20012. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: Insights from anatomical magnetic resonance imaging. Neuroscience and Biobehavioral Reviews. 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Malkova L, Heuer E, Saunders RC. Longitudinal magnetic resonance imaging study of rhesus monkey brain development. European Journal of Neuroscience. 2006;24:3204–3212. doi: 10.1111/j.1460-9568.2006.05175.x. [DOI] [PubMed] [Google Scholar]
- Martin RD. Human brain evolution in an ecological context. New York: American Museum of Natural History; Fifty-second James Arthur Lecture on the Evolution of the Human Brain. 1983
- Matsuzawa J, Matsui M, Konishi T, Noguchi K, Gur RC, Bilker W, Miyawaki T. Age-related volumetric changes of brain gray and white matter in healthy infants and children. Cerebral Cortex. 2001;11:335–342. doi: 10.1093/cercor/11.4.335. [DOI] [PubMed] [Google Scholar]
- Mouton PR. Principles and Practices of Unbiased Stereology. Baltimore: The Johns Hopkins University Press; 2002. [Google Scholar]
- Noppeney U, Friston KJ, Ashburner J, Frackowiak R, Price CJ. Early visual deprivation induces structural plasticity in gray and white matter. Current Biology. 2005;15:R488–R490. doi: 10.1016/j.cub.2005.06.053. [DOI] [PubMed] [Google Scholar]
- Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, Rapoport JL, Evans AC. Structural maturation of neural pathways in children and adolescents: In vivo study. Science. 1999;283:1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging of changes in brain morphology from infancy to late adulthood. Archives of Neurology. 1994;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Phillips KA, Sherwood CC, Lilak AL. Corpus callosum morphology in capuchin monkeys is influenced by sex and handedness. PLoS ONE. 2007;2(8):e792. doi: 10.1371/journal.pone.0000792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol J, Soriano-Mas C, Ortiz H, Sebastián-Gallés N, Losilla JM, Deus J. Myelination of language-related areas in the developing brain. Neurology. 2006;66:339–343. doi: 10.1212/01.wnl.0000201049.66073.8d. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB. Brain development, gender and IQ in children. A volumetric imaging study. Brain. 1996;119:1763–1774. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Insel TR. The primate neocortex in comparative perspective using magnetic resonance imaging. Journal of Human Evolution. 1999;37:191–223. doi: 10.1006/jhev.1999.0313. [DOI] [PubMed] [Google Scholar]
- Schaefer GB, Thompson JN, Jr, Bodensteiner JB, Hamza M, Tucker RR, Marks W, Gay C, Wilson D. Quantitative morphometric analysis of brain growth using magnetic resonance imaging. Journal of Child Neurology. 1990;5:127–130. doi: 10.1177/088307389000500211. [DOI] [PubMed] [Google Scholar]
- Semendeferi K, Damasio H, Frank R, Van Hoesen GW. The evolution of the frontal lobes: a volumetric analysis based on three-dimensional reconstructions of magnetic resonance scans of human and ape brains. Journal of Human Evolution. 1997;32:375–388. doi: 10.1006/jhev.1996.0099. [DOI] [PubMed] [Google Scholar]
- Serrien DJ, Ivry RB, Swinnen SP. Dynamics of hemispheric specialization and integration in the context of motor control. Nature Reviews Neuroscience. 2006;7:160–167. doi: 10.1038/nrn1849. [DOI] [PubMed] [Google Scholar]
- Sherwood CC, Cranfield MR, Mehlman PT, Lilly AA, Garbe JAL, Whittier CA, Nutter FB, Rein TR, Bruner HJ, Holloway RL, Tang CY, Naidich TP, Delman BN, Steklis HD, Erwin JM, Hof PR. Brain structure variation in great apes, with attention to the mountain gorilla (Gorilla beringei beringei) American Journal of Primatology. 2004;63:149–164. doi: 10.1002/ajp.20048. [DOI] [PubMed] [Google Scholar]
- Smith RJ. Relative size versus controlling for size. Current Anthropology. 2005;46:249–273. [Google Scholar]
- Sowell ER, Trauner DA, Gamst A, Jernigan TL. Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Developmental Medicine & Child Neurology. 2002;44:4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- Stevens B, Porta S, Haak LL, Gallo V, Fields RD. Adenosine: A neuron-glial transmitter promoting myelination in the CNS in response to action potentials. Neuron. 2002;36:855–868. doi: 10.1016/s0896-6273(02)01067-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Giedd JN, Woods RP, MacDonald D, Evans AC, Toga AW. Growth patterns in the developing brain detected by using continuum mechanical tensor maps. Nature. 2000;404:190–193. doi: 10.1038/35004593. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM, Sowell ER. Mapping brain maturation. Trends in Neuroscence. 2006;29:148–159. doi: 10.1016/j.tins.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Knaap MS, Valk J. MR imaging of the various stages of normal myelination during the first year if life. Neuroradiology. 1990;31:459–470. doi: 10.1007/BF00340123. [DOI] [PubMed] [Google Scholar]
- Vinicius L. Human encephalization and developmental timing. Journal of Human Evolution. 2005;49:762–776. doi: 10.1016/j.jhevol.2005.08.001. [DOI] [PubMed] [Google Scholar]