Abstract
Peroxynitrite (ONOO−) is formed endogenously by the reaction of nitric oxide (NO) and superoxide (O2−). To examine the hypothesis that OONO− cause renal vasodilation at low concentrations but cause vasoconstriction at higher concentrations, we examined renal responses to intra-arterial infusion of incremental doses of OONO− (10, 20, and 40 μg·kg−1·min−1; 45 min each) in anesthetized rats. Renal blood flow (RBF) and glomerular filtration rate (GFR) were determined by PAH and inulin clearance. In control rats (n = 6), low dose (10 μg·kg−1·min−1) of OONO− increased RBF by 10 ± 3% and GFR by 15 ± 5%. The higher doses (20 and 40 μg·kg−1·min−1) mostly reversed these responses which were −7 ± 4 and −27 ± 7% (P < 0.05) in RBF and −0.1 ± 4.8 and −14 ± 12% in GFR, respectively. There were no appreciable changes in urine flow (V) and sodium excretion (UNaV) during OONO− infusion. However, in rats pretreated with NO synthase (NOS) inhibitor, l-NAME (50 μg·kg−1·min−1; n = 5), these doses of ONOO− significantly reduced RBF (−26 ± 7, −27 ± 6, and −44 ± 3%) and GFR (−21 ± 6, −25 ± 8, and −32 ± 12%) with variable increases in V or UNaV. Long-term infusion of OONO− (10 μg·kg−1·min−1 for 75 min) in another set of control rats (n = 5) also showed similar vasodilator and hyperfiltration responses. These data indicate that ONOO− acts as an oxidant at high concentration but provides renoprotective function at low concentration that depends on intact NOS activity.
Keywords: nitric oxide, superoxide, oxidative stress, renal blood flow, glomerular filtration rate
nitric oxide (NO) reacts with superoxide (O2−) nonenzymatically to generate peroxynitrite (ONOO−), a powerful oxidant molecule. Although the discovery of ONOO− was reported many years ago (23), its biologic oxidant activity has been recognized only in recent years (3). ONOO− can induce cytotoxic effects by several mechanisms, including sulfhydryl oxidation, protein tyrosine nitration, membrane lipid peroxidation, as well as DNA damage leading to cellular injury and death (6, 32, 33). Indeed, the overproduction of ONOO− may occur in a variety of pathologic conditions causing major cytotoxic effects either by direct or indirect oxidant mechanisms (1, 4, 36). In contrast, it has also been proposed that, under physiological conditions, the production of ONOO− will be low and oxidative damage minimized by endogenous antioxidant defense mechanisms (34).
Prior animal experiments addressing mechanisms of oxidative stress associated with renal dysfunction and hypertension have strongly suggested a renoprotective role for the NO-O2− interaction (13, 17, 20–22, 27, 28), but the exact mechanism associated with this protection has not yet been determined. It is hypothesized that ONOO− is a major factor involved in this protection, although its cytotoxic effects would argue against a renoprotective role. However, cytotoxic effects usually occur at high concentrations (micromolar to millimolar) as shown in in vitro studies (31). At low concentrations (nanomolar to low micromolar), ONOO− induces vascular relaxation, which may be physiologically more relevant, as previously demonstrated by in vivo studies (19, 30). It is possible that the vasodilatory effects of ONOO− at low concentrations are mediated either by a reverse nonenzymatic reaction of ONOO− to NO and O2−, or by the conversion of ONOO− to a NO donor compound (31). It is postulated that nitrite, a major end product of ONOO−, acts as a NO donor by its reduction to NO (14). This reaction occurs in certain specific conditions and is mediated by a nitrite reductase pathway involving the endothelium (35).
Although the biological activity of ONOO− has been extensively investigated in recent years, few in vivo studies have been conducted to examine its direct effects on hemodynamics and organ function. In particular, the role of direct ONOO− administration on renal excretory function has not been reported. Thus, the present study was designed to evaluate renal hemodynamic and excretory responses to acute ONOO− infusion at different doses directly into the renal artery, in the presence or absence of the NO synthase (NOS) enzyme inhibitor nitro-l-arginine methyl ester (l-NAME) in rats.
METHODS
Experiments were performed on male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) weighing 300 to 350 g. The protocol was approved by the Tulane University Animal Care and Use Committee. The rats were given food and tap water ad libitum and a minimum of 1 wk was allowed for them to adjust to the animal care facility before conducting acute renal clearance experiments to determine renal responses to infusions of incremental doses of ONOO−. Solutions of ONOO− in different concentrations were prepared from a stock solution of sodium peroxynitrite (purchased from Cayman Chemicals, Ann Arbor, MI) which was supplied as a solution in sodium hydroxide. This solution remained stable for ∼1 mo when stored at −80°C. The concentration of ONOO− was determined by diluting an aliquot of the stock solution 40-fold with cold 0.3 M NaOH and measuring the absorbance of ONOO− at 302 nm with 0.3 M NaOH as a blank in a SmartSpec 3000 spectrophotometer (Bio-Rad Laboratories, Hercules, CA). Immediately before use, stock solution concentration was calculated using the extinction coefficient for ONOO− of 1,670 M/cm. Before intra-arterial infusion, an aliquot of the ONOO− stock solution (generally in the range of 25 to 35 nM) was diluted with cold NaOH solution to maintain an alkaline pH and infused with a cold glass syringe. The pH of the final infusion solution was 8.0. To prevent degradation of ONOO− in solution before infusion into the renal artery, syringe and infusion catheters were kept in ice-jacket during the period of infusion. At the end of each experiment, the activity of residual ONOO− was tested spectrophotometrically.
Experiments
Protocol 1.
Rats were randomly divided into two groups, based on pretreatment with or without NOS inhibitor l-NAME (Sigma, St. Louis, MO). Rats in group 1 were not given l-NAME (control rats; n = 6) and rats in group 2 were pretreated with l-NAME (l-NAME-treated rats; n = 5) before infusions of ONOO− doses. On the day of experiment, rats were anesthetized with thiobutabarbital sodium (100 mg/kg ip Inactin; Sigma) and placed on a heating pad to maintain rectal temperature at 37°C throughout the study. After tracheal intubation, the right jugular vein was catheterized for intravenous administration of saline solutions containing albumin (bovine serum, Calbiochem, La Jolla, CA), para-aminohippuric acid (PAH; MP Biomedicals, Aurora, OH), and inulin (Inutest; Laevosan, Linz/Donau, Austria). The right femoral artery was cannulated for continuous monitoring of arterial pressure, using a Biopac MP100 data-acquisition system, and for arterial blood samplings. Thereafter, the left kidney was exposed via a flank incision and placed in a Lucite cup, the renal artery was isolated, and the left ureter was cannulated for urine collection. A tapered PE-10 catheter was then inserted into the renal artery via the left femoral artery to allow intra-arterial administration of drugs (ONOO− doses) directly into the kidney (20, 22). This catheter was kept patent by a continuous infusion of heparinized isotonic saline at a rate of 5 μl/min.
After a 60-min period for stabilization following completion of surgical procedures, the experimental protocol was started with a 30-min control clearance period to assess baseline renal hemodynamic and excretory parameters. This initial period was considered the baseline period in group 1. In group 2, following 30-min initial control clearance period, l-NAME was infused intra-arterially for 60 min and then stopped. The baseline period for ONOO− infusion in this group was considered the last 30-min period of l-NAME infusion. In both groups, ONOO− was then infused in three incremental doses of 10, 20, and 40 μg·kg−1·min−1 administered intra-arterially. At the beginning of each rate of infusion, a minimum of 15 min was allowed for stabilization followed by a 30-min clearance period for sample collections. In each experiment, a new ONOO− solution was prepared immediately before each period of infusion.
These doses of ONOO− were selected based on results from pilot studies using a wide range of doses. These selected doses were within the range that showed a stable systemic blood pressure and physical state of the animals during these intrarenal administrations. It is of note that the highest dose of ONOO− (100 μg·kg−1·min−1) tested in these pilot experiments caused severe hematologic disturbances such as hemolysis, renal artery thrombosis, and hematuria. Additionally, to verify the activity of the ONOO− solution, further experiments were carried out with ONOO− solutions that had been allowed to degrade for 1 to 3 wk at room temperature. The doses administered for degraded ONOO− were exactly the same as the doses of active stable ONOO− at 10, 20, and 40 μg·kg−1·min−1. These degraded solutions were also prepared from the stock solution of degraded compound, which was the same concentration as of the stable compound. The solutions of degraded ONOO− also had a similar pH (8.0) as stable compounds. These experiments were used as the time control experiments with proper vehicle treatment. In these experiments, the degraded ONOO− had no significant renal or systemic effects. Since infusion of the degraded compound did not cause any significant change in renal parameters, the data indicate that the use of small volume of alkaline solutions (infusion rate 0.5 μl/min) was effectively buffered in the plasma during intra-arterial administration. Furthermore, a few time-controlled experiments were performed in l-NAME-pretreated animals (n = 3) without administration of the ONOO− solution. In these experiments, l-NAME was infused for 60 min as previously described and then saline, instead of ONOO−, was infused for more than a 90-min period. These time control experiments were conducted to evaluate the responses to ONOO− infusion observed in l-NAME-treated rats.
Protocol 2.
To explore a possible or delayed effect on the kidney, a low dose of ONOO− (10 μg·kg−1·min−1) was administered in control rats (n = 5) for a period of 75 min longer than that used in protocol 1. These control rats were subjected to the same surgical procedures as described earlier. Additionally, an ultrasonic flow probe (Transonic System) was placed around the left renal artery to continuously monitor renal blood flow (RBF) and further confirm the RBF responses to ONOO− infusion observed using the PAH clearance technique. In these experiments, the protocol was started with two 30-min control clearance periods to assess baseline hemodynamic and excretory parameters. ONOO− was then infused intra-arterially for a 75-min period. After the initiation of ONOO− infusion, a 15-min period was allowed for stabilization followed by two 30-min periods for clearance collections. As in the other experiment protocol, a new ONOO− solution was prepared immediately before each period of infusion.
Analytical Procedures
The collected blood and urine samples were analyzed for inulin, PAH, and sodium/potassium concentrations. Inulin and PAH concentrations were determined by spectrophotometry and sodium/potassium concentrations were determined by flame photometry. The value for inulin clearance was considered as glomerular filtration rate (GFR) and the value for PAH clearance was considered as renal plasma flow (RPF). The formulas for measurements of GFR and RPF are as follows: GFR (inulin clearance) = (urinary conc. of inulin × urine volume)/plasma inulin conc.; RPF (PAH clearance) = [(urinary conc. of PAH × urine volume)/plasma PAH conc.]. RBF was calculated from RPF and hematocrit (Hct) value [RBF = RPF/(1 − Hct)]. Renal vascular resistance (RVR) was calculated by dividing the value of mean arterial pressure (MAP) with the value of RBF. Urinary concentration of 8-isoprostane was measured by enzyme immunoassay (Cayman Chemical) and nitrate/nitrite were determined using a colorimetric assay kit (Cayman Chemical). All values were normalized per gram of kidney weight.
Statistical Analysis
Data are expressed as means ± SE. In experiments in protocol 1, comparisons between baseline values and the values obtained during infusion of ONOO− doses in both groups were made using one-way repeated-measures ANOVA followed by a Bonferroni test for post hoc comparisons. Comparison of the percent responses to ONOO− infusion among the experimental groups (control and l-NAME-treated) was made using unpaired Student's t-test. In experiments in protocol 2, responses to single-dose ONOO− infusion were compared with baseline values using paired Student's t-test. P < 0.05 was considered to be statistically significant.
RESULTS
Protocol 1 Experiments
Tables 1 and 2 show the absolute responses to ONOO− infusion in control (group 1) and in l-NAME-treated (group 2) rats. As the baseline values before ONOO− infusions are different between group 1 and group 2, the responses to ONOO− are normalized and expressed as percent change from the baseline value which is taken as 100%. Figures 1, 2, 3, and 4 illustrate the percent responses to ONOO− in both groups of animals. l-NAME administration in group 2 decreased RBF and increased MAP and RVR without significant changes in GFR, urine flow (V), sodium excretion (UNaV), or fractional excretion of sodium (FENa; Table 2). l-NAME infusion decreased urinary excretion rate of nitrite/nitrate (UNOxV) and increased 8-isoprostane excretion rate (UISOV) as reported earlier (21, 22, 27, 28).
Table 1.
Responses to incremental doses of ONOO− in intact rats (n = 6) untreated with l-NAME (group 1, protocol 1)
| Baseline |
ONOO−, μg·kg−1·min−1 |
|||
|---|---|---|---|---|
| 10 | 20 | 40 | ||
| MAP, mmHg | 128±3 | 131±2 | 130±3 | 132±3 |
| RBF, ml·min−1·g−1 | 6.6±0.6 | 7.3±0.7 | 6.1±0.5 | 4.4±0.8* |
| RVR, mmHg·ml−1·min·g | 20.3±2.1 | 18.9±2.2 | 22.1±2.0 | 35.1±6.3* |
| GFR, ml·min−1·g−1 | 1.02±0.12 | 1.17±0.14 | 1.00±0.12 | 0.84±0.14 |
| V, μl·min−1·g−1 | 10.2±3.5 | 10.9±3.3 | 8.6±2.0 | 10.7±3.4 |
| UNaV, μmol·min−1·g−1 | 0.9±0.3 | 0.8±0.3 | 0.7±0.2 | 1.0±0.3 |
| UNOxV, nmol·min−1·g−1 (n = 3) | 1.2±0.6 | 1.8±0.7 | 2.0±0.7 | 2.7±1.3 |
| UISOV, pg·min−1·g−1 (n = 3) | 5.0±1.5 | 4.1±1.4 | 5.5±1.6 | 5.9±1.7 |
Values are means ± SE; statistical analyses were conducted using one-way repeated-measures ANOVA followed by a Bonferroni test for post hoc comparisons. ONOO−, peroxynitrite; l-NAME, NG-nitro-l-arginine methyl ester; MAP, mean arterial pressure; RBF, renal blood flow; RVR, renal vascular resistance; GFR, glomerular filtration rate; V, urine flow; UNaV, urinary sodium excretion rate; UNOxV, urinary nitrite/nitrate excretion rate; UISOV, urinary 8-isoprostane excretion rate.
P < 0.05 vs. baseline.
Table 2.
Responses to incremental doses of ONOO− in l-NAME-treated rats (n = 5; group 2, protocol 2)
| Control | Baseline, 50 μg·kg−1·min−1l-NAME |
ONOO−, μg·kg−1·min−1 |
|||
|---|---|---|---|---|---|
| 10 | 20 | 40 | |||
| MAP, mmHg | 123±2 | 130±2* | 131±2 | 131±2 | 130±3 |
| RBF, ml·min−1·g−1 | 7.3±0.7 | 5.5±0.3* | 4.2±0.4† | 4.2±0.4† | 3.4±0.5† |
| RVR, mmHg·ml−1·min·g | 17.4±1.7 | 23.7±1.5* | 32.4±3.8† | 32.3±4.1† | 40.2±5.9† |
| GFR, ml·min−1·g−1 | 1.35±0.10 | 1.24±0.06 | 1.00±0.11† | 0.94±0.12† | 0.85±0.17† |
| V, μl·min−1·g−1 | 7.4±1.0 | 8.0±2.0 | 14.6±6.2 | 12.8±4.0 | 12.4±3.5 |
| UNaV, μmol·min−1·g−1 | 0.7±0.2 | 0.7±0.2 | 1.3±0.4 | 1.5±0.3 | 1.6±0.3† |
| UNOxV, nmol·min−1·g−1 | 1.7±0.2 | 1.2±0.3* | 1.7±0.6 | 2.5±1.0 | 2.6±1.0 |
| UISOV, pg·min−1·g−1 | 3.9±1.6 | 5.4±1.9* | 4.9±1.7 | 5.0±1.5 | 4.4±1.1 |
Values are means ± SE; statistical analyses were conducted using one-way repeated-measures ANOVA followed by a Bonferroni test for post hoc comparisons.
P < 0.05 vs. control.
P < 0.05 vs. baseline values before ONOO− infusion (l-NAME period).
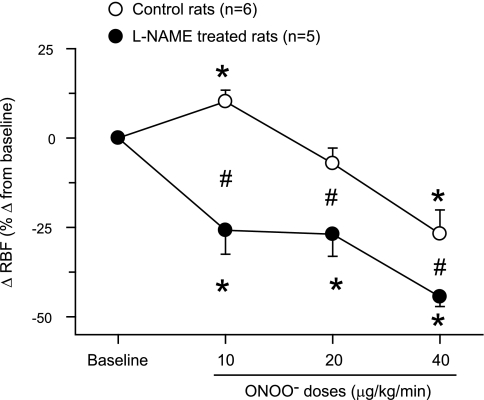
Fig. 1.
Changes in renal blood flow (RBF) in response to intra-arterial infusion of peroxynitrite (ONOO−) doses in rats. These responses are expressed as percent changes from the baseline value taken as 100%. *P < 0.05 vs. baseline values. #P < 0.05 vs. corresponding values in control rats.
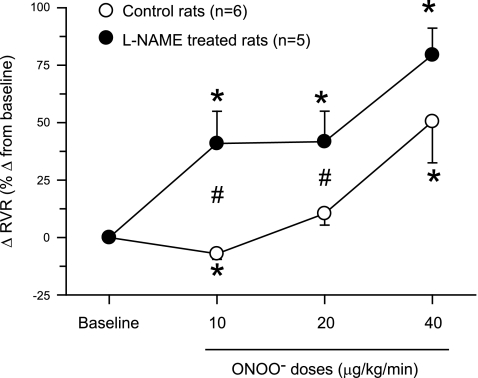
Fig. 2.
Changes in renal vascular resistance (RVR) in response to intra-arterial infusion of ONOO− doses in rats. These responses are expressed as percent changes from the baseline value taken as 100%. *P < 0.05 vs. baseline values. #P < 0.05 vs. corresponding values in control rats.
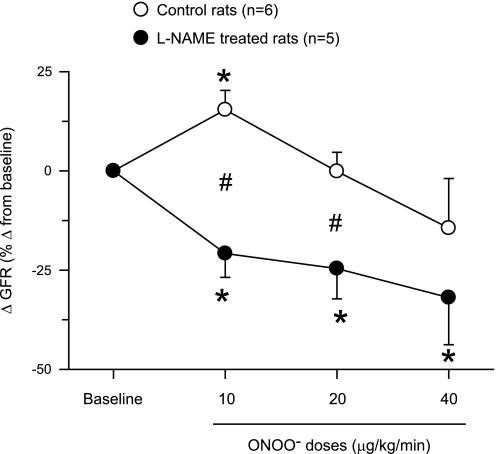
Fig. 3.
Changes in glomerular filtration rate (GFR) in response to intra-arterial infusion of ONOO− doses in rats. These responses are expressed as percent changes from the baseline value taken as 100%. *P < 0.05 vs. baseline values. #P < 0.05 vs. corresponding values in control rats.
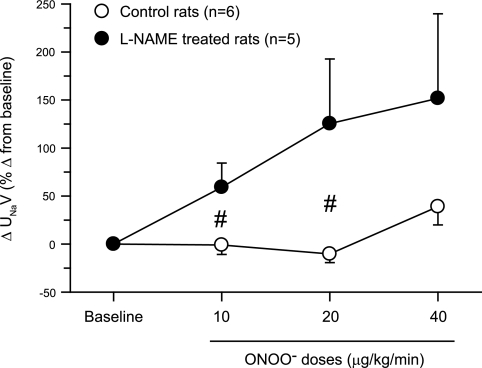
Fig. 4.
Changes in urinary sodium excretion (UNaV) in response to intra-arterial infusion of ONOO− doses in rats. These responses are expressed as percent changes from the baseline value taken as 100%. *P < 0.05 vs. baseline values. #P < 0.05 vs. corresponding values in control rats.
ONOO− infusion directly in the renal artery did not cause a change in MAP in both groups of rats. However, low-dose infusion of ONOO− (10 μg·kg−1·min−1) in control rats caused significant increases of 10 ± 3% in RBF and of 15 ± 5% in GFR with a decrease of 7 ± 2% in RVR as illustrated in Figs. 1–3. There were no significant changes in RBF (−7 ± 4%), GFR (−0.1 ± 4.8%), or RVR (10 ± 5%) at the mid-dose infusion of ONOO− (20 μg·kg−1·min−1). With high-dose (40 μg·kg−1·min−1) infusion, ONOO− caused a significant reduction in RBF (−27 ± 7%) and an increase in RVR (50 ± 18%) without a significant change in GFR (−14 ± 12%; Figs. 1–3). There were no significant changes in V (18 ± 12, 8 ± 17, and 13 ± 10%), UNaV (−1 ± 10, −10 ± 9, and 39 ± 19%), or in FENa (−11 ± 12, −9 ± 14, and 79 ± 41%) during infusion of increasing doses of ONOO−. In the experiments in group 1, UNOxV and UISOV were measured in three rats as urine samples in the initial three rats were not collected for these purposes. UNOxV showed increasing trend with increasing doses of ONOO− (67 ± 19, 100 ± 62, and 189 ± 136%). In contrast, UISOV was decreased (−20 ± 10%) with the lowest dose of ONOO− but had an increasing trend (13 ± 12 and 22 ± 10%) with the higher doses.
In l-NAME-treated rats (group 2), ONOO− infusion elicited significant decreases in RBF (−26 ± 7, −27 ± 6, and −44 ± 3%) and GFR (−21 ± 6, −25 ± 8, and −32 ± 12%) and increases in RVR (41 ± 14, 42 ± 13, and 79 ± 12%) with increasing doses of ONOO− infused (Figs. 1–3). However, ONOO− infusion in l-NAME-treated rats produced variable increases in V (60 ± 24, 58 ± 27, and 57 ± 27%), UNaV (59 ± 25, 125 ± 67, and 152 ± 88%), and FENa (110 ± 47, 230 ± 113, and 409 ± 283%). Figure 4 illustrates the percent changes in UNaV in these rats. Incremental doses of ONOO− infusion also produced variable increases in UNOxV (39 ± 21, 92 ± 64, and 108 ± 68%). Although UISOV increased significantly due to l-NAME treatment, doses of ONOO− infusion did not cause further significant changes in UISOV (Table 2). Compared with these results with infusion of ONOO− doses, infusion of the vehicle in l-NAME-treated rats was without such marked changes in these hemodynamic and excretory parameters. During these three collection periods during vehicle infusion in l-NAME-treated rats (n = 3), the changes in mean absolute values are as follows: RBF 5.6 ± 0.7 to 5.6 ± 0.9, 4.8 ± 0.5, 4.9 ± 0.5 ml·min−1·g−1; GFR 1.0 ± 0.2 to 0.94 ± 0.2, 1.0 ± 0.2, 1.1 ± 0.1 ml·min−1·g−1; RVR 28.8 ± 9.1 to 33.3 ± 12.2, 32.3 ± 7.9, 30.7 ± 6.3 mmHg·ml−1·min·g; V 5.7 ± 1.5 to 7.3 ± 3.0, 6.6 ± 2.1, 5.2 ± 0.8 μl·min−1·g−1; and UNaV 0.5 ± 0.2 to 0.6 ± 0.4, 0.6 ± 0.3, 0.5 ± 0.1 μmol·min−1·g−1. These findings as well as results from the experiments with degraded ONOO− solutions infusion do not indicate a time-dependent effect in the observed responses to low doses of ONOO− administration. In our laboratory, time control experiments with intra-arterial saline infusions in anesthetized rats have been conducted in many previous studies (20–22) and demonstrate a stable preparation with insignificant changes in arterial pressure, renal hemodynamics, or excretory parameters for at least 4 h following completion of surgical procedures. Thus, the responses to ONOO− administration reported in the present study are not due to time-dependent effects.
Protocol 2 Experiments
When the low dose of ONOO− (10 μg·kg−1·min−1) was infused for a longer period of time (75 min) in rats, similar results were observed as with protocol 1. These results are summarized in Table 3. RBF values were measured by the renal flow probe in these experiments. Prolonged administration of ONOO− at 10 μg·kg−1·min−1 significantly increased RBF by 8 ± 2% and GFR by 17 ± 5% and decreased RVR by 5 ± 1% and is similar to the changes that were observed with short-term infusion as in protocol 1. The changes in V, UNaV, FENa, and UNaV were not statistically different. However, UNOxV increased and UISOV decreased significantly with this low-dose infusion of ONOO− (Table 3).
Table 3.
Responses to prolonged administration of low-dose (10 μg·kg−1·min−1) ONOO− in rats (n = 5; protocol 2)
| Baseline | ONOO−, 10 μg·kg−1·min−1 | |
|---|---|---|
| MAP, mmHg | 122±2 | 124±2 |
| RBF, ml·min−1·g−1 | 7.1±0.7 | 7.6±0.8* |
| RVR, mmHg·ml−1·min·g | 13.5±1.5 | 12.8±1.3* |
| GFR, ml·min−1·g−1 | 1.08±0.08 | 1.25±0.06* |
| V, μl·min−1·g−1 | 9.8±1.9 | 11.7±2.0 |
| UNaV, μmol·min−1·g−1 | 0.7±0.2 | 0.9±0.2 |
| UNOxV, nmol·min−1·g−1 | 0.7±0.2 | 2.3±0.3* |
| UISOV, pg·min−1·g−1 | 7.7±0.7 | 6.8±0.6* |
Values are means ± SE; statistical analyses were conducted using Student's paired t-test.
P < 0.05 vs. baseline.
DISCUSSION
In the present study, the acute effects of increasing doses of peroxynitrite on the kidney were investigated. Our most important finding is that a low-dose (10 μg·kg−1·min−1) infusion of ONOO−, both in short term and in long term, resulted in increases in RBF and GFR, while the higher doses (20 and 40 μg·kg−1·min−1) progressively decreased these renal hemodynamic parameters (Figs. 1–3). While the responses to higher doses of ONOO− could possibly be influenced by the cumulative effect of additional doses of ONOO− in the same animal, this is unlikely because the biological half-life of ONOO− is less than 1 s (31). Thus, recovery experiment with clearance collections after ONOO− infusion was not carried out in the present study. However, we observed that when the infusion of ONOO− was stopped and replaced by saline infusion at the end of long-term experiments (protocol 2), the RBF returned back to near control levels within 2–3 min indicating a short half-life of the ONOO− during infusion. Thus, the cumulative effect of additional doses of ONOO−, if any, would be very minimal and unlikely to influence the overall response pattern in these experiments.
The vasodilator response to low-dose infusion of ONOO− is associated with an increase in UNOxV and a decrease in UISOV (Table 3). These data support our hypothesis that ONOO− at low concentration produces a vasodilator effect. Although the concentration range of normal plasma ONOO− has not been determined experimentally, it can be assumed that a low plasma level of ONOO− exists in a physiological state since NO-O2− interaction occurs normally in biological systems. As mentioned earlier, this low-dose infusion of ONOO− achieved a plasma level that could be physiologically more relevant than concentrations achieved with higher doses representing a pathophysiological condition. Interestingly, in the present experiments, it was observed that continuous ONOO− infusion at low dose produced a renal vasodilatory response as opposed to a vasoconstrictor response induced by higher doses. The vasodilatory response to ONOO− was abolished in rats pretreated with l-NAME, suggesting that the presence of NOS is required to facilitate this vasodilatory action. It is also interesting to note that infusion of ONOO− doses markedly decreased GFR in NOS-inhibited rats but not in control rats indicating that intact NOS activity provides a protective function against the actions of ONOO− in the kidney.
Peroxynitrite is formed by the spontaneous reaction of NO with O2−. This nonenzymatic reaction is a key element in understanding the contrasting roles of ONOO− in physiology and in pathology. ONOO− is a powerful oxidant and may contribute to various diseases affecting humans (31). As an oxidant radical, ONOO− can directly induce cytocytoxic effects such as sulfhydryl oxidation, protein tyrosine nitration, membrane lipid peroxidation, and DNA damage (6, 32, 33). These effects lead to dysfunction of important cellular processes causing disruption of cell signaling pathways, and the induction of cell death through both apoptosis (11) and necrosis (36). On the other hand, it has also been reported that ONOO− administration causes vascular relaxation in several organ systems including coronary (25) and systemic arteries of dogs (19), rabbit aortic strips (29), and the hindlimb vascular bed of the rat (30). Systemic bolus administration of ONOO− solution in rats (5) at doses 1–10 μmol/kg injected into the femoral vein also elicited vasorelaxant effects in the hindquarter and mesenteric vascular beds with a minimal change in renal vascular bed that could be related to its dose reaching the kidney. Furthermore, ONOO− provided protection against ischemia-reperfusion injury in vivo (24) and did not impair vasodilator responses to acetylcholine or vasoconstrictor responses to ANG II (30). Indeed, under physiologic conditions, the production of ONOO− should be low and oxidative damage minimized by endogenous antioxidant defenses (34). However, in pathologic conditions and with a moderate increase in ONOO− formation over long periods of time, substantial oxidation, dysfunction, and destruction of host cellular constituents can occur (31).
Although previous studies provided evidence that the interaction between NO and O2−, and thus, the formation of ONOO− is very important in providing a protective role in the kidney (13, 17, 20–22, 27, 28), the mechanisms associated with this protective effect are not yet understood. It is hypothesized that this renoprotective effect may be due to the conversion of ONOO− to a NO donor-like substance, which would prolong the vasorelaxant properties of NO. In this regard, several hypotheses have been proposed, including increased formation of cGMP and nitrite by ONOO−, and the reverse reaction of ONOO− has been reported to increase cGMP causing vascular relaxation by nitrosylating tissue and reducing glutathione or other thiols forming NO donors that subsequently release NO over prolonged periods of time (37). Another possible explanation is that the major end product of ONOO− oxidation, the anion nitrite, would be reduced to NO (14). The results of our present study show increased urinary excretion of nitrite following ONOO− administration in both control and l-NAME-treated rats. Circulating nitrite anion has been reported to have cytoprotective effects in pathologic conditions by acting as a direct vasodilator (10) or as a NO compound donor (12). It is reported that NOS, acting as a nitrite reductase, has the capacity to reduce nitrite to NO under anoxic condition in vitro (14). Although the activity of nitrite reductases is well-described in bacteria, some in vitro studies showed that certain mammalian enzymes may also have nitrite reductase activity in addition to their normal physiologic function (7, 9, 15, 18, 26). The present findings that the vasodilatory response to ONOO− was abolished in rats pretreated with l-NAME allow us to hypothesize that NOS may act as nitrite reductase involved in the reduction of ONOO− to NO. To demonstrate the in vivo activity of nitrite, a recent study showed that infusions of sodium nitrite into the human circulation produced significant vasodilation that appeared to be mediated by a nitrite reductase activity of deoxygenated hemoglobin (8). An alternative explanation for the vasodilatory effects of ONOO− is the reverse reaction, a nonenzymatic reaction that causes the conversion of ONOO− to NO and O2−. This reaction is well-described in a recent review by Pacher et al. (31), showing that the degradation of both NO and O2− produced during the reverse reaction yields nitrite as the most important end product. Although the reverse reaction is much slower than the forward reaction, it appears to be significant in physiologic conditions. It was reported that inhibition of NOS enhances endogenous O2− activity (28). The finding that UISOV increased after l-NAME administration in the present study also indicates an increase in endogenous O2− level. In this regard, it is possible that the absence of a vasodilator action of ONOO− in l-NAME-treated rats could be due to enhanced O2− activity that inactivates NO and prevents its vasodilator action. Further experiments both in vivo as well as in vitro will be required to test the involvement of this putative mechanism(s) in the renal vasodilator action of ONOO−.
In the present study, infusion of ONOO− doses did not produce appreciable changes in V or UNaV in control rats. However, variable increases in V and UNaV were noted in response to ONOO− doses in l-NAME-treated rats. It seems interesting if we consider the fact that RBF was markedly decreased in that group of NOS-inhibited rats. Intra-arterial administration of sodium nitrate doses also showed variable increases in UNaV in NOS-inhibited dogs (16). The exact mechanism involved in such NOS-dependant or -independent actions of ONOO− is not yet clear. However, results from an earlier in vitro study (38) indicate that at a nanomolar concentration, ANG II inhibits Na+-K+-ATPase activity in rat proximal tubules and such inhibition is mediated by ONOO− formation. These data suggest that ONOO− may be involved in the diuretic and natriuretic responses to a high-dose administration of ANG II. It is possible that an amplification of ANG II actions following l-NAME administration (2) alters the reactivity of ONOO− on the renal tubules which results in such variable increases in UNaV. Further comprehensive studies are needed to investigate the direct action of ONOO− on renal tubular reabsorptive function.
In conclusion, we showed in rats that ONOO− at low infusion rate produces renal vasodilation, but higher infusion rates cause vasoconstriction. In addition, infusion of ONOO− in rats pretreated with l-NAME does not cause vasodilatory effects but rather produces a vasoconstrictor response. These findings indicate that intact NOS activity provides a protective function against the actions of ONOO− in the kidney.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-66432 and HL-18426.
Acknowledgments
The authors thank A. Castillo for technical assistance. A portion of this work was presented at the 2007 meeting of the American Society of Nephrology held in San Francisco, CA.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Alvarez S, Radi R. Peroxynitrite reactivity with amino acids and proteins. Amino Acids 25: 295–311, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Baylis C, Harvey J, Engels K. Acute nitric oxide blockade amplifies the renal vasoconstrictor actions of angiotensin II. J Am Soc Nephrol 5: 211–214, 1994. [DOI] [PubMed] [Google Scholar]
- 3.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA 87: 1620–1624, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beckman JS, Koppenol WH. Nitric oxide, superoxide, peroxynitrite: the good, the bad, ugly. Am J Physiol Cell Physiol 271: C1424–C1437, 1996. [DOI] [PubMed] [Google Scholar]
- 5.Benkusky NA, Lewis SJ, Kooy NW. Attenuation of vascular relaxation after development of tachyphylaxis to peroxynitrite in vivo. Am J Physiol Heart Circ Physiol 275: H501–H508, 1998. [DOI] [PubMed] [Google Scholar]
- 6.Burney S, Niles JC, Dedon PC, Tannembaum SR. DNA damage in deoxynucleosides and oligonucleotides treated with peroxynitrite. Chem Res Toxicol 12: 513–520, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Castello PR, David PS, McClure T, Crook Z, Poyton RO. Mitochondrial cytochrome oxidase produces nitric oxide under hypoxic conditions: implications for oxygen sensing and hypoxic signaling in eukaryotes. Cell Metab 3: 277–287, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon III RO, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med 9: 1498–1505, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Delaforge M, Servent D, Wirsta P, Ducrocq C, Mansuy D, Lenfant M. Particular ability of cytochrome P-450 CYP3A to reduce glyceryl trinitrate in rat liver microsomes: subsequent formation of nitric oxide. Chem Biol Interact 86: 103–117, 1993. [DOI] [PubMed] [Google Scholar]
- 10.Demoncheaux EA, Higenbottam T, Foster P, Borland C, Smith A, Marriot H, Bee D, Akamine S, Davies M. Circulating nitrite anions are a directly acting vasodilator and are donors for nitric oxide. Clin Sci (Colch) 102: 77–83, 2002. [PubMed] [Google Scholar]
- 11.Dickhout JG, Hossain GS, Pozza LM, Zhou J, Lhoták S, Austin RC. Peroxynitrite causes endoplasmic reticulum stress and apoptosis in human vascular endothelium: implications in atherogenesis. Arterioscler Thromb Vasc Biol 25: 2623–2639, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Duransky MR, Greer J, Dejam A, Jaganmohan S, Hogg N, Langston W, Patel R, Yet S, Wang X, Kevil C, Gladwin M, Lefer D. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J Clin Invest 115: 1232–1240, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dutta UK, Lane J, Roberts II LJ, Majid DS. Superoxide formation and interaction with nitric oxide modulate systemic arterial pressure and renal function in salt-depleted dogs. Exp Biol Med 231: 269–276, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Gautier C, van Faassen EE, Mikula I, Martasek P, Slama-Schwok A. Endothelial nitric oxide synthase reduces nitrite anions to NO under anoxia. Biochem Biophys Res Commun 341: 816–821, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Godber BL, Doel JJ, Sapkota GP, Blake DR, Stevens CR, Eisenthal R, Harrison R. Reduction of nitrite to nitric oxide catalyzed by xanthine oxidoreductase. J Biol Chem 275: 7757–7763, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Godfrey M, Majid DS. Renal handling of circulating nitrates in anesthetized dogs. Am J Physiol Renal Physiol 275: F68–F73, 1998. [DOI] [PubMed] [Google Scholar]
- 17.Haque MZ, Majid DS. Assessment of renal functional phenotype in mice lacking gp91PHOX subunit of NAD(P)H oxidase. Hypertension 43: 335–340, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Hill KE, Hunt RW Jr, Hoover RL, Burk RF. Metabolism of nitroglycerin by smooth muscle cells. Involvement of glutathione and glutathione S-transferase. Biochem Pharmacol 43: 561–566, 1992. [DOI] [PubMed] [Google Scholar]
- 19.Katori T, Donzelli S, Tocchetti CG, Miranda KM, Cormaci G, Thomas DD, Ketener EA, Lee MJ, Mancardi D, Wink DA, Kass DA, Paolocci N. Peroxynitrite and myocardial contractility: in vivo versus in vitro effects. Free Radic Biol Med 41: 1606–1618, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Kopkan L, Castillo A, Navar LG, Majid DS. Enhanced superoxide generation modulates renal function in ANG II-induced hypertensive rats. Am J Physiol Renal Physiol 290: F80–F86, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Kopkan L, Majid DS. Superoxide contributes to development of salt sensitivity and hypertension induced by nitric oxide deficiency. Hypertension 46: 1026–1031, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Kopkan L, Majid DS. Enhanced superoxide activity modulates renal function in NO-deficient hypertensive rats. Hypertension 47: 568–572, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Koppenol WH 100 Years of peroxynitrite chemistry and 11 years of peroxynitrite biochemistry. Redox Rep 6: 339–341, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Lefer DJ, Scalia R, Campbell B, Nossuli T, Hayward R, Salomon M, Grayson J, Lefer AM. Peroxynitrite inhibits leukocyte-endothelial cell interactions and protects against ischemia-reperfusion injury in rats. J Clin Invest 99: 684–691, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu S, Beckman JS, Ku DD. Peroxynitrite, a product of superoxide and nitric oxide, produces coronary vasorelaxation in dogs. J Pharmacol Exp Ther 268: 1114–1121, 1994. [PubMed] [Google Scholar]
- 26.Luchsinger BP, Rich EN, Yan Y, Williams EM, Stamler JS, Singel DJ. Assessments of the chemistry and vasodilatory activity of nitrite with hemoglobin under physiologically relevant conditions. J Inorg Biochem 99: 912–921, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Majid DS, Nishiyama A. Nitric oxide blockade enhances renal responses to superoxide dismutase inhibition in dogs. Hypertension 39: 293–297, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Majid DS, Nishiyama A, Jackson KE, Castillo A. Inhibition of nitric oxide synthase enhances superoxide activity in canine kidney. Am J Physiol Regul Integr Comp Physiol 287: R27–R32, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Moro MA, Darley-Usmar VM, Lizasoain I, Su Y, Knowles RG, Radomski MW, Moncada S. The formation of nitric oxide donors from peroxynitrite. Br J Pharmacol 116: 1999–2004, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nossaman BD, Dabisch PA, Liles JT, Baber SR, Champion HC, Kaye AD, Feng CJ, Anwar M, Bivalacqua TJ, Santiago JA, De Witt BJ, Kadowitz PJ. Peroxynitrite does not impair pulmonary and systemic vascular responses. J Appl Physiol 96: 455–462, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87: 315–424, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Radi R, Beckman JS, Bush KM, Freeman BA. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J Biol Chem 266: 4244–4250, 1991. [PubMed] [Google Scholar]
- 33.Radi R, Beckman JS, Bush KM, Freeman BA. Peroxynitrite-induced membrane lipid peroxidation: the cytotoxic potential of superoxide and nitric oxide. Arch Biochem Biophys 288: 481–487, 1991. [DOI] [PubMed] [Google Scholar]
- 34.Radi R, Cassina A, Hodara R, Quijano C, Castro L. Peroxynitrite reactions and formation in mitochondria. Free Radic Biol Med 33: 1451–1464, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Vanin AF, Bevers LM, Slama-Schwok A, van Faassen EE. Nitric oxide synthase reduces nitrite to NO under anoxia. Cell Mol Life Sci 64: 96–103, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Virag L, Szabo E, Gergely P, Szabo C. Peroxynitrite-induced cytotoxicity: mechanisms and opportunities for intervention. Toxicol Lett 140–141: 113–124, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Wu M, Pritchard KA, Kaminski PM, Fayngersh RP, Hintze TH, Wolin MS. Involvement of nitric oxide and nitrosothiols in relaxation of pulmonary arteries to peroxynitrite. Am J Physiol Heart Circ Physiol 266: H2108–H2113, 1994. [DOI] [PubMed] [Google Scholar]
- 38.Zhang C, Imam SZ, Ali SF, Mayeux PR. Peroxynitrite and the regulation of Na+,K+-ATPase activity by angiotensin II in the rat proximal tubule. Nitric Oxide 7: 30–35, 2002. [DOI] [PubMed] [Google Scholar]