Abstract
Triglyceride-rich lipoprotein (TGRL) lipolysis products provide a pro-inflammatory stimulus that can alter endothelial barrier function. To probe the mechanism of this lipolysis-induced event, we evaluated the pro-inflammatory potential of lipid classes derived from human postprandial TGRL by lipoprotein lipase (LpL). Incubation of TGRL with LpL for 30 min increased the saturated and unsaturated FFA content of the incubation solutions significantly. Furthermore, concentrations of the hydroxylated linoleates 9-hydroxy ocatadecadienoic acid (9-HODE) and 13-HODE were elevated by LpL lipolysis, more than other measured oxylipids. The FFA fractions elicited pro-inflammatory responses inducing TNFα and intracellular adhesion molecule expression and reactive oxygen species (ROS) production in human aortic endothelial cells (HAECs). The FFA-mediated increase in ROS was blocked by both the cytochrome P450 2C9 inhibitor sulfaphenazole and NADPH oxidase inhibitors. Compared with linoleate, 13-HODE was found to be a more potent inducer of ROS production in HAECs, an activity that was insensitive to both NADPH oxidase and cytochrome P450 inhibitors. Therefore, although the oxidative metabolism of FFA in endothelial cells can produce inflammatory responses, TGRL lipolysis can also release preformed mediators of oxidative stress (e.g., HODEs) that may influence endothelial cell function in vivo by stimulating intracellular ROS production.
Keywords: endothelial dysfunction, fatty acids, oxidized lipids
Triglyceride-rich lipoproteins (TGRLs) may have important pro-atherogenic and pro-inflammatory properties. Circulating TGRLs, including chylomicrons (CMs), VLDLs, and their remnant particles, constitute a heterogeneous family of triglyceride-rich apolipoprotein B (apoB)-containing lipoproteins. Elevation of TGRL in the postprandial state is associated with increased endothelial cell inflammation and complications of atherosclerosis (1) and is an independent predictor of coronary heart disease (2–4).
Increases in plasma triglyceride concentrations are associated with impaired endothelial cell function in vitro (5) and in vivo (5, 6). For instance, increased lipolysis of TGRL in proximity to the endothelium increases endothelial layer permeability (7) and causes a pro-inflammatory state in endothelial cells, manifested by increased TNFα secretion, adhesion molecule expression, and oxidative stress induction (8). In vivo, TGRLs are hydrolyzed by lipoprotein lipase (LpL), an enzyme anchored to endothelial cells, releasing FFAs into the blood in immediate proximity to endothelial cells. Thus, endothelial cells are routinely exposed to high concentrations of FFAs, compounds that may play an important role in endothelial function and dysfunction (9–11). For instance, linoleic acid (LA) increases the permeability of human aortic endothelial cells (HAECs) in culture (12–14), whereas decreased endothelial nitric oxide synthase activity is produced by palmitate and oleate, but not linoleate (15). These results suggest that elevations in FAs may contribute to endothelial dysfunction in hypertriglyceridemia and emphasize that various FAs may affect the endothelium differently. However, these studies have primarily examined the ability of single purified FFAs to induce endothelial cell injury, which may not adequately represent the complexity of the in vivo state. Notably, the constituents of the LpL-liberated chemical pool are not routinely characterized and are likely to be influenced by health and nutritional status.
Previous studies from our group have shown that TGRL lipolysis products cause endothelial dysfunction with increased endothelial layer permeability (7, 8, 16, 17). The mechanisms underlying TGRL-dependent pro-inflammatory changes in the endothelium are still unclear. Our recent findings suggest that changes in lipid raft morphology and composition may modulate endothelial reactive oxygen species (ROS) production and contribute to TGRL lipolysis-mediated endothelial cell injury (18). We also showed that FFA concentrations in TGRL medium increased 10-fold after 30 min incubation with LpL, whereas triglyceride, cholesteryl ester, and phospholipid concentrations remained unchanged (18). Although endothelial dysfunction in hypertriglyceridemia is partially caused by lipolysis products, and FFAs are the suspected culprits, changes in cholesteryl esters as well as mono- and diacylglycerides are also possible sources of these effects. By evaluating the ability of chemical classes of TGRL lipolysis products to alter endothelial function, we can focus on the TGRL lipolysis lipid components capable of eliciting an inflammatory response. Here we report the specific chemical class responsible for the elevated postlipolysis inflammatory state and, using pharmacological tools, have shown distinct modes of inflammatory signaling between LA and its oxidation product, 13-hydroxy ocatadecadienoic acid (13-HODE).
MATERIALS AND METHODS
Lipoprotein isolation and characterization
Postprandial blood samples were obtained from healthy volunteers 3.5 h after consumption of a standard moderately high-fat meal (40% calories from fat). This time point coincided with the peak elevation in plasma triglyceride concentrations. All procedures were conducted under a protocol approved by the Human Subject Review Committee at the University of California Davis. Written informed consent was obtained from all study subjects before participating. The blood was drawn in EDTA-Na tubes, and the plasma from four volunteers was separated and pooled. TGRLs were isolated from human plasma at d < 1.0063 g/ml by aspiration with a narrow-bore pipette following 18 h centrifugation at 40,000 rpm in a SW41 Ti swinging bucket rotor (Beckman Coulter, Sunnyvale, CA) held at 14°C within a Beckman L-90K ultracentrifuge. The top fraction TGRLs (i.e., CM, VLDL, and their remnant particles) was collected and exhaustively dialyzed in Spectra/Por® membrane tubing (molecular weight cutoff 3,500; Spectrum Medical Industries, Los Angeles, CA) at 4°C overnight against a saline solution containing 0.01% EDTA. When necessary, TGRLs were subfractioned into CMs and VLDLs by spinning at 25,000 rpm in a SW41 Ti swinging bucket rotor (Beckman) held at 14°C within a Beckman L-90K L8-70M ultracentrifuge (19). The purity of TGRL was confirmed by analytical SDS-PAGE with Coomassie staining and lipid analyses. SDS electrophoresis showed the exclusive presence of apoB-48 in CM and apoB-100 in VLDL. Plasma triglyceride (TG) and cholesterol levels were determined enzymatically (Sigma-Aldrich, St. Louis, MO).
LpL incubations
To evaluate the impact of TGRL lipolysis on cultured HAECs, isolated postprandial TGRL, CM, or VLDL [2.5 mg TG for all treatments] was subjected to enzymatic lipolysis with 2 U/ml bovine LpL (Sigma-Aldrich). Diluted lipoprotein fractions were preincubated with LpL for 30 min at 37°C, and reactions were stopped by placing the tubes on wet ice. Fractions were either used immediately for incubations with cells in culture or were stored frozen at −80°C until analysis for lipids.
To validate the efficiency of LpL incubations to release esterified fatty acids (EFAs) as NEFAs, VLDLs isolated from pooled volunteer plasma (n = 4) were used. Sub-aliquots containing 20 μg triglycerides were prepared and spiked with 0.17 mg/ml butylated hydroxytoluene (BHT). The NEFA concentrations were quantified using direct extractive methylation with ethereal diazomethane (20) and pentadecenoic acid (Nucheck Prep, Elysian, MN) as an analytical surrogate. The total EFA + NEFA content of an equivalent VLDL aliquot was determined using triheptadecanoin (Nucheck Prep) as an analytical surrogate after sequential transesterification in methanolic potassium hydroxide and methylation in methanolic HCl (21). Fatty acid methyl esters (FAMEs) were analyzed on an Agilent 6890 gas chromatograph equipped with a 30 m × 0.25 mm × 0.2 μm film DB225ms column (Agilent, Santa Clara, CA) interfaced with an Agilent 5973N mass spectral detector. Samples were ionized by electron impact, and data were acquired with simultaneous selected ion monitoring/full scan modes. Data analysis and quantification of SIM data were performed using Agilent Chemstation Software. Seven-point calibration curves prepared from authentic FAME standards (Nucheck Prep) bracketed all residues. Full scan mass spectra were used to confirm FA identity.
Total lipid extraction
To prepare lipid isolates for cell incubations, lipids were extracted from TGRL, with and without LpL pretreatment, by using 60 mg Oasis HLB solid-phase extraction cartridges (Waters, Milford, MA). The columns were precleaned with methanol and conditioned by 0.1% acetic acid in 5% methanol. Sample aliquots were mixed with 0.17 mg/ml BHT in the column reservoir and loaded onto the column packing by gravity. Loaded columns were washed with conditioning solution, dried, and eluted with 0.5 ml methanol and 1.5 ml ethyl acetate. The organic extracts were dried using Genevac EZ-2 evaporation system (Genevac Inc., UK), and residues were reconstituted in 100 μl chloroform. Analysis of the aqueous wash indicated that analyte breakthrough did not occur. For the oxylipid analyses described below, equivalent protocols were followed, except that aliquots were spiked with analytical surrogates prior to extraction and the final extract was reconstituted in 50 μl methanol containing internal standards for surrogate recovery determinations as previously reported for plasma analyses (22).
Lipid class separation
To investigate the differential effects of lipid classes on cell responses in culture, total lipid extracts were fractionated into neutral lipids, FFAs, and phospholipids using aminopropyl solid phase extraction (SPE) columns using slight modifications of published procedures (23, 24). Briefly, the total lipid extract, dissolved in 100 μl chloroform, was loaded onto a 500 mg aminopropyl column (Supelco, Bellefonte, PA), previously washed and activated with 4 ml hexane. Neutral lipids were eluted with 4 ml chloroform, followed by FFAs eluted with 4 ml diethyl ether-acetic acid (98:2; v/v), and phospholipids eluted with 4 ml methanol. To verify the performance of these separations, total and fractional FFA, phospholipid, triglyceride, and total cholesterol levels were determined using clinical lipid kits from Wako Chemicals USA, Inc. (Richmond, VA). The recoveries and relative purities of selected lipid fractions are shown in Table 1. Recoveries of lipid classes in specific fractions were >85% and equivalent with and without the addition of LpL. The neutral lipid fraction was subdivided further into triglycerides, diglycerides, monoglycerides, cholesteryl esters, and free cholesterol. Briefly, the isolated neutral lipid fraction was dried, resuspended in 100 μl hexane, and applied to a freshly prepared aminopropyl column. Cholesteryl esters were eluted in 4 ml hexane, followed by triglycerides eluted in 6 ml hexane containing 1% diethyl ether and 10% methylene chloride. The free cholesterol fraction was then eluted with 12 ml of 5% ethyl acetate in hexane. Diglycerides and monoglycerides were finally eluted with 4 ml of 15% ethyl acetate in hexane and chloroform-methanol (2:1; v/v), respectively.
TABLE 1.
Recovery of lipid fractions isolated from TGRL and TGRL + LpL (n = 5)
| Lipid Fraction | TGRL Recovery | TGRL + LpL Recovery | |
|---|---|---|---|
| % | |||
| FFAs | 94 ± 3.2 | 96 ± 3.0 | |
| Phospholipids | 93 ± 2.0 | 94 ± 2.4 | |
| Triglycerides | 87 ± 5.6 | 90 ± 3.6 | |
| Cholesteryl ester | 89 ± 6.8 | 86 ± 3.3 | |
TGRL, triglyceride-rich lipoprotein; LpL, lipoprotein lipase. Values are presented as percentage of recovery.
HPLC/MS/MS lipid determinations
The background oxidation state of the TGRL was characterized by quantification of epoxides, diols, and alcohols derived from LA and arachidonic acid (AA) using previously described HPLC/MS/MS-based procedures (22) adapted for use on a Quattro Micro tandem mass spectrometer and Acquity UPLC equipped with a 2.1 × 150 mm BEH-C18 reverse-phase column (Waters). Specifically, a 10 μl aliquot of the methanolic total lipid extract was separated by reverse-phase HPLC and analyzed by negative-mode electrospray ionization and tandem mass spectroscopy. The LA oxidation products epoxy octadecenoic acid, dihydroxy octadecanoic acid, HODE, and oxo octadecanoic acid, as well as the AA oxidation products epoxy eicosatrienoic acids, dihydroxy eicosatrienoic acid, hydroxy eicosatetraenoic acid (HETE), and oxo eicosatetraenoic acid were determined. Signals were also acquired for LA [m/z 279.2 > 261.2, cone voltage (CV) = 30, collision energy (CE) = 19, retention time (tR) = 18.66 min]; α-linoleic acid (m/z 277.2 > 259.2, CV = 30, CE = 19, tR = 17.61 min); AA (m/z 303.2 > 259.2, CV = 28, CE = 14; tR = 18.44 min); eicosapentaenoic acid (EPA: m/z 301.2 > 257.2, CV = 28, CE = 14, tR = 17.43 min); and docosahexaenoic acid (DHA: m/z 303.2 > 259.2, CV = 28, CE = 14, tR = 18.13 min). Oxylipids were quantified using deuterated surrogates and internal standard methodologies against a minimum five-point calibration curve bracketing all reported concentrations. The free LA and AA were quantified with six-point calibration curves using the d15-HETE recoveries to correct for extraction losses. The n-3 FAs were pseudo-quantified using the response factors produced by the calibration curves of n-6 FAs with equivalent carbon numbers and should only be viewed as indications of LpL-dependent increases.
Cell culture
HAECs were purchased from Cascade Bioscience, Inc. (Winchester, MA) and cultured in Medium 200 (Cascade Bioscience) supplemented with low-serum (2% FBS) growth supplement and penicillin, streptomycin, and amphotericin B at 37°C in a humidified atmosphere of 5% CO2. HAECs between passages 4 and 6 were grown in T-75 flasks until confluent.
HAEC treatment
To assess the potential impact of LpL-released components of TGRL on endothelium, HAEC cultures were treated with media only (untreated control), TGRL, or TGRL plus LpL in saline. Neither LpL itself nor heat-inactivated LpL caused an inflammatory response in HAEC cultures (data not shown). In a preliminary study of lipoprotein exposure, we treated endothelial cells with CM, VLDL, or TGRL and found that CMs produced highly variable endothelial cell responses. Conversely, VLDL incubations produced a more predictable induction of endothelial cell injury; thus, VLDL was selected as the primary TG source for the remaining experiments.
TNFα and intracellular adhesion molecule determination
HAECs were treated with lipid fractions extracted from 50 mg/dl VLDL, with or without LpL, for 2 h and their culture media was harvested. TNFα and intracellular adhesion molecule (ICAM) production was detected using ELISA kits from BD Bioscience.
ROS determination
ROS generation was probed by quantifying the oxidative transformation of 2,7-dichlorofluorescein diacetate (DCFDA; Invitrogen, Carlsbad CA) to the highly fluorescent product dichlorofluorescein (25). Confluent HAECs (104 cells/well) in 96-well plates were preincubated for 30 min with 10 μM DCFDA. Excess DCFDA-containing media was removed. Cells were washed twice with PBS and then incubated for 2 h with lipid fractions (15 μl), separated from VLDL (50 mg/dl TG), without (14 μmol/l FFA) or with LpL (196.5 μmol/l FFA), and incubated for 30 min at 37°C. After removal of medium from wells, cells were washed three times with PBS, and emission fluorescence density at 538 nm was measured after a 485 nm excitation using a fluorescence FLA 5100 micro plate reader (FUJIFLIM, Stamford, CT). To investigate the role of various oxidant-generating enzymes on VLDL + LpL-induced ROS generation, assays were repeated in the presence of either the xanthine oxidase inhibitor allopurinol (100 μM; Sigma), the NADPH oxidase inhibitors apocynin (100 μM; Sigma) and diphenylene iodonium (DPI) (50 μM; Sigma), or the cytochrome P450-2C9 inhibitor sulfaphenazole (10 μM; Sigma). To evaluate the relative impact of oxidized and neutral FAs on lipolysis-induced oxidative stress, assays were also repeated with 70 μM stearic acid (20 μg/ml), 71 μM LA, or 67 μM 13-HODE (Cayman Chemical, Ann Arbor, MI) with or without inhibitors. 4β-Phorbol 12-myristate 13-acetate (10 μM; Sigma) was used as a positive control because of its ability to stimulate oxidative activity.
Statistical analysis
All experiments were performed in triplicate in at least three independent trials. Values were expressed as means ± SEM unless otherwise noted. Comparisons among treatments were evaluated with t-tests. P < 0.05 was considered statistically significant.
RESULTS
LpL-induced FFA release from VLDL
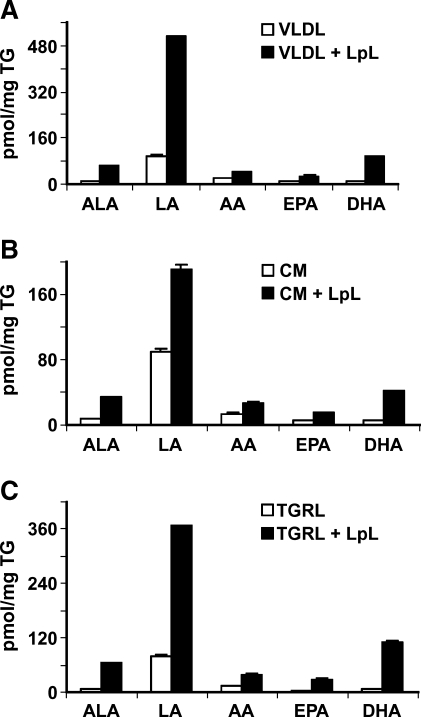
The VLDL EFA and NEFA content, as well as the NEFA content after LpL incubation of samples from four healthy volunteers, is shown in (Table 2). In this experiment, the 30 min incubation of VLDL with LpL released 1.3 ± 0.1% of the available esterified saturated fatty acids (SFAs), MUFAs, and PUFAs. Although the VLDL lipolysate nonesterified MUFA/SFA and n3/n6 PUFA ratios were not significantly altered by LpL, the free concentration of LA was increased 1.5-fold (P = 0.03) when VLDL was preincubated with LpL.
TABLE 2.
Effect of LpL on FFA composition of human VLDL fractions
| VLDL EFA | VLDL NEFAa | VLDL+LpL NEFAa | LpL Released NEFA | LpL Released NEFA | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| nmol/mg TG | % | |||||||||
| Saturated fatty acid | ||||||||||
| C14:0 | 694 ± 13 | 8.43 ± 1.2 | 16.7 ± 0.98b | 8.27 ± 0.98 | 1 | |||||
| C15:0 | 75.1 ± 1.6 | 0.622 ± 0.22 | 1.78 ± 0.16b | 1.16 ± 0.16 | 2 | |||||
| C16:0 | 9240 ± 410 | 148 ± 22 | 268 ± 19b | 120 ± 19 | 1 | |||||
| C17:0 | 64.7 ± 1.9 | ND | ND | ND | 1 | |||||
| C18:0 | 1350 ± 59 | 38.3 ± 7.1 | 62 ± 6.3b | 23.8 ± 6.3 | 2 | |||||
| C19:0 | 13.2 ± 0.23 | ND | ND | ND | ND | |||||
| C20:0 | 10.9 ± 0.43 | ND | ND | ND | ND | |||||
| C21:0 | 1.71 ± 0.12 | ND | ND | ND | ND | |||||
| C22:0 | 5.15 ± 0.25 | ND | ND | ND | ND | |||||
| C24:0 | 3.94 ± 0.24 | ND | ND | ND | ND | |||||
| MUFA | ||||||||||
| C14:1n5 | 50 ± 1.6 | 0.951 ± 0.071 | 1.31 ± 0.072b | 0.361 ± 0.072 | 1 | |||||
| C16:1n7t | 140 ± 5.2 | 0.564 ± 0.042 | 2.57 ± 0.29b | 2.01 ± 0.29 | 1 | |||||
| C16:1n7 | 1,030 ± 61 | 10.7 ± 1.7 | 19.9 ± 1.5b | 9.18 ± 1.5 | 1 | |||||
| C18:1n9t | 65.6 ± 3.7 | 2.07 ± 0.66 | 4.39 ± 0.65b | 2.32 ± 0.65 | 4 | |||||
| C18:1n9/1n7t | 7020 ± 300 | 98.7 ± 13 | 167 ± 10b | 67.8 ± 10 | 1 | |||||
| C18:1n7/1n6 | 1,170 ± 49 | 18.2 ± 2.7 | 30.8 ± 2.3b | 12.6 ± 2.3 | 1 | |||||
| C20:1n9t/1n12 | 8.24 ± 0.55 | ND | ND | ND | ND | |||||
| C20:1n9 | 28.3 ± 0.81 | 1.17 ± 0.088 | 1.58 ± 0.073b | 0.411 ± 0.072 | 1 | |||||
| PUFA | ||||||||||
| C18:2(9t,12t)n6 | 42.8 ± 0.72 | 0.164 ± 0.063 | 0.681 ± 0.089b | 0.518 ± 0.089 | 1 | |||||
| C18:2n6 | 6340 ± 270 | 86.1 ± 13 | 134 ± 11b | 48.2 ± 11 | 1 | |||||
| C18:3n6 | 121 ± 2.1 | 1.69 ± 0.16 | 2.15 ± 0.14 | 0.465 ± 0.14 | 0 | |||||
| C18:2(9ct,11t)n6 | 115 ± 2.7 | 0.226 ± 0.046 | 1.37 ± 0.24b | 1.15 ± 0.24 | 1 | |||||
| C18:3n3 | 213 ± 4.2 | ND | ND | 2.31 ± 0.37 | 1 | |||||
| C18:4n3 | 10.9 ± 0.37 | ND | ND | ND | ND | |||||
| C20:2n6 | 34.1 ± 0.63 | ND | ND | ND | ND | |||||
| C20:3n6 | 190 ± 4.6 | 4.96 ± 1 | 7.66 ± 1.1 | 2.7 ± 1.1 | 1 | |||||
| C20:4n6 | 732 ± 29 | 28 ± 5 | 36.8 ± 4.5 | 8.83 ± 4.5 | 1 | |||||
| C20:3n3 | 4.78 ± 0.25 | ND | ND | ND | ND | |||||
| C20:3n9 | 34.6 ± 1 | ND | ND | ND | ND | |||||
| C20:4n3 | 11.8 ± 0.3 | 0.628 ± 0.033 | 0.741 ± 0.038 | 0.113 ± 0.038 | 1 | |||||
| C20:5n3 | 56.2 ± 1.1 | 2.04 ± 0.19 | 2.42 ± 0.21 | 0.38 ± 0.21 | 1 | |||||
| C22:4n6 | 43.6 ± 1.1 | 1.28 ± 0.15 | 1.81 ± 0.15b | 0.535 ± 0.15 | 1 | |||||
| C22:5n6 | 37.7 ± 0.79 | 1.85 ± 0.22 | 2.27 ± 0.19 | 0.578 ± 0.15 | 2 | |||||
| C22:5n3 | 70.2 ± 2.1 | 2.18 ± 0.25 | 2.75 ± 0.21 | 0.568 ± 0.21 | 1 | |||||
| C22:6n3 | 195 ± 5.5 | 8.65 ± 1.5 | 11.7 ± 1.3 | 3.05 ± 1.3 | 2 | |||||
| C24:6n3 | 5.78 ± 0.25 | 0.447 ± 0.039 | 0.479 ± 0.041 | 0.097 ± 0.029 | 2 | |||||
TG, triglyceride; EFA, esterified fatty acid. Values are presented as blank corrected mean ± SEM (n = 4). Data were compared by complete weighted least-squares means analysis.
Multiplying NEFA nmol/mg TG concentrations by 4 will yield solution concentrations in μM.
Significant release of EFA (P < 0.05).
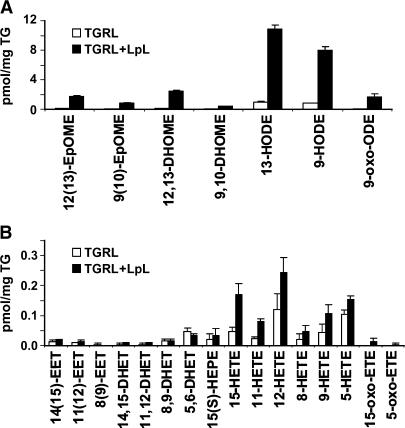
Using VLDL from an independent group of healthy volunteers (n = 4), LpL incubations were seen to increase not only PUFA (Fig. 1) but also the levels of an array of oxylipids in the FFA fraction (Fig. 2). In this instance, TGRL lipolysis produced a >9-fold increase in LA-derived oxidation products, including 13-HODE, the major oxidized lipid in oxidized LDL (26), when compared with TGRL (Fig. 2A). There were also increases in AA-derived oxylipids, but at concentrations much lower than observed for the LA products (Fig. 2B).
Fig. 1.
PUFAs from triglyceride-rich lipoprotein (TGRL) incubated with or without lipoprotein lipase (LpL). Human postprandial VLDL (A), chylomicron (CM) (B), or TGRL (C) [2.5 mg triglyceride (TG) for all treatments] with or without LpL were used for lipid extraction and oxidized lipids measurement. For LpL-dependent lipolysis, LpL was preincubated with CM, VLDL, or TGRL for 30 min at 37°C, and the reaction was stopped by transferring the tubes on ice prior to sample extraction and FA analysis. The concentration of linoleic acid (LA) and arachidonic acid (AA), as well as the n-3 FAs α-linoleic acid (ALA), eicosapentenoic acid (EPA), and docosahexaenoic acid (DHA) in VLDL, CM, and TGRL increased with LpL treatment. a Lipids were pseudo-quantified and adjusted by relative response factors to estimate their concentrations and allow consistent graphical representation of these data. Data are mean ± SEM (n = 3).
Fig. 2.
Oxidized lipids from VLDL incubated with or without LpL. Concentrations of oxylipids were increased by LpL treatment. Human postprandial VLDLs (2.5 mg TG) with or without LpL were used for lipid extraction and oxidized lipids measurement. For LpL-dependent VLDL lipolysis, LpL was preincubated with VLDL for 30 min at 37°C, and the reaction was stopped by transferring the tubes on ice prior to sample extraction. The LA oxidation products (A) epoxy octadecenoic acid (EpOME), dihydroxy octadecanoic acid (DHOME), hydroxy ocatadecadienoic acid (HODE), and oxo octadecanoic acid (oxo-ODE), as well as the AA oxidation products (B) epoxy eicosatrienoic acid (EET), dihydroxy eicosatrienoic acid (DHET), hydroxy eicosatetraenoic acid (HETE), and oxo eicosatetraenoic acid (oxo-ETE) were determined. Data are mean ± SEM (n = 3).
TGRL lipolysis fractions induced expression of inflammatory mediators
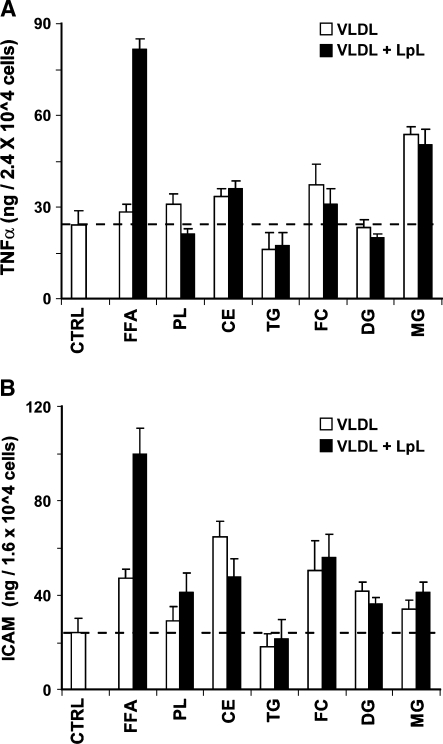
Lipid fractions isolated from VLDL, with or without LpL treatment, were evaluated for the ability to induce endothelial cell inflammatory reactions by measuring the production of the inflammatory cytokine TNFα and the cellular adhesion molecule ICAM. As shown in Fig. 3A, treatment with either monoacylglycerols (MGs) or post-LpL FFA fractions from VLDL significantly increased TNFα production, compared with control and other lipid subclass treatments. However, lipolysis only changed the FFA fraction-induced TNFα production.
Fig. 3.
VLDL and its LpL lipolysis-derived fractions increase endothelial cell TNFα production (A) and intracellular adhesion molecule (ICAM) expression (B). TNFα level and ICAM expression on human aortic endothelial cells were measured by ELISA. Endothelial cell cultures in 12-well plates were treated for 2 h with individual lipids isolated from VLDL (50 mg/dl TG) with or without 30 min incubation with LpL (2 U/ml). Each fraction was isolated from VLDL, evaporated, redissolved, and delivered to cells in a PBS vehicle (5 μl). TNFα level or ICAM expression stimulated with TNFα (10 ng/ml, 16 h) was measured. Excess FFA released from lipolysis increased TNFα expression and TNF-induced ICAM expression on HAECs. Data are mean ± SEM (n = 6). CTRL, control; FFA, free fatty acid; PL, phospholipid; CE, cholesteryl ester; TG, triglyceride; FC, free cholesterol; DG, diglyceride; MG, monoacylglycerol.
ICAM expression induced by these fractions was variable, with several lipid fractions, including FFA, cholesteryl ester, free cholesterol, diglyceride, and MG treatments increasing ICAM expression after treatment (Fig. 3B). However, as seen with TNFα, only the FFA fraction-induced ICAM expression response was significantly influenced by VLDL lipolysis (P < 0.01).
TGRL lipolysis products induced ROS generation
The effect of each lipid fraction on oxidative stress in HAECs was assessed by DCFDA oxidation. Exposure of HAECs to FFA for 2 h significantly increased ROS generation, compared with control treatment or other fractions (P < 0.05; Fig. 4). Therefore, FFAs isolated from VLDL lipolysis were chosen to explore the mechanisms of LpL-dependent changes. Our albumin dose-response experiment showed that VLDL lipolysis-induced ROS production was significantly decreased when the concentration of albumin in medium was greater than 50 mg/ml. In most of our experiments, we used 20 mg/ml albumin (2% FBS) in the medium.
Fig. 4.
Effects of lipid fractions isolated from VLDL on reactive oxygen species (ROS) production in HAECs. Each lipid fraction was isolated from VLDL (50 mg/dl TG), evaporated, redissolved, and delivered to cells in a PBS vehicle (5 μl). HAECs were preincubated for 30 min with the H2O2-sensitive fluorescence probe DCF-AM (10 μM), followed by incubation for 2 h with individual lipids isolated from VLDL and 30 min incubation with LpL (2 U/ml). Fluorescence intensity of cells was measured with a fluorescence microplate reader. Fluorescence distribution of DCF-AM oxidation was expressed as fluorescence units (FU) and expressed as the mean ± SEM (n = 6). * P < 0.01. CTRL, control; FFA, free fatty acid; PL, phospholipid; CE, cholesteryl ester; TG, triglyceride; FC, free cholesterol; DG, diglyceride; MG, monoacylglycerol.
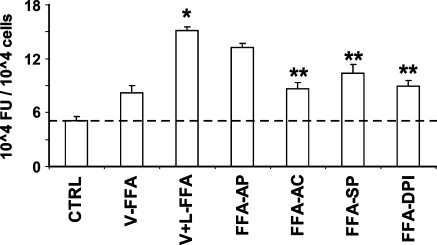
To identify pathways involved in FFA-induced ROS generation, we measured the effects of NADPH oxidase inhibitors (apocynin and DPI), the cytochrome P450 inhibitor (sulfaphenazole), and the NO synthase/xanthine oxidase inhibitor (allopurinol) on FFA-induced ROS production. As shown in Fig. 5, the oxidant production by FFA in HAECs was significantly suppressed (P < 0.01) by apocynin (100 μM), DPI (50 μM), and sulfaphenazole (10 μM), but not by allopurinol (100 μM).
Fig. 5.
Effects of FFA isolated from VLDL (V-FFA) and VLDL lipolysis (V + L-FFA) on ROS production in HAECs in the absence or presence of enzyme inhibitors. Each lipid fraction was isolated from VLDL (50 mg/dl TG), evaporated, redissolved, and delivered to cells in a PBS vehicle (5 μl). HAECs were preincubated for 30 min with the fluorescence probe DCF-AM (10 μM), followed by incubation for 2 h with FFA isolated from VLDL with or without 30 min incubation with LpL (2 U/ml) in the absence or presence of allopurinol (FFA-AP) (100 μM), apocynin (FFA-AC) (100 μM), sulfaphenazole (FFA-SP) (10 μM), or DPI (FFA-DPI) (50 μM). Fluorescence intensity of cells was measured with a fluorescence microplate reader. Fluorescence distribution of DCF-AM oxidation was expressed as fluorescence units (FU) and expressed as the mean ± SEM (n = 6). Significant differences in means were determined by 2-tailed Student's t-test (* P < 0.05 vs. V-FFA; **P < 0.05 vs. V+L-FFA).
Oxidized lipids induced oxidative stress
To evaluate the relative impact of oxidized and neutral FAs on lipolysis-induced oxidative stress, we measured lipid-induced ROS generation. In preliminary experiments, HAECs treated with 20 μg/ml of either stearic acid (70 μM) or LA (71 μM) showed no significant difference in ROS production (data not shown). However, 2 h incubation with 20 μg/ml 13-HODE (67 μM) significantly induced ROS generation in treated HAECs (4.2 ± 0.09 units/cell) compared with either LA (2.7 ± 0.3 units/cell) or vehicle controls (2.3 ± 0.15 units/cell). Treatment of HAECs with apocynin (100 μM), DPI (50 μM), and sulfaphenazole (10 μM) did not affect the 13-HODE-induced ROS production.
DISCUSSION
Postprandial increases in lipids and carbohydrates have been shown to increase oxidative stress, a state implicated in the pathogenesis of cardiovascular disease and diabetic complications (27, 28). More specifically, atherosclerosis is correlated with postprandial hyperlipidemia, which is characterized by elevated TGRL levels in the blood, and there is increasing evidence that postprandial TGRLs are more atherogenic than fasting TGRLs (3, 29–31). TGRL lipolysis during the postprandial state releases excess FFAs in the immediate proximity of the endothelium, which may cause endothelial injury (11) and subsequent endothelial dysfunction (32). In addition, TGRL remnants derived from TGRL lipolysis are themselves atherogenic (33, 34), with properties similar to oxidized LDL (35–37). In recent studies, we found that postprandial TGRLs, lipolyzed in vitro by purified bovine milk LpL, injured HAECs, induced the expression of inflammatory factors and cytokines (8), stimulated membrane microdomain aggregation, and increased the production of ROS (18). Thus, the current literature suggests that both FFAs and oxidant-bearing/stimulating remnants may contribute to TGRL lipolysis-induced endothelial cell injury. Here, we incubated freshly isolated human postprandial TGRL with LpL to generate a mixture of lipolysis products that approximates TGRL lipolysis in blood. We then investigated which of the soluble lipid classes generated during TGRL hydrolysis could mediate endothelial cell injury and explored the composition of the responsible class.
Using the inflammatory mediators TNFα and ICAM as markers of endothelial cell injury and inflammation, TGRL lipolysates were found to produce more injury than naive TGRL or TGRL incubated with LpL that was partially inactivated by incubation at 70°C for 30 min prior to use (data not shown). Separation of each TGRL lipid fraction before and after LpL-mediated lipolysis provided a unique set of materials for evaluating the independent effects of these complex mixtures. Using these fractions, we found that only the FFA fraction showed significant LpL-mediated increases in TNFα and ICAM production and ROS formation in cultured endothelial cells. Although the FFA fractions from both TGRL and TGRL lipolysates induced oxidative stress, the FFA fractions from TGRL lipolysates elicited more ROS generation from treated cells.
Lipolysis of TGRL by LpL is reported to have both pro- (38) and/or anti-atherogenic (39) effects. In a prior study, Ziouzenkova et al. (39) showed that LpL-mediated TGRL lipolysis has an anti-inflammatory role; however, there are several key differences in their paper as compared with ours. First, Ziouzenkova et al. induced endothelial cell injury with TNFα, whereas we did not. Second, the LpL concentration was 100-fold (200 U/ml) greater than the concentration used in our studies (2 U/ml). Human serum contains ∼66 ng/ml LpL after treatment with heparin (40). In comparison, Ziouzenkova et al. used LpL at 5,300–35,000 ng/ml. Third, Ziouzenkova et al. mixed VLDL and LpL to treat the cells, whereas we used LpL to pretreat VLDL (enzymatic lipolysis) for 30 min at 37°C and then treat the endothelial cells. Thus, there are major differences between our study and those of Ziouzenkova et al., starting with the stimulus to endothelial cell injury. Moreover, these differences make the present results difficult to compare with those of Ziouzenkova et al., because in a previous study, we showed that when endothelial cells were treated with TNFα, LpL (3 U/ml) itself had an anti-inflammatory effect (41). Regardless, these studies complement each other, with each supporting the hypothesis that lipolytically releasable bioactive agents are carried within the VLDL compartment.
To further explore the underlying mechanisms of the observed FFA-mediated endothelial injury, we next considered the content of the FFA fractions. The effect of FFAs on oxidative stress and endothelial dysfunction has been extensively investigated and found to vary depending on the specific FA tested. For instance, in HAEC cultures, the saturated FAs palmitate and stearate have been found to be pro-apoptotic, whereas unsaturated FAs are anti-apoptotic (42). However, high levels of serum PUFAs, when insufficiently protected from peroxidation, may increase the risk of atherosclerotic complications (43). Other studies have demonstrated that exposure to oleic acid and LA increased human LDL transfer across cultured endothelial monolayers, whereas equivalent doses of palmitic, linolenic, arachidonic, and eicosapentaenoic acids did not (13). Furthermore, the selective enrichment of serum FA mixtures with LA, but not various SFAs, induced disruption of endothelial barrier function (44). LA potentiates TNFα-mediated oxidative stress (45), and mediates pro-inflammatory effects in vascular endothelial cells (46). In contrast, EPA and DHAs attenuate adhesion molecule expression (47, 48) and inhibit inflammatory cytokine production (49), and recent studies suggest that DHA release could reduce intracellular ROS production by reducing p47phox membrane translocation, thus diminishing NADPH oxidase activity (50). Therefore, within the polyunsaturated class of FAs, distinct actions are apparent and the composition of dietary PUFA may be an important factor in determining the potential for TGRL-dependent endothelial injury in vivo.
In the present study, we measured TGRL and TGRL lipolysate FAs and found significant increases in free concentrations of many lipids, including various PUFAs. Although some discrepancies exist in the magnitude of release and the NEFA concentrations observed in the naive VLDL between the two independent VLDL isolates used in this study, the initial experiment described in Table 2 suggests that LpL uniformly releases the available esterified FAs. Notably, in experimental lipolysates used for HAEC incubations, evidence for excessive liberation of PUFAs, including LA, EPA, and DHA was observed. Considering the opposing effects of LA, EPA, and DHA on cellular inflammation, endothelial cell responses elicited by VLDL lipolysate exposures will reflect the balance between these “inflammatory” and “anti-inflammatory” lipids released from the particles. However, the neutral lipids are only one species of FA present in these particles with the ability to elicit an anti-inflammatory response.
Oxidized lipids such as 13-HODE, 9-HODE, and corresponding epoxides and diols are major components of oxidized LDL (26) and VLDL (51), which can be esterified into various lipid pools. Such oxidized lipids are generated by the interaction of unsaturated FAs with ROS either free in solution or coordinated by enzymes including the di-iron oxidases (e.g., lipoxygenase) and heme-monoxygenases (e.g., cytochrome P450s; CYP). Various oxidized lipids, including oxidized cholesterol (52) and oxidized-1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine (53), are reported to induce cellular responses that can promote the generation of ROS. Although LDLs are prominent targets for postprandial oxidative modification in humans, the balance of factors controlling LDL oxidation in vivo remains controversial. A small component of atherogenic oxidized lipoprotein lipids is derived directly from the diet (31, 54), whereas others are formed in situ in the circulation. Although a high monounsaturated fat intake is reported to reduce lipoprotein oxidizability (55), a prolonged elevation in TGRL, associated with decreased CM clearance, results in a restriction of vitamin E transfer to LDL along with a greater susceptibility to oxidation (56). However, Reaven et al. (57), studying the effect of different FAs on lipoproteins, showed that only the percentage of LA in LDL was strongly associated with the extent of lipoprotein oxidization. Regardless, oxidized lipids are clearly present and may participate actively in the development of atherosclerosis.
Our study showed that TGRL exposure to LpL produced significant increases in free oxidized lipids compared with TGRL only. In the VLDL fractions used for HAEC treatments, the LA-derived oxidized lipids 13-HODE and 9-HODE were elevated >10-fold, whereas LA increased ∼3-fold during the 30 min lipolysis process. In a recent study, Goodfriend et al. (58) observed a remarkable increase in nonesterified oxylipids in plasma from subjects who received heparin, suggesting that human TGRLs contain these oxidized lipids in vivo, as observed in rats (51). Therefore, it appears that significant amounts of oxidized lipids are released during TGRL lipolysis, and may be involved in eliciting responses in exposed endothelium. In the context of the present study, it is interesting that the morphology of endothelial cell monolayers was perturbed by lipolyzed but not oxidized lipoproteins and the perturbation could be prevented by vitamin E (11), arguing for a reactive oxygen-mediated event.
TGRL remnants, resulting from the lipolysis of TGRLs, are a major contributor to the atherogenicity of postprandial TGRL (59). Previous studies have shown that TGRL remnants have pro-atherogenic and pro-inflammatory properties similar to those of oxidized LDL (36, 37, 60, 61). Chung et al. (35) observed that lipolytic remnants of TGRL were cytotoxic to macrophages, and neither FFA nor lysolecthin from TGRL alone could account for this cytotoxicity. However, considering our findings, oxidized lipids in remnant particles may contribute to their cytotoxicity. We found that oxidative stress was induced by the lipolysis-derived FFA fractions and 13-HODE but not by the FFAs from naive TGRL, stearate, or linoleate. Moreover, a 20 μg/ml (71 μM) dose of LA did not induce HAEC oxidative stress, whereas 13-HODE increased oxidative stress 1.8-fold compared, with control. However, both LA and 13-HODE produced cytotoxicity at 40 μg/ml (LA, 142 μM; 13-HODE, 135 μM). Interestingly, in a study by Hennig and colleagues (46), 90 μM of LA induced a pro-inflammatory response in cultured human umbilical vein endothelial cells.
Because LpL is anchored immediately adjacent to the endothelium, concentrations of TGRL lipolysis products are expected to be higher at the blood-endothelial cell interface relative to the average plasma concentration. Therefore, 13-HODE, as well as other species liberated by lipolytic release from TGRL, could have pathophysiological actions on vascular endothelium. Moreover, TGRL lipolysis is rapid, causing a dramatic increase in the concentrations of such lipolysis products, leaving the cells little time to mount a protective response.
Previous studies have shown that CYP2C9-dependent metabolism of PUFA is associated with the production of linoleate-derived epoxide protoxicant (62), significant intracellular ROS production, and inflammation in the vascular endothelium (63). Consistent with these reports, the CYP2C9 inhibitor sulfaphenazole reduced the reactive oxygen stress induced by FA fractions derived from TGRL, but not the13-HODE-stimulated ROS production, suggesting that this compound is not a substrate for this enzyme. Inhibitors of NADPH oxidase, however, reduced the production of reactive oxygen stimulated by TGRL NEFA. As shown previously (64), FA-induced activation of NADPH oxidase in plasma membranes of human neutrophils depends on neutrophil cytosol and is potentiated by stable guanine nucleotides. Various FAs can activate NADPH oxidase, whereas PUFAs are substrates of the CYP2C family that produce superoxide in vascular cell types (63). Our results clearly show that these two independent pathways, i.e., NADPH oxidase and CYP450, contribute to lipid-mediated oxidative stress and the production of reactive oxygen stimulated by TGRL lipolysis.
In summary, the present study demonstrates that FFA fractions released during lipolysis contain a host of lipids, both neutral and oxygenated, that activate NADPH oxidase and cytochrome P450-mediated mechanisms of ROS production within endothelial cells.
Acknowledgments
The authors thank Danielle Baute and William Keyes for technical assistance.
Abbreviations
AA, arachidonic acid
apoB, apolipoprotein B
BHT, butylated hydroxytoluene
CM, chylomicron
DCFDA, dichlorofluorescein diacetate
DHA, docosahexaenoic acid
EFA, esterified fatty acid
DPI, diphenylene iodonium
EPA, eicosapentaenoic acid
FAME, fatty acid methyl ester
HAEC, human aortic endothelial cell
HETE, hydroxy eicosatetraenoic acid
HODE, hydroxy ocatadecadienoic acid
ICAM, intracellular adhesion molecule
LA, linoleic acid
LpL, lipoprotein lipase
MG, monoacylglycerol
ROS, reactive oxygen species
SFA, saturated fatty acid
TGRL, triglyceride-rich lipoprotein
Published, JLR Papers in Press, September 23, 2008.
Footnotes
This study was supported by National Institutes of Health Grants HL-78615 and HL-55665 and the Richard A. and Nora Eccles Harrison Endowed Chair in Diabetes Research. Additional support for this research was provided by United States Department of Agriculture Agricultural Research Service Project #5306-51530-016-00D, and by National Institute of Environmental Health Sciences Grant P42 ES04699.
References
- 1.Vogel R. A., M. C. Corretti, and G. D. Plotnick. 1997. Effect of a single high-fat meal on endothelial function in healthy subjects. Am. J. Cardiol. 79 350–354. [DOI] [PubMed] [Google Scholar]
- 2.Karpe F. 1997. Postprandial lipid metabolism in relation to coronary heart disease. Proc. Nutr. Soc. 56 671–678. [DOI] [PubMed] [Google Scholar]
- 3.Zilversmit D. B. 1979. Atherogenesis: a postprandial phenomenon. Circulation. 60 473–485. [DOI] [PubMed] [Google Scholar]
- 4.Zilversmit D. B. 1995. Atherogenic nature of triglycerides, postprandial lipidemia, and triglyceride-rich remnant lipoproteins. Clin. Chem. 41 153–158. [PubMed] [Google Scholar]
- 5.Lundman P., M. J. Eriksson, M. Stuhlinger, J. P. Cooke, A. Hamsten, and P. Tornvall. 2001. Mild-to-moderate hypertriglyceridemia in young men is associated with endothelial dysfunction and increased plasma concentrations of asymmetric dimethylarginine. J. Am. Coll. Cardiol. 38 111–116. [DOI] [PubMed] [Google Scholar]
- 6.Lundman P., M. Eriksson, K. Schenck-Gustafsson, F. Karpe, and P. Tornvall. 1997. Transient triglyceridemia decreases vascular reactivity in young, healthy men without risk factors for coronary heart disease. Circulation. 96 3266–3268. [DOI] [PubMed] [Google Scholar]
- 7.Rutledge J. C., A. E. Mullick, G. Gardner, and I. J. Goldberg. 2000. Direct visualization of lipid deposition and reverse lipid transport in a perfused artery: roles of VLDL and HDL. Circ. Res. 86 768–773. [DOI] [PubMed] [Google Scholar]
- 8.Eiselein L., D. W. Wilson, M. W. Lame, and J. C. Rutledge. 2007. Lipolysis products from triglyceride-rich lipoproteins increase endothelial permeability, perturb zonula occludens-1 and F-actin, and induce apoptosis. Am. J. Physiol. Heart Circ. Physiol. 292 H2745–H2753. [DOI] [PubMed] [Google Scholar]
- 9.Steinberg H. O., M. Tarshoby, R. Monestel, G. Hook, J. Cronin, A. Johnson, B. Bayazeed, and A. D. Baron. 1997. Elevated circulating free fatty acid levels impair endothelium-dependent vasodilation. J. Clin. Invest. 100 1230–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung B. H., B. Hennig, B. H. Cho, and B. E. Darnell. 1998. Effect of the fat composition of a single meal on the composition and cytotoxic potencies of lipolytically-releasable free fatty acids in postprandial plasma. Atherosclerosis. 141 321–332. [DOI] [PubMed] [Google Scholar]
- 11.Hennig B., B. H. Chung, B. A. Watkins, and A. Alvarado. 1992. Disruption of endothelial barrier function by lipolytic remnants of triglyceride-rich lipoproteins. Atherosclerosis. 95 235–247. [DOI] [PubMed] [Google Scholar]
- 12.Hennig B., D. M. Shasby, A. B. Fulton, and A. A. Spector. 1984. Exposure to free fatty acid increases the transfer of albumin across cultured endothelial monolayers. Arteriosclerosis. 4 489–497. [DOI] [PubMed] [Google Scholar]
- 13.Hennig B., D. M. Shasby, and A. A. Spector. 1985. Exposure to fatty acid increases human low density lipoprotein transfer across cultured endothelial monolayers. Circ. Res. 57 776–780. [DOI] [PubMed] [Google Scholar]
- 14.Hennig B., and B. A. Watkins. 1989. Linoleic acid and linolenic acid: effect on permeability properties of cultured endothelial cell monolayers. Am. J. Clin. Nutr. 49 301–305. [DOI] [PubMed] [Google Scholar]
- 15.Halle M., P. Eriksson, and P. Tornvall. 2005. Effects of free fatty acids and a triglyceride-rich fat emulsion on endothelial nitric oxide synthase. Eur. J. Clin. Invest. 35 154–155. [DOI] [PubMed] [Google Scholar]
- 16.Rutledge J. C., and I. J. Goldberg. 1994. Lipoprotein lipase (LpL) affects low density lipoprotein (LDL) flux through vascular tissue: evidence that LpL increases LDL accumulation in vascular tissue. J. Lipid Res. 35 1152–1160. [PubMed] [Google Scholar]
- 17.Rutledge J. C., M. M. Woo, A. A. Rezai, L. K. Curtiss, and I. J. Goldberg. 1997. Lipoprotein lipase increases lipoprotein binding to the artery wall and increases endothelial layer permeability by formation of lipolysis products. Circ. Res. 80 819–828. [DOI] [PubMed] [Google Scholar]
- 18.Wang L., A. R. Sapuri-Butti, H. H. Aung, A. N. Parikh, and J. C. Rutledge. 2008. Triglyceride-rich lipoprotein lipolysis increases aggregation of endothelial cell membrane microdomains and produces reactive oxygen species. Am. J. Physiol. Heart Circ. Physiol. 295 H237–H244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Redgrave T. G., and L. A. Carlson. 1979. Changes in plasma very low density and low density lipoprotein content, composition, and size after a fatty meal in normo- and hypertriglyceridemic man. J. Lipid Res. 20 217–229. [PubMed] [Google Scholar]
- 20.Pace-Asciak C. R. 1989. One-step rapid extractive methylation of plasma nonesterified fatty acids for gas chromatographic analysis. J. Lipid Res. 30 451–454. [PubMed] [Google Scholar]
- 21.Kelley D. S., G. J. Nelson, J. E. Love, L. B. Branch, P. C. Taylor, P. C. Schmidt, B. E. Mackey, and J. M. Iacono. 1993. Dietary alpha-linolenic acid alters tissue fatty acid composition, but not blood lipids, lipoproteins or coagulation status in humans. Lipids. 28 533–537. [DOI] [PubMed] [Google Scholar]
- 22.Luria A., S. M. Weldon, A. K. Kabcenell, R. H. Ingraham, D. Matera, H. Jiang, R. Gill, C. Morisseau, J. W. Newman, and B. D. Hammock. 2007. Compensatory mechanism for homeostatic blood pressure regulation in Ephx2 gene-disrupted mice. J. Biol. Chem. 282 2891–2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaluzny M. A., L. A. Duncan, M. V. Merritt, and D. E. Epps. 1985. Rapid separation of lipid classes in high yield and purity using bonded phase columns. J. Lipid Res. 26 135–140. [PubMed] [Google Scholar]
- 24.Pinkart H. C., R. Devereux, and P. J. Chapman. 1998. Rapid separation of microbial lipids using solid phase extraction columns. J. Microbiol. Methods. 34 9–15. [Google Scholar]
- 25.Cathcart R., E. Schwiers, and B. N. Ames. 1983. Detection of picomole levels of hydroperoxides using a fluorescent dichlorofluorescein assay. Anal. Biochem. 134 111–116. [DOI] [PubMed] [Google Scholar]
- 26.Lenz M. L., H. Hughes, J. R. Mitchell, D. P. Via, J. R. Guyton, A. A. Taylor, A. M. Gotto, Jr., and C. V. Smith. 1990. Lipid hydroperoxy and hydroxy derivatives in copper-catalyzed oxidation of low density lipoprotein. J. Lipid Res. 31 1043–1050. [PubMed] [Google Scholar]
- 27.Bae J. H., E. Bassenge, H. J. Lee, K. R. Park, C. G. Park, K. Y. Park, M. S. Lee, and M. Schwemmer. 2001. Impact of postprandial hypertriglyceridemia on vascular responses in patients with coronary artery disease: effects of ACE inhibitors and fibrates. Atherosclerosis. 158 165–171. [DOI] [PubMed] [Google Scholar]
- 28.Ceriello A. 2005. Postprandial hyperglycemia and diabetes complications: is it time to treat? Diabetes. 54 1–7. [DOI] [PubMed] [Google Scholar]
- 29.Karpe F. 2002. Postprandial lipemia–effect of lipid-lowering drugs. Atheroscler. Suppl. 3 41–46. [DOI] [PubMed] [Google Scholar]
- 30.Martins I. J., and T. G. Redgrave. 2004. Obesity and post-prandial lipid metabolism. Feast or famine? J. Nutr. Biochem. 15 130–141. [DOI] [PubMed] [Google Scholar]
- 31.Staprans I., X. M. Pan, J. H. Rapp, and K. R. Feingold. 2005. The role of dietary oxidized cholesterol and oxidized fatty acids in the development of atherosclerosis. Mol. Nutr. Food Res. 49 1075–1082. [DOI] [PubMed] [Google Scholar]
- 32.Hennig B., M. Toborek, C. J. McClain, and J. N. Diana. 1996. Nutritional implications in vascular endothelial cell metabolism. J. Am. Coll. Nutr. 15 345–358. [DOI] [PubMed] [Google Scholar]
- 33.Cohn J. S., C. Marcoux, and J. Davignon. 1999. Detection, quantification, and characterization of potentially atherogenic triglyceride-rich remnant lipoproteins. Arterioscler. Thromb. Vasc. Biol. 19 2474–2486. [DOI] [PubMed] [Google Scholar]
- 34.Fukushima H., S. Sugiyama, O. Honda, S. Koide, S. Nakamura, T. Sakamoto, M. Yoshimura, H. Ogawa, D. Fujioka, and K. Kugiyama. 2004. Prognostic value of remnant-like lipoprotein particle levels in patients with coronary artery disease and type II diabetes mellitus. J. Am. Coll. Cardiol. 43 2219–2224. [DOI] [PubMed] [Google Scholar]
- 35.Chung B. H., J. P. Segrest, K. Smith, F. M. Griffin, and C. G. Brouillette. 1989. Lipolytic surface remnants of triglyceride-rich lipoproteins are cytotoxic to macrophages but not in the presence of high density lipoprotein. A possible mechanism of atherogenesis? J. Clin. Invest. 83 1363–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park S. Y., J. H. Lee, Y. K. Kim, C. D. Kim, B. Y. Rhim, W. S. Lee, and K. W. Hong. 2005. Cilostazol prevents remnant lipoprotein particle-induced monocyte adhesion to endothelial cells by suppression of adhesion molecules and monocyte chemoattractant protein-1 expression via lectin-like receptor for oxidized low-density lipoprotein receptor activation. J. Pharmacol. Exp. Ther. 312 1241–1248. [DOI] [PubMed] [Google Scholar]
- 37.Shin H. K., Y. K. Kim, K. Y. Kim, J. H. Lee, and K. W. Hong. 2004. Remnant lipoprotein particles induce apoptosis in endothelial cells by NAD(P)H oxidase-mediated production of superoxide and cytokines via lectin-like oxidized low-density lipoprotein receptor-1 activation: prevention by cilostazol. Circulation. 109 1022–1028. [DOI] [PubMed] [Google Scholar]
- 38.Saxena U., N. M. Kulkarni, E. Ferguson, and R. S. Newton. 1992. Lipoprotein lipase-mediated lipolysis of very low density lipoproteins increases monocyte adhesion to aortic endothelial cells. Biochem. Biophys. Res. Commun. 189 1653–1658. [DOI] [PubMed] [Google Scholar]
- 39.Ziouzenkova O., S. Perrey, L. Asatryan, J. Hwang, K. L. MacNaul, D. E. Moller, D. J. Rader, A. Sevanian, R. Zechner, G. Hoefler, et al. 2003. Lipolysis of triglyceride-rich lipoproteins generates PPAR ligands: evidence for an antiinflammatory role for lipoprotein lipase. Proc. Natl. Acad. Sci. USA. 100 2730–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rip J., M. C. Nierman, N. J. Wareham, R. Luben, S. A. Bingham, N. E. Day, J. N. van Miert, B. A. Hutten, J. J. Kastelein, J. A. Kuivenhoven, et al. 2006. Serum lipoprotein lipase concentration and risk for future coronary artery disease: the EPIC-Norfolk prospective population study. Arterioscler. Thromb. Vasc. Biol. 26 637–642. [DOI] [PubMed] [Google Scholar]
- 41.Kota R. S., C. V. Ramana, F. A. Tenorio, R. I. Enelow, and J. C. Rutledge. 2005. Differential effects of lipoprotein lipase on tumor necrosis factor-alpha and interferon-gamma-mediated gene expression in human endothelial cells. J. Biol. Chem. 280 31076–31084. [DOI] [PubMed] [Google Scholar]
- 42.Staiger K., H. Staiger, C. Weigert, C. Haas, H. U. Haring, and M. Kellerer. 2006. Saturated, but not unsaturated, fatty acids induce apoptosis of human coronary artery endothelial cells via nuclear factor-kappaB activation. Diabetes. 55 3121–3126. [DOI] [PubMed] [Google Scholar]
- 43.Kok F. J., G. van Poppel, J. Melse, E. Verheul, E. G. Schouten, D. H. Kruyssen, and A. Hofman. 1991. Do antioxidants and polyunsaturated fatty acids have a combined association with coronary atherosclerosis? Atherosclerosis. 86 85–90. [DOI] [PubMed] [Google Scholar]
- 44.Hennig B., S. Ramasamy, A. Alvarado, N. C. Shantha, G. A. Boissonneault, E. A. Decker, and B. A. Watkins. 1993. Selective disruption of endothelial barrier function in culture by pure fatty acids and fatty acids derived from animal and plant fats. J. Nutr. 123 1208–1216. [DOI] [PubMed] [Google Scholar]
- 45.Toborek M., E. M. Blanc, S. Kaiser, M. P. Mattson, and B. Hennig. 1997. Linoleic acid potentiates TNF-mediated oxidative stress, disruption of calcium homeostasis, and apoptosis of cultured vascular endothelial cells. J. Lipid Res. 38 2155–2167. [PubMed] [Google Scholar]
- 46.Saraswathi V., G. Wu, M. Toborek, and B. Hennig. 2004. Linoleic acid-induced endothelial activation: role of calcium and peroxynitrite signaling. J. Lipid Res. 45 794–804. [DOI] [PubMed] [Google Scholar]
- 47.Goua M., S. Mulgrew, J. Frank, D. Rees, A. A. Sneddon, and K. W. Wahle. 2008. Regulation of adhesion molecule expression in human endothelial and smooth muscle cells by omega-3 fatty acids and conjugated linoleic acids: involvement of the transcription factor NF-kappaB? Prostaglandins Leukot. Essent. Fatty Acids. 78 33–43. [DOI] [PubMed] [Google Scholar]
- 48.Weber C., W. Erl, A. Pietsch, U. Danesch, and P. C. Weber. 1995. Docosahexaenoic acid selectively attenuates induction of vascular cell adhesion molecule-1 and subsequent monocytic cell adhesion to human endothelial cells stimulated by tumor necrosis factor-alpha. Arterioscler. Thromb. Vasc. Biol. 15 622–628. [DOI] [PubMed] [Google Scholar]
- 49.Khalfoun B., F. Thibault, H. Watier, P. Bardos, and Y. Lebranchu. 1997. Docosahexaenoic and eicosapentaenoic acids inhibit in vitro human endothelial cell production of interleukin-6. Adv. Exp. Med. Biol. 400B 589–597. [PubMed] [Google Scholar]
- 50.Massaro M., A. Habib, L. Lubrano, S. Del Turco, G. Lazzerini, T. Bourcier, B. B. Weksler, and R. De Caterina. 2006. The omega-3 fatty acid docosahexaenoate attenuates endothelial cyclooxygenase-2 induction through both NADP(H) oxidase and PKC epsilon inhibition. Proc. Natl. Acad. Sci. USA. 103 15184–15189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Newman J. W., G. A. Kaysen, B. D. Hammock, and G. C. Shearer. 2007. Proteinuria increases oxylipid concentrations in VLDL and HDL but not LDL particles in the rat. J. Lipid Res. 48 1792–1800. [DOI] [PubMed] [Google Scholar]
- 52.Staprans I., X. M. Pan, J. H. Rapp, and K. R. Feingold. 1998. Oxidized cholesterol in the diet accelerates the development of aortic atherosclerosis in cholesterol-fed rabbits. Arterioscler. Thromb. Vasc. Biol. 18 977–983. [DOI] [PubMed] [Google Scholar]
- 53.Rouhanizadeh M., J. Hwang, R. E. Clempus, L. Marcu, B. Lassegue, A. Sevanian, and T. K. Hsiai. 2005. Oxidized-1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine induces vascular endothelial superoxide production: implication of NADPH oxidase. Free Radic. Biol. Med. 39 1512–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Staprans I., J. H. Rapp, X. M. Pan, K. Y. Kim, and K. R. Feingold. 1994. Oxidized lipids in the diet are a source of oxidized lipid in chylomicrons of human serum. Arterioscler. Thromb. 14 1900–1905. [DOI] [PubMed] [Google Scholar]
- 55.Turpeinen A. M., G. Alfthan, L. Valsta, E. Hietanen, J. T. Salonen, H. Schunk, K. Nyyssonen, and M. Mutanen. 1995. Plasma and lipoprotein lipid peroxidation in humans on sunflower and rapeseed oil diets. Lipids. 30 485–492. [DOI] [PubMed] [Google Scholar]
- 56.Couderc R., J. Peynet, M. Cambillaud, F. Tallet, C. Cosson, G. Lefevre, and V. Atger. 1998. Effects of postprandial hyperlipemia on the vitamin E content of lipoproteins. GERBAP section Lipoproteines. Groupe d'Evaluation et de Recherche de l'Assistance Publique des Hopitaux de Paris. Clin. Chim. Acta. 277 141–152. [DOI] [PubMed] [Google Scholar]
- 57.Reaven P., S. Parthasarathy, B. J. Grasse, E. Miller, F. Almazan, F. H. Mattson, J. C. Khoo, D. Steinberg, and J. L. Witztum. 1991. Feasibility of using an oleate-rich diet to reduce the susceptibility of low-density lipoprotein to oxidative modification in humans. Am. J. Clin. Nutr. 54 701–706. [DOI] [PubMed] [Google Scholar]
- 58.Goodfriend T. L., T. L. Pedersen, R. J. Grekin, B. D. Hammock, D. L. Ball, and A. Vollmer. 2007. Heparin, lipoproteins, and oxygenated fatty acids in blood: a cautionary note. Prostaglandins Leukot. Essent. Fatty Acids. 77 363–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nakajima K., T. Nakano, and A. Tanaka. 2006. The oxidative modification hypothesis of atherosclerosis: the comparison of atherogenic effects on oxidized LDL and remnant lipoproteins in plasma. Clin. Chim. Acta. 367 36–47. [DOI] [PubMed] [Google Scholar]
- 60.Saniabadi A. R., K. Umemura, M. Shimoyama, M. Adachi, M. Nakano, and M. Nakashima. 1997. Aggregation of human blood platelets by remnant like lipoprotein particles of plasma chylomicrons and very low density lipoproteins. Thromb. Haemost. 77 996–1001. [PubMed] [Google Scholar]
- 61.Stiko-Rahm A., A. Hultgardh-Nilsson, J. Regnstrom, A. Hamsten, and J. Nilsson. 1992. Native and oxidized LDL enhances production of PDGF AA and the surface expression of PDGF receptors in cultured human smooth muscle cells. Arterioscler. Thromb. 12 1099–1109. [DOI] [PubMed] [Google Scholar]
- 62.Slim R., B. D. Hammock, M. Toborek, L. W. Robertson, J. W. Newman, C. H. Morisseau, B. A. Watkins, V. Saraswathi, and B. Hennig. 2001. The role of methyl-linoleic acid epoxide and diol metabolites in the amplified toxicity of linoleic acid and polychlorinated biphenyls to vascular endothelial cells. Toxicol. Appl. Pharmacol. 171 184–193. [DOI] [PubMed] [Google Scholar]
- 63.Viswanathan S., B. D. Hammock, J. W. Newman, P. Meerarani, M. Toborek, and B. Hennig. 2003. Involvement of CYP 2C9 in mediating the proinflammatory effects of linoleic acid in vascular endothelial cells. J. Am. Coll. Nutr. 22 502–510. [DOI] [PubMed] [Google Scholar]
- 64.Seifert R., and G. Schultz. 1987. Fatty-acid-induced activation of NADPH oxidase in plasma membranes of human neutrophils depends on neutrophil cytosol and is potentiated by stable guanine nucleotides. Eur. J. Biochem. 162 563–569. [DOI] [PubMed] [Google Scholar]