Abstract
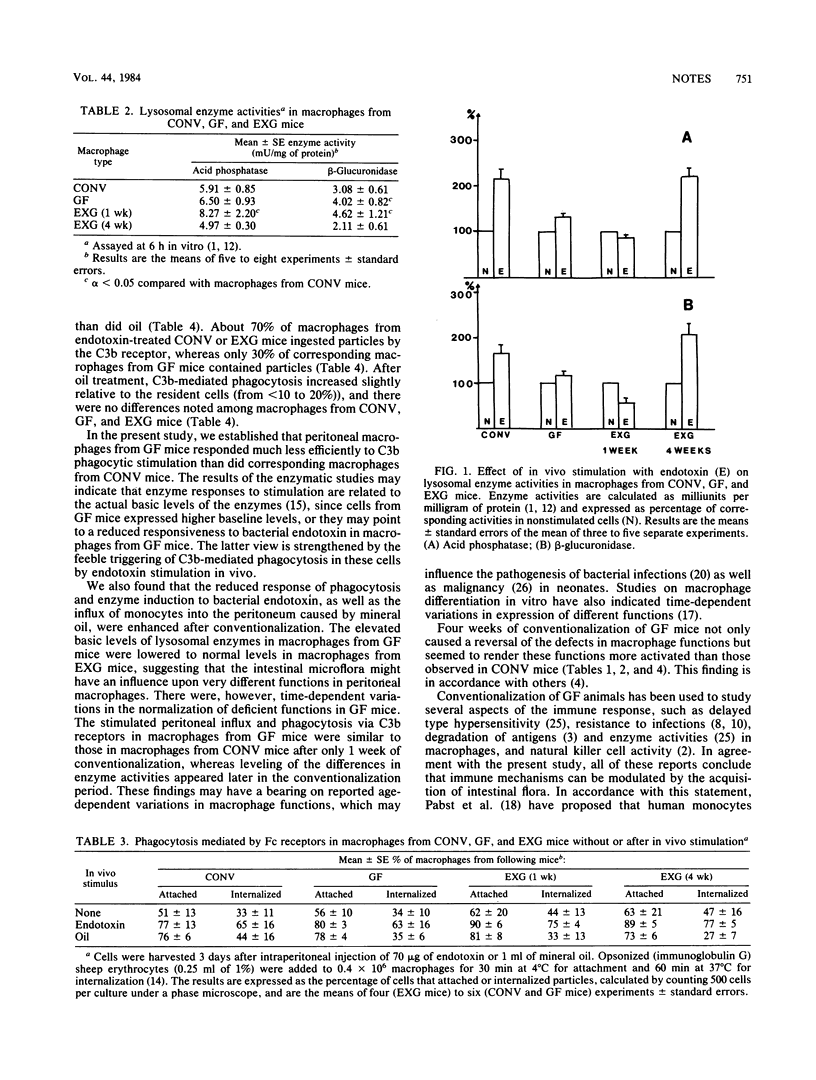
Peritoneal macrophages from germfree mice showed a higher basic activity of lysosomal enzymes than did macrophages from conventional mice, whereas oil-induced peritoneal influx, induction of lysosomal enzymes, and phagocytosis via the C3b receptor after endotoxin stimulation were reduced or absent. After germfree mice had been housed with conventional mice for 1 week, peritoneal influx and C3b receptor-mediated phagocytosis reached normal levels; after 4 weeks, enzyme activities also reached normal levels.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartizal K. F., Salkowski C., Balish E. The influence of a gastrointestinal microflora on natural killer cell activity. J Reticuloendothel Soc. 1983 May;33(5):381–390. [PubMed] [Google Scholar]
- Bauer H., Paronetto F., Burns W. A., Einheber A. The enhancing effect of the microbial flora on macrophage function and the immune response. A study in germfree mice. J Exp Med. 1966 Jun 1;123(6):1013–1024. doi: 10.1084/jem.123.6.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman B. L., Gershwin M. E., Scates S. S., Ohsugi Y. Immunobiology of germfree mice infected with Nocardia asteroides. Infect Immun. 1980 Aug;29(2):733–743. doi: 10.1128/iai.29.2.733-743.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A., BENSON B. THE DIFFERENTIATION OF MONONUCLEAR PHAGOCYTES. MORPHOLOGY, CYTOCHEMISTRY, AND BIOCHEMISTRY. J Exp Med. 1965 Jan 1;121:153–170. doi: 10.1084/jem.121.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUSTAFSSON B. E. Lightweight stainless steel systems for rearing germfree animals. Ann N Y Acad Sci. 1959 May 8;78:17–28. doi: 10.1111/j.1749-6632.1959.tb53092.x. [DOI] [PubMed] [Google Scholar]
- Johnson W. J., Balish E. Macrophage function in germ-free, athymic (nu/nu), and conventional-flora (nu/+) mice. J Reticuloendothel Soc. 1980 Jul;28(1):55–66. [PubMed] [Google Scholar]
- Jungi T. W., McGregor D. D. Impaired chemotactic responsiveness of macrophages from gnotobiotic rats. Infect Immun. 1978 Feb;19(2):553–561. doi: 10.1128/iai.19.2.553-561.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee K. W., Balish E. Effect of T-cells and intestinal bacteria on resistance of mice to candidosis. J Reticuloendothel Soc. 1982 Mar;31(3):233–240. [PubMed] [Google Scholar]
- Lev M., Battisto J. R. Impaired delayed hypersensitivity in germ-free guinea-pigs. Immunology. 1970 Jul;19(1):47–54. [PMC free article] [PubMed] [Google Scholar]
- Meltzer M. S. Tumoricidal responses in vitro of peritoneal macrophages from conventionally housed and germ-free nude mice. Cell Immunol. 1976 Mar 1;22(1):176–181. doi: 10.1016/0008-8749(76)90018-6. [DOI] [PubMed] [Google Scholar]
- Morland B., Kaplan G. Macrophage activation in vivo and in vitro. Exp Cell Res. 1977 Sep;108(2):279–288. doi: 10.1016/s0014-4827(77)80035-9. [DOI] [PubMed] [Google Scholar]
- Mørland B., Kaplan G. Properties of a murine monocytic tumor cell line J-774 in vitro. II. Enzyme activities. Exp Cell Res. 1978 Aug;115(1):63–72. doi: 10.1016/0014-4827(78)90402-0. [DOI] [PubMed] [Google Scholar]
- Mørland B., Smievoll A. I., Midtvedt T. Comparison of peritoneal macrophages from germfree and conventional mice. Infect Immun. 1979 Dec;26(3):1129–1136. doi: 10.1128/iai.26.3.1129-1136.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann C., Sorg C. Sequential expression of functions during macrophage differentiation in murine bone marrow liquid cultures. Eur J Immunol. 1980 Nov;10(11):834–840. doi: 10.1002/eji.1830101107. [DOI] [PubMed] [Google Scholar]
- Pabst M. J., Hedegaard H. B., Johnston R. B., Jr Cultured human monocytes require exposure to bacterial products to maintain an optimal oxygen radical response. J Immunol. 1982 Jan;128(1):123–128. [PubMed] [Google Scholar]
- Rabellino E. M., Ross G. D., Trang H. T., Williams N., Metcalf D. Membrane receptors of mouse leukocytes. II. Sequential expression of membrane receptors and phagocytic capacity during leukocyte differentiation. J Exp Med. 1978 Feb 1;147(2):434–445. doi: 10.1084/jem.147.2.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuit K. E. Macrophages and resistance of newborn rats to infection. J Reticuloendothel Soc. 1981 Nov;30(5):341–346. [PubMed] [Google Scholar]
- Smith C. S., Pilgrim H. I., Steinmuller D. The survival of skin allografts and xenografts in germ-free mice. Transplantation. 1972 Jan;13(1):38–41. doi: 10.1097/00007890-197201000-00009. [DOI] [PubMed] [Google Scholar]
- Starling J. R., Balish E. Lysosomal enzyme activity in pulmonary alveolar macrophages from conventional, germfree, monoassociated, and conventionalized rats. J Reticuloendothel Soc. 1981 Dec;30(6):497–505. [PubMed] [Google Scholar]
- Stashak P. W., Baker P. J., Roberson B. S. The serum antibody response to bacteriophage phi chi 174 in germ-free and conventionally reared mice. II. Kinetics of the serum antibody response following primary immunization. Immunology. 1970 Feb;18(2):307–317. [PMC free article] [PubMed] [Google Scholar]
- Ueda K., Yamazaki S., Someya S. Impairment and restoration of the delayed type hypersensitivity in germ-free mice. Jpn J Microbiol. 1973 Nov;17(6):533–536. doi: 10.1111/j.1348-0421.1973.tb00942.x. [DOI] [PubMed] [Google Scholar]
- Ueda K., Yamazaki S., Someya S. Studies on tubercle bacillus infection in germ-free mice. J Reticuloendothel Soc. 1972 Nov;12(5):545–563. [PubMed] [Google Scholar]
- Weeks B. A., Kavas A. F. Macrophage chemotaxis and phagocytosis in guinea pigs: influence of age and nutrition. J Reticuloendothel Soc. 1979 Nov;26(5):501–506. [PubMed] [Google Scholar]


