Abstract
Inflammation plays a role in trans-10, cis-12 (10,12)-conjugated linoleic acid (CLA)-mediated delipidation and insulin resistance in adipocytes. Given the anti-inflammatory role of resveratrol (RSV), we hypothesized that RSV would attenuate inflammation and insulin resistance caused by 10,12 CLA in human adipocytes. RSV blocked 10,12 CLA induction of the inflammatory response by preventing activation of extracellular signal-related kinase and induction of inflammatory gene expression (i.e., IL-6, IL-8, IL-1β) within 12 h. Similarly, RSV suppressed 10,12 CLA-mediated activation of the inflammatory prostaglandin pathway involving phospholipase A2, cyclooxygenase-2, and PGF2α. In addition, RSV attenuated 10,12 CLA increase of intracellular calcium and reactive oxygen species associated with cellular stress, and activation of stress-related proteins (i.e., activating transcription factor 3, JNK) within 12 h. 10,12 CLA-mediated insulin resistance and suppression of fatty acid uptake and triglyceride content were attenuated by RSV. Finally, 10,12 CLA-mediated decrease of peroxisome proliferator-activated receptor γ (PPARγ) protein levels and activation of a peroxisome proliferator response element (PPRE) reporter were prevented by RSV. RSV increased the basal activity of PPRE, suggesting that RSV increases PPARγ activity. Collectively, these data demonstrate for the first time that RSV prevents 10,12 CLA-mediated insulin resistance and delipidation in human adipocytes by attenuating inflammation and cellular stress and increasing PPARγ activity.
Keywords: stress, anti-inflammatory, delipidation
Feeding a mixture of conjugated linoleic acid (CLA) isomers [i.e., trans-10, cis-12 (10,12) CLA and cis-9, trans-11 (9,11) CLA] reduces adiposity in animals (1) and some humans (2). The triglyceride (TG)-lowering properties of CLA appear to be due exclusively to the 10,12 isomer (3–5), and involve decreased uptake and metabolism of glucose and fatty acids (FA)s (6), and increased lipolysis (7) in adipocytes. These anti-obesity properties of 10,12 CLA are dependent on the activation of mitogen-activated protein kinase kinase/extracellular signal-related kinase (MEK/ERK) (6) and nuclear factor κB (NFκB) (8, 9) in adipocytes. These signaling pathways induced by 10,12 CLA are linked to the induction and secretion of cytokines (8, 9), which are known to antagonize peroxisome proliferator-activated receptor γ (PPARγ) target gene expression and insulin sensitivity (10–15). Consistent with these data, 10,12 CLA supplementation of humans is associated with hyperglycemia, insulin resistance, elevated levels of inflammatory prostaglandins (PGs) and cytokines, and dyslipidemia (16–18).
Recently, supplementation of mice and 3T3-L1 adipocytes with 10,12 CLA has been shown to activate the integrated stress response (ISR) pathway (19), which is linked to inflammation, insulin resistance, and endoplasmic reticulum (ER) stress (20). Cellular stress can be caused by a relatively disproportional influx of macronutrients that adversely affect organelle function, including the mitochondria and ER. This cellular stress increases the release of calcium and reactive oxygen species (ROS), which leads to inflammation and/or insulin resistance (20). These stressors can impair the adipocyte's ability to synthesize and/or store FAs as TG, causing lipids to accumulate in nonadipocytes (e.g., hepatocytes, myotubes) and resulting in disorders like steatosis and insulin resistance, respectively (14). Therefore, it is possible that 10,12 CLA, a trans-conjugated FA not normally abundant in the diet, causes cellular stress in adipocytes, initiating a signaling cascade that adversely affects adipocyte function. These issues raise concern about the safe and effective use of supplements containing 10,12 CLA as a dietary strategy for weight loss.
Resveratrol (RSV), a phenolic phytochemical found in grapes, berries, and peanuts, has been shown to inhibit tumor necrosis factor α (TNFα)-induced inflammatory gene expression and secretion in 3T3-L1 adipocytes (21). In mice, supplementing a high-saturated-FA diet with RSV increased lifespan, motor activity, AMP kinase (AMPK), and insulin sensitivity (22) and reduced adipocyte size and metabolic disease (23), compared with a high-saturated-FA diet alone. These and other studies (24) suggest that low micromolar blood levels of RSV protect against inflammatory and stress-related diseases. However, the mechanism(s) through which RSV protects against the development of these diseases are not fully understood, and its impact on CLA-mediated inflammation is unknown.
Therefore, we wanted to determine the extent to which RSV prevented some of the side effects (i.e., inflammation, cellular stress, insulin resistance) associated with CLA supplementation. To begin to answer this question, we examined the isomer-specific influence of CLA in the absence and presence of RSV on 1) the induction or activation of genes, proteins, PGs, ROS, and intracellular calcium levels [Ca+2]i associated with inflammation and cellular stress, 2) insulin resistance, 3) FA uptake and TG content, and 4) the protein levels and activity of PPARγ.
MATERIALS AND METHODS
Materials
All cell culture ware was purchased from Fisher Scientific (Norcross, GA). Western Lightning Chemiluminescence Substrate was purchased from Perkin Elmer Life Science (Boston, MA). Immunoblotting buffers and precast gels were purchased from Invitrogen (Carlsbad, CA). DNA-free was purchased from Ambion (Austin, TX). Gene-specific primers were purchased from Applied Biosystems (Foster City, CA). Polyclonal antibodies for anti-GAPDH (sc20357), activating transcription factor 3 (ATF3; sc-188), and β-actin (sc1616) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-P (Ser505) phospholipase A2 (PLA2), anti-P (Thr183/185) c-Jun-NH2-terminal kinase (JNK), and anti-P (Thr-202/204) and total ERK1/2 antibodies were purchased from Cell Signaling Technologies (Beverly, MA). PGF2α levels were measured in conditioned media using an Enzyme Immunoassay (EIA) kit from Caymen Chemicals (Ann Arbor, MI). FBS was purchased from Hyclone (Logan, UT). The thiazolidinedione (TZD) BRL (rosiglitazone) was a generous gift from Dr. Per Sauerberg, Novo Nordisk, Denmark. Isomers of CLA (+98% pure) were purchased from Matreya (Pleasant Gap, PA) or Natural ASA (Hovdebygda, Norway). The Nucleofector and Dual Glo luciferase kits were obtained from Amaxa (Cologne, Germany) and Promega (Madison, WI), respectively. Dichlorofluorescein (DCF) and fluo-3 acetoxymethyl ester (fluo-3 AM) were purchased from Molecular Probes (Eugene, OR). All other reagents and chemicals were purchased from Sigma Chemical (St. Louis, MO) unless otherwise stated.
Culturing of human primary adipocytes
Abdominal white adipose tissue (WAT) was obtained from nondiabetic females between the ages of 20 and 50 years with a body mass index ≤30 during abdominoplasty. Consent was obtained from the Institutional Review Board at the University of North Carolina at Greensboro. Tissue was digested using collagenase, and stromal vascular cells were isolated as previously described (6). Cultures containing ∼50% preadipocytes and ∼50% adipocytes were treated between days 6 to 12 of differentiation. Each experiment was repeated at least twice at different times using a mixture of cells from 2–3 subjects unless otherwise indicated.
Preparation of FAs
Both isomers of CLA were complexed to FA-free (≥98%) BSA (Sigma #A-7030) at a 4:1 molar ratio using 1 mmol/l BSA stocks. This specific type of BSA has a relatively low inflammatory capacity compared with at least 10 different types of BSA we have tested in our lab.
Immunoblotting
Immunoblotting was conducted as we previously described (6).
Measuring [Ca2+]i levels
[Ca2+]i levels were measured using fluo-3 AM. Briefly, cells were preloaded with 5 μM fluo-3 AM and 10% Pluronic F-127, an anionic detergent, at 25°C for 30 min in the dark. Cells were then washed with a buffer consisting of HBSS, CaCl2, and probenecid, which prevents fluo-3 leakage from cells, and baseline fluorescence was measured using a Synergy Multi-detection Microplate Reader (Bio-Tek, Inc., Winooski, VT). Cells were then treated in the absence or presence of CLA or BSA vehicle in the absence and presence of RSV. Fluorescence was monitored for 10 s intervals for 5 min. Excitation wavelength was 485 nm, and fluorescence was collected at 528 nm. Changes in the ratio of calcium-dependent fluorescence over pre-stimulus background fluorescence (F/F0) are plotted over time in single representative experiments.
Measuring ROS levels
For the DCF assay, primary human adipocytes were seeded in 96-well plates and differentiated for 6 days. On day 6, media was changed to serum- and phenol red-free media for 24 h. After 24 h, cells were treated with various treatments for 3 h. Cells were then spiked and incubated with 5 μM DCF and kept at 37°C for 1 h. Cells were then washed once with HBSS, and fluorescence was immediately measured in a plate reader with an excitation/emission wavelength of 485/528 nm. DCF values were calculated after normalizing background fluorescence levels of DCF.
Transient transfections of human adipocytes
For measuring PPARγ activity, primary human adipocytes were transiently transfected with the multimerized peroxisome proliferator response element (PPRE)-responsive luciferase (luc) reporter construct pTK-PPRE3×-luc (25) using the Amaxa Nucleofector as previously described (8). On day 6 of differentiation, 1 × 106 cells from a 60 mm plate were trypsinized and resuspended in 100 μl of nucleofector solution (Amaxa) and mixed with 2 μg of pTK-PPRE3×-luc and 25 ng pRL-CMV for each sample. Electroporation was performed using the V-33 nucleofector program (Amaxa). Cells were re-plated in 96-well plates after 10 min recovery in calcium-free RPMI media. Firefly luciferase activity was measured using the Dual-Glo luciferase kit and normalized to Renilla luciferase activity from the cotransfected control pRL-CMV vector. All luciferase data are presented as a ratio of firefly luciferase to Renilla luciferase activity.
RNA isolation and real-time PCR
Total RNA was isolated from the cultures using Tri Reagent purchased from Molecular Research Center (Cincinnati, OH) according to the manufacturer's protocol. For real-time quantitative PCR (qPCR), 2.0 μg total RNA was converted into first-strand cDNA using Applied Biosystems High-Capacity cDNA Archive Kit. qPCR was performed in an Applied Biosystems 7500 FAST Real Time PCR System using Taqman Gene Expression Assays. To account for possible variation related to cDNA input or the presence of PCR inhibitors, the endogenous reference gene GAPDH was simultaneously quantified for each sample, and data were normalized accordingly.
Lipid staining
Lipid staining was conducted using Oil Red O as previously described (5). The TG levels were measured using a modified, commercially available TG assay as previously described (5). The protein content was determined using the BioRad BCA assay.
2-[3H]deoxyglucose and [14C]oleic acid uptake
Following the experimental treatments for 48 h, insulin-stimulated uptake of 2-[3H]deoxyglucose and [14C]oleic acid was measured following a 90 min incubation in the presence of 100 nmol/l human insulin as described previously (6).
Statistical analysis
Statistical analyses were performed for data in Figs. 1D, 2B, 3D, 4A, B, and 5B, C by testing the main effects of CLA (BSA, CLA) and RSV (− or + RSV) and their interaction (CLA × RSV) using two-way ANOVA (JMP version 6.03; SAS Institute, Cary, NC). For the data shown in Fig. 2C, a two-way ANOVA of the main effects Treatment (BSA, 9,11 CLA, 10,12 CLA, 10,12 CLA+RSV) and Time (6, 12, 24, 48 h) and their interactions for each dosage was conducted. A one-way ANOVA was conducted for data shown in Figs. 1C, 3B, and 6B, C. Student's t-tests were used to compute individual pairwise comparisons of least-square means (P < 0.05). Data are expressed as means ± SE.
Fig. 1.
Resveratrol (RSV) attenuates 10,12-conjugated linoleic acid (CLA) activation of ERK1/2 and induction of cytokines. Cultures of newly differentiated human adipocytes were serum starved for ∼24 h and then treated for 12 h with BSA vehicle or 50 μM 10,12 CLA in the absence (−) or presence (+) of 0, 10, 25, or 50 μM RSV (R). Subsequently, cultures were harvested for the determination of the protein levels of P-ERK1/2 and total (T)-ERK1/2 by immunoblot (A, B) or mRNA levels for IL-6, IL-8, and IL-1β by real-time quantitative PCR (qPCR) (C, D). A, B: Data are representative of one (A) or at least three (B) independent experiments. C, D: Data are representative of one (C) or at least three (D) independent experiments. Means (±SE; n = 2 for C, and n = 3 for D) not sharing a lower-case letter differ significantly (P < 0.05). The RSV dose in B and D was 50 μM.
Fig. 2.
RSV attenuates 10,12 CLA activation of the inflammatory prostaglandin pathway [A, PLA2; B, cyclooxygenase-2 (COX-2); C, PGF2α]. Cultures of newly differentiated human adipocytes were serum starved for ∼24 h and then treated for either 12 h (A and B) or 6, 12, 24, or 48 h (C) with BSA vehicle or 50 μM 10,12 CLA in the absence (−) or presence (+) of 50 μM RSV (R). Subsequently, cultures were harvested for the determination of the protein levels of P-PLA2 and total (T)-PLA2 by immunoblot (A), mRNA levels for COX-2 by real-time qPCR (B), or the secreted levels of PGF2α in conditioned media by enzyme immunoassay (C). A: Data are representative of at least three independent experiments. B: Means (±SE; n = 3) not sharing a lower-case letter differ significantly (P < 0.05), and are representative of at least three independent experiments. C: Means (±SE; n = 4) with asterisks (*) differ significantly (P < 0.05) from the BSA controls at each time point, and are representative of at least two independent experiments. Means with number sign (#) are significantly lower than cultures treated with 10,12 CLA alone.
Fig. 3.
RSV attenuates 10,12 CLA increase in intracellular calcium and indicators of cellular stress. A: Cultures of newly differentiated human adipocytes were serum starved for ∼24 h and then preloaded with 5 μM fluo-3 acetoxymethyl ester. Subsequently, cultures were injected with 30 μM 10,12 CLA (closed triangle), 30 μM 10,12 CLA + 50 μM RSV (CLA+R, open triangle), BSA vehicle (BSA, closed circle), or BSA+RSV (BSA+R, open circle). Emitted fluorescence intensities were collected over time using a multi-detection microplate reader. Excitation wavelength was 485 nm and fluorescence was collected at 528 nm. Means (±SE; n = 12) are representative of three independent experiments. B: Cultures of newly differentiated human adipocytes were serum starved for ∼24 h and then treated with 50 μM (open bar) or 150 μM (closed bar) BSA, 9,11 CLA(9), 10,12 CLA(10), 10,12 CLA + 50 μM RSV (10+R), or 50 μM RSV alone(R) for 3 h. Cultures were then loaded with dichlorofluorescein (DCF) for 1 h, and emitted fluorescence intensities were measured using a multi-detection microplate reader. Excitation wavelength was 485 nm and fluorescence was collected at 528 nm. Means (±SE; n = 3–12) are representative of three independent experiments. C, D: Cultures of newly differentiated human adipocytes were serum starved for ∼24 h and then treated for 12 h with BSA vehicle or 50 μM 10,12 CLA in the absence (−) or presence (+) of 50 μM RSV (R). Subsequently, cultures were harvested for the determination of the protein levels of P-JNK, total (T)-JNK, activating transcription factor 3 (ATF3), and β-actin by immunoblot (C) or mRNA levels for ATF3 by real-time qPCR (D). C: Data are representative of at least three independent experiments. D: Means (±SE; n = 3) not sharing a lower-case letter differ significantly (P < 0.05), and are representative of at least two independent experiments.
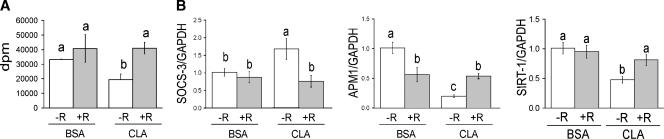
Fig. 4.
RSV blocks 10,12 CLA-mediated insulin resistance. Cultures of newly differentiated human adipocytes were serum starved for ∼24 h and then treated for either 48 h (A) or 24 h (B) with BSA vehicle or 50 μM 10,12 CLA in the absence (−) or presence (+) of 50 μM RSV (R). A: Insulin-stimulated glucose uptake using 2-[3H]deoxyglucose was measured after a 90 min incubation in the presence of insulin. Means (±SE; n = 6) not sharing a lower-case letter differ significantly (P < 0.05). B: The mRNA levels of suppressor of SOCS-3, adiponectin (APM-1), and sirtuin 1 (SIRT-1) were measured by real-time qPCR. Means (±SE; n = 3) not sharing a lower-case letter differ significantly (P < 0.05) and are representative of at least two independent experiments.
Fig. 5.
RSV attenuates delipidation by 10,12 CLA. Cultures of newly differentiated human adipocytes were treated in adipocyte media for 7 days with BSA vehicle or 50 μM 10,12 CLA in the absence (−) or presence (+) of 50 μM RSV (R). Fresh media containing treatments were changed every 2 days. Cultures were then either stained with Oil Red O and phase-contrast photomicrographs were taken using an Olympus inverted microscope with a 10× objective (A) or their triglyceride (TG) content was measured using a commercially available TG assay kit (B). A: Data are representative of two independent experiments. B: Means (±SE; n = 6) not sharing a lower-case letter differ significantly (P < 0.05). C: Cultures of newly differentiated human adipocytes were serum starved for ∼24 h and then treated for 48 h with BSA vehicle or 50 μM 10,12 CLA in the absence (−) or presence (+) of 50 μM RSV (R). [14C]oleic acid uptake was measured after 90 min incubation in the presence of insulin. Means (±SE; n = 3) not sharing a lower-case letter differ significantly (P < 0.05).
Fig. 6.
RSV inhibits 10,12 CLA suppression of peroxisome proliferator-activated receptor γ (PPARγ) activity. Cultures of newly differentiated human adipocytes were serum starved for ∼24 h and then treated for 24 h with BSA vehicle or 50 μM 10,12 CLA in the absence (−) or presence (+) of 50 μM RSV (R). Subsequently, cultures were harvested for the determination of PPARγ and β-actin protein levels by immunoblot (A). B: Cultures of newly differentiated human adipocytes were transfected on day 6 with pTK-PPRE3×-luc and pRL-CMV. Transfected cells were treated with BSA vehicle, 50 μM 10,12 CLA, or 50 μM 10,12 CLA + 50 μM RSV (R) for 24 h. Subsequently, cultures were treated with 0.1 μM BRL for 28 h, and then the luciferase activation of the reporter was measured using a luminometer (±SE; n = 3). C: Transfected cells were treated with 1, 10, or 50 μM RSV for 24 h, and then the luciferase was measured as in B (±SE; n = 3). Means (±SE; n = 3) not sharing a lower-case letter differ significantly (P < 0.05). Data in A–C are representative of at least two independent experiments.
RESULTS
RSV blocks 10,12 CLA induction of inflammation and stress-related signaling
A preliminary dose response study showed that 50 μM RSV decreased most effectively the activation of ERK1/2 (Fig. 1A) and JNK (data not shown), and expression of inflammatory genes (Fig. 1C) caused by 10,12 CLA. Thus, we examined the extent to which 50 μM RSV prevented inflammation caused by 50 μM 10,12 CLA in human adipocytes. RSV attenuated 10,12 CLA activation of ERK1/2 (Fig. 1B) and induction of IL-6, IL-8, and IL-1β gene expression (Fig. 1D) within 12 h. Similarly, RSV blocked 10,12 CLA induction of the inflammatory PG pathway [Fig. 2A, PLA2; Fig. 2B, cyclooxygenase-2 (COX-2); Fig. 2C, PGF2α]. Next, we examined the influence of RSV on [Ca+2]i and ROS levels in CLA-treated cultures. RSV attenuated the rapid, 10,12 CLA increase in [Ca+2]i and ROS (Fig. 3A, [Ca+2]i, Fig. 3B, ROS), events directly linked to cellular stress, inflammation, and insulin resistance (20, 26). Similarly, RSV attenuated 10,12 CLA activation of the stress-related proteins ATF3 and JNK (Fig. 3C), and the mRNA levels of ATF3 (Fig. 3D). RSV alone increased the levels of ATF3 mRNA and protein. Taken together, these data demonstrate that RSV attenuates 10,12 CLA-mediated inflammation and cellular stress in cultures of human adipocytes.
10,12 CLA suppression of insulin sensitivity, FA uptake, and TG content are prevented by RSV
We previously demonstrated that 10,12 CLA causes insulin resistance, dependent on the activation of ERK1/2 (6) and NFκB (8). Given the insulin-sensitizing effects reported for RSV in rodents (22, 23, 27), we speculated that RSV would improve insulin sensitivity in cultures of human adipocytes treated with 10,12 CLA. Indeed, 10,12 CLA-treated cultures co-supplemented with 50 μM RSV had higher levels of insulin-stimulated glucose uptake, compared with cultures treated with 10,12 CLA alone (Fig. 4A). Consistent with these data, RSV blocked 10,12 CLA induction of SOCS-3, a protein that causes insulin resistance through serine phosphorylation of IRS-1. RSV also attenuated 10,12 CLA suppression of adiponectin and sirtuin 1 (SIRT1) mRNA levels, compared with cultures treated with 10,12 CLA alone, suggesting that RSV enhances glucose and/or FA metabolism in these cultures (Fig. 4B). Similarly, RSV co-supplementation attenuated delipidation and suppression of FA uptake by 10,12 CLA (Fig. 5A–C). Thus, RSV may enhance insulin-stimulated glucose and FA uptake in CLA-treated cultures by upregulating genes that stimulate metabolism.
10,12 CLA suppression of PPARγ inhibited by RSV
To determine the mechanism by which RSV improves insulin sensitivity and reduces delipidation, we examined the extent to which RSV enhanced the protein levels and activity of PPARγ, a transcription factor that enhances glucose and FA uptake and utilization. RSV prevented CLA-mediated decrease of PPARγ protein levels (Fig. 6A). To determine whether RSV blocks CLA suppression of PPARγ activity, the ligand-induced activation of a PPRE-luciferase reporter construct was measured. 10,12 CLA-mediated suppression of the BRL-activated PPRE reporter was prevented by co-supplementation with RSV (Fig. 6B), suggesting that RSV prevents CLA suppression of ligand-stimulated PPARγ activity. Concordantly, RSV increased the activation of the PPRE reporter in a dose-dependent manner in the absence of BRL (Fig. 6C). Collectively, these data support our hypothesis that RSV enhances insulin sensitivity and TG content of 10,12 CLA-treated human adipocytes by increasing the activity and/or protein expression of PPARγ.
DISCUSSION
Feeding mixed isomers of CLA, or 10,12 CLA alone, has been shown to reduce body fat and the TG content of adipocytes, especially in murine models (1). However, adverse metabolic complications (i.e., inflammation, ISR, insulin resistance) have been reported with CLA supplementation of humans, particularly for the 10,12 isomer (16–19). In this article, we demonstrate for the first time that RSV, a phytoalexin with antioxidant properties, attenuates markers of inflammation, cellular stress, ROS production, insulin resistance, and delipidation in cultures of newly differentiated human adipocytes treated with 10,12 CLA. Central to this mechanism is our discovery that RSV blocks 10,12 CLA-mediated 1) increase in [Ca+2]i, which is essential for CLA-mediated inflammation and cellular stress (28), and 2) suppression of PPARγ activity in primary cultures of human adipocytes treated with 10,12 CLA.
On the basis of these and our previously (un)published data, we propose the following working model (Fig. 7) by which RSV prevents 10,12 CLA-mediated inflammation (Figs. 1, 2), cellular stress (Fig. 3), insulin resistance (Fig. 4), and, ultimately, delipidation (Fig. 5). We speculate that RSV initially blocks CLA-mediated ROS production or accumulation, and release of calcium from the ER. This prevents cellular stress initiated by ROS and calcium signaling. Without these signals to activate MAPKs (e.g., ERK, JNK) and other inflammatory proteins (e.g., NFκB, COX-2, PLA2) or PGs (e.g., PGF2α), 1) inflammatory cytokines (e.g., IL-6, IL-1β) and chemokines (IL-8) are not induced, 2) insulin signaling is not disrupted, and 3) the protein levels and activity of PPARγ and the TG levels are preserved (Figs. 5, 6). This allows for normal insulin signaling, glucose and FA uptake and metabolism, and TG accumulation in adipocytes.
Fig. 7.
Working model. RSV initially blocks 10,12 CLA-mediated increase in the levels of reactive oxygen species (ROS) and intracellular calcium ([Ca+2]i), thereby preventing ROS and calcium signaling. Without these signals to activate ERK, JNK, nuclear factor κB (NFκB), inflammatory cytokines, and prostaglandins, PPARγ activity is not suppressed. This allows for normal insulin signaling, glucose and FA uptake and metabolism, and TG accumulation in adipocytes. RSV may also directly activate PPARγ.
Consistent with our data in human adipocytes, 50–150 μM RSV has been shown in vitro to reduce inflammation in murine 3T3-L1 adipocytes (21), to reduce oxidative stress in human lung epithelial cells (29, 30), to reduce ER stress in mouse macrophages (31), to decrease TNFα-mediated NFκB activation in hepatocytes (32) and coronary arterial endothelial cells (33), and to enhance glucose transport in muscle (34). Thus, RSV reduces inflammation and enhances glucose and FA utilization in vivo and in vitro, although the mechanism is unknown.
Further support for our working model comes from studies showing that RSV decreases [Ca+2]i levels following stimulation with various inflammatory agents or disease states. For example, elevated levels of [Ca+2]i induced by severe acute pancreatitis were attenuated by RSV (35). Similarly, RSV reduced [Ca+2]i levels in stress-induced oxygen-glucose deprivation/reperfusion in primary neurons of neonatal rats (36). Finally, trans-RSV prevented platelet aggregation by inhibiting elevated [Ca+2]i levels (37). Thus, RSV suppression of the levels of ROS and [Ca+2]i is an important mechanism through which RSV prevents these deleterious side effects of 10,12 CLA.
Alternatively, RSV may suppress inflammation by activating PPARγ. Support for this hypothesis comes from studies showing that RSV activates PPARγ in CaCo2 cells (38) and macrophages (39). Additionally, our data show that RSV robustly induces PPARγ activity and prevents CLA-mediated suppression of TZD-induced PPARγ activation (Fig. 6). Further support comes from studies showing that compounds that enhance PPARγ activity and insulin sensitivity antagonize NFκB-mediated signaling, and vice versa (10, 11, 40, 41). For example, TZDs, which are high-affinity PPARγ ligands, suppress inflammation (40, 41). In contrast, PPARγ depletion via RNA interference enhances the inflammatory responses of TNFα (42). Consistent with these data, rosiglitazone prevents CLA-mediated insulin resistance and hepatic steatosis in rats (14, 15) and delipidation of human adipocytes (13).
One proposed mechanism for the anti-inflammatory actions of PPARγ ligands is via (trans)repression of inflammatory gene transcription (43–46). Activation of PPARγ can repress the transcriptional activation of inflammatory genes by 1) direct interaction with the mediator of transcription (i.e., NFκB, JNK), 2) inhibition of its co-activator recruitment, or 3) inhibition of its co-repressor clearance. For an example of transrepression, ligand-activated PPARγ becomes SUMOylated, and then binds to co-repressor complexes at inflammatory gene promoters, thereby inhibiting dismissal of nuclear receptor corepressor/histone deacetylase NCoR/HDAC, which blocks inflammatory gene transcription (43, 44). Notably, this pathway does not interfere with transactivation of PPARγ-responsive genes (43, 44). Studies are under way to determine whether this is the mechanism through which RSV prevents inflammation in CLA-treated adipocytes.
RSV has also been shown to enhance insulin sensitivity and glucose uptake in C2C12 myotubes by enhancing AMPK activity (40). AMPK is activated by adiponectin, and by SIRT1 and PGC1α (23, 27). These events are directly linked to mitochondrial biogenesis and oxidative metabolism, which are positively regulated by PPARα. Consistent with these data, RSV prevented or attenuated insulin resistance and the suppression of SIRT1 and adiponectin mRNA levels in CLA-treated adipocytes (Fig. 4). This effect of RSV may be responsible for increasing glucose and FA uptake for oxidative metabolism, which we have previously shown is suppressed by 10,12 CLA (6). However, we do not know whether this was due to increased SIRT1 or PGC-1α activation by RSV.
SIRT1 activation appears to be an important means through which RSV enhances glucose and FA utilization, at least in muscle. SIRT1 increases the activation of PGC-1α and FOXO1-C, thereby increasing mitochondrial biogenesis and oxidative metabolism (47). Furthermore, several studies have demonstrated that feeding 20–400 mg/kg body weight/day of RSV prevents insulin resistance and adiposity in mice fed a high-fat diet (22, 23). These studies suggest that RSV shifts excess calories away from storage in WAT and toward oxidation in muscle and brown adipose tissue, in part by activating SIRT1. In contrast to our hypothesis that RSV activates PPARγ, SIRT1 overexpression in murine adipocytes decreased PPARγ activity, and treatment of murine adipocytes with 50–100 μM RSV enhanced FA mobilization and release. However, PPARγ activities in WAT of mice or in cultures of adipocytes treated with RSV were not investigated (48). Thus, SIRT1 activation by RSV is clearly linked to enhanced glucose and FA metabolism in muscle, and involves AMPK activation. However, the effects of RSV on SIRT1 activation and adipocyte metabolism are still unclear, and differences in SIRT1 regulation by RSV between mouse and human adipocytes are unknown.
Taken together, these data demonstrate that RSV prevents 10,12 CLA-mediated inflammation, insulin resistance, and delipidation of human adipocytes. Potential anti-inflammatory mechanisms for RSV include preventing CLA-mediated ROS accumulation and release of calcium from the ER, which are associated with cellular stress and inflammation, and antagonism of PPARγ activity. Alternatively, RSV may be directly activating PPARγ, which has the potential to (trans)repress inflammatory gene transcription. Studies are under way to examine these proposed mechanisms.
Abbreviations
AMPK, AMP kinase
ATF3, activating transcription factor 3
[Ca+2]i, intracellular calcium
CLA, conjugated linoleic acid
COX, cyclooxygenase
DCF, dichlorofluorescein
ER, endoplasmic reticulum
ERK, extracellular signal-related kinase
fluo-3 AM, fluo-3 acetoxymethyl ester
ISR, integrated stress response
JNK, c-Jun-NH2-terminal kinase
NFκB, nuclear factor κB
PG, prostaglandin
PLA2, phospholipase A2
PPAR, peroxisome proliferator-activated receptor
PPRE, peroxisome proliferator response element
ROS, reactive oxygen species
RSV, resveratrol
SIRT1, sirtuin 1
TG, triglyceride
TZD, thiazolidinedione
WAT, white adipose tissue
Published, JLR Papers in Press, September 5, 2008.
Footnotes
This work was supported by National Institutes of Health Grant 5R01 DK-063070-06, North Carolina Agriculture Research Service Grant NCARS 06771, and Cognis GmbH (M.M.), a fellowship award from the National Institutes of Health (F31DK076208), and the United Negro College Fund-Merck (A.K.).
References
- 1.House R., J. Cassady, E. Eisen, M. McIntosh, and J. Odle. 2005. Conjugated linoleic acid evokes delipidation through the regulation of genes controlling lipid metabolism in adipose tissue and liver. Obes. Rev. 6 247–258. [DOI] [PubMed] [Google Scholar]
- 2.Whigman L., A. Watras, and D. Schoeller. 2007. Efficacy of conjugated linoleic acid for reducing fat mass: a meta-analysis in humans. Am. J. Clin. Nutr. 85 1203–1211. [DOI] [PubMed] [Google Scholar]
- 3.Park Y., J. Storkson, K. Albright, W. Liu, and M. Pariza. 1999. Evidence that trans-10, cis-12 isomer of conjugated linoleic acid induces body composition changes in mice. Lipids. 34 235–241. [DOI] [PubMed] [Google Scholar]
- 4.Brown J. M., Y. D. Halverson, R. Lea-Currie, C. Geigerman, and M. McIntosh. 2001. Trans-10, cis-12, but not cis-9, trans-11, conjugated linoleic acid attenuates lipogenesis in primary cultures of stromal vascular cells from human adipose tissue. J. Nutr. 131 2316–2321. [DOI] [PubMed] [Google Scholar]
- 5.Brown M., M. Sandberg-Boysen, S. Skov, R. Morrison, J. Storkson, R. Lea-Currie, M. Pariza, S. Mandrup, and M. McIntosh. 2003. Isomer-specific regulation of metabolism and PPARγ by conjugated linoleic acid (CLA) in human preadipocytes. J. Lipid Res. 44 1287–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown J. M., M. Sandberg-Boysen, S. Chung, O. Fabiyi, R. Morrision, S. Mandrup, and M. McIntosh. 2004. Conjugated linoleic acid (CLA) induces human adipocyte delipidation: autocrine/paracrine regulation of MEK/ERK signaling by adipocytokines. J. Biol. Chem. 279 26735–26747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung S., J. M. Brown, and M. McIntosh. 2005. Trans-10, cis-12 CLA increases adipocyte lipolysis and alters lipid droplet-associated proteins: role of mTOR and ERK signaling. J. Lipid Res. 46 885–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung S., J. M. Brown, J. N. Provo, R. Hopkins, and M. McIntosh. 2005. Conjugated linoleic acid promotes human adipocyte insulin resistance through NFκB-dependent cytokine production. J. Biol. Chem. 280 38445–38456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poirier H., J. Shapiro, R. Kim, and M. Lazar. 2006. Nutritional supplementation with trans-10, cis-12 conjugated linoleic acid induces inflammation of white adipose tissue. Diabetes. 55 1634–1641. [DOI] [PubMed] [Google Scholar]
- 10.Suzawa M., I. Takada, J. Yanagisawa, F. Ohtake, S. Ogawa, T. Yamauchi, T. Kadowaki, Y. Takeuchi, H. Shibuya, Y. Gotoh, et al. 2003. Cytokines suppress adipogenesis and PPAR-gamma function through the TAK1/TAB1/NIK cascade. Nat. Cell Biol. 5 224–230. [DOI] [PubMed] [Google Scholar]
- 11.Adams M., M. Reginato, D. Shao, M. Lazar, and V. Chatterjee. 1997. Transcriptional activation by peroxisome proliferator activated receptor gamma is inhibited by phosphorylation at a consensus mitogen-activated protein kinase site. J. Biol. Chem. 272 5128–5132. [DOI] [PubMed] [Google Scholar]
- 12.Chung S., K. LaPoint, A. Kennedy, K. Martinez, M. Boysen-Sandberg, and M. McIntosh. 2006. Preadipocytes mediate lipopolysaccharide-induced inflammation and insulin resistance in primary cultures of newly differentiated human adipocytes. Endocrinology. 147 5340–5351. [DOI] [PubMed] [Google Scholar]
- 13.Kennedy A., S. Chung, K. LaPoint, O. Fabiyi, and M. McIntosh. 2008. Trans-10, cis-12 conjugated linoleic acid antagonizes ligand-dependent PPARγ activity in primary cultures of human adipocytes. J. Nutr. 138 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lui L-F., A. Purushotham, A. Wendel, and M. Belury. 2007. Combined effects of rosiglitazone and conjugated linoleic acid on adiposity, insulin sensitivity, and heptatic steatosis in high fat-fed mice. Am. J. Physiol. 292 G1671–G1682. [DOI] [PubMed] [Google Scholar]
- 15.Purushotham A., A. Wendel, L-F. Lui, and M. Belury. 2007. Maintenance of adiponectin attenuates insulin resistance induced by dietary conjugated linoleic acid. J. Lipid Res. 48 444–452. [DOI] [PubMed] [Google Scholar]
- 16.Riserus U., S. Basu, S. Jovinge, G. Fredrickson, J. Arnlov, and B. Vessby. 2002. Supplementation with conjugated linoleic acid causes isomer-dependent oxidative stress and elevated C-reactive protein. Circulation. 106 1925–1929. [DOI] [PubMed] [Google Scholar]
- 17.Riserus U., P. Arner, K. Brismar, and B. Vessby. 2002. Treatment with dietary trans-10 cis-12 conjugated linoleic acid causes isomer-specific insulin resistance in obese men with the metabolic syndrome. Diabetes Care. 25 1516–1521. [DOI] [PubMed] [Google Scholar]
- 18.Tholstrup, T., M. Raff, E. Straarup, P. Lund, J. Bruun, and S. Basur. 2007. Opposing effects of trans-10, cis-12 conjugated linoleic acid and cis-9, trans-11 conjugated linoleic acid on risk markers for coronary heart disease. FASEB J. 21: 701.4, A728.
- 19.LaRosa P., J. Riethoven, H. Chen, Y. Xia, Y. Xhou, M. Chen, J. Miner, and M. Fromm. 2007. Trans-10, cis-12 conjugated linoleic acid activates the integrated stress response pathway in adipocytes. Physiol. Genomics. 31 544–553. [DOI] [PubMed] [Google Scholar]
- 20.Gregor M., and G. Hotamisligil. 2007. Adipocyte stress: the endoplasmic reticulum and metabolic disease. J. Lipid Res. 48 1905–1914. [DOI] [PubMed] [Google Scholar]
- 21.Ahn J., H. Lee, S. Kim, and T. Ha. 2007. Resveratrol inhibits TNFa-induced changes in adipokines in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 364 972–977. [DOI] [PubMed] [Google Scholar]
- 22.Baur J., K. Pearson, N. Price, H. Jamieson, C. Lerin, A. Kalra, V. V. Prabhu, J. S. Allard, G. Lopez-Lluch, K. Lewis, et al. 2006. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 444 337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lagouge M., C. Argmann, Z. Gerhart-Hines, H. Meziane, C. Lerin, F. Daussin, N. Messadeq, J. Milne, P. Lambert, P. Elliott, et al. 2006. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell. 127 1109–1122. [DOI] [PubMed] [Google Scholar]
- 24.Saiko P., A. Szakmary, W. Jaeger, and T. Szekeres. 2008. Resveratrol and its analogs: defense against cancer, coronary disease and neurodegenerative maladies or just a fad? Mutat. Res. 658 68–94. [DOI] [PubMed] [Google Scholar]
- 25.Kliewer S., K. Umesono, D. Noonan, R. Heyman, and R. Evans. 1992. Convergence of 9-cis retinoic acid and peroxisome proliferators signaling pathways through heterodimer formation of their receptors. Nature. 358 771–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Houstis N., E. Rosen, and E. Lander. 2006. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 440 944–948. [DOI] [PubMed] [Google Scholar]
- 27.Sun C., F. Zhang, X. Ge, T. Yan, X. Chen, X. Shi, and Q. Zhai. 2007. SIRT1 improves insulin sensitivity under insulin resistance conditions by repressing PTP1B. Cell Metab. 6 307–319. [DOI] [PubMed] [Google Scholar]
- 28.Kennedy A., S. Chung, R. Hopkins, K. LaPoint, and M. McIntosh. 2007. Inflammation and delipidation induced by trans-10, cis-12 conjugated linoleic acid (CLA) are linked to intracellular calcium accumulation in primary cultures of human adipocytes (Abstract). FASEB J. 21 703. [Google Scholar]
- 29.Kode A., S. Rajendrasozhan, S. Caito, S. Yang, L. Megson, and I. Rahman. 2008. Resveratrol induces glutathione synthesis by activation of Nrf2 and protects against cigarette smoke-mediated oxidative stress in human lung epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 294 L478–L488. [DOI] [PubMed] [Google Scholar]
- 30.Robb E., M. Page, B. Wiens, and J. Stuart. 2008. Molecular mechanisms of oxidative stress induced by resveratrol: specific and progressive induction of MnSOD. Biochem. Biophys. Res. Commun. 376 406–412. [DOI] [PubMed] [Google Scholar]
- 31.Tabata Y., K. Takano, T. Ito, M. Iinuma, T. Yoshimoto, H. Miura, Y. Kitao, S. Ogawa, and O. Hori. 2007. Vaticanol B. a resveratrol tetramer, regulates endoplasmic stress and inflammation. Am. J. Physiol. Cell Physiol. 293 C411–C418. [DOI] [PubMed] [Google Scholar]
- 32.Yu H., C. Pan, S. Zhao, Z. Wang, H. Zhang, and W. Wu. 2008. Resveratrol inhibits tumor necrosis factor-alpha-mediated matrix metalloproteinase-9 expression and invasion of human heptacellular carcinoma cells. Biomed. Phamacol. 62 366–372. [DOI] [PubMed] [Google Scholar]
- 33.Csiszar A., K. Smith, N. Labinskyy, Z. Orosz, A. Rivera, and Z. Ungvari. 2006. Resveratrol attenuates TNF-alpha induced activation of coronary arterial endothelial cells: role of NF-kB inhibition. Am. J. Physiol. 291 H1694–H1699. [DOI] [PubMed] [Google Scholar]
- 34.Park C., M. Kim, H. Lee, B. Min, H. Bae, W. Choe, S. Kim, and J. Ha. 2007. Resveratrol stimulates glucose transport in C2C12 myotubes by activating AMP-activated protein kinase. Exp. Mol. Med. 39 222–229. [DOI] [PubMed] [Google Scholar]
- 35.Wang L., Q. Ma, X. Chen, H. Sha, and Z. Ma. 2008. Effects of resveratrol on calcium regulation in rats with severe acute pancreatitis. Eur. J. Pharmacol. 580 271–276. [DOI] [PubMed] [Google Scholar]
- 36.Gong Q. H., Q. Wang, J. S. Shi, X. N. Huang, Q. Liu, and H. Ma. 2007. Inhibition of caspases and intracellular free Ca2+ concentrations are involved in resveratrol protection against apoptosis in rat primary neuron cultures. Acta Pharmacol. Sin. 28 1724–1730. [DOI] [PubMed] [Google Scholar]
- 37.Dobrydneva Y., R. Williams, and P. Blackmore. 1999. trans-Resveratrol inhibits calcium influx in thrombin-stimulated human platelets. Br. J. Pharmacol. 128 149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ulrich S., S. Loitsch, O. Rau, A. Von Knethen, B. Brune, M. Zsilavecz, and J. Stein. 2006. Peroxisome proliferators-activated receptor gamma as a molecular target of resveratrol-induced modulation of polamine metabolism. Cancer Res. 66 7348–7354. [DOI] [PubMed] [Google Scholar]
- 39.Ge H., J. F. Zhang, B. S. Guo, Q. He, B. Y. Wang, B. He, and C. Q. Wang. 2006. Resveratrol inhibits macrophage expression of EMMPRIN by activating PPARγ. Vascul. Pharmacol. 46 114–121. [DOI] [PubMed] [Google Scholar]
- 40.Ruan H., H. Pownall, and H. Lodish. 2003. Troglitazone antagonizes tumor necrosis factor-a induced reprogramming of adipocyte gene expression by inhibiting the transcriptional regulatory functions of NFkB. J. Biol. Chem. 278 28181–28192. [DOI] [PubMed] [Google Scholar]
- 41.Nie M., L. Corbett, A. Knox, and L. Pang. 2005. Differential regulation of chemokine expression by peroxisome proliferator-activated receptor gamma agonists: interactions with glucocorticoids and beta2-agonists. J. Biol. Chem. 280 2550–2561. [DOI] [PubMed] [Google Scholar]
- 42.Liao W., M. Nguyen, T. Yoshizaki, S. Favelyukis, D. Patsouris, T. Imamura, I. M. Verma, and J. M. Olefsky. 2007. Suppression of PPARgamma attenuates insulin-stimulated glucose uptake by affecting both GLUT1 and GLUT4 in 3T3-L1 adipocytes. Am. J. Physiol. Endocrinol. Metab. 293 E219–E227. [DOI] [PubMed] [Google Scholar]
- 43.Pascual G., A. Fong, S. Ogawa, A. Gamliel, A. C. Li, V. Perissi, D. W. Rose, T. M. Willson, M. G. Rosenfeld, and C. K. Glass. 2005. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPARgamma. Nature. 437 759–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ricote M., and C. Glass. 2007. PPARs and molecular mechanisms of transrepression. Biochim. Biophys. Acta. 1771 926–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Straus D., and C. Glass. 2007. Anti-inflammatory actions of PPAR ligands: new insights on cellular and molecular mechanisms. Trends Immunol. 28 551–558. [DOI] [PubMed] [Google Scholar]
- 46.Ghisletti S., W. Huang, S. Ogawa, G. Pascual, M. E. Lin, T. M. Wilson, M. G. Rosenfield, and C. K. Glass. 2007. Parallel SUMOylation-dependent pathways mediate gene- and signal-specific transrepression by LXRs and PPARgamma. Mol. Cell. 25 57–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qiao L., and J. Shao. 2006. SIRT1 regulates adiponectin gene expression through Foxo1-C/enhancer-binding protein alpha transcriptional complex. J. Biol. Chem. 281 39915–39924. [DOI] [PubMed] [Google Scholar]
- 48.Picard F., M. Kurtev, N. Chung, A. Topark-Ngarm, T. Senawong, R. Machado De Oliveira, M. Leid, M. W. McBurney, and L. Guarente. 2004. SIRT1 promotes fat mobilization in white adipocytes by repressing PPARgamma. Nature. 429 771–776. [DOI] [PMC free article] [PubMed] [Google Scholar]