Abstract
Angiostatin, a proteolytic fragment of plasminogen, is a potent anti-angiogenic factor recently shown also to have an inhibitory effect on leukocyte recruitment and macrophage migration. Because both angiogenesis and inflammation play key roles in the progression of chronic kidney disease, we evaluated the effect of angiostatin treatment in the rat remnant kidney model. Rats were pretreated for 4 wk with recombinant adeno-associated viruses expressing either angiostatin or green fluorescence protein. Chronic renal disease was then induced by a subtotal nephrectomy, and rats were killed 8 wk later for analysis. Angiostatin treatment was associated with significantly less proteinuria but no alterations in serum creatinine, creatinine clearance, and blood urea nitrogen levels. Treatment with angiostatin reduced renal peritubular capillary number and decreased urinary nitric oxide levels. Despite reducing capillary density, angiostatin diminished interstitial fibrosis in association with reduced macrophage and T-cell infiltration and renal monocyte chemoattractant protein-1 mRNA levels. In conclusion, angiostatin overexpression was associated with attenuated renal disease progression in a model of chronic kidney injury, likely because of its anti-inflammatory actions. However, its anti-angiogenic actions suggest countering effects that could partially offset its benefit in chronic kidney diseases.
Keywords: angiogenesis, gene therapy, inflammation, macrophage, vascular growth factors
the late stages of kidney progression are characterized by the development of glomerulosclerosis and interstitial fibrosis (17). Two key processes that contribute to kidney disease pathophysiology are alterations in renal angiogenesis (24) and inflammation (43). A chronic loss of renal interstitial capillaries occurs in both human nephropathies (40) and animal models of chronic kidney damage (23), while inflammatory biomarkers (such as C-reactive protein and white blood cell counts) correlate with disease progression in patients with end-stage renal disease (43). Several studies have directed novel therapies to either block inflammation or stimulate angiogenesis (22, 31). Administration of the pro-angiogenic molecule vascular endothelial growth factor A (VEGF-A) attenuated renal fibrosis and stabilized renal function in the rat remnant kidney model (22). Interleukin-10 (IL-10) gene therapy in the remnant kidney model reduced renal inflammatory changes and improved renal function and lessened fibrosis (31).
However, stimulating angiogenesis can also be associated with increased inflammation. Indeed, the recruitment of macrophages is required for many angiogenic processes (2, 32). VEGF-A, for example, can cause potent inflammatory effects itself by binding to VEGF receptor-1 (VEGFR1) expressed on leukocytes (39). Likewise, inflammatory cytokines such as TNF-α can regulate the expression of VEGFR2 on human vascular endothelial cells (18).
One molecule that could affect the balance between renal angiogenesis and inflammation is angiostatin, the proteolytic fragment of plasminogen (kringle 1 to 4) that exhibits anti-tumor activity in murine models (35, 36). Angiostatin is a potent angiogenic inhibitor that blocks proliferation, induces apoptosis, and prevents migration of endothelial cells in vitro (7, 12, 45). In addition, angiostatin has anti-inflammatory actions by inhibiting leukocyte recruitment (8) and both neutrophil and macrophage migration (5, 37).
The role of angiostatin in kidney disease has been largely unexplored. One could postulate that the anti-inflammatory properties might be beneficial, whereas the anti-angiogenic actions might have countering effects. In kidney disease, levels of angiostatin are elevated in ischemia-reperfusion injury in which endothelial injury and capillary loss are prominent (3). On the other hand, adenoviral-mediated delivery of angiostatin alleviated albuminuria and glomerular hypertrophy in streptozocin-induced diabetic nephropathy (50). However, this is a model in which excessive VEGF-A expression and angiogenesis appear to be involved in the disease process (34). Angiostatin treatment was also effective in preventing the growth of tumors in a mouse model of renal cell carcinoma by decreasing blood vessel number and flow velocity (30).
Therefore, to better understand the role of angiostatin in chronic kidney disease, we overexpressed angiostatin systemically and determined its effects in the classic model of renal progression, the remnant kidney disease model.
MATERIALS AND METHODS
Vector construction and production.
Recombinant adeno-associated virus (rAAV) vectors expressing angiostatin (K1K3) (38) or green fluorescence protein (GFP) were generated and purified as described (52). The vector cassette consisted of a cytomegalovirus chicken β-actin hybrid promoter, rat K1K3 or GFP cDNAs, and a simian virus 40 polyadenylation sequence, flanked by AAV2 inverted terminal repeats. Vectors were transcapsidated using the helper plasmid pXYZ1 to provide capsids for the AAV1 serotype and purified by iodixanol density gradient centrifugation. DNA dot blot assays were performed to quantify the titer of AAV (52). This viral system allows gene expression to be elevated for the lifespan of the animals (6). Previous studies with IL-10 using the same rAAV serotype demonstrated overexpression for the duration of the whole study in both rat remnant kidney (31) and aortic transplant models (9).
Experimental protocol.
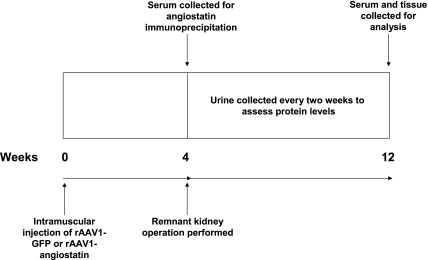
The experimental protocol is outlined in Fig. 1. Procedures were approved by the University of Florida Institutional Animal Care and Use Committee. Male Sprague-Dawley rats (n = 20; Charles River Laboratories, Wilmington, MA) weighing between 150 and 175 g were injected with 100 μl of saline containing either 1 × 1010 infectious units of rAAV1-GFP (n = 10) or rAAV1-K1K3 (n = 10) intramuscularly into the caudal muscle of the hindlimb. Four weeks after injection, angiostatin serum levels were measured, and remnant kidney operations were performed on all animals by resecting the right kidney with surgical extirpation of the upper and lower thirds of the left kidney (31). One week after surgery, six animals in each group were chosen for further study based on the percentage of remnant kidney weight removed (31), urinary protein excretion, and blood urea nitrogen (BUN) levels. Overnight urine samples were collected from animals in metabolic cages at 2, 4, 6, and 8 wk after surgery. Eight weeks after remnant kidney operation, all animals were killed for biochemical and histological analysis.
Fig. 1.
Experimental protocol. GFP, green fluorescence protein; rAAV, recombinant adeno-associated virus.
Immunoprecipitation for serum levels of angiostatin.
Rabbit anti-angiostatin antibody (R&D Systems, Minneapolis, MN) was immobilized to protein-A beads in PBS at room temperature for 30 min. The beads were washed in PBS and incubated with 10% rabbit serum to prevent nonspecific binding. Five hundred microliters of rat serum collected from animals 4 wk after either rAAV1-GFP or rAAV1-K1K3 injection (or 5 and 10 μg of recombinant angiostatin protein as a positive control; R&D Systems) were added to the beads, and the samples were incubated at 4°C overnight. The beads were washed four times with PBS, and 50 μl of Western blotting loading buffer (Bio-Rad Laboratories, Hercules, CA) were added. Beads were denatured by heating at 95°C for 5 min. Twenty microliters of supernatant were separated on 10% polyacrylamide electrophoresis gels, and proteins were transferred to nitrocellulose membranes (Invitrogen, Carlsbad, CA) by electroblotting (Bio-Rad Laboratories). Membranes were then blocked with 5% fat-free milk in PBS for 30 min at room temperature followed by incubation with rabbit anti-angiostatin antibody (R&D Systems; 1:250) at 4°C overnight. Membranes were washed in 0.05% Tween-20 in PBS and incubated at room temperature for 30 min with goat anti-rabbit antibody labeled with horseradish peroxidase (Dako, Carpinteria, CA). Bands were detected by chemiluminescence (Bio-Rad Laboratories), proteins were sized with Rainbow markers (Bio-Rad Laboratories), and the intensities of the angiostatin band were measured by densitometry.
Proteinuria and renal function.
Twenty-four-hour urinary protein excretion was measured by use of a protein assay kit (Bio-Rad Laboratories). Serum BUN and serum and urinary creatinine were determined by the VetACE biochemistry machine (Alfa Wassermann, West Caldwell, NJ).
Histological and immunohistological studies.
Renal tissues were fixed in Methyl Carnoy's fixative, and 4-μm paraffin sections were stained with periodic acid-Schiff reagent and hematoxylin for histological assessment. The percentage of glomeruli exhibiting focal or global glomerulosclerosis was determined by evaluation of all the glomeruli present in each biopsy. Glomerulosclerosis was defined as segmental increases in the glomerular matrix, segmental collapse, obliteration of capillary lumina, and accumulation of hyaline, often with synechial attachment to Bowman's capsule (31). Cortical tubulointerstitial injury (defined as inflammatory cell infiltration, tubular dilation and/or atrophy, or interstitial fibrosis) was graded semiquantitatively in at least 20 fields at ×200 magnification in every sample by a blinded observer using the following scoring system (31): 0 = changes in <10% of the cortex; 1+ = changes in 10–20% of the cortex; 2+ = changes in 20–40% of the cortex; 3+ = changes in 40–60% of the cortex; 4+ = changes in 60–80% of the cortex; 5+ = changes in >80% of the cortex.
For immunohistochemistry, renal tissues were fixed in 4% (wt/vol) buffered paraformaldehyde and frozen at −70°C. Cryosections (4 μm) were incubated overnight at 4°C with either rat anti-mouse monoclonal antibodies against CD4, CD8, ED-1, OX62 (BD Pharmingen, San Diego, CA), and JG-12 (a gift from Dr. Dontscho Kerjaschki, University of Vienna, Austria) or goat anti-human polyclonal antibody to collagen I (Southern Biotech, Birmingham, AL). Sections were washed in PBS, and endogenous peroxidase was quenched by 0.3% H2O2 in methanol. Sections were then labeled with appropriate secondary antibodies (Rockland Immunochemicals, Gilbertsville, PA), followed by mouse peroxidase anti-peroxidase (DAKO), and developed with 3,3′-diaminobenzidine substrate kit (Vector Laboratories, Burlingame, CA). Quantification of immunohistochemistry was performed using an Axioplan 2 imaging microscope (Carl Zeiss, Munich, Germany), CR5 digitized color camera and computer software (Axiovision 4.1, Carl Zeiss). For CD4, CD8, ED-1, and OX62 glomerular staining, the number of positive cells at ×400 magnification was counted. To assess all interstitial parameters, the diaminobenzidine-positive pixels in each field were evaluated at ×200 magnification. For each parameter, at least 20 fields were evaluated in every sample.
RNA extraction, reverse transcription, and real-time PCR.
Whole kidney tissue collected at the time of death was homogenized in tissue lysis buffer (Promega, Madison, WI). Total RNA was isolated using the SV Total RNA Isolation kit (Promega) according to the manufacturer's protocol and quantified by a spectrophotometer. Reverse transcription was performed using the iScript cDNA Synthesis kit (Bio-Rad Laboratories) according to the manufacturer's protocols. Reactions were incubated at 25°C for 5 min, 42°C for 30 min, and 85°C for 5 min and cooled at 4°C in a thermocycler (Eppendorf, Hamburg, Germany). Primers (Table 1) were designed by Genetool software (BioTools, Edmonton, AB, Canada), and oligonucleotides were synthesized (Sigma-Genosys, St. Louis, MO). Real-time PCR was performed using the SYBR Green Master Mix kit (Bio-Rad Laboratories) on the Opticon PCR machine (MJ Research, Waltham, MA). PCR was performed as follows: 94°C for 2 min followed by 40 cycles of denaturation, annealing, and extension at 94°C for 15 s, 64°C for 30 s, and 72°C for 45 s, respectively, and a final extension step at 72°C for 10 min. All reactions were performed in duplicate, and gene expression was normalized to GAPDH, previously used as a housekeeping gene for real-time PCR experiments in the remnant kidney model (20, 29), and not significantly altered between rAAV1-GFP or rAAV1-K1K3 groups in this study (data not shown). Primer specificity was confirmed by running the PCR products on agarose gels.
Table 1.
Primer sequences for real-time PCR
| Gene | Sequence | Size, bp |
|---|---|---|
| ICAM-1 | Fwd: GCCCGGAGGATCACAAACGAC | 186 |
| Rev: CCTGGGGCTGGCATGTAAGAGT | ||
| VCAM-1 | Fwd: GGGGGCCAAGTCCGTTCTGA | 158 |
| Rev: GGGGGCCACTGAATTGAATCTC | ||
| E-selectin | Fwd: CCCTGACCATGGAAGCCTGAAC | 163 |
| Rev: ATGGCAGGCAGGAGCAGGTG | ||
| P-selectin | Fwd: CGGCGGAGGCTGAGAACTG | 191 |
| Rev: GCGCTCCCCTTGGCTGTT | ||
| Collagen IVα5 | Fwd: AAGGGGGAGCCAGGCAGTATAA | 118 |
| Rev: TTGGCCCTGATACACCCTTGG | ||
| TGF-β1 | Fwd: ATACGCCTGAGTGGCTGTCT | 153 |
| Rev: TGGGACTGATCCCATTGATT | ||
| VEGF-A | Fwd: TGCGGATCAAACCTCACCAAA | 147 |
| Rev: TGCGCTTTCGTTTTTGACCC | ||
| VEGF-C | Fwd: CCACAGTGTCAGGCAGCTAA | 157 |
| Rev: ACTCCTTGTTGGGTCCACAG | ||
| VEGFR1 | Fwd: TGCAAGCGGGCCAGACTC | 163 |
| Rev: CGCCATGTTCAAGGTCAAGGT | ||
| VEGFR2 | Fwd: GCCGGCATGGTCTTCTGTGAG | 109 |
| Rev: GGGGGCTCAGGACCACATCATA | ||
| Angiopoietin 1 | Fwd: TGCCGTGAGAGTGCGACAGA | 181 |
| Rev: GGGCCATCTCCGACTTCATATT | ||
| Angiopoietin 2 | Fwd: GGAGGAGGGTGGACGGTCATC | 150 |
| Rev: GCGATGCCCACTGGTCAGCT | ||
| Tie-2 | Fwd: CCACACACATTTGGCAGGACCT | 245 |
| Rev: AGCCCTTGCCATCCTTGAGAGC | ||
| MCP-1 | Fwd: AGCCCAGAAACCAGCCAACTC | 196 |
| Rev: GCCGACTCATTGGGATCATCTT | ||
| IL-2 | Fwd: TGCAGCGTGTGTTGGATTTGAC | 144 |
| Rev: TTGCTGGCTCATCATCGAATTG | ||
| IFN-γ | Fwd: AACCAGGCCATCAGCAACAACA | 214 |
| Rev: ACCGACTCCTTTTCCGCTTCCT | ||
| GAPDH | Fwd: GCCAAAAGGGTCATCATCTC | 229 |
| Rev: GGCCATCCACAGTCTTCT |
ICAM, intercellular adhesion molecule; VCAM, vascular cell adhesion molecule; TGF, transforming growth factor; VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor; MCP, monocyte chemoattractant protein; IFN, interferon; Fwd, forward; Rev, reverse.
Urine nitric oxide measurement (nitrite/nitrate).
Urinary nitric oxide (NO) was analyzed by chemiluminescence using the Sievers NO analyzer (Ionics Instruments, Boulder, CO) as previously described (26). Urine samples were centrifuged, and duplicate 20-μl aliquots were injected into the NO analyzer. Standard curves, ranging from 50 nM to 50 μM, were prepared in nitrite-free deionized water for both NaNO3 and NaNO2 (Sigma) (for the measurement of nitrate- and nitrite-derived NO, respectively). Preliminary experiments determined that, under the current experimental conditions, only negligible amounts of NO3 were generated (data not shown). Therefore, in the actual experiments, only NO2-derived NO was analyzed and quantified against an NaNO2-constructed standard curve.
Statistical analysis.
All data are presented as means ± SD. Differences in various parameters between groups were evaluated by single-factor ANOVA. Differences in parameters at each time point after remnant kidney surgery were compared by paired t-test. Correlation between different factors was evaluated by Pearson bivariate correlation. Significance was defined as P < 0.05.
RESULTS
Elevation of serum angiostatin in rats injected with rAAV1-K1K3.
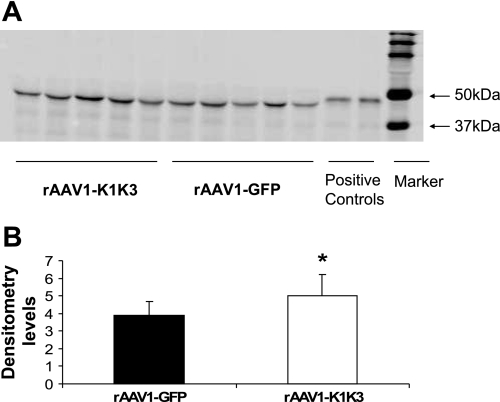
To confirm increased levels of angiostatin, serum samples were collected 4 wk after AAV injection, and immunoprecipitation was performed. Angiostatin levels were 1.3-fold higher in the rats that received rAAV1-K1K3 compared with those receiving rAAV1-GFP (P < 0.05, Fig. 2).
Fig. 2.
Immunoprecipitation analysis for serum angiostatin. A: positive signals were detected in the serum of all groups 4 wk after AAV injection at ∼50 kDa. B: densitometry results demonstrated that serum angiostatin levels were 1.3-fold higher in the rats treated with rAAV1-K1K3 compared with animals administered rAAV1-GFP. *P < 0.05 between groups.
Angiostatin attenuates proteinuria in the remnant kidney.
rAAV1-K1K3-treated rats had less urine protein excretion beginning at 4 wk after remnant kidney operation compared with rAAV1-GFP rats (Fig. 3). At the end of the experiment, rAAV1-K1K3 animals had nonsignificant alterations in serum creatinine (0.98 ± 0.3 mg/dl vs. 0.74 ± 0.1 in rAAV1-GFP and rAAV1-K1K3 animals, respectively), creatinine clearance (0.20 ± 0.01 ml·min−1·100 g body wt−1 vs. 0.23 ± 0.01 in AAV1-GFP and AAV1-K1K3 animals, respectively), and BUN levels (41.6 ± 11.4 mg/dl vs. 33.5 ± 3.8 in rAAV1-GFP and rAAV1-K1K3 animals, respectively).
Fig. 3.
Proteinuria. rAAV1-K1K3-treated animals had significantly less urine protein excretion at 4, 6, and 8 wk following remnant kidney surgery compared with animals administered rAAV1-GFP. *P < 0.05 between groups.
Angiostatin suppresses renal inflammation in the remnant kidney.
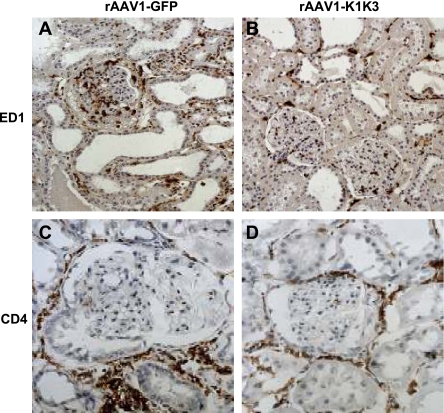
Eight weeks after remnant kidney surgery, rats administered rAAV1-K1K3 showed significant reductions in interstitial macrophages (ED-1 positive; Fig. 4, A and B; Table 2; P < 0.05) and dendritic cells (OX62 positive, Table 2, P < 0.05) compared with rAAV1-GFP rats. There were no differences in interstitial T-cell infiltration between rAAV1-K1K3 and rAAV1-GFP rats (Table 2). In glomeruli, ED-1-positive cells were significantly decreased in rAAV1-K1K3 rats compared with animals administered rAAV1-GFP (Fig. 4, A and B; Table 2; P < 0.05). In addition, there was a 40% reduction in T-cell infiltration in the glomeruli of animals administered rAAV-K1K3 (Fig. 4, C and D; Table 2; P < 0.05). Using quantitative real-time PCR, mRNA levels of a battery of cytokines and chemokines were assessed in whole kidney specimens 8 wk following remnant kidney surgery. mRNA levels of monocyte chemoattractant protein (MCP-1) were significantly reduced in rAAV1-K1K3-treated rats compared with rAAV1-GFP animals (Table 3, P < 0.05). There was no difference in the mRNA expression of IL-12 and γ-interferon (Table 3).
Fig. 4.
Assessment of inflammatory cells. Macrophages (ED-1-positive cells) were observed in the glomerulus and tubulointerstitium of animals treated with rAAV1-GFP 8 wk following remnant kidney surgery (A). The no. of macrophages was significantly decreased in rats treated with AAV1-K1K3 (B). T cells were evaluated by CD4 immunohistochemistry. Animals treated with rAAV1-GFP (C) contained positive cells in the glomerulus and tubulointerstitium. In rats treated with rAAV1-K1K3, there was a significant decrease in the no. of positive T cells in the glomerulus (D).
Table 2.
Evaluation of immunohistochemical parameters
| rAAV1-GFP | rAAV1-K1K3 | |
|---|---|---|
| ED-1 | ||
| TI | 1,005±194 | 625±73* |
| G | 32±5 | 19±3* |
| CD4 | ||
| TI | 1,720±470 | 1,526±427 |
| G | 27±6 | 15±2* |
| CD8 | ||
| TI | 621±110 | 513±68 |
| G | 9±2 | 5±2* |
| OX62 | ||
| TI | 552±93 | 341±58* |
| Collagen I | ||
| TI | 23,380±2,232 | 14,735±1,799* |
| JG-12 | ||
| TI | 16,430±1,219 | 13,857±1,367* |
Data are expressed as mean positive pixels per field ± SD; n = 6. TI, tubulointerstitial; G, glomerular; rAAV, recombinant adeno-associated virus; GFP, green fluorescence protein.
P < 0.05 vs. rAAV1-GFP rats.
Table 3.
mRNA expression in rat kidney
| rAAV1-GFP | rAAV1-K1K3 | |||
|---|---|---|---|---|
| Adhesion molecules | ||||
| ICAM-1 | 0.124±0.045 | 0.106±0.016 | ||
| VCAM-1 | 0.018±0.005 | 0.014±0.004 | ||
| E-selectin | 0.031±0.014 | 0.015±0.003 | ||
| P-selectin | 0.079±0.033 | 0.022±0.011 | ||
| Extracellular matrix molecules | ||||
| Collagen IVα5 | 0.047±0.007 | 0.032±0.003* | ||
| TGF-β | 0.022±0.01 | 0.011±0.002 | ||
| Angiogenesis molecules | ||||
| VEGF-A | 0.090±0.009 | 0.087±0.009 | ||
| VEGF-C | 0.007±0.002 | 0.006±0.001 | ||
| VEGFR1 (Flt-1) | 0.011±0.001 | 0.007±0.001* | ||
| VEGFR2 (KDR) | 0.020±0.003 | 0.017±0.002 | ||
| Angiopoietin-1 | 0.017±0.003 | 0.019±0.004 | ||
| Angiopoietin-2 | 0.008±0.002 | 0.005±0.002 | ||
| Tie-2 | 0.005±0.001 | 0.003±0.001* | ||
| Cytokines and chemokine | ||||
| MCP-1 | 0.046±0.01 | 0.027±0.002* | ||
| IL-2 | 0.022±0.006 | 0.023±0.009 | ||
| INF γ | 0.031±0.007 | 0.028±0.01 | ||
Data are expressed as mean gene expression/GAPDH ratio ± SD; n = 6.
P < 0.05 vs. rAAV1-GFP rats.
Effect of angiostatin on the endothelium in the remnant kidney.
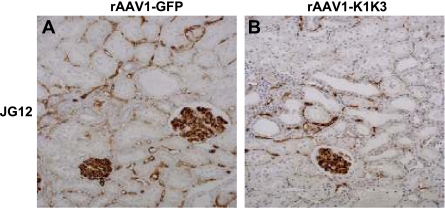
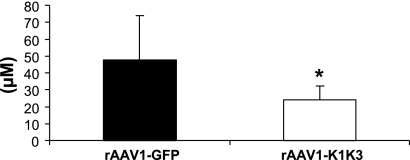
Rats treated with rAAV1-K1K3 had a significant reduction in peritubular capillaries 8 wk after surgery as assessed by JG-12 immunostaining compared with those administered rAAV1-GFP (Fig. 5, Table 2, P < 0.05). Urinary NO levels were also 50% lower in rats administered rAAV1-K1K3 compared with rAAV1-GFP animals (Fig. 6, P < 0.05). We assessed renal levels of the VEGF and angiopoietin families. There was no significant difference in the mRNA levels of VEGF-A, VEGF-C, and VEGFR2 between rAAV1-K1K3- or rAAV1-GFP-treated rats (Table 3), but levels of VEGFR1 were significantly reduced in animals overexpressing angiostatin (Table 3, P < 0.01). Levels of the Tie-2 receptor were also reduced in rats administered rAAV1-K1K3 compared with rAAV1-GFP animals (Table 3, P < 0.05), but there was no difference in either angiopoietin-1 or -2 (Table 3). Renal mRNA levels of the leukocyte adhesion molecules E-selectin, P-selectin, intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) also showed a nonsignificant reduction in rats treated with rAAV1-K1K3 compared with those administered rAAV1-GFP (Table 3).
Fig. 5.
Assessment of peritubular capillaries. In rats treated with rAAV1-GFP, peritubular capillaries were observed by JG-12 immunostaining surrounding the majority of tubules 8 wk following remnant kidney surgery (A). Treatment with rAAV1-K1K3 led to a significant reduction in peritubular capillary no. (B).
Fig. 6.
Urinary nitric oxide levels. rAAV1-K1K3-treated animals had significantly less urinary nitric oxide 8 wk following remnant kidney surgery compared with animals administered rAAV1-GFP. *P < 0.05 between groups.
In previous studies, we have found a tight inverse correlation of macrophage infiltration with peritubular capillary density (23). Because angiostatin was found to reduce both capillary density and inflammation, we expected that this relationship would no longer be present. Indeed, the relationship between macrophage infiltration and peritubular capillary density was not significant (r = −0.2915, P = 0.6).
Effect of angiostatin on renal fibrosis in the remnant kidney.
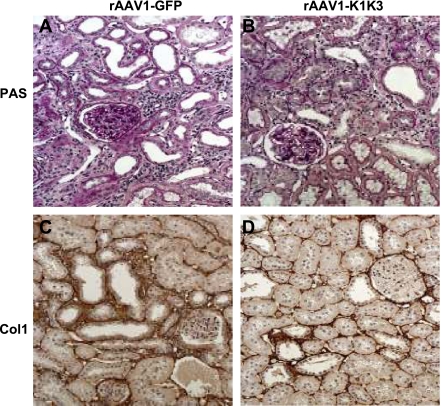
Following remnant kidney surgery, both groups developed progressive renal disease with focal glomerular scarring and interstitial fibrosis. Eight weeks after remnant kidney surgery, rats treated with angiostatin showed a significant attenuation of interstitial fibrosis (3.4 ± 0.7% in rAAV1-GFP-treated animals vs. 2.8 ± 0.3% in rAAV1-K1K3 rats; P < 0.05; Fig. 7, A and B) and glomerulosclerosis (32.9 ± 12.3% in rAAV1-GFP-treated animals vs. 20.0 ± 10.6% in rAAV1-K1K3 rats; P < 0.05; Fig. 7, A and B) and an ∼30% reduction in collagen I protein (Fig. 7, C and D; Table 2; P < 0.05) and collagen IVα5 mRNA (Table 3, P < 0.05).
Fig. 7.
Assessment of fibrosis. In rats treated with rAAV1-GFP, glomerulosclerosis and tubulointerstitial injury was observed 8 wk following remnant kidney surgery (A). Both of these parameters were significantly reduced in animals treated with AAV1-K1K3 (B). Animals treated with rAAV1-GFP (C) had considerable collagen I expression surrounding the cortical tubules. In rats treated with rAAV1-K1K3, there was a significant decrease in the amount of collagen I in the renal cortex (D).
DISCUSSION
This study provides the first evidence that angiostatin administration is associated with the attenuation of kidney disease progression in the rat remnant kidney model. Angiostatin treatment before surgery further enhanced the loss of peritubular capillaries in the model, but prevented the infiltration of inflammatory cells and led to an improvement in proteinuria. Angiostatin has been implicated in several animal models of kidney disease. Basile et al. (3) demonstrated that renal angiostatin protein levels were elevated in the first 3 days following ischemia-reperfusion injury. When the kidney recovered in the following weeks, angiostatin protein levels declined but remained higher than those observed in sham-operated controls (3). In a model of type I diabetic nephropathy induced in rats by streptozotocin, angiostatin levels were downregulated (50). Administration of angiostatin in this model by adenovirus had a therapeutic benefit by reducing albuminuria and attenuating glomerular hypertrophy (50). In addition, angiostatin reduced levels of VEGF-A and transforming growth factor-β, key players in the progression of diabetic kidney disease (34, 49).
Administration of angiostatin before remnant kidney operation led to a decrease in peritubular capillary number and a loss in mRNA expression of the angiopoietin receptor Tie-2. The loss of renal endothelium in this study was expected, as angiostatin is a potent angiogenic inhibitor (7, 12, 45). Indeed, administration of another anti-angiogenic agent, soluble VEGFR1, also leads to an inhibition of vascular density in the peritubular capillaries of adult mice (21). We also observed a loss in urinary NO levels, which may be a consequence of the renal vascular drop-out, although the effects directly on renal hemodynamics or blood pressure were not investigated in this study. The mechanism by which angiostatin can bind to and alter endothelia biology is uncertain, with many hypotheses currently postulated. In vitro studies have indicated that angiostatin inhibits endothelial cell proliferation by the downregulation of cyclin-dependent kinase-5 (cdk5) (41). Alternatively, angiostatin can induce endothelial cell apoptosis either by preventing ATP synthesis (46) or modulating the activity of FasL, p53, or Bax (11). Finally, angiostatin can bind to the cell surface receptor angiomotin, leading to the inhibition of endothelial cell migration (45). Mutant mice lacking angiomotin have general vascular defects, including dilated brain vessels and a defective intersomitic vasculature (1).
Angiostatin treatment before remnant kidney operation also led to anti-inflammatory effects with reduced interstitial macrophages and dendritic cells, lower numbers of glomerular macrophages and T cells, and a reduction in renal MCP-1. There are several potential explanations for this finding. First, angiostatin administration may cause decreased levels of cell adhesion molecules expressed on the endothelium (25). Indeed, we observed a nonsignificant decrease in renal mRNA levels of E-selectin, P-selectin, ICAM-1 and VCAM-1, molecules all previously demonstrated to be upregulated in the rat remnant kidney model (19, 44, 48). A loss of these molecules would prevent the migration of leukocytes to the damaged kidney and hence decrease levels of inflammation. The loss of cell adhesion molecules could also be due to the loss of endothelial cells or a direct effect of angiostatin on these molecules, as has been observed in vitro in the case of E-selectin (28). Alternatively, angiostatin could affect the biology of inflammatory cells themselves. In vitro, angiostatin directly inhibits integrin-β1 and -β2 expressed on leukocytes, which normally facilitate the binding of leukocytes to endothelial adhesion molecules (8). Angiostatin can also prevent the chemotaxis of both macrophages and neutrophils in vitro (5, 37). Interestingly, Falcone et al. (16) have shown that inflammatory cells themselves can generate angiostatin, which could have contributed to the effects observed in the remnant kidney model.
We investigated the potential relationship between angiostatin and other vascular growth factors. VEGF-A is a key player in the initiation of vasculogenesis and altered in many models of renal disease (33). In rats with oxygen-induced retinopathy or streptozotocin-induced diabetes, angiostatin downregulates VEGF-A alongside defects in retinal vascular permeability in rats (42). In vitro, angiostatin is also able to block VEGF-induced endothelial proliferation (10). In our studies, angiostatin administration had no effect on VEGF-A mRNA levels 8 wk after remnant kidney operation. Renal VEGF-A levels are elevated 2 wk following rat remnant kidney operation, and this is associated with an increase in peritubular capillary number (23). This pro-angiogenic response is not maintained, and several weeks later capillary number is decreased alongside a reduction in renal VEGF-A protein (23). One possibility is that angiostatin treatment suppresses VEGF-A expression in the early stages of remnant kidney injury. This would prevent the initial angiogenic response in this model and subsequently lead to a more severe loss in peritubular capillary number 8 wk after the operation. Kidney VEGFR1 mRNA expression was significantly suppressed in rats administered angiostatin, with no significant difference in renal VEGFR2 mRNA. Interestingly, VEGFR1 is not only expressed on endothelial cells but also macrophages (39); therefore, the reduction in mRNA levels of this gene could reflect the overall decrease in renal inflammatory cell number in animals administered angiostatin before remnant kidney operation. We also investigated mRNA levels of the angiopoietins, which are important in the remodeling of established blood vessels and have also been implicated in several animal models of kidney disease (47). We observed no alteration in either angiopoietin-1 or angiopoietin-2 mRNA in animals treated with angiostatin, but there was a significant decrease in the receptor Tie-2. The loss of Tie-2 but not VEGFR2 suggests that angiostatin exerts its actions through several vascular growth factor families and also may primarily act on the remodeling of capillaries. In addition, recent data have suggested that the expression of Tie-2 is not restricted to endothelia but also occurs on macrophages (14, 27), and, therefore, a loss of Tie-2 may be explained by the decreased inflammatory cell number following angiostatin treatment.
The different effects of angiostatin on inflammation and endothelial function may explain why a profound effect on renal function was not observed in this model, in contrast to changes in proteinuria. Another consideration is whether angiostatin could have direct effects on glomerular biology itself and contribute to glomerular integrity, as described for other angiogenic molecules such as VEGF (15) and angiopoietin-2 (13). Future studies could examine glomerular morphology and proteinuria in normal and diseased kidneys following angiostatin treatment using detailed electron microscopy analysis.
The finding that angiostatin reduced proteinuria in the remnant kidney model may have important clinical implications regarding cancer therapy. Recent studies have shown that cancer patients receiving the anti-angiogenic drug bevacizumab can develop hypertension and proteinuria as a side effect (15, 51). There are few data on the renal effects of angiostatin therapy in cancer patients, with one published study (4) showing that 2 of 24 patients receiving recombinant angiostatin twice daily for a median treatment period of 98 days developed hypertension, although proteinuria was not measured in these patients. Our study suggests that systemic administration of angiostatin may be beneficial in preventing proteinuria. However, these data should be interpreted with caution, as the systemic increase in our study of angiostatin 5 wk after AAV injection was only 1.3-fold (measured by immunoprecipitation), whereas patients receiving angiostatin for several months have at least a 10-fold increase in serum levels (4). The increase in angiostatin in our study is modest and may be due to the immunoprecipitation assay being limited in sensitivity and not accounting for changes in serum composition or water content between animals; therefore, higher levels might have been discerned by other methods such as ELISA. Therefore, it is possible that this mild elevation in angiostatin may not account for the differences observed in measured parameters compared with rAAV1-GFP animals, and other factors may be involved. However, overexpression of angiostatin did lead to biological effects, as noted by decreased peritubular capillary number and less inflammation in the renal lesions, consistent with known biological actions of angiostatin (7, 8, 10, 37), and this strongly suggests that the experiment succeeded in determining the effect of increased levels of angiostatin on the disease process.
GRANTS
Support for this research is provided by National Institutes of Health Grants DK-52121 and HL-68607 and Gatorade funds (R. J. Johnson). D. A. Long is supported by a Kidney Research UK Senior Non-Clinical Fellowship.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Aase K, Ernkvist M, Ebarasi L, Jakobsson L, Majumdar A, Yi C, Birot O, Ming Y, Kvanta A, Edholm D, Aspenström P, Kissil J, Claesson-Welsh L, Shimono A, Holmgren L. Angiomotin regulates endothelial cell migration during embryonic angiogenesis. Genes Dev 21: 2055–2068, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arras M, Ito WD, Scholz D, Winkler B, Schaper J, Schaper W. Monocyte activation in angiogenesis and collateral growth in the rabbit hindlimb. J Clin Invest 101: 40–50, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basile DP, Fredrich K, Weihrauch D, Hattan N, Chilian WM. Angiostatin and matrix metalloprotease expression following ischemic acute renal failure. Am J Physiol Renal Physiol 286: F893–F902, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Beerepoot LV, Witteveen EO, Groenewegen G, Fogler WE, Sim BK, Sidor C, Zonnenberg BA, Schramel F, Gebbink MF, Voest EE. Recombinant human angiostatin by twice-daily subcutaneous injection in advanced cancer: a pharmacokinetic and long-term safety study. Clin Cancer Res 15: 4025–4033, 2003. [PubMed] [Google Scholar]
- 5.Benelli R, Morini M, Carrozzino F, Ferrari N, Minghelli S, Santi L, Cassatella M, Noonan DM, Albini A. Neutrophils as a key cellular target for angiostatin: implications for regulation of angiogenesis and inflammation. FASEB J 16: 267–269, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Buch PK, Bainbridge JW, Ali RR. AAV-mediated gene therapy for retinal disorders: from mouse to man. Gene Ther 15: 849–857, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Cao Y, Ji RW, Davidson D, Schaller J, Marti D, Söhndel S, McCance SG, O'Reilly MS, Llinás M, Folkman J. Kringle domains of human angiostatin. Characterization of the anti-proliferative activity on endothelial cells. J Biol Chem 271: 29461–29467, 1996. [DOI] [PubMed] [Google Scholar]
- 8.Chavakis T, Athanasopoulos A, Rhee JS, Orlova V, Schmidt-Wöll T, Bierhaus A, May AE, Celik I, Nawroth PP, Preissner KT. Angiostatin is a novel anti-inflammatory factor by inhibiting leukocyte recruitment. Blood 105: 1036–1043, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Chen S, Kapturczak MH, Wasserfall C, Glushakova OY, Campbell-Thompson M, Deshane JS, Joseph R, Cruz PE, Hauswirth WW, Madsen KM, Croker BP, Berns KI, Atkinson MA, Flotte TR, Tisher CC, Agarwal A. Interleukin 10 attenuates neointimal proliferation and inflammation in aortic allografts by a heme oxygenase-dependent pathway. Proc Natl Acad Sci USA 102: 7251–7256, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen YH, Wu HL, Chen CK, Huang YH, Yang BC, Wu LW. Angiostatin antagonizes the action of VEGF-A in human endothelial cells via two distinct pathways. Biochem Biophys Res Commun 310: 804–810, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Chen YH, Wu HL, Li C, Huang YH, Chiang CW, Wu MP, Wu LW. Anti-angiogenesis mediated by angiostatin K1-3, K1-4 and K1-4.5. Involvement of p53, FasL, AKT and mRNA deregulation. Thromb Haemost 95: 668–677, 2006. [PubMed] [Google Scholar]
- 12.Claesson-Welsh L, Welsh M, Ito N, Anand-Apte B, Soker S, Zetter B, O'Reilly M, Folkman J. Angiostatin induces endothelial cell apoptosis and activation of focal adhesion kinase independently of the integrin-binding motif RGD. Proc Natl Acad Sci USA 95: 5579–5583, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis B, Dei Cas A, Long DA, White KE, Hayward A, Ku CH, Woolf AS, Bilous R, Viberti G, Gnudi L. Podocyte-specific expression of angiopoietin-2 causes proteinuria and apoptosis of glomerular endothelia. J Am Soc Nephrol 18: 2320–2329, 2007. [DOI] [PubMed] [Google Scholar]
- 14.De Palma M, Venneri MA, Galli R, Sergi Sergi L, Politi LS, Sampaolesi M, Naldini L. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell 8: 211–226, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, Weisstuch J, Richardson C, Kopp JE, Kabir MG, Backx PH, Gerber HP, Ferrara N, Barisoni L, Alpers CE, Quaggin SE. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med 13: 1129–1136, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Falcone DJ, Khan KM, Layne T, Fernandes L. Macrophage formation of angiostatin during inflammation. A byproduct of the activation of plasminogen. J Biol Chem 273: 31480–31485, 1998. [DOI] [PubMed] [Google Scholar]
- 17.Fogo AB Mechanisms of progression of chronic kidney disease. Pediatr Nephrol 22: 2011–2022, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giraudo E, Primo L, Audero E, Gerber HP, Koolwijk P, Soker S, Klagsbrun M, Ferrara N, Bussolino F. Tumor necrosis factor-alpha regulates expression of vascular endothelial growth factor receptor-2 and of its co-receptor neuropilin-1 in human vascular endothelial cells. J Biol Chem 273: 22128–22135, 1998. [DOI] [PubMed] [Google Scholar]
- 19.Gong R, Rifai A, Dworkin LD. Anti-inflammatory effect of hepatocyte growth factor in chronic kidney disease: targeting the inflamed vascular endothelium. J Am Soc Nephrol 17: 2464–2473, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Hou CC, Wang W, Huang XR, Fu P, Chen TH, Sheikh-Hamad D, Lan HY. Ultrasound-microbubble-mediated gene transfer of inducible Smad7 blocks transforming growth factor-beta signaling and fibrosis in rat remnant kidney. Am J Pathol 166: 761–771, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamba T, Tam BY, Hashizume H, Haskell A, Sennino B, Mancuso MR, Norberg SM, O'Brien SM, Davis RB, Gowen LC, Anderson KD, Thurston G, Joho S, Springer ML, Kuo CJ, McDonald DM. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol 290: H560–H576, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Kang DH, Hughes J, Mazzali M, Schreiner GF, Johnson RJ. Impaired angiogenesis in the remnant kidney model: II. Vascular endothelial growth factor administration reduces renal fibrosis and stabilizes renal function. J Am Soc Nephrol 12: 1448–1457, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Kang DH, Joly AH, Oh SW, Hugo C, Kerjaschki D, Gordon KL, Mazzali M, Jefferson JA, Hughes J, Madsen KM, Schreiner GF, Johnson RJ. Impaired angiogenesis in the remnant kidney model: I. Potential role of vascular endothelial growth factor and thrombospondin-1. J Am Soc Nephrol 12: 1434–1447, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Kang DH, Kanellis J, Hugo C, Truong L, Anderson S, Kerjaschki D, Schreiner GF, Johnson RJ. Role of the microvascular endothelium in progressive renal disease. J Am Soc Nephrol 13: 806–816, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Ley K The role of selectins in inflammation and disease. Trends Mol Med 9: 263–268, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Long DA, Newaz MA, Prabhakar SS, Price KL, Truong LD, Feng L, Mu W, Oyekan AO, Johnson RJ. Loss of nitric oxide and endothelial-derived hyperpolarizing factor-mediated responses in aging. Kidney Int 68: 2154–2163, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Long DA, Price KL, Ioffe E, Gannon CM, Gnudi L, White KE, Yancopoulos GD, Rudge JS, Woolf AS. Angiopoietin-1 therapy maintains kidney peritubular capillaries but enhances fibrosis and inflammation after folic acid-induced acute renal failure. Kidney Int 74: 300–309, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Luo J, Lin J, Paranya G, Bischoff J. Angiostatin upregulates E-selectin in proliferating endothelial cells. Biochem Biophys Res Commun 245: 906–911, 1998. [DOI] [PubMed] [Google Scholar]
- 29.Matsuguma K, Ueda S, Yamagishi S, Matsumoto Y, Kaneyuki U, Shibata R, Fujimura T, Matsuoka H, Kimoto M, Kato S, Imaizumi T, Okuda S. Molecular mechanism for elevation of asymmetric dimethylarginine and its role for hypertension in chronic kidney disease. J Am Soc Nephrol 17: 2176–2183, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Mellon MJ, Ahn M, Jiménez JA, Kao C, Gardner TA. Anti-angiogenic gene therapy for metastatic renal cell carcinoma produces tumor growth suppression in an athymic nude mouse model. J Urol 179: 737–742, 2008. [DOI] [PubMed] [Google Scholar]
- 31.Mu W, Ouyang X, Agarwal A, Zhang L, Long DA, Cruz PE, Roncal CA, Glushakova OY, Chiodo VA, Atkinson MA, Hauswirth WW, Flotte TR, Rodriguez-Iturbe B, Johnson RJ. IL-10 suppresses chemokines, inflammation, and fibrosis in a model of chronic renal disease. J Am Soc Nephrol 16: 3651–3660, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Murdoch C, Giannoudis A, Lewis CE. Mechanisms regulating the recruitment of macrophages into hypoxic areas of tumors and other ischemic tissues. Blood 104: 2224–2234, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Nakagawa T Uncoupling of the VEGF-endothelial nitric oxide axis in diabetic nephropathy: an explanation for the paradoxical effects of VEGF in renal disease. Am J Physiol Renal Physiol 292: F1665–F1672, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Nakagawa T, Sato W, Sautin YY, Glushakova O, Croker B, Atkinson MA, Tisher CC, Johnson RJ. Uncoupling of vascular endothelial growth factor with nitric oxide as a mechanism for diabetic vasculopathy. J Am Soc Nephrol 17: 736–745, 2006. [DOI] [PubMed] [Google Scholar]
- 35.O'Reilly MS, Holmgren L, Chen C, Folkman J. Angiostatin induces and sustains dormancy of human primary tumors in mice. Nat Med 2: 689–692, 1996. [DOI] [PubMed] [Google Scholar]
- 36.O'Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Moses M, Lane WS, Cao Y, Sage EH, Folkman J. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell 79: 315–328, 1994. [DOI] [PubMed] [Google Scholar]
- 37.Perri SR, Annabi B, Galipeau J. Angiostatin inhibits monocyte/macrophage migration via disruption of actin cytoskeleton. FASEB J 21: 3928–3936, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Raisler BJ, Berns KI, Grant MB, Beliaev D, Hauswirth WW. Adeno-associated virus type-2 expression of pigmented epithelium-derived factor or Kringles 1–3 of angiostatin reduce retinal neovascularization. Proc Natl Acad Sci USA 99: 8909–8914, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sawano A, Iwai S, Sakurai Y, Ito M, Shitara K, Nakahata T, Shibuya M. Flt-1, vascular endothelial growth factor receptor 1, is a novel cell surface marker for the lineage of monocyte-macrophages in humans. Blood 97: 785–791, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Serón D, Alexopoulos E, Raftery MJ, Hartley B, Cameron JS. Number of interstitial capillary cross-sections assessed by monoclonal antibodies: relation to interstitial damage. Nephrol Dial Transplant 5: 889–893, 1990. [DOI] [PubMed] [Google Scholar]
- 41.Sharma MR, Tuszynski GP, Sharma MC. Angiostatin-induced inhibition of endothelial cell proliferation/apoptosis is associated with the down-regulation of cell cycle regulatory protein cdk5. J Cell Biochem 91: 398–409, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Sima J, Zhang SX, Shao C, Fant J, Ma JX. The effect of angiostatin on vascular leakage and VEGF expression in rat retina. FEBS Lett 564: 19–23, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Stenvinkel P, Ketteler M, Johnson RJ, Lindholm B, Pecoits-Filho R, Riella M, Heimbürger O, Cederholm T, Girndt M. IL-10, IL-6, and TNF-alpha: central factors in the altered cytokine network of uremia–the good, the bad, and the ugly. Kidney Int 67: 1216–1233, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Taal MW, Zandi-Nejad K, Weening B, Shahsafaei A, Kato S, Lee KW, Ziai F, Jiang T, Brenner BM, MacKenzie HS. Proinflammatory gene expression and macrophage recruitment in the rat remnant kidney. Kidney Int 58: 1664–1676, 2000. [DOI] [PubMed] [Google Scholar]
- 45.Troyanovsky B, Levchenko T, Månsson G, Matvijenko O, Holmgren L. Angiomotin: an angiostatin binding protein that regulates endothelial cell migration and tube formation. J Cell Biol 152: 1247–1254, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Veitonmäki N, Cao R, Wu LH, Moser TL, Li B, Pizzo SV, Zhivotovsky B, Cao Y. Endothelial cell surface ATP synthase-triggered caspase-apoptotic pathway is essential for k1-5-induced antiangiogenesis. Cancer Res 64: 3679–3686, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Woolf AS, Gnudi L, Long DA. Roles of the angiopoietins in kidney development and disease. J Am Soc Nephrol. 2008. Sep 17. (Epub ahead of print) PMID: 18799719. [DOI] [PubMed]
- 48.Wu K, Zhou T, Sun G, Wang W, Zhang Y, Zhang Y, Hao L, Chen N. Valsartan inhibited the accumulation of dendritic cells in rat fibrotic renal tissue. Cell Mol Immunol 3: 213–220, 2006. [PubMed] [Google Scholar]
- 49.Young BA, Johnson RJ, Alpers CE, Eng E, Gordon K, Floege J, Couser WG, Seidel K. Cellular events in the evolution of experimental diabetic nephropathy. Kidney Int 47: 935–944, 1995. [DOI] [PubMed] [Google Scholar]
- 50.Zhang SX, Wang JJ, Lu K, Mott R, Longeras R, Ma JX. Therapeutic potential of angiostatin in diabetic nephropathy. J Am Soc Nephrol 17: 475–486, 2006. [DOI] [PubMed] [Google Scholar]
- 51.Zhu X, Wu S, Dahut WL, Parikh CR. Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: systematic review and meta-analysis. Am J Kidney Dis 49: 186–193, 2007. [DOI] [PubMed] [Google Scholar]
- 52.Zolotukhin S, Potter M, Zolotukhin I, Sakai Y, Loiler S, Fraites TJ Jr, Chiodo VA, Phillipsberg T, Muzyczka N, Hauswirth WW, Flotte TR, Byrne BJ, Snyder RO. Production and purification of serotype 1, 2, and 5 recombinant adeno-associated viral vectors. Methods 28: 158–167, 2002. [DOI] [PubMed] [Google Scholar]