Abstract
Among the known mechanisms of reverse cholesterol transport (RCT), ATP binding cassette transporter G1 (ABCG1)-mediated free cholesterol (FC) transport is the most recent and least studied. Here, we have characterized the efficiencies of different acceptors using baby hamster kidney (BHK) cells transfected with human ABCG1 cDNA, which is inducible upon treatment with mifepristone. When normalized on particle number and particle surface area, the acceptor efficiency for FC efflux was as follows: small unilamellar vesicles (SUV)>LDL>reconstituted HDL>HDL2 = HDL3. Based on phospholipid content, the order was reversed. ABCG1 also mediated phospholipid efflux to human serum and HDL3. ABCG1-mediated FC efflux correlated significantly with a number of HDL subfractions and components in serum collected from 25 normolipidemic individuals: apolipoprotein A-II (apoA-II) (r2 = 0.7), apolipoprotein A-I (apoA-I) (r2 = 0.5), HDL-C (r2 = 0.4), HDL-PL (r2 = 0.4), α-2 HDL (r2 = 0.4), and preβ HDL (r2 = 0.2). ABCG1 did not enhance influx of FC or cholesteryl oleyl ether (COE) when cells were incubated with radiolabeled HDL3. ABCG1 expression did not increase the association of HDL3 with cells. Compared with control cells, ABCG1 expression significantly increased the FC pool available for efflux and the rate constant for efflux. In conclusion, composition and particle size determine the acceptor efficiency for ABCG1-mediated efflux. ABCG1 increases cell membrane FC pools and changes its rate of desorption into the aqueous phase without enhancing the association with the acceptor.
Keywords: apolipoproteins, ATP binding cassette transporter G1, BHK cells, binding, high density lipoprotein, influx, phospholipid efflux, time course
The release of cholesterol from cells in the vessel wall, primarily macrophage-derived foam cells, is the first step in the process termed “reverse cholesterol transport” (RCT) (1–3). It is believed that in RCT, the excess cholesterol present in peripheral cells is incorporated into HDL, which then, either directly or indirectly by transfer to LDL, delivers the excess cholesterol to the liver for excretion. A number of different mechanisms have been shown to participate in the movement of cholesterol from cells to extracellular acceptor lipoproteins. These pathways for cholesterol efflux include unmediated diffusion (4, 5) or protein-mediated transport linked to the presence in cells of scavenger receptor BI (SR-BI) (6, 7) and ATP binding cassette transporter A1 (ABCA1) (8–10). These pathways have been discussed in detail in a number of recent reviews (4, 8, 11–13). More recently, another cell protein, ATP binding cassette transporter G1 (ABCG1), has been shown to influence the efflux of cholesterol from cells (8, 14–18). Whereas much is known about the nature of the efflux process mediated by aqueous diffusion, SR-BI, and ABCA1, little has been established about the nature of the efflux process mediated by ABCG1. It has been established that this protein is present in a variety of tissues, where it can enhance the efflux of cell cholesterol and oxysterols (15, 19, 20), and it is very promiscuous in terms of the particles that can serve as cholesterol acceptors (21). However, in contrast to the situation with ABCA1, lipid-free apolipoprotein A-I (apoA-I) does not serve as an acceptor of cell cholesterol provided by ABCG1 (16, 21). Based on this broad array of potential acceptors and the distribution of ABCG1 throughout the cell (22), it has been proposed that the protein may not serve as a direct transporter of membrane cholesterol to acceptors, but rather play a role by enriching the cell membrane with cholesterol that can then be incorporated into a variety of acceptor particles (8, 14, 22).
The purpose of the present investigation was to study in detail the role of different acceptors in mediating cell cholesterol efflux, and in this way to gain further understanding of the ABCG1-mediated cholesterol efflux process. The experimental cell system used in the present study has been previously described in detail (22, 23). Briefly, baby hamster kidney (BHK) cells were stably transfected with an N-terminal FLAG-tagged human ABCG1 cDNA that was inducible by treatment with mifepristone. This inducible system allows for the direct assessment of the impact of ABCG1 expression on cell cholesterol flux and was used for a detailed study characterizing a number of aspects of ABCG1 and its role in cholesterol efflux (22).
MATERIALS AND METHODS
Tissue culture plastic wares were obtained from Falcon (Becton Dickinson Labware, Lincoln, NJ) and from Corning Inc. (Corning, NY). Cell culture media, PBS, Dulbecco's phosphate buffered saline were purchased from Mediatech Cellgro (Manassas, VA), and FBS, calf serum, gentamycin, DNases 1, sodium choleate, and heparin from Sigma-Aldrich (St. Louis, MO). BSA was obtained from Cellianxe (Toronto, Canada). [3H] cholesterol and [3H] cholesterol oleyl ether (COE) were from Perkin-Elmer Analytical Sciences (Boston, MA). Acyl-CoA:cholesterol acyl transferase inhibitor (ACAT inhibitor), compound CP113, 818 was kindly provided by Pfizer Pharmaceuticals (Groton, CT). Bovine brain sphingomyelin (SM), 1, 2-dimyristoyl-sn-glycerophosphocholine (DMPC), egg glycerophosphocholine (egg PC) were obtained from Avanti Polar Lipids (Alabaster, AL). Cholesteryl ester transfer protein (CETP) was purchased from Cardiovascular Targets, Inc. (Audubon Biomedical Center, NY). All other reagents and organic solvents were purchased from Fisher Scientific (Pittsburgh, PA).
Cultured cells
BHK cells expressing high levels of human ABCG1 were generated using the mifepristone-inducible GeneSwitch system as described in detail previously (22, 23). Although mifepristone is a progesterone antagonist and progesterone has been shown to inhibit cellular cholesterol trafficking, the concentration of mifepristone used in our studies was 3,000-fold lower than those that affect cholesterol trafficking and had no effect on cholesterol metabolism in BHK control cells. However, we cannot exclude the possibility that even these low levels of mifepristone may have some effect on ABCG1-dependent cholesterol efflux. Cells were grown and maintained in DMEM containing 10% FBS except during experimental treatments. ABCG1 was induced by incubating cells for 18 h in DMEM containing 0.2% BSA and 10 nM mifepristone. Studies were carried out on standard 12-well tissue-culture plates unless otherwise stated.
Phospholipid vesicle preparation and HDL phospholipid modification
HDL2 (d, 1.066–1.125 g/ml) and HDL3 (d, 1.125–1.210 g/ml) were isolated from human plasma by sequential ultracentrifugation (24). ApoA-I was purified from delipidated HDL as described previously (25). Prior to use, apoA-I, HDL2, HDL3, and LDL were extensively dialyzed against 0.15 M NaCl (pH 7.4) and sterilized by filtration using a 0.45 μm Millipore filter. Egg PC small unilamellar vesicles (SUV) were made as previously described (26).
Enrichment of HDL3 with phospholipid, DMPC, or SM, multilamellar vesicles (MLVs) was done as described (27, 28). For DMPC enrichment, 1–2 mg of HDL3 protein/ml was incubated with increasing amounts of DMPC MLVs (1.5-, 2-, and 4-fold the amount of native HDL3 phospholipid) for 2 h at the gel to liquid crystal phase transition temperature of 24°C. For SM enrichment, MLVs were added at increasing amounts (5-, 10-, and 20-fold the SM content of native HDL3, which is estimated to be 12% of total HDL3 phospholipid), and the mixture was incubated for 1 h at 37°C. Because SM has broad phase transition temperature, the mixture was warmed to 42°C and then allowed to cool slowly to 25°C over 5 h. During DMPC or SM enrichment of HDL3, as a control, native HDL3 was also incubated similarly but without addition of MLVs. After incubation of HDL3 with MLVs, any unreacted MLVs were removed by sequentially filtering the HDL3-MLV mixture through 0.45 and 0.22 μm filters. The mixture was then stored at 4°C overnight, and any remaining MLVs were removed from HDL3 by centrifugation for 30 min at 3,000 g. Phospholipid and protein concentrations were measured by phosphorus (29) and a modified Lowry (30) assays, respectively. At the end of the phospholipid enrichment procedure, the phospholipid to protein ratio of enriched HDL3 particles used in these experiments ranged from 0.48–2.05 in case of DMPC and 0.45–1.25 in case of SM. The HDL particles having the maximum enrichment of either DMPC or SM were fractionated by fast-protein liquid chromatography (FPLC) using a Superdex HR200 column. No difference in elution volume was observed indicating that the enrichment with phospholipid had no significant effect on HDL particle size.
Preparation of discoidal reconstituted HDL particles
Reconstituted HDL (rHDL) discs containing human apoA-I or apoA-II and egg PC were prepared by the cholate dialysis method (31). Briefly, egg PC in chloroform was dried down under nitrogen in a 15 ml conical tube. The egg PC was vortexed in TBS (pH 7.4) to generate MLVs and incubated with sodium cholate at 37°C for 1.5 h to generate detergent-PC mixed micelles. ApoA-I (egg PC to apoA-I weight ratio of 2:1 and egg PC/protein mole ratio of 79:1) or apoA-II (egg PC to apoA-II weight ratio of 2.6:1 and egg PC/protein mole ratio of 58:1), freshly dialyzed from 6 M guanidine-HCl in TBS, was added to the lipid-detergent mixture. The mixture was then incubated for 1 h at 37°C followed by extensive dialysis against TBS at 4°C to remove sodium cholate. ApoA-II-containing discs were fractionated by FPLC using a Superdex HR200 column (32), and particles having the same size as that of apo A-I rHDL discs were used.
Analysis of HDL3 association to ABCG1
HDL3 particles were dialyzed against PBS, 0.25 mM EDTA (pH 7.4), and iodinated with Na [125I] using the modified iodine monochloride method previously described (33). The sample was then passed through a PD-10 column equilibrated with PBS, 0.25 mM EDTA (pH 7.4), and dialyzed against PBS, 0.25 mM EDT, 100 mM KI (pH 7.4), followed by three changes of PBS, 0.25 mM EDTA (pH 7.4) at 4°C. The specific activity of [125I] HDL3 was 111 cpm/ng of protein.
For analysis of the association of [125I] HDL3, BHK cells transfected with ABCG1 were plated in a 12-well plate and after 24 h one set of cells were upregulated with mifepristone for 18 h. The cells were then washed three times with MEM-HEPES medium containing 1% BSA and were incubated for 2 h at 37°C with the iodinated HDL3 particles (0.5 ml) at different concentrations. At the end of the incubation, the medium was removed from the cells while the plates were on ice and the cell monolayers were washed three times with ice cold MEM-HEPES containing 1% BSA, followed by three washes with ice cold PBS. The cells were then solubilized in 1 ml of 0.1 M NaOH at room temperature for 15 min. Aliquots of the samples were then taken for γ-counting and for total protein measurement.
Cholesterol efflux and influx
To measure cellular cholesterol efflux, cells were plated for a day and then labeled for 24 h with 3 μCi/ml of [3H] free cholesterol (FC) in the presence of 2.5% FBS. Cells were later treated with or without mifepristone for 18 h in DMEM medium containing 0.2% BSA. Efflux of FC was induced by incubation with respective acceptors for 4 h. Efflux was calculated by measuring the release of radiolabeled FC into the medium, as previously described (34, 35). All the experiments were performed in the presence of the ACAT inhibitor CP113, 818 unless otherwise mentioned. In a separate experiment, the BHK control and ABCG1-upregulated cells were grown and labeled in 10% FBS and then incubated with 25 μg/ml HDL3 for 8 h and 18 h. The incubation media were removed, and the amount of label cholesterol and cholesterol mass were determined. Cholesterol mass in the media was quantitated flourimetrically using the Amplex Red Cholesterol Assay Kit (Molecular probes, Eugene, OR).
To measure influx of HDL cholesterol in to cells, HDL3 particles were labeled by incubation with [3H] FC (100 μCi/mg HDL3 protein) or with CETP (50 μg/ml) and [3H] COE (100 μCi/mg HDL3 protein) that had been dried on the glass wall of a test tube under N2. After incubation of HDL3 with either [3H] FC or [3H] COE overnight at 4°C, the particles were sterilized by filtration through a 0.45 μm filter. The radiolabeled HDL3 particles were diluted with DMEM and incubated with untreated and mifepristone-treated cells for 4 h in case of FC and 6 h for COE at 37°C. At the end of the incubation, the medium was removed and the cells were washed two times with Dulbecco's phosphate buffered saline. The cell lipids were extracted with isopropyl alcohol and the [3H] FC or [3H] COE present in the total lipid extract was measured by liquid scintillation counting.
Serum and lipoproteins
Human serum was collected from 25 normolipidemic healthy individuals with approved consent and used individually or pooled. Polyethylene glycol (PEG) supernatants of individual sera were prepared by precipitating apoB-containing lipoproteins from serum as previously described (36), by adding 4 parts 20% PEG 8000 (Sigma P-2139) in 200 mM glycine (pH = 7.4) to 10 parts serum. After 20 min incubation at room temperature the solution was centrifuged (15,000 rpm, 20 min, 4°C). Agarose gel electrophoresis (Paragon Electrophoresis system, Beckman Coulter, Fullerton, CA) of the isolated supernatants confirmed the removal of apoB-containing lipoproteins from the serum fraction.
Serum HDL-cholesterol and -phospholipid levels were measured enzymatically on a Cobas Fara II (Roche Diagnostic Systems, Inc.) using Sigma reagents (Sigma Chemical Co.). Human serum apoA-I and apoA-II levels were quantified using an immunoturbidimetric assay (Sigma Chemical Co.) and Wako Pure Chemical Industries, respectively) on the Cobas Fara.
Nondenaturing two-dimensional gel electrophoresis
ApoA-I containing human HDL subfractions were determined by nondenaturing two-dimensional gel electrophoresis, immunoblotting, and image analysis as described (36). Briefly, 4 μl of plasma was applied and electrophoresed on a vertical-slab agarose gel (0.7%) in the first dimension at 250 V until the α-mobility front moved 3.5 cm from the origin. The agarose gel was sliced, and the strips were applied onto 3–35% nondenaturing concave gradient polyacrylamide gels. In the second dimension, gels were electrophoresed to completion at 250 V for 24 h at 10°C followed by electrotransfer to nitrocellulose membranes at 30 V for 24 h at 10°C. ApoA-I was immunolocalized on the membrane with monospecific goat anti-human primary and [125I]labeled secondary antibodies [immunopurified rabbit F (ab')2 fraction against goat IgG]. The bound [125I]labeled secondary antibody was quantified in a FluoroImager (Molecular Dynamics, CA).
Data analysis
Statistical and kinetic analyses were performed using Prism (4.0), GraphPad Inc. (San Diego, CA). All the experiments were conducted in triplicate, and data were expressed as mean ± SD unless otherwise indicated. Linear correlation coefficients were used to describe relations between cholesterol efflux and various serum parameters. Binding parameters (Bmax and Kd) were obtained by nonlinear regression using a one-site binding analysis. Time courses of FC efflux from cells were analyzed using nonlinear regression and one phase exponential decay model (37) to derive the rate constant (ke) for cellular cholesterol efflux and the fraction of cellular cholesterol involved in the efflux. See figure legends for details of the calculation of particle number concentration and particle size.
RESULTS
Using the ABCG1-expressing BHK cell system (22, 23), we have tested a variety of acceptors for their efficiencies in mediating cell cholesterol efflux. The different acceptors included HDL2, HDL3, LDL, rHDL discs, and egg PC SUVs at a range of concentrations (Fig. 1). Because these acceptors differ in composition, efflux data are normalized on the basis of their protein content (Fig. 1A), phospholipid content (Fig. 1B), particle number concentration (Fig. 1C), and particle size (Fig. 1D). All of these particles, with the exception of lipid-free apoA-I (data not shown) act as cholesterol acceptors. The most efficient acceptor when normalized on the basis of protein was rHDL, and HDL2 and HDL3 were equally effective (Fig. 1A), while HDL3 proved to be the best acceptor when normalized on phospholipid concentration (Fig. 1B). The Km values for HDL3 and HDL2 were 47 ± 8 μg/ml and 52 ± 12 μg/ml, respectively, which were generally similar to the Km value for HDL (31 μg/ml) obtained by Vaughan and Oram (22). At a given protein or phospholipid concentration, the smaller acceptor particlesm such as rHDL disc, HDL3, and HDL2, were present in higher numbers and therefore gave higher ABCG1-mediated efflux compared with large particles such as LDL and SUV. However, at a similar particle number (Fig. 1C), and when particle size was taken into account (Fig. 1D), the larger SUV and LDL particles proved to be the most effective.
Fig. 1.
ATP binding cassette GI (ABCG1)-mediated free cholesterol efflux to different acceptors. Baby hamster kidney (BHK) cells transfected with ABCG1 were radiolabeled with [3H] free cholesterol (FC) for 24 h and were treated with or without mifepristone for 18 h. The cells were incubated with various acceptors at the indicated concentrations for 4 h, and % of efflux was measured. The different acceptors were normalized based on their protein (A), phospholipid (B), particle number concentration calculated from particle compositions and molecular weights (C; (39, 56), and a function of particle size (D; (38). The ABCG1-mediated FC efflux data (i.e., the difference in efflux between mifepristone-treated and control cells) are fitted to the Michaelis-Menten equation. The efflux values are mean ± SD of triplicate measurements. Reconstituted HDL disc (rHDL disc), ♦; HDL3, ▴; HDL2, ▾; LDL, ▪; SUV, •.
We next examined the effect of enrichment of HDL3 with DMPC or SM on ABCG1-mediated FC efflux (Fig. 2) by enriching HDL3 with SM (5-, 10-, and 20-fold, Fig. 2A), or DMPC (1.5-, 2-, and 4-fold, Fig. 2B). The FC efflux for untreated and mifepristone-treated cells at a fixed HDL protein concentration increased in parallel with increasing degrees of phospholipid enrichment (PC and SM). However, the ABCG1-mediated FC efflux (the difference between the mifepristone-treated and untreated cells) did not change regardless of the type of phospholipid or the level of the enriched phospholipid (Fig. 2A, B).
Fig. 2.
ABCG1-mediated free cholesterol efflux to phospholipid-enriched HDL3. Radiolabeled BHK cells were treated with or without mifepristone for 18 h and were incubated with HDL3 (25 μg protein/ml), enriched with different levels of either sphingomyelin (SM; 5-, 10-, 20-fold; A), or 1, 2-dimyristoyl-sn-glycerophosphocholine (DMPC; 1.5-, 2-, 4-fold; B) for 4 h (the control HDL3 PL/protein ratio is 0.45 for SM and 0.48 for DMPC). ABCG1-mediated efflux was the difference between the values for untreated and mifepristone-treated cells. The efflux values are mean ± SD of triplicate measurements (the error bars are inside the symbols). Untreated, □; mifepristone, ▴; ABCG1-specific, •.
We also examined ABCG1-mediated phospholipid efflux (Fig. 3) after labeling BHK cells with [3H] choline for 24 h followed by treatment of cells with or without mifepristone. Whole serum and isolated HDL stimulated ABCG1-mediated efflux, although the fractional efflux of phospholipid was considerably less than that observed for cholesterol (Fig. 3). Lipid-free apoA-I (20 μg/ml) was completely inactive with these cells, which lack ABCA1 (Fig. 3).
Fig 3.
ABCG1-mediated phospholipid efflux to different acceptors. BHK cells transfected with ABCG1 were labeled with [3H] choline for 24 h before being treated with or without mifepristone for 18 h. The cells were then incubated with different acceptors for 4 h, and the % of choline-containing phospholipid efflux was measured. The values are mean ± SD of triplicate measurements. The ABCG1-mediated phospholipid efflux with 2.5% human serum was statistically higher (P < 0.05) than with the other acceptors. Untreated, □; mifepristone, gray bars; ABCG1-specific, ▪.
It is clear that expression of ABCG1 stimulates cell cholesterol efflux; however, no information is available on this transporter's ability to enhance influx of either FC or COE from HDL3 particles. As shown in Fig. 4, the influx of FC and COE were the same for untreated and mifepristone-treated cells, indicating that ABCG1 expression did not enhance either FC or COE influx at any HDL3 concentration. The enhanced cholesterol efflux mediated by ABCG1 without a parallel increase in FC and cholesteryl ester (CE) influx, as indicated by the isotopic data, should result in a greater net loss of cell cholesterol when exposed to HDL. To confirm the isotopic data, we compared the FC content of media supplemented with 25 μg/ml HDL3 that had been incubated with BHK control and ABCG1-upregulated cells for 8 h and 18 h. The results are presented in Table 1. Expression of ABCG1 produced an increase in cell cholesterol efflux and an increase in the labeled cholesterol recovered in the media. Importantly, the mass of FC in the incubation media from upregulated cells was greater than the media from control cells, and this difference was evident after 8 h and 18 h of exposure to HDL3. This greater cholesterol mass in the media is consistent with the data derived from isotopic assays.
Fig. 4.
ABCG1-mediated free cholesterol or cholesteryl oleyl ether (COE) influx from HDL3. BHK cells expressing ABCG1 were treated with or without mifepristone for 18 h and subsequently incubated with increasing concentrations of HDL3 labeled with either [3H] FC (A) or [3H] COE (B) for 4 h and 6 h, respectively. The values are mean ± SD of triplicate measurements. Untreated, □; mifepristone, ▴.
TABLE 1.
Differences in the medium cholesterol collected from ABCG1 positive and negative BHK cells incubated with HDL3 (25 μg/ml)
| 8 h
|
18 h
|
|||
|---|---|---|---|---|
| Untreated | Mifepristone | Untreated | Mifepristone | |
| Media FC (μg/well, n = 9)a | 1.30 ± 0.10 | 1.80 ± 0.11 | 1.49 ± 0.07 | 2.15 ± 0.09 |
| Media cpm/well (×103, n = 3)a | 42.75 ± 0.57 | 76.50 ± 1.66 | 71.01 ± 2.22 | 116.18 ± 1.22 |
| % Eflux/well (n = 3)a | 3.84 ± 0.05 | 7.35 ± 0.17 | 6.05 ± 0.21 | 11.11 ± 0.12 |
BHK cells transfected with ABCG1 were labeled with [3H] free cholesterol in 10% FBS for 24 h and upregulated with or without 10 nM mifepristone for 18 h. The cells were then incubated with HDL3 (25 μg/ml) for 8 h and 18 h, and the media were collected for measuring the radioactivity and FC mass. Statistical significance (P < 0.05) was assessed between the untreated and mifepristone-treated (ABCG1) cells at each incubation time point.
P < 0.0001 between the medium of untreated and mifepristone-treated cells at 8 h and 18 h incubation period.
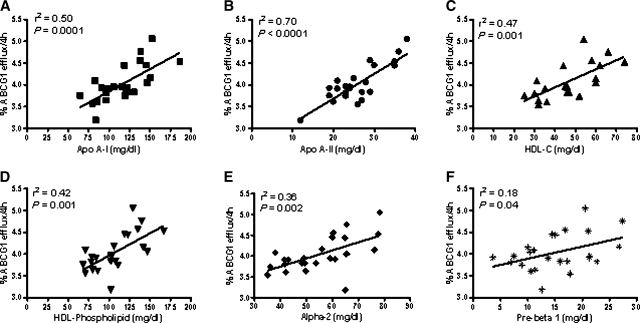
Although ABCG1 mediates FC efflux to all phospholipid-containing acceptors, HDL is thought to be the main acceptor for ABCG1-mediated efflux (14, 16, 22). We further examined what subfractions and components of HDL correlate with the ABCG1-mediated efflux by using PEG supernatants of 25 individual human sera collected from clinically healthy, normo-lipidemic subjects. Control and ABCG1-upregulated cells were incubated with the PEG supernatants for 4 h, and the ABCG1-mediated efflux was measured. We quantified the HDL components such as cholesterol, phospholipid, and apolipoproteins; and using 2D gel analysis, we characterized and measured the HDL subfractions present in the individual PEG samples. The ABCG1-mediated efflux correlated with the parameters shown in Fig. 5. All HDL components (apoA-I, apoA-II, phospholipids, and cholesterol) were significantly correlated to ABCG1 efflux. Among the HDL subfractions, the α-2 HDL subfraction demonstrated a significant correlation (Fig. 5E). A modest but significant correlation was also observed between ABCG1-mediated efflux and preβ HDL (Fig. 5F).
Fig. 5.
Correlation of ABCG1-mediated free cholesterol efflux to HDL components in 25 individual human sera. BHK cells expressing ABCG1 were incubated for 4 h with the polyethylene glycol (PEG) supernatants (3.5%) of serum samples obtained from 25 healthy individuals, and the FC efflux was measured. The HDL subfractions of the 25 PEG supernatants were determined using a 2D gel assay (see Materials and Methods). The correlations between the ABCG1-mediated efflux and HDL components were assessed using linear regression. ApoA-I, ▪ (A); apoA-II, • (B); HDL-C, ▴ (C); HDL-PL, ▾ (D); α-2, ♦ (E); preβ, asterisk (F).
We further compared the efficiencies of apoA-II and apoA-I in promoting ABCG1-mediated FC efflux by incubating the cells with increasing concentrations of either apoA-I or apoA-II rHDL discs (Fig. 6). Because there was some difference in particle size, we obtained by FPLC apoA-II-containing discs having the same size and composition as the apoA-I disc. The data were analyzed using the Michaelis-Menten equation to provide Km and Vmax values. At low acceptor concentrations (2–8 μg/ml), the apoA-I and apoA-II rHDL discs gave rise to similar fractional FC efflux, but at high concentrations (8–100 μg/ml) the apoA-II-containing discs demonstrated somewhat greater efflux. The Vmax was greater for the apoA-II particle (8.0% FC efflux/6 h vs. 4.7% FC efflux/6 h) (Fig. 6). On the other hand, the Km values were 17 μg/ml for particles containing apoA-I vs. 32 μg/ml for the apoA-II particles, indicating that ABCG1-mediated FC efflux to the apoA-I containing particles is a high affinity process. The catalytic efficiency (Vmax/Km) of FC efflux was essentially the same for both particles (apoA-I = 0.27% FC. ml/6 h.μg; apoA-II = 0.25% FC. ml/6 h.μg).
Fig. 6.
ABCG1-mediated free cholesterol efflux to rHDL discs made with apoA-I or apoA-II. BHK cells, after radiolabeling with [3H] FC for 24 h and treatment with or without mifepristone, were incubated for 6 h with reconstituted HDL (rHDL) discs made with apoA-I or apoA-II. The data are fitted to the Michaelis-Menten equation. The values are mean ± SD of triplicate measurements. ApoA-II, ▴; apoA-I, □.
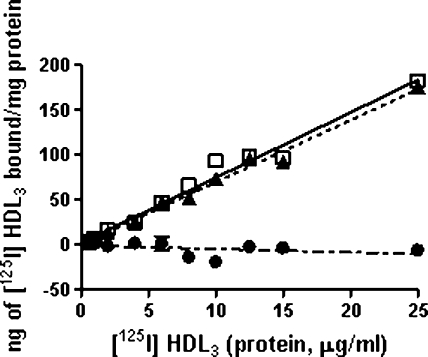
The mechanism of ABCG1-mediated FC and phospholipid efflux to HDL is unknown. To explore the role of HDL association to the transporter, BHK control and upregulated cells were incubated at 37°C for 2 h with increasing concentrations of HDL3 labeled with [125I]. As shown in Fig. 7, the amount of cell-associated [125I] HDL radioactivity was the same for control and mifepristone-treated cells. Thus, the upregulation of ABCG1 had no impact on HDL-cell association, consistent with a lack of specific binding of HDL to ABCG1.
Fig. 7.
Influence of ABCG1 expression on HDL3 association with cells. BHK cells were treated with or without mifepristone for 18 h after which they were incubated with increasing concentrations of HDL3 labeled with [125I] for 2 h at 37°C. The cell monolayers were solubilized in 0.1 M NaOH, after being washed with MEM-HEPES containing 1% BSA (3×) followed by washing with PBS (2×). Radioactivity and protein concentrations were then measured. ABCG1-specific association with [125I] HDL3 was calculated by subtracting the values for untreated from mifepristone-treated cells. The values are mean ± SD of triplicate measurements. Data were fitted by linear regression (P ≤ 0.05). Untreated, □; mifepristone, ▴; ABCG1-specific, •.
We further analyzed the ABCG1-mediated efflux reaction by monitoring the kinetics of FC efflux to HDL3 (Fig. 8). The FC efflux time courses from control and mifepristone-treated cells fitted single-phase exponential decay curves that yielded values for the efflux rate constant (ke) and pool size. The fitting of the efflux values were consistent with one kinetic pool of FC being available for efflux, and the expression of ABCG1 induced a statistically significant (P = 0.01) increase in the size of this FC pool available for efflux from 22.0 ± 1.3% to 28.1 ± 1.4%. The ke for control cells was 0.018 ± 0.001 h−1 (t1/2 = 38 h), while that for mifepristone-treated cells was 0.025 ± 0.002 h−1 (t1/2 = 28 h) (mean ± SEM, n = 6); these values are significantly different from one another (P = 0.01). Thus, the ke and pool size for FC efflux were increased upon expression of ABCG1.
Fig. 8.
Kinetics of FC efflux from control and ABCG1-upregulated cells to HDL3. After radiolabeling for 24 h with [3H] FC (15 μCi/ml), BHK cells were treated with or without mifepristone for 18 h. The cells were then incubated with HDL3 (100 μg/ml) for 8 h and the medium was collected and radioactivity measured at intervals over this period. The efflux curves for untreated (control) and mifepristone-treated cells were fitted to a mono exponential decay equation as described in Materials and Methods (r2 = 0.997). The data points are mean ± SEM (n = 6) and the error bars are contained within the symbols. Untreated, □; mifepristone, ▴.
DISCUSSION
A variety of mechanisms have been shown to be involved in the efflux of cholesterol from cells (13). These include 1) unmediated aqueous diffusion, 2) SR-BI-mediated efflux, 3) efflux via the participation of ABCA1, and most recently 4) ABCG1-mediated efflux. In the present study we have collected data that aid in the elucidation of the mechanism by which ABCG1 enhances the removal of FC from cells.
Acceptor effects
Particle size
The effects of acceptor particle size on the efflux of cellular FC have been studied previously in this laboratory (38–40). Generally, when different types of acceptor particles are compared on the basis of their phospholipid contents, smaller particles are more efficient at a given phospholipid concentration. The results in Fig. 1B show that this finding also applies to ABCG1-mediated FC efflux whereby HDL particles are much more effective than SUV. This effect occurs with fibroblast-type cells (38, 39) whereby FC efflux occurs primarily by the aqueous diffusion mechanism (5) and with cells whereby efflux involves diffusion that is facilitated by SR-BI (39, 40). These observations, combined with the results in Fig. 1C showing that larger particles are better acceptors at a given particle number concentration, are consistent with the aqueous diffusion mechanism being involved in ABCG1-mediated efflux.
From considerations of colloid theory and the aqueous diffusion mechanism of cellular FC efflux, it can be shown that the collision frequency between acceptor particles and cholesterol molecules that have desorbed from the plasma membrane of cells into a given volume of extracellular medium is a function of acceptor concentration and particle size (38). Thus, the relative efflux of cellular cholesterol to different acceptors should be normalized when expressed in terms of the product (particle number concentration x particle radius). As shown in Fig. 1D, in agreement with prior work (38), this prediction holds for SUV, LDL, and the rHDL disc, supporting the notion that the ABCG1-mediated efflux proceeds via the aqueous diffusion mechanism. Interestingly, the HDL2 and HDL3 efflux curves in Fig. 1D do not coincide with the curves for the other types of acceptor particles. The higher collision frequency required with the HDL2 and HDL3 particles to achieve a given efflux may be due to their high protein surface coverage; the lower amount of phospholipid surface could reduce the number of productive collisions by which cholesterol molecules diffusing in the aqueous phase are absorbed into the acceptor particle.
Acceptor composition
To address further the role of phospholipid in ABCG1-mediated efflux, we conducted an assay using HDL3 particles that had been enriched with either SM or PC using a published protocol in which the HDL is incubated with multilamellar phospholipid vesicles (27, 28). As can be seen from Fig. 2, keeping the particle number constant (i.e., constant HDL protein concentration) and increasing the phospholipid to protein ratio increased efflux from the control BHK and the ABCG1-upregulated cells, as has been seen with other cell systems (27, 28). However, the difference between control and mifepristone-treated cells, which represents the ABCG1 contribution to efflux, is essentially unchanged; this is in marked contrast to SR-BI-facilitated FC efflux, which is highly sensitive to HDL phospholipid composition (28, 41). Thus, increasing the phospholipid content of the medium stimulated ABCG1-mediated efflux (Fig. 1B), but changing the phospholipid content of HDL3 while maintaining a constant particle number, had no effect on ABCG1-mediated FC efflux (Fig. 2). The enrichment with phospholipid would primarily affect the surface of the HDL and did not significantly change lipoprotein size and therefore collision frequency with desorbed cholesterol molecules; these results are also consistent with ABCG1 promoting efflux by an aqueous diffusion mechanism.
Because ABCG1 is capable, to varying degrees, of releasing cellular cholesterol to all phospholipid-containing extracellular acceptors, we determined if there are specific HDL components or subfractions that are preferential acceptors of cell cholesterol provided via the ABCG1 pathway. Because apoA-II is present in HDL subfractions that also contain apoA-I (apoA-I/A-II particles), the results illustrated in Fig. 5E suggest that these particles, when present in whole serum, are efficient acceptors of cholesterol supplied by ABCG1. It should also be noted that α-2 particles carry the majority of apoA-II, and previously we have found that this particle has an important role in ABCA1 and SR-BI-mediated cholesterol efflux (36, 42). We further determined if there is an intrinsic difference between apoA-I and apoA-II in mediating ABCG1 cholesterol efflux by preparing reconstituted discs containing phospholipid and either apoA-I or apoA-II. Though the apoA-II had higher fractional of FC efflux compared with apoA-I at concentration >8 μg/ml, the catalytic efficiencies of both rHDL discs were the same (Fig. 6).
Association of HDL with ABCG1
The binding of extracellular acceptor particles plays a role in cholesterol efflux mediated by ABCA1 and SR-BI. To determine if there is a binding component in ABCG1-mediated efflux to HDL, we examined the association of [125I] HDL3 with control and mifepristone-treated cells. As can be seen from Fig. 7, there is a linear increase in the association of the radiolabeled HDL with the cells as the concentration of the HDL is increased; however, there is no difference in this association between control and ABCG1-expressing cells. Our finding of no association of HDL with ABCG1 disagrees with previously published data (43), whereby they have shown that reduced ABCG1 expression diminishes HDL binding with the RAW264.7 cell. This discrepancy may be attributed to the different cell types and experimental conditions that have been used. However, our data are consistent with the observation by Wang et al. (16), who also observed a lack of binding.
Mechanism of ABCG1-mediated efflux
Kinetics of FC efflux
An analysis of the efflux of cell cholesterol over time yielded rate constants (ke) and pool sizes for control and ABCG1-expressing BHK cells (Fig. 8). The data are consistent with a model in which the rate of desorption of cholesterol from the cell membrane is increased by ABCG1; in this experiment, the pool size of cholesterol available for efflux was increased by 6%. In a series of similar studies, the increase in size of the FC pool generated by the expression of ABCG1 was 6%–12%. This observation is consistent with previous studies that proposed that the enhanced efflux observed with cells expressing ABCG1 is linked to this protein's ability to enrich the membrane pool of cholesterol that undergoes efflux (8, 15). Further evidence that ABCG1 increases the availability of cholesterol in the plasma membrane comes from the observation that the cholesterol oxidase-sensitive pool of membrane cholesterol is expanded upon ABCG1 expression (22). Thus, the major role of ABCG1 in this BHK cell system is to enrich the plasma membrane with cholesterol, perhaps in specific pools in the membrane.
Phospholipid efflux
In addition to the efflux of cholesterol, ABCG1 expression also stimulates the release of cellular phospholipids to HDL3 and to human serum (Fig. 3). Kobayashi et al. (44) have shown that ABCG1 in HEK293 cells can mediate the efflux of cholesterol as well as choline phospholipids to HDL3, and that ABCG1 differs from ABCA1 in the type of phospholipid secreted. We have measured choline-phospholipid efflux to different acceptors (Fig. 3). We observed phospholipid efflux to whole serum and isolated HDL; however, as with cholesterol efflux, incubation with lipid-free apoA-I had no ability to promote phospholipid efflux. The fractional release of phospholipid is considerably less than that obtained with cholesterol (Fig. 3 and Fig. 1A). The reason for this probably reflects the lower aqueous solubility of phospholipid compared with cholesterol consistent with the aqueous diffusion mechanism (5) and the distribution of phospholipid in plasma and internal membranes, whereas cholesterol is enriched in the plasma membrane and thus more available for efflux (45, 46). The extent to which ABCG1-mediated phospholipid release to lipoproteins can modify the composition of the acceptor particles remains to be determined.
Influx
The general pattern of ABCG1-mediated efflux is similar to that obtained with cells expressing high levels of SR-BI. SR-BI expression enhances the passive efflux of cell FC and also stimulates the influx of FC, and the selective uptake of HDL CE (47, 48). ABCG1 activity promotes FC efflux; thus we determined if such expression also stimulates the influx of either HDL FC or CE. The results presented in Fig. 4 demonstrate that, unlike SR-BI, expression of ABCG1 has no impact on the influx of either form of cholesterol. Incubating SR-BI-expressing cells with HDL can result in either net influx or net efflux, depending on the cholesterol gradient between the HDL and the cells (4, 13). In contrast, and on a theoretical basis, if ABCG1 were the only protein participating in cholesterol flux, its expression would result in only greater net efflux when the cells were exposed to HDL. The cholesterol mass data presented in Table 1 are consistent with the isotopic data.
Physiological implications of findings
It has become increasingly obvious that there are a number of pathways for the flux of cholesterol between cells and serum lipoproteins. At least three protein-mediated pathways have been identified, and these proteins demonstrate similarities and differences. Among them are:
Acceptor specificity
ABCG1 and SR-BI require cholesterol acceptors that contain phospholipid, and the efficiency of the acceptor is determined by the composition and size of the extracellular particle. In contrast, phospholipid-free or phospholipid-poor apoproteins or helical amphipathic peptides serve as cholesterol acceptors via the ABCA1 pathway.
Binding
Cholesterol efflux via ABCA1 has an absolute requirement for the binding of apoprotein to the cell, with recent evidence indicating the efficient efflux requires binding directly to the ABCA1 protein and indirectly to other membrane domains (49, 50). There is a binding component to the SR-BI-mediated efflux of cholesterol to HDL (40, 51), particularly at low acceptor concentrations; however, there is also evidence that some cholesterol efflux occurs via aqueous diffusion (52). Our present study and the results of Wang et al. (16) indicate that acceptor binding to the donor cell is not a requirement for efflux via the ABCG1 pathway. However, in all cases the expression of efflux transporter results in changes in the organization of the lipids in the plasma membrane as evidenced by an increased susceptibility of plasma membrane cholesterol to cholesterol oxidase (22).
Bidirectional flux
The two ABC transporters participate in unidirectional flux of cholesterol between cells and extracellular acceptors, even though the nature of the acceptors differs. In contrast, SR-BI expression enhances the efflux of cell FC and also the influx of lipoprotein FC and CE.
Physiological significance
The role of cholesterol efflux in RCT has largely focused on macrophages and macrophage-derived foam cells. All three transporters are present in macrophages, although the level of expression and the contribution to efflux of SR-BI remains controversial (12, 53, 54). However, it is now well established that macrophages express ABCA1 and ABCG1, and the expression of these proteins is increased in cholesterol-enriched macrophages. There is evidence that ABCA1 and ABCG1 function coordinately, with ABCA1 initially providing phospholipid to lipid-poor apoproteins (pre-β HDL) that then become further enriched with phospholipid and cholesterol through the action of ABCG1 (21, 23, 55). It can be speculated that SR-BI, if present, could also contribute to the lipidation of newly generated HDL; however it is apparent that SR-BI plays a major role in the flux of cholesterol between HDL and hepatocytes and endocrine cells, whereas the ABC transporters provide the major pathways for removal of excess cholesterol from macrophages.
Abbreviations
ABC, ATP binding cassette
apoA-I, apolipoprotein A-I
apoA-II, apolipoprotein A-II
BHK, baby hamster kidney
CE, cholesteryl ester
CETP, cholesteryl ester transfer protein
COE, cholesteryl oleyl ether
FC, free cholesterol
FPLC, fast-protein liquid chromatography
DMPC, 1, 2-dimyristoyl-sn-glycerophosphocholine
egg PC, egg glycerophosphocholine
MLV, multilamellar vesicle
PEG, polyethylene glycol
RCT, reverse cholesterol transport
rHDL, reconstituted HDL disc
SM, sphingomyelin
SR-BI, scavenger receptor class B type I
SUV, small unilamellar vesicles
Published, JLR Papers in Press, September 30, 2008.
Footnotes
This work was supported by HL-22633 (G.H.R., M.C.P., S.L.K.), HL-63768 (G.H.R.), RO1HL055362 (J.O.) from National Institute of Health/National Heart, Lung, and Blood Institute, and Contract 53-3K06-5-10 (B.A.) from US Department of Agriculture.
References
- 1.Movva R., and D. J. Rader. 2008. Laboratory assessment of HDL heterogeneity and function. Clin. Chem. 54 788–800. [DOI] [PubMed] [Google Scholar]
- 2.Angelin B., P. Parini, and M. Eriksson. 2002. Reverse cholesterol transport in man: promotion of fecal steroid excretion by infusion of reconstituted HDL. Atheroscler. Suppl. 3 23–30. [DOI] [PubMed] [Google Scholar]
- 3.Badimon J. J., V. Fuster, and L. Badimon. 1992. Role of high density lipoproteins in the regression of atherosclerosis. Circulation. 86 86–94. [PubMed] [Google Scholar]
- 4.Rothblat G. H., M. de la Llera-Moya, V. Atger, G. Kellner-Weibel, D. L. Williams, and M. C. Phillips. 1999. Cell cholesterol efflux: integration of old and new observations provides new insights. J. Lipid Res. 40 781–796. [PubMed] [Google Scholar]
- 5.Phillips M. C., W. J. Johnson, and G. H. Rothblat. 1987. Mechanism and consequence of cellular cholesterol exchange and transfer. Biochim. Biophys. Acta. 906 223–276. [DOI] [PubMed] [Google Scholar]
- 6.Ji Y., B. Jian, N. Wang, Y. Sun, M. de la Llera Moya, M. C. Phillips, G. H. Rothblat, J. B. Swaney, and A. R. Tall. 1997. Scavenger receptor B1 promotes high density lipoprotein-mediated cellular cholesterol efflux. J. Biol. Chem. 272 20982–20985. [DOI] [PubMed] [Google Scholar]
- 7.Trigatti B. L., M. Krieger, and A. Rigotti. 2003. Influence of the HDL receptor SR-BI on lipoprotein metabolism and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 23 1732–1738. [DOI] [PubMed] [Google Scholar]
- 8.Oram J. F., and A. M. Vaughan. 2006. ATP-binding cassette cholesterol transporters and cardiovascular disease. Circ. Res. 99 1031–1043. [DOI] [PubMed] [Google Scholar]
- 9.Lee J. Y., and J. S. Parks. 2005. ATP-binding cassette transporter A1 and its role in HDL formation. Curr. Opin. Lipidol. 16 19–25. [DOI] [PubMed] [Google Scholar]
- 10.Yokoyama S. 2005. Assembly of high density lipoprotein by the ABCA1/apolipoprotein pathway. Curr. Opin. Lipidol. 16 269–279. [DOI] [PubMed] [Google Scholar]
- 11.Zannis V. I., A. Chroni, and M. Krieger. 2006. Role of apoA-I, ABCA1, LCAT, and SR-BI in the biogenesis of HDL. J. Mol. Med. 84 276–294. [DOI] [PubMed] [Google Scholar]
- 12.Van Eck M., M. Pennings, M. Hoekstra, R. Out, and T. J. C. van Berkel. 2005. Scavenger receptor BI and ATP-binding cassette transporter A1 in reverse cholesterol transport and atherosclerosis. Curr. Opin. Lipidol. 16 307–315. [DOI] [PubMed] [Google Scholar]
- 13.Yancey P. G., A. E. Bortnick, G. Kellner-Weibel, M. de la Llera-Moya, M. C. Phillips, and G. H. Rothblat. 2003. Importance of different pathways of cellular cholesterol efflux. Arterioscler. Thromb. Vasc. Biol. 23 712–719. [DOI] [PubMed] [Google Scholar]
- 14.Baldan A., P. Tarr, R. Lee, and P. A. Edwards. 2006. ATP-binding cassette transporter G1 and lipid homeostasis. Curr. Opin. Lipidol. 17 227–232. [DOI] [PubMed] [Google Scholar]
- 15.Jessup W., I. C. Gelissen, K. Gaus, and L. Kritharides. 2006. Roles of ATP binding cassette transporters A1 and G1, scavenger receptor BI and membrane lipid domains in cholesterol export from macrophages. Curr. Opin. Lipidol. 17 247–257. [DOI] [PubMed] [Google Scholar]
- 16.Wang N., D. Lan, W. Chen, F. Matsuura, and A. R. Tall. 2004. ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoprotein. Proc. Natl. Acad. Sci. USA. 101 9774–9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kennedy M. A., G. C. Barrera, K. Nakamura, A. Baldan, P. T. Tarr, M. C. Fishbein, J. Frank, O. L. Francone, and P. A. Edwards. 2005. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 1 121–129. [DOI] [PubMed] [Google Scholar]
- 18.Out R., W. Jessup, W. Le Goff, M. Hoekstra, I. C. Gelissen, Y. Zhao, L. Kritharides, G. Chimini, J. Kuiper, M. J. Chapman, et al. 2008. Coexistence of foam cells and hypocholesterolemia in mice lacking the ABC transporters AI and GI. Circ. Res. 102 113–120. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura K., M. Kennedy, A. Baldan, D. Bojanic, K. Lyons, and P. Edwards. 2004. Expression and regulation of multiple murine ATP-binding cassette transporter G1 mRNAs/isoforms that stimulate cellular cholesterol efflux to high density lipoprotein. J. Biol. Chem. 279 45980–45989. [DOI] [PubMed] [Google Scholar]
- 20.Terasaka N., N. Wang, L. Yvan-Charvet, and A. R. Tall. 2007. High-density lipoprotein protects macrophages from oxidized low-density lipoprotein-induced apoptosis by promoting efflux of 7-ketocholesterol via ABCG1. Proc. Natl. Acad. Sci. USA. 104 15093–15098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gelissen I. C., M. Harris, K. A. Rye, C. Quinn, A. J. Brown, M. Kockx, S. Cartland, M. Packiananthan, L. Kritharides, and W. Jessup. 2006. ABCA1 and ABCG1 synergize to mediate cholesterol export to apo A-I. Arterioscler. Thromb. Vasc. Biol. 26 534–540. [DOI] [PubMed] [Google Scholar]
- 22.Vaughan A. M., and J. F. Oram. 2005. ABCG1 redistributes cell cholesterol to domains removable by high density lipoprotein but not by lipid-depleted apolipoproteins. J. Biol. Chem. 280 30150–30157. [DOI] [PubMed] [Google Scholar]
- 23.Vaughan A. M., and J. F. Oram. 2006. ABCA1 and ABCG1 or ABCG4 act sequentially to remove cellular cholesterol and generate cholesterol-rich HDL. J. Lipid Res. 47 2433–2443. [DOI] [PubMed] [Google Scholar]
- 24.Hatch F. T., and R. S. Lees. 1968. Practical methods for plasma lipoprotein analysis. Adv. Lipid Res. 6 1–68. [PubMed] [Google Scholar]
- 25.Weisweiler P., C. Friedl, and M. Ungar. 1987. Isolation and quantitation of apolipoprotein A-I and A-II from human high density lipoproteins by fast-protein liquid chromatography. Clin. Chim. Acta. 169 249–254. [DOI] [PubMed] [Google Scholar]
- 26.Rothblat G. H., and M. C. Phillips. 1982. Mechanism of cholesterol efflux from cells: Effects of acceptor structure and concentration. J. Biol. Chem. 257 4775–4782. [PubMed] [Google Scholar]
- 27.Jian B., M. de la Llera-Moya, L. Royer, G. Rothblat, O. Francone, and J. B. Swaney. 1997. Modification of the cholesterol efflux properties of human serum by enrichment with phospholipid. J. Lipid Res. 38 734–744. [PubMed] [Google Scholar]
- 28.Yancey P. G., M. de la Llera-Moya, S. Swarnakar, P. Monzo, S. M. Klein, M. A. Connelly, W. J. Johnson, D. L. Williams, and G. H. Rothblat. 2000. HDL phospholipid composition is a major determinant of the bi-directional flux and net movement of cellular free cholesterol mediated by scavenger receptor-BI (SR-BI). J. Biol. Chem. 275 36596–36604. [DOI] [PubMed] [Google Scholar]
- 29.Sokoloff L., and G. H. Rothblat. 1974. Sterol to phospholipid molar ratios of L-cells with qualitative and quantitative variations of cellular sterol. Proc. Soc. Exp. Biol. Med. 146 1166–1172. [DOI] [PubMed] [Google Scholar]
- 30.Markwell M. A. K., S. M. Haas, L. L. Bieber, and N. E. Tolbert. 1978. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal. Biochem. 87 206–210. [DOI] [PubMed] [Google Scholar]
- 31.Sparks D. L., M. C. Phillips, and S. Lund-Katz. 1992. The conformation of apolipoprotein A-I discoidal and spherical recombinant high density lipoprotein particles. J. Biol. Chem. 267 25830–25838. [PubMed] [Google Scholar]
- 32.Duong P. T., G. L. Weibel, S. Lund-Katz, G. H. Rothblat, and M. C. Phillips. 2008. Characterization and properties of preß-HDL particles formed by ABCA1-mediated cellular lipid efflux to apo A-I. J. Lipid Res. 49 1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldstein J. L., S. K. Basu, and M. S. Brown. 1983. Receptor-mediated endocytosis of low-density lipoprotein in cultured cells. Methods Enzymol. 98 241–260. [DOI] [PubMed] [Google Scholar]
- 34.Bates S. R., and G. H. Rothblat. 1975. Effect of mixtures of human serum lipoproteins on cellular sterol metabolism. Artery. 1 480–494. [Google Scholar]
- 35.Duong M-N., W. Jin, I. Zanotti, E. Favari, and G. H. Rothblat. 2006. The relative contributions of ABCA1 and SR-BI to cholesterol efflux to serum from fibroblasts and macrophages. Arterioscler. Thromb. Vasc. Biol. 26 541–547. [DOI] [PubMed] [Google Scholar]
- 36.Asztalos B. F., M. de la Llera-Moya, G. E. Dallal, K. V. Horvath, E. J. Schaefer, and G. H. Rothblat. 2005. Differential effects of HDL subpopulations on cellular ABCA1- and SR-BI-mediated cholesterol efflux. J. Lipid Res. 46 2246–2253. [DOI] [PubMed] [Google Scholar]
- 37.Johnson W. J., M. J. Bamberger, M. J. Latta, R. A. Rapp, M. C. Phillips, and G. H. Rothblat. 1986. The bidirectional flux of cholesterol between cells and lipoproteins. J. Biol. Chem. 261 5766–5776. [PubMed] [Google Scholar]
- 38.DeLamatre J., G. Wolfbauer, M. C. Phillips, and G. H. Rothblat. 1986. Role of apolipoproteins in cellular cholesterol efflux. Biochim. Biophys. Acta. 875 419–428. [DOI] [PubMed] [Google Scholar]
- 39.Davidson W. S., W. V. Rodrigueza, S. Lund-Katz, W. J. Johnson, G. H. Rothblat, and M. C. Phillips. 1995. Effects of acceptor particle size on the efflux of cellular free cholesterol. J. Biol. Chem. 270 17106–17113. [DOI] [PubMed] [Google Scholar]
- 40.Thuahnai S. T., S. Lund-Katz, P. Dhanasekaran, M. de la Llera-Moya, M. A. Connelly, D. L. Williams, G. H. Rothblat, and M. C. Phillips. 2004. Scavenger receptor class B type I-mediated cholesteryl ester-selective uptake and efflux of unesterified cholesterol: Influence of high density lipoprotein size and structure. J. Biol. Chem. 279 12448–12455. [DOI] [PubMed] [Google Scholar]
- 41.Yancey P. G., M. Kawashiri, R. Moore, J. M. Glick, D. L. Williams, M. A. Connelly, D. J. Rader, and G. H. Rothblat. 2004. In vivo modulation of HDL phospholipid has opposing effects on SR-BI- an ABCA1-mediated cholesterol efflux. J. Lipid Res. 45 337–346. [DOI] [PubMed] [Google Scholar]
- 42.Yancey P. G., B. F. Asztalos, N. Stettler, D. Piccoli, D. L. Williams, M. A. Connelly, and G. H. Rothblat. 2004. SR-BI- and ABCA1-mediated cholesterol efflux to serum from patients with Alagille syndrome. J. Lipid Res. 45 1724–1732. [DOI] [PubMed] [Google Scholar]
- 43.Lorenzi I., A. von Eckardstein, S. Radosavljevic, and L. Rohrer. 2008. Lipidation of apolipoprotein A-I by ATP-binding cassette transporter (ABC) AI generates an interaction partner for ABCG1 but not for scavenger receptor BI. Biochim. Biophys. Acta. 1781 306–313. [DOI] [PubMed] [Google Scholar]
- 44.Kobayashi A., Y. Takanezawa, T. Hirata, Y. Shimizu, K. Misasa, N. Kioka, H. Arai, K. Ueda, and M. Matsuo. 2006. Efflux of sphingomyelin, cholesterol, and phosphatidylcholine by ABCG1. J. Lipid Res. 47 1791–1802. [DOI] [PubMed] [Google Scholar]
- 45.Lange Y., M. H. Swaisgood, B. V. Ramos, and T. L. Steck. 1989. Plasma membranes contain half the phospholipid and 90% of the cholesterol and sphingomyelin in cultured human fibroblasts. J. Biol. Chem. 264 3786–3793. [PubMed] [Google Scholar]
- 46.Lange Y., J. Ye, M. Rigney, and T. L. Steck. 1999. Regulation of endoplasmic reticulum cholesterol by plasma membrane cholesterol. J. Lipid Res. 40 2264–2270. [PubMed] [Google Scholar]
- 47.Williams D. L., M. A. Connelly, R. E. Temel, S. Swanakar, M. C. Phillips, M. de la Llera-Moya, and G. H. Rothblat. 1999. Scavenger receptor BI and cholesterol trafficking. Curr. Opin. Lipidol. 10 329–339. [DOI] [PubMed] [Google Scholar]
- 48.Tall A. R., P. Coster, and N. Wang. 2002. Regulation and mechanisms of macrophage cholesterol efflux. J. Clin. Invest. 110 899–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vedhachalam C., P. T. Duong, M. Nickel, D. Nguyen, P. Dhanasekaran, H. Saito, G. H. Rothblat, S. Lund-Katz, and M. C. Phillips. 2007. Mechanism of ATP-binding cassette transporter A1-mediated cellular lipid efflux to apolipoprotein A-l and formation of high density lipoprotein particles. J. Biol. Chem. 282 25123–25130. [DOI] [PubMed] [Google Scholar]
- 50.Hassan H. H., M. Denis, D-Y. D. Lee, I. Iatan, D. Nyholt, I. Ruel, L. Krimbou, and J. Genest. 2007. Identification of an ABCA1-dependent phospholipid-rich plasma membrane apolipoprotein A-I binding site for nascent HDL formation: implications for current models of HDL biogenesis. J. Lipid Res. 48 2428–2442. [DOI] [PubMed] [Google Scholar]
- 51.Xu S., M. Laccotripe, X. Huang, A. Rigotti, V. I. Zannis, and M. Krieger. 1997. Apolipoproteins of HDL can directly mediate binding to the Scavenger Receptor SR-B1, an HDL receptor that mediates selective lipid uptake. J. Lipid Res. 38 1289–1298. [PubMed] [Google Scholar]
- 52.de la Llera-Moya M., G. H. Rothblat, M. A. Connelly, G. Kellner-Weibel, S. W. Sakr, M. C. Phillips, and D. L. Williams. 1999. Scavenger receptor BI (SR-BI) mediates free cholesterol flux independently of HDL tethering to the cell surface. J. Lipid Res. 40 575–580. [PubMed] [Google Scholar]
- 53.Adorni M. P., F. Zimetti, J. T. Billheimer, N. Wang, D. J. Rader, M. C. Phillips, and G. H. Rothblat. 2007. The role of different pathways in the release of cholesterol from macrophages. J. Lipid Res. 48 2453–2462. [DOI] [PubMed] [Google Scholar]
- 54.Wang X., H. L. Collins, M. Ramalletta, I. V. Fuki, J. T. Billheimer, G. H. Rothblat, A. Tall, and D. J. Rader. 2007. Macrophage ABCA1 and ABCG1, but not SR-BI, promote macrophage reverse cholesterol transport in vivo. J. Clin. Invest. 117 2216–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tall A. R., L. Yvan-Charvet, N. Terasaka, T. A. Pagler, and N. Wang. 2008. HDL, ABC transporters, and cholesterol efflux: implications for the treatment of atherosclerosis. Cell Metab. 7 365–375. [DOI] [PubMed] [Google Scholar]
- 56.Shen B. W., A. M. Scanu, and F. J. Kezdy. 1977. Structure of human serum lipoproteins inferred from compositional analysis. Proc. Natl. Acad. Sci. USA. 74 837–841. [DOI] [PMC free article] [PubMed] [Google Scholar]