Abstract
Oxidized-1-palmitoyl-2-arachidonyl-sn-glycerol-3-phosphocholine (Ox-PAPC) has been demonstrated to accumulate in atherosclerotic lesions and regulates expression of more than 1,000 genes in human aortic endothelial cell (HAEC). Among the most highly induced is heme oxygenase-1 (HO-1), a cell-protective antioxidant enzyme, which is sensitively induced by oxidative stress. To identify the pathway by which Ox-PAPC induces HO-1, we focused on the plasma membrane electron transport (PMET) complex, which contains ecto-NADH oxidase 1 (eNOX1) and NADPH:quinone oxidoreductase 1 (NQO1) and affects cellular redox status by regulating levels of NAD(P)H. We demonstrated that Ox-PAPC and its active components stimulated electron transfer through the PMET complex in HAECs from inside to outside [as determined by extracellular 2-(4-iodophenyl)-3-(44-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium (WST-1) reduction] and from outside to inside of the cell (as determined by intracellular NBT reduction). Chemical inhibitors of PMET system and siRNAs to PMET components (NQO1 and eNOX1) significantly decreased HO-1 induction by Ox-PAPC. We present evidence that Ox-PAPC activation of nuclear factor (erythroid-derived 2)-like 2 (Nrf2) in HAEC plays an important role in the induction of HO-1 and PMET inhibitors blocked Nrf2 activation by Ox-PAPC. We hypothesized that PMET activation by Ox-PAPC causes intracellular NAD(P)H depletion, which leads to the increased oxidative stress and HO-1 induction. Supporting this hypothesis, cotreatment of cells with exogenous NAD(P)H and Ox-PAPC significantly decreased oxidative stress and HO-1 induction by Ox-PAPC. Taken together, we demonstrated that the PMET system in HAEC plays an important role in the regulation of cellular redox status and HO-1 expression by Ox-PAPC.
Keywords: oxidized-1-palmitoyl-2-arachidonyl-sn-glycerol-3-phosphocholine, plasma membrane electron transport, Nrf2, atherosclerosis, endothelium
Oxidation product of phospholipids with arachidonic acid at the sn2 position accumulate in the atherosclerotic lesions and other chronic inflammatory sites (1, 2). Minimally modified low-density lipoprotein whose bioactive component is oxidized-1-palmitoyl-2-arachidonyl-sn-glycerol-3-phosphocholine (Ox-PAPC) was shown to activate monocyte-endothelial cell interaction (2, 3). We recently demonstrated that a 4 h treatment with Ox-PAPC regulates more than 1,000 pro- and antiatherogenic genes in human aortic endothelial cell (HAEC) (4, 5). These include genes shown to control inflammation, sterol synthesis, unfolded protein response, thrombosis, and redox status (4, 5). Previously, we identified active components of Ox-PAPC namely 1-palmitoyl-2-(5,6-epoxyisoprostane E2-sn-glycerol-3-phosphocholine) (PEIPC), 1-palmitoyl-2-glutaroyl-sn-glycerol-3-phosphocholine (PGPC), and POVPC (1-palmitoyl-2-oxovaleroyl-sn-glycerol-3-phosphocholine) with PEIPC being the most active component (2, 3, 6). We and others have shown that heme oxygenase-1 (HO-1) is strongly induced by Ox-PAPC and PEIPC in endothelial cells (5, 7, 8). HO-1 is sensitively induced by oxidative stress and has been shown to be cell-protective and antiatherogenic enzyme in endothelial cells (9, 10). The expression of HO-1 is regulated by several transcriptional factors including nuclear factor (erythroid-derived 2)-like 2 (Nrf2) (7, 11). In the absence of stimulation, Nrf2 is associated with kelch-like ECH-associated protein 1 (Keap1), which is a negative regulator of Nrf2; however increased oxidative stress induces Nrf2 to be released from keap1 and translocated into the nucleus (12, 13). However the mechanism for the induction of oxidative stress in the cells and subsequent Nrf2 activation by Ox-PAPC in HAEC is not known. Our studies present evidence that plasma membrane electron transport (PMET) system plays a role in Nrf2 activation by Ox-PAPC.
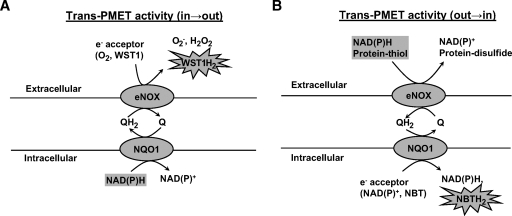
The PMET system transfers electrons from intracellular electron source (NAD(P)H) to the extracellular acceptors such as oxygen or artificial probe 2-(4-iodophenyl)-3-(44-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium (WST-1) (14, 15); or electrons can be transported from the extracellular source like exogenous donors such as NAD(P)H to the intracellular NAD(P)+ or artificial acceptor nitrobluetetrazolium (NBT) (16) (Fig. 1). Additionally electrons from exogenous NAD(P)H can be transferred to the extracellular electron acceptor by the cell-surface PMET system (17). The PMET system is involved in the regulation of cellular redox status by regulating NAD(P)H levels in the cells (18). NADH has direct antioxidant effects and NADPH is an essential cofactor for the regeneration of glutathione and thioredoxin, which are important antioxidant free thiol systems in the cells (19). The PMET complex has been shown to be composed of ecto-NADH oxidase (eNOX) at the extracellular surface of the plasma membrane, a recycling quinone molecule in the intermembrane space transferring electrons between NQO1 and eNOX, and NAD(P)H:quinone oxidoreductase 1 (NQO1) at the inner surface of plasma membrane (18, 20, 21) (Fig. 1). Three subtypes of eNOX have been identified, namely ecto-NADH oxidase 1 (eNOX1), eNOX2, and aging-related NADH oxidase (arNOX) (20). Recently, arNOX was shown to produce extracellular superoxide in the lymphocyte and platelets from aged person suggesting a role of PMET system with age-related diseases (22, 23). It was originally thought that PMET activity is passively regulated by intracellular NAD(P)H level. However, recently it has been shown that homocysteine increased PMET activity in endothelial cells, suggesting that the PMET system may be regulated by molecules (24). In the current study we demonstrated that, in HAEC, Ox-PAPC activates the PMET system inducing oxidative stress and leading to HO-1 induction.
Fig. 1.
Schematic diagram of trans plasma membrane electron transport (PMET) activities directed from inside to outside (in→out) and from outside to inside (out→in) of the cells. PMET complex is composed of extracellular surface component ecto-NADH oxidase 1 (eNOX1), and intermembrane recycling quinone, and intracellular surface component NADPH:quinone oxidoreductase 1 (NQO1). A: Trans-PMET activity directed from the intracellular NAD(P)H to the extracellular electron acceptor such as O2 or artificial probe 2-(4-iodophenyl)-3-(44-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium (WST-1), which is cell-impermeable. B: Trans-PMET activity directed from the extracellular electron source, such as protein-thiols or exogenous NAD(P)H, to the intracellular electron acceptor, such as NAD(P)+ or artificial electron acceptor nitrobluetetrazolium (NBT). WST-1 and NBT develop color changes by reduction.
MATERIALS AND METHODS
Materials and reagents
PAPC, POVPC, and PGPC were purchased from Avanti Polar Lipids. PAPC was oxidized by air exposure for 48 h and the composition of Ox-PAPC was analyzed by electrospray ionization-mass spectrometry as described (6). PEIPC was prepared as described (25). The Nrf2 antibody was purchased from Santa Cruz. Antibodies for GAPDH, NQO1, and histone were purchased from Cell Signaling. Diphenyleneiodonium (DPI), NBT, protease inhibitor, and phosphatase inhibitor cocktails were purchased from Sigma. Dicoumarol (DC) was purchased from Calbiochem. 2-(4-Iodophenyl)-3-(44-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium (WST-1) and 1-methoxy-5-methylphenazinium, methylsulfate (mPMS) were purchased from Dojindo. Minimally modified LDL was prepared by oxidation of human LDL as described (26).
Cell culture
Postconfluent HAECs passage 4–8 were used for this study. HAEC were isolated and maintained as described (27). For gene regulation, HAECs were incubated in M199 media containing 1% FBS for 4 h. For the activation of transcription factor Nrf2, cells were treated for 2 hrs with stimuli in M199 containing 0.2% FBS. Unless otherwise specified, cells were pretreated with inhibitors for 1 h and cotreated with stimuli for specified times.
Gene silencing by siRNA-transfection
Scrambled control and siRNAs specific to eNOX1 (Hs_FLJ10094_1_HP, Hs_FLJ10094_4_HP), NQO1 (Hs_NQO1_1_HP, Hs_NQO1_8_HP), and Nrf2 (Hs_NFE2L2_4_HP) were purchased from Qiagen. HAECs were transfected with siRNA using Lipofectamine 2000 (Invitrogen) following the recommended protocol by the manufacturer (28). After 2–6 days of culture, silencing of target genes was confirmed by QRT-PCR and Western blotting. Two siRNAs were used for each gene to control off-target effects.
QRT-PCR
Total RNA and cDNA were prepared using RNA extraction and cDNA synthesis kit (Bio-Rad). SYBR® green master mixture and PCR amplification system from Applied Biosystems were used for quantitative PCR. The primer sequences used for Quantitative RT-PCR (QRT-PCR) were: GAPDH, F:5′-CCT CAA GAT CAT CAG CAA TGC CTC CT-3′, R:3′-GGT CAT GAG TCC TTC CAC GAT ACC AA-5′; HO-1, F:5′-ATA GAT GTG GTA CAG GGA GGC CAT CA-3′, R:5′-GGC AGA GAA TGC TGA GTT CAT GAG GA-3′; IL-8: F:5′-ACC ACA CTG CGC CAA CAC AGA AAT-3′, R:5′-TCC AGA CAG AGC TCT CTT CCA TCA GA-3′; eNOX1, F: 5′-TGA GCA AGA GAT GGA GGA AGC CAA -3′, R:5′-ATT CCG GAG CTC CTC AGA ATG CTT-3′; eNOX2, F:5′-AGT ATT TGT GGG TGG TCT GCC TGA-3′, R: 5′-ACC ATG TAC TCC TCA GCA AAG CGA-3′; NQO1, F: 5′-AAG GAT GGA AGA AAC GCC TGG AGA -3′, R:5′-GGC CCA CAG AAA GGC CAA ATT TCT-3′; NQO2: F:5′-AGT GGA AAC CCA CGA AGC CTA CAA-3′, R:5′-TGA ACC AGT ACA GCG GGA ACT GAA-3′; Nrf2: F: 5′-AGC ATG CCC TCA CCT GCT ACT TTA-3′, R: 5′-ACT GAG TGT TCT GGT GAT GCC ACA-3′.
Immunoblotting (Western blot)
Laemmli buffer (Bio-Rad) containing protease and phosphatase inhibitor cocktails and PMSF (1 mM) was used for total cell lysate preparation. Nuclear fraction was isolated as described in the previous report (29). The protein samples were separated on 4–20% Tris-glycine SDS-PAGE gel (ISC Bioexpress) using standard protocol for gel running and blotting (28, 30). Blots were incubated with antibodies in 5% fat-free milk or 3% BSA in TBST (Tris-buffered saline, 0.1% Tween 20) for 1 hr at room temperature or overnight at 4°C. Blots were developed and the image was acquired using chemiluminescence kit (Amersham) and VersaDoc Imaging System-Model 5000 (Bio-Rad). Quantity One® program was used to measure band densities in the gel image.
Assay for trans-PMET activity
To measure the trans-PMET activity (in→out), which transfer electrons from the intracellular electron source NAD(P)H to the extracellular acceptor, the method described in the previous report was used (17). Briefly, HAECs in 96-well formats were incubated with stimuli in Hank's Balance Salt Solution containing WST-1 (500 μM) and mPMS (1 μM) in total volume of 100 μl. WST-1 accepts electrons from PMET complex only in the presence of an electron mediator mPMS and develops a yellow color (17). After incubation for the specified time, the absorbance at 440 nm was measured as an indicator of trans-PMET activity (in→out). A blank test was performed in the absence of cells but in the presence of stimuli, and the blank values were subtracted from the values with cells. For the measurement of trans-PMET activity directed from the exogenous NAD(P)H to the intracellular electron acceptor (out→in), a cell-permeable artificial electron acceptor NBT was employed. Briefly, after washing twice with PBS confluent HAECs in 96-well dishes were incubated with stimuli in Hank's Balance Salt Solution containing NBT (400 μM) and NADH (100 μM) or NADPH (500 μM) in total volume of 100 μl. NBT forms blue formazan upon reduction in the cells and NAD(P)H is generally regarded as cell-impermeable (18, 31). After washing twice with PBS, unincorporated NBT was discarded by washing with methanol (150 μl). Formazan in the cells was dissolved in 60 μl of 2M KOH and 90 μl of DMSO, and the absorbance at 690 nm was measured as an indicator of formazan formation in the cells.
Detection of intracellular reactive oxygen species formation
For the detection of reactive oxygen species in the cells, a DCF assay was employed. Briefly cells were grown in 4 well-glass chamber slides to confluence. Cells were preincubated with DCF (5 μM, molecular probes) for 30 min in assay media (M199 plus 1% FBS) at 37°C in CO2 incubator. After removing DCF solution, cells were treated with stimuli (e.g., Ox-PAPC) in assay media. The green fluorescence (excitation: 488 nm, detection: 505 nm) was measured using an inverted confocal microscope. Readings were analyzed at the times shown. The PASCAL program was used for imaging and analysis of the signal. Minimum laser intensity and exposure time were used to prevent nonspecific bleaching of the cells during the observation. For the quantification of the signal, the mean signal intensity of 10 cells randomly chosen was used. The same intensity of laser and settings for image acquiring were used for the comparison of multiple observations.
Statistical analysis
Two-tailed unpaired Student's t-test was used to evaluate the difference between two groups. The P value <0.05 was regarded as a statistically significant.
RESULTS
Ox-PAPC increased trans-PMET activity (in→out) in HAEC
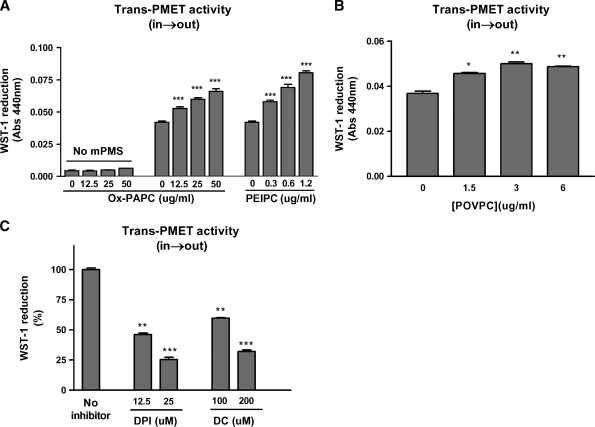
As shown in Table 1, HAECs express components of PMET system (eNOX1, eNOX2, NQO1, and NQO2) with NQO1 being a highly abundant protein. To measure the trans-PMET activity (in→out), we employed artificial electron acceptor WST-1 which is a cell-impermeable and develops a yellow color upon reduction (16). The PMET complex cannot transfer electrons directly to WST-1 without mediator mPMS (18). As shown in Fig. 2A, Ox-PAPC dose-dependently increased trans-PMET activity (in→out) in HAEC. In the absence of mPMS, WST-1 reduction by Ox-PAPC itself was negligible (Fig. 2A) indicating that our assay is a true measure of trans-PMET (in→out) activity in the cells. The bioactive components of Ox-PAPC, PEIPC (Fig. 2A), and, to a much smaller extent, POVPC (Fig. 2B) also significantly increased trans-PMET activity (in→out) in HAEC. Minimally modified LDL, which contains Ox-PAPC, also significantly increased trans-PMET activity (in→out); normal LDL was without effect (data not shown). Previous reports showed that DPI inhibits PMET activity in cells (18). DPI is an efficient inhibitor of flavoenzyme, including NQO1, a PMET component (32). As shown in Fig. 2C, DPI efficiently inhibited Ox-PAPC-induced trans-PMET (in→out) activity, likely acting on the flavoprotein NQO1. A specific inhibitor of NQO1 DC (33, 34) also dose-dependently inhibited Ox-PAPC-induced trans-PMET activity (in→out) in HAEC (Fig. 2C). In the absence of cells, Ox-PAPC, PEIPC, and POVPC, DPI and DC did not show significant interactions with WST-1 in the range of concentrations used in the assay.
TABLE 1.
Relative mRNA levels of eNOX1, eNOX2, NQO1, and NQO2 in human aortic endothelial cell (HAEC)
| Gene | GAPDH | eNOX1 | eNOX2 | NQO1 | NQO2 |
|---|---|---|---|---|---|
| Ct value | 13.83 ± 0.153 | 24.33 ± 0.141 | 23.73 ± 0.141 | 15.93 ± 0.153 | 21.0 ± 0.00 |
| Relative mRNA copy numbera | 1,000 | 0.69 | 1.05 | 233.94 | 6.98 |
Value means mRNA copy number per thousand copies of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA in HAEC. Copy numbers were calculated from Ct value of QRT-PCR. Each primer set showed ideal double expansion in PCR reactions as determined by standard reactions using known amounts of templates in the reactions. There was no formation of primer dimers, which was determined by melting dissociation curve. Values are mean ± SD of triplicate PCR reactions.
Fig. 2.
Oxidized-1-palmitoyl-2-arachidonyl-sn-glycerol-3-phosphocholine (Ox-PAPC), PEIPC, and POVPC increase trans-PMET (in→out) activities. A: The trans-PMET activity (in→out) induced by various concentration of Ox-PAPC and PEIPC was measured using WST-1 as probe for 4 hr incubation in the presence or absence of electron mediator 1-methoxy-5-methylphenazinium, methylsulfate (mPMS) (1uM). B: The activation of trans-PMET (in→out) system by POVPC was measured for 4 h as in A. C: Human aortic endothelial cells (HAECs) were pretreated with the indicated concentrations of diphenyleneiodonium (DPI) and dicoumarol (DC) for 1 hr, and trans-PMET activity (in→out) was measured in the presence of Ox-PAPC (50 μg/ml) and the same levels of DPI or DC for 2.5 h additional incubation. All values are mean ± SEM of 4–6 reactions per treatment. * P < 0.05, ** P < 0.01, *** P < 0.001 compared with the control values (no Ox-PAPC or no inhibitor).
Ox-PAPC activated trans-PMET activity (out→in) in HAEC
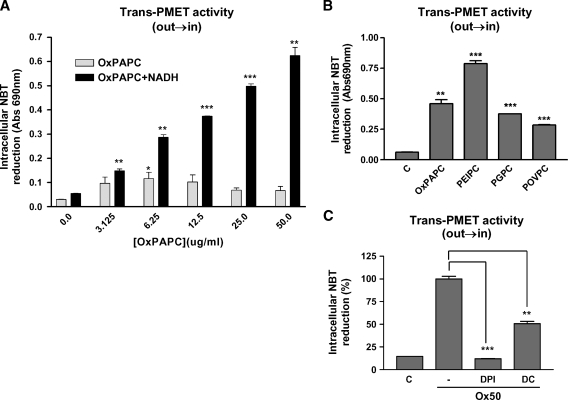
Interestingly, Ox-PAPC dramatically increased electron flow from exogenously added NAD(P)H into the cells as determined by the reduction of cell-permeable probe NBT (Fig. 3A). The effect of NADH on intracellular NBT reduction was slightly more potent than that effect of NADPH (NADPH data not shown). In the absence of NADH, Ox-PAPC alone increased intracellular NBT reduction with a peak at about 5 ug/ml (Fig. 3A, light bars) with a bell-shape curve. However, in the presence of NADH, Ox-PAPC increased NBT reduction about 10-fold (Fig. 3A, dark bars), which was dose-dependent up to about 100 ug/ml. Three bioactive components of Ox-PAPC also increased intracellular NBT reduction in HAEC (Fig. 3B) with PEIPC being the most potent stimulator. PEIPC (0.1 ug/ml) induced more NBT reduction than 50 ug/ml of Ox-PAPC, PGPC, or POVPC. PMET inhibitors DPI and DC significantly inhibited Ox-PAPC-induced intracellular NBT reduction in the cells (Fig. 3C). Importantly NAD(P)H alone did not induce any significant intracellular NBT reduction in the cells without Ox-PAPC. Ox-PAPC and its components, DPI, and DC did not show significant interactions with NBT without cells.
Fig. 3.
Ox-PAPC and its active components increased trans-PMET activities (out→in). A, B: HAECs were incubated for 1 hr with Hank's Balanced Salt Solution containing NBT (400 μM) and NADH (100 μM) in the presence of various levels of Ox-PAPC (A), or Ox-PAPC (50 μg/ml), PEIPC (0.1μg/ml), 1-palmitoyl-2-glutaroyl-sn-glycerol-3-phosphocholine (PGPC) (50 μg/ml), POVPC (50 μg/ml) (B). Cells were rinsed, lysed, and levels of reduced NBT were determined. C: HAECs were pretreated with vehicle, DPI (6.5 μM), and DC (200 μM) for 1 hr. Trans-PMET activity (out→in) was measured as in A and B after addition of Ox-PAPC (50 μg/ml) for 1 hr. Values were mean ± SD of duplicate reactions. * P < 0.05, ** P < 0.01, *** P < 0.001 compared with the control values (no Ox-PAPC or no inhibitor). These experiments are representatives of three giving similar results.
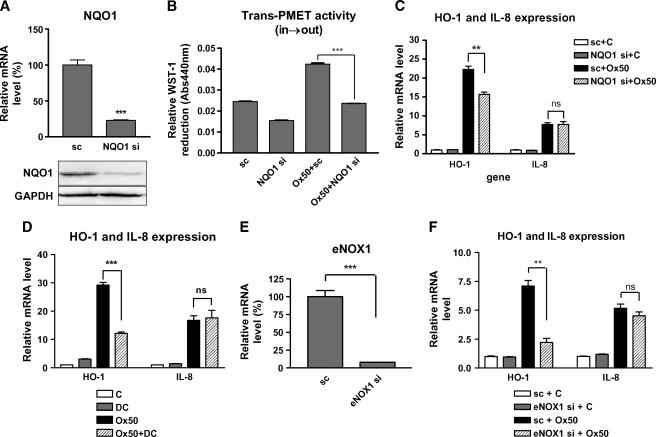
PMET components (NQO1 and eNOX1) are involved in the induction of HO-1 by Ox-PAPC. In order to determine the role of PMET system in regulation of HO-1 by Ox-PAPC, we silenced two PMET components using siRNA transfection. As shown in Fig. 4A, NQO1 mRNA level was decreased by 75% by siRNA transfection. As was seen for dicumarol (Fig. 3C), NQO1 gene silencing significantly decreased extracellular WST-1 reduction by Ox-PAPC thus blocking trans-PMET (in→out) activity in the cells (Fig. 4B). The basal level of WST-1 reduction was also decreased by NQO1 silencing. Previous reports showed that Ox-PAPC decreased intracellular NAD(P)H level in bovine vascular endothelial cells (35), suggesting that NAD(P)H depletion might be an important factor for oxidative stress induced by Ox-PAPC. We hypothesized that trans-PMET (in→out) activity induced depletion of intracellular NAD(P)H levels, causing an increase of cellular oxidative stress. Because HO-1 is sensitively induced by oxidative stress, we examined the silencing effects of PMET components on HO-induction by Ox-PAPC. Interestingly, NQO1 gene silencing significantly decreased HO-1 induction by Ox-PAPC (Fig. 4C); however, NQO1 silencing did not affect the induction of interleukin-8 (IL-8), which is a representative proinflammatory and proatherogenic cytokine induced by Ox-PAPC in HAEC. As shown in Fig. 4D, NQO1 inhibitor DC also significantly decreased HO-1 induction by Ox-PAPC in HAEC; however, IL-8 induction by Ox-PAPC was also not affected by dicoumarol. We tested the effects of the silencing of eNOX1, which is another component of PMET complex. We relied on the QRT-PCR to confirm the silencing of eNOX1 because of the lack of eNOX1-specific antibody. As shown in Fig. 4D, the eNOX1 mRNA level was decreased substantially by siRNA to eNOX1. Importantly eNOX1 gene silencing also significantly inhibited the induction of HO-1 but not of IL-8 by Ox-PAPC (Fig. 4E). We concluded that HO-1 expression is regulated by trans-PMET activity (in→out) in the presence of Ox-PAPC.
Fig. 4.
NQO1 and eNOX1 regulate Ox-PAPC-induced heme oxygenase-1(HO-1) expression in HAEC. A: Scrambled (sc) or siRNAs specific to NQO1 (NQO1 si) were transfected into HAEC. After 2 days, mRNA and protein levels of NQO1 were measured using QRT-PCR and Western blotting as described in the procedure. B: Cells treated with siRNA to NQO1 or scrambled control were used for measurement of trans-PMET activity (in→out) induced by Ox-PAPC (50 μg/ml, Ox50) as determined by WST-1 reduction. C: Cells were treated as in B, and HO-1 and interleukin-8 (IL-8) transcriptional levels induced by Ox-PAPC (50 μg/ml, Ox50) were determined by QRT-PCR. D: HAECs were pretreated with DC (20 μM) for 1 hr and cotreated with Ox-PAPC (50 μg/ml, Ox50) for 4 hrs. HO-1 and IL-8 mRNA levels induced by Ox-PAPC were determined by QRT-PCR. E: Cells were transfected with scrambled (sc) or siRNA specific to eNOX1 (eNOX si). After 5–6 days, mRNA levels of eNOX1 were measured using QRT-PCR. F: Cells were treated as in E, and HO-1 and IL-8 transcriptional levels induced by Ox-PAPC (50 μg/ml, Ox50) were then determined by QRT-PCR. Values are mean ± SEM of triplicate reactions. ** P < 0.01, *** P < 0.001; ns: not significant.
Exogenous NAD(P)H decreased intracellular oxidative stress and HO-1 induction by Ox-PAPC in HAEC
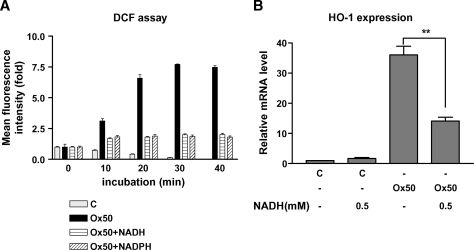
Our data suggest that the supplementation of cells with NAD(P)H in the cells blocks the increase of oxidative stress by Ox-PAPC. We therefore tested the effects of exogenously NAD(P)H on the induction of oxidative stress and HO-1 expression by Ox-PAPC in HAEC. As shown in Fig. 5A, exogenous NAD(P)H in HAEC efficiently decreased intracellular reactive oxygen species formation in the cells as determined by DCF assay that measures the formation of hydrogen peroxide (H2O2) and peroxinitrite (ONOO-) in the cells. Correspondingly, exogenous NADH also significantly decreased HO-1 induction by Ox-PAPC (Fig. 5B). Because NAD(P)H is cell-impermeable, trans-PMET activity (out→in) is likely to be involved in this process. Conclusively the electrons derived from the extracellular NAD(P)H may be transported into the cells through trans-PMET activity (out→in) and reduce oxidative stress in the cells.
Fig. 5.
NAD(P)H decreased oxidative stress and HO-1 induction by Ox-PAPC in HAEC. (A) The formation of intracellular reactive oxygen species was measured by DCF assay as described in method section. After 30 min incubation with DCF, HAECs were treated with media (C); Ox-PAPC (50 μg/ml, Ox50), alone or plus NADH (0.5 mM); or NADPH (0.5 mM) for 40 min. Relative mean fluorescence intensity from 10 randomly chosen cells was used for graph with fold increase from the basal signal. B: HAECs were untreated or treated with Ox-PAPC (50 μg/ml, Ox50) with or without NADH (0.5 mM) for 4 hrs, and HO-1 transcriptional levels were determined by QRT-PCR as described in Materials and Methods. NAD(P)H (0.5 mM) did not induce any cell toxicity or change of cell morphology. Values are mean ± SEM of triplicate reactions. ** P < 0.01.
Activation of Nrf2 plays a role in the Ox-PAPC-mediated regulation of HO-1 by the PMET system
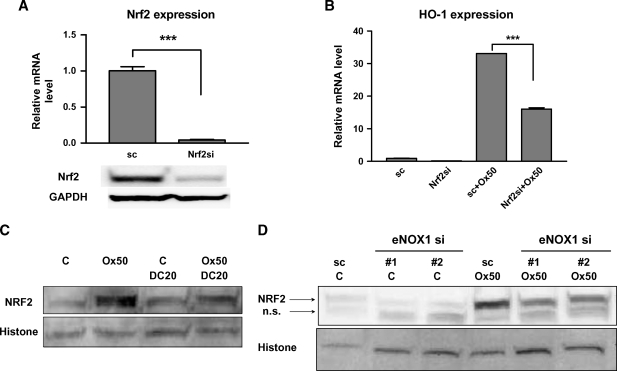
Since Nrf2 had previously been shown to be a strong regulator of HO-1 transcription in other cell types, we tested the importance of Nrf2 for the induction of HO-1 in response to Ox-PAPC. The transfection of siRNA to Nrf2 substantially decreased Nrf2 expression levels in HAEC (Fig. 6A). Nrf2 silencing significantly downregulated HO-1 induction by Ox-PAPC (Fig. 6B). In Fig. 4D and 4F, we demonstrated that NQO1 inhibitor DC and eNOX1 silencing significantly inhibited HO-1 induction by Ox-PAPC in HAEC. DC (Fig. 6C) as well as eNOX1 silencing (Fig. 6D) significantly inhibited Nrf2 activation by Ox-PAPC in the cells as shown by the blocking of nuclear transport of Nrf2. We employed two siRNAs with different nucleotide sequences for eNOX1 silencing in the cells and both were active. We conclude that PMET activation by Ox-PAPC regulates Nrf2 activation.
Fig. 6.
Nuclear factor (erythroid-derived 2)-like 2 (Nrf2) regulates HO-1 induction by Ox-PAPC and Ox-PAPC activation of Nrf2 is mediated by PMET. A: Nrf2-specific siRNA efficiently silenced Nrf2 in HAEC as determined by QRT-PCR (A) and Western blotting of Nrf2 using total cell lysates. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) protein levels were determined to normalize band density. B: Nrf2 silencing decreased HO-1 induction by Ox-PAPC (50 μg/ml, Ox50) (4 hrs) as determined by QRT-PCR. Values are mean ± SEM of triplicate reactions. *** P < 0.001. C: HAECs were pretreated with DC (20 μM) for 1 h and cotreated with or without Ox-PAPC (50 μg/ml, Ox50) for an additional 2 hrs. Nuclear fractions were isolated and Nrf2 levels in the nuclear fractions were determined by Western blotting using Nrf2 antibody. Histone levels were determined for normalization of Nrf2 bands. This Western blot was a representative of at least three repeated experiments with similar results. D: HAECs were transfected with scrambled (sc) or two sets of eNOX1 siRNAs (#1 and #2) for 5–6 days. Cells were then incubated with or without Ox-PAPC (50 μg/ml, Ox50) for 2 hrs. Nuclear fractions were isolated and Nrf2 levels in the nuclear fractions were determined as in C. The Nrf2 antibody showed a nonspecific as well as specific band in Western blot assay. The lower band is nonspecific (n.s.). We confirmed the identity of the top band as Nrf2 by showing reduction of band intensity by silencing as shown in A. This Western blot was a representative of at least three repeated experiments with similar results.
DISCUSSION
These studies were directed at identifying the mechanism of HO-1 transcriptional regulation by Ox-PAPC. HO-1 levels have been shown to be regulated by oxidative stress in a number of different systems. We sought to identify the oxidative stress signaling pathway(s) involved in Ox-PAPC regulation of HO-1 transcription. In the current study, we present evidence that the trans-PMET activity is a key factor in induction of HO-1 in HAEC. HAEC were shown to contain PMET proteins with NQO1 being a highly abundant protein (Table 1). We demonstrated that Ox-PAPC activated movement of electrons from inside of the cell to the extracellular electron acceptor WST-1. The most active component of Ox-PAPC, PEIPC, also stimulated trans-PMET (in→out) activity in HAEC. In an artificial system, where cell-impermeable NAD(P)H was added to the cell, Ox-PAPC was able to stimulate the movement of electrons from outside to inside of the cells as determined by increase of NBT reduction in the cells.
We hypothesized that the Ox-PAPC induced a decrease in NAD(P)H by PMET (in→out) leading to HO-1 induction. This hypothesis was based on several published observations. In tumor cells, in to out stimulation has been shown to reduce NAD(P)H levels in the cells (15, 18). Furthermore, previous studies showed that NAD(P)H was decreased by treatment of endothelial cells with Ox-PAPC and that oxidative stress was increased (35). We also observed an increase in oxidative stress with Ox-PAPC treatment in HAEC (Fig. 5A). In support of this hypothesis, silencing of eNOX1 and NQO1 as well as use of NQO1 inhibitor DC (Figs. 4C, D, F) decreased HO-1 induction by Ox-PAPC. The siRNA to NQO1 was probably less effective because NQO1 levels are high in HAEC and we could not achieve complete protein knockdown in the cells. DPI, a general flavoprotein inhibitor, which can inhibit the effect of NQO1, was also effective in inhibiting HO-1 induction by Ox-PAPC (data not shown). A role for PMET and NAD(P)H depletion in HO-1 induction by Ox-PAPC was confirmed by showing that addition of NAD(P)H to the cells (which would be expected to raise NAD(P)H levels by PMET out to in) decreased oxidative stress and HO-1 induction (Fig. 5). Although NAD(P)H is a cofactor of NADPH oxidase (NOX) potentially increasing oxidative stress, the overall effect in HAEC and in some other cell types is antioxidant (19). DC showed similar effects in DCF assay (data not shown). Interestingly, the PMET system did not regulate the proinflammatory effects of Ox-PAPC as shown by a lack of effect of the silencing of eNOX1, NQO1, and DC on IL-8 induction by Ox-PAPC. We have previously observed that different signaling pathways mediate HO-1 and IL-8 induction in HAEC (4, 28, 30, 36).
We also gained some insight into the mechanism by which PMET activation by Ox-PAPC increased HO-1 transcription. We demonstrated that activation of Nrf2 played an important role in the induction of HO-1 by Ox-PAPC since siRNA to Nrf2 decreased HO-1 induction by 50% (Fig. 6B). We further demonstrated that siRNA to eNOX1 and DC both decreased Nrf2 activation by Ox-PAPC (Figs. 6C–D). The most likely mechanism for Ox-PAPC activation of Nrf2 by the PMET system relates to the decrease in NAD(P)H caused by PMET in to out. This decrease would be expected to increase oxidative stress, with subsequent activation of Nrf2. Previous studies have demonstrated the activation of Nrf2 by oxidative stress. Although Ox-PAPC can activate electron movement from NAD(P)H both in and out of cells, based on our data, the dominant direction of movement in response to Ox-PAPC (without electron donor in the medium) is in to out. The inhibition by NQO1, eNOX1 silencing, and DC of HO-1 induction by Ox-PAPC was only partially suggesting additional transcriptional factors might be involved in regulating HO-1 expression by Ox-PAPC in HAEC. Previous reports showed that PPARγ and CREB are also involved in regulating HO-1 induction in some types of endothelial cells (7, 37, 38). The relationship between the PMET system and PPARγ and CREB in the action of Ox-PAPC will be an important area to address in the future.
The activation mechanism of the PMET complex by Ox-PAPC is not identified in the current study, but the characteristics of the activation have been shown. Ox-PAPC activates trans-PMET activities in both directions (in→out and out→in), depending on the location of electron sources NAD(P)H. This suggested that Ox-PAPC has a general effect on the PMET system to increase the efficiency of electron flow and the direction of electron movement seems to be determined by the location of electron sources. These data also suggest that Ox-PAPC may increase the quinone recycling in the plasma membrane to increase the efficiency of electron flow across the plasma membrane via the PMET system. We previously reported that Ox-PAPC causes depletion of cholesterol in the plasma membrane in HAEC (39), which may alter membrane fluidity and thus recycling efficiency of quinone molecule. Previous reports also showed that the incorporation of oxidized phospholipids into the membrane can alter membrane structure (40, 41) and fluidity (42). To our knowledge, no studies on the effects of changes in membrane fluidity on the PMET system have been reported. However, several groups showed that membrane fluidity is an important factor for the efficiency of electron transport by quinone recycling as shown in mitochondrial electron transport system (43, 44).
In summary our studies present evidence that the PMET system is activated by Ox-PAPC and involved in the regulation of HO-1 induction by Ox-PAPC through activation of Nrf2. These studies suggest that PMET system is an important regulator of redox status in endothelial cells and a potential regulator of atherogenesis in the vascular system.
Abbreviations
DC, dicoumarol
DPI, diphenyleneiodonium
eNOX1, ecto-NADH oxidase 1
HAEC, human aortic endothelial cell
HO-1, heme oxygenase-1
IL-8, interleukin-8
mPMS, 1-methoxy-5-methylphenazinium, methylsulfate
NBT, nitrobluetetrazolium
NQO1, NAD(P)H:quinone oxidoreductase 1
Nrf2, nuclear factor (erythroid-derived 2)-like 2
Ox-PAPC, oxidized-1-palmitoyl-2-arachidonyl-sn-glycerol-3-phosphocholine
PEIPC, 1-palmitoyl-2-(5,6-epoxyisoprostane E2]-sn-glycerol-3-phosphocholine
PGPC, 1-palmitoyl-2-glutaroyl-sn-glycerol-3-phosphocholine
PMET, plasma membrane electron transport
WST-1, 2-(4-iodophenyl)-3-(44-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium
Published, JLR Papers in Press, August 29, 2008.
Footnotes
This study was supported by National Institute of Health (NIH) grants HL30568 and HL064731 (J.A.B.) and postdoctoral fellowship from American Heart Association (S.L.).
References
- 1.Yla-Herttuala S., W. Palinski, M. E. Rosenfeld, S. Parthasarathy, T. E. Carew, S. Butler, J. L. Witztum, and D. Steinberg. 1989. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J. Clin. Invest. 84 1086–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Subbanagounder G., N. Leitinger, D. C. Schwenke, J. W. Wong, H. Lee, C. Rizza, A. D. Watson, K. F. Faull, A. M. Fogelman, and J. A. Berliner. 2000. Determinants of bioactivity of oxidized phospholipids. Specific oxidized fatty acyl groups at the sn-2 position. Arterioscler. Thromb. Vasc. Biol. 20 2248–2254. [DOI] [PubMed] [Google Scholar]
- 3.Watson A. D., G. Subbanagounder, D. S. Welsbie, K. F. Faull, M. Navab, M. E. Jung, A. M. Fogelman, and J. A. Berliner. 1999. Structural identification of a novel pro-inflammatory epoxyisoprostane phospholipid in mildly oxidized low density lipoprotein. J. Biol. Chem. 274 24787–24798. [DOI] [PubMed] [Google Scholar]
- 4.Gargalovic P. S., M. Imura, B. Zhang, N. M. Gharavi, M. J. Clark, J. Pagnon, W. P. Yang, A. He, A. Truong, S. Patel, et al. 2006. Identification of inflammatory gene modules based on variations of human endothelial cell responses to oxidized lipids. Proc. Natl. Acad. Sci. USA. 103 12741–12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gargalovic P. S., N. M. Gharavi, M. J. Clark, J. Pagnon, W. P. Yang, A. He, A. Truong, T. Baruch-Oren, J. A. Berliner, T. G. Kirchgessner, et al. 2006. The unfolded protein response is an important regulator of inflammatory genes in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 26 2490–2496. [DOI] [PubMed] [Google Scholar]
- 6.Subbanagounder G., J. W. Wong, H. Lee, K. F. Faull, E. Miller, J. L. Witztum, and J. A. Berliner. 2002. Epoxyisoprostane and epoxycyclopentenone phospholipids regulate monocyte chemotactic protein-1 and interleukin-8 synthesis. Formation of these oxidized phospholipids in response to interleukin-1beta. J. Biol. Chem. 277 7271–7281. [DOI] [PubMed] [Google Scholar]
- 7.Kronke G., V. N. Bochkov, J. Huber, F. Gruber, S. Bluml, A. Furnkranz, A. Kadl, B. R. Binder, and N. Leitinger. 2003. Oxidized phospholipids induce expression of human heme oxygenase-1 involving activation of cAMP-responsive element-binding protein. J. Biol. Chem. 278 51006–51014. [DOI] [PubMed] [Google Scholar]
- 8.Jyrkkanen H. K., E. Kansanen, M. Inkala, A. M. Kivela, H. Hurttila, S. E. Heinonen, G. Goldsteins, S. Jauhiainen, S. Tiainen, H. Makkonen, et al. 2008. Nrf2 regulates antioxidant gene expression evoked by oxidized phospholipids in endothelial cells and murine arteries in vivo. Circ. Res. 103 e1–e9. [DOI] [PubMed] [Google Scholar]
- 9.Morse D., and A. M. Choi. 2005. Heme oxygenase-1: from bench to bedside. Am. J. Respir. Crit. Care Med. 172 660–670. [DOI] [PubMed] [Google Scholar]
- 10.Gharavi N. M., P. S. Gargalovic, I. Chang, J. A. Araujo, M. J. Clark, W. L. Szeto, A. D. Watson, A. J. Lusis, and J. A. Berliner. 2007. High-density lipoprotein modulates oxidized phospholipid signaling in human endothelial cells from proinflammatory to anti-inflammatory. Arterioscler. Thromb. Vasc. Biol. 27 1346–1353. [DOI] [PubMed] [Google Scholar]
- 11.Srisook K., C. Kim, and Y. N. Cha. 2005. Molecular mechanisms involved in enhancing HO-1 expression: de-repression by heme and activation by Nrf2, the “one-two” punch. Antioxid. Redox Signal. 7 1674–1687. [DOI] [PubMed] [Google Scholar]
- 12.Kang K. W., S. J. Lee, and S. G. Kim. 2005. Molecular mechanism of nrf2 activation by oxidative stress. Antioxid. Redox Signal. 7 1664–1673. [DOI] [PubMed] [Google Scholar]
- 13.Sun Z., S. Zhang, J. Y. Chan, and D. D. Zhang. 2007. Keap1 controls postinduction repression of the Nrf2-mediated antioxidant response by escorting nuclear export of Nrf2. Mol. Cell. Biol. 27 6334–6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herst P. M., A. S. Tan, D. J. Scarlett, and M. V. Berridge. 2004. Cell surface oxygen consumption by mitochondrial gene knockout cells. Biochim. Biophys. Acta. 1656 79–87. [DOI] [PubMed] [Google Scholar]
- 15.Herst P. M., and M. V. Berridge. 2007. Cell surface oxygen consumption: a major contributor to cellular oxygen consumption in glycolytic cancer cell lines. Biochim. Biophys. Acta. 1767 170–177. [DOI] [PubMed] [Google Scholar]
- 16.Berridge M. V., P. M. Herst, and A. S. Tan. 2005. Tetrazolium dyes as tools in cell biology: new insights into their cellular reduction. Biotechnol. Annu. Rev. 11 127–152. [DOI] [PubMed] [Google Scholar]
- 17.Scarlett D. J., P. M. Herst, and M. V. Berridge. 2005. Multiple proteins with single activities or a single protein with multiple activities: the conundrum of cell surface NADH oxidoreductases. Biochim. Biophys. Acta. 1708 108–119. [DOI] [PubMed] [Google Scholar]
- 18.Herst P. M., and M. V. Berridge. 2006. Plasma membrane electron transport: a new target for cancer drug development. Curr. Mol. Med. 6 895–904. [DOI] [PubMed] [Google Scholar]
- 19.Ying W. 2008. NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences. Antioxid. Redox Signal. 10 179–206. [DOI] [PubMed] [Google Scholar]
- 20.Morre D. J. 2004. Quinone oxidoreductases of the plasma membrane. Methods Enzymol. 378 179–199. [DOI] [PubMed] [Google Scholar]
- 21.Sun I. L., E. E. Sun, F. L. Crane, D. J. Morre, A. Lindgren, and H. Low. 1992. Requirement for coenzyme Q in plasma membrane electron transport. Proc. Natl. Acad. Sci. USA. 89 11126–11130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morre D. J., and D. M. Morre. 2006. Aging-related cell surface ECTO-NOX protein, arNOX, a preventive target to reduce atherogenic risk in the elderly. Rejuvenation Res. 9 231–236. [DOI] [PubMed] [Google Scholar]
- 23.Morre D. M., F. Guo, and D. J. Morre. 2003. An aging-related cell surface NADH oxidase (arNOX) generates superoxide and is inhibited by coenzyme Q. Mol. Cell. Biochem. 254 101–109. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez-Alonso J., R. Montanez, L. Rodriguez-Caso, and M. A. Medina. 2008. Homocysteine is a potent modulator of plasma membrane electron transport systems. J. Bioenerg. Biomembr. 40 45–51. [DOI] [PubMed] [Google Scholar]
- 25.Jung M. E., J. A. Berliner, D. Angst, D. Yue, L. Koroniak, A. D. Watson, and R. Li. 2005. Total synthesis of the epoxy isoprostane phospholipids PEIPC and PECPC. Org. Lett. 7 3933–3935. [DOI] [PubMed] [Google Scholar]
- 26.Liao F., J. A. Berliner, M. Mehrabian, M. Navab, L. L. Demer, A. J. Lusis, and A. M. Fogelman. 1991. Minimally modified low density lipoprotein is biologically active in vivo in mice. J. Clin. Invest. 87 2253–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Navab M., G. P. Hough, L. W. Stevenson, D. C. Drinkwater, H. Laks, and A. M. Fogelman. 1988. Monocyte migration into the subendothelial space of a coculture of adult human aortic endothelial and smooth muscle cells. J. Clin. Invest. 82 1853–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zimman A., K. P. Mouillesseaux, T. Le, N. M. Gharavi, A. Ryvkin, T. G. Graeber, T. T. Chen, A. D. Watson, and J. A. Berliner. 2007. Vascular endothelial growth factor receptor 2 plays a role in the activation of aortic endothelial cells by oxidized phospholipids. Arterioscler. Thromb. Vasc. Biol. 27 332–338. [DOI] [PubMed] [Google Scholar]
- 29.Li R., W. Chen, R. Yanes, S. Lee, and J. A. Berliner. 2007. OKL38 is an oxidative stress response gene stimulated by oxidized phospholipids. J. Lipid Res. 48 709–715. [DOI] [PubMed] [Google Scholar]
- 30.Gharavi N. M., N. A. Baker, K. P. Mouillesseaux, W. Yeung, H. M. Honda, X. Hsieh, M. Yeh, E. J. Smart, and J. A. Berliner. 2006. Role of endothelial nitric oxide synthase in the regulation of SREBP activation by oxidized phospholipids. Circ. Res. 98 768–776. [DOI] [PubMed] [Google Scholar]
- 31.Dikalov S., K. K. Griendling, and D. G. Harrison. 2007. Measurement of reactive oxygen species in cardiovascular studies. Hypertension. 49 717–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chakraborty S., and V. Massey. 2002. Reaction of reduced flavins and flavoproteins with diphenyliodonium chloride. J. Biol. Chem. 277 41507–41516. [DOI] [PubMed] [Google Scholar]
- 33.Asher G., O. Dym, P. Tsvetkov, J. Adler, and Y. Shaul. 2006. The crystal structure of NAD(P)H quinone oxidoreductase 1 in complex with its potent inhibitor dicoumarol. Biochemistry. 45 6372–6378. [DOI] [PubMed] [Google Scholar]
- 34.Forthoffer N., C. Gomez-Diaz, R. I. Bello, M. I. Buron, S. F. Martin, J. C. Rodriguez-Aguilera, P. Navas, and J. M. Villalba. 2002. A novel plasma membrane quinone reductase and NAD(P)H:quinone oxidoreductase 1 are upregulated by serum withdrawal in human promyelocytic HL-60 cells. J. Bioenerg. Biomembr. 34 209–219. [DOI] [PubMed] [Google Scholar]
- 35.Rouhanizadeh M., J. Hwang, R. E. Clempus, L. Marcu, B. Lassegue, A. Sevanian, and T. K. Hsiai. 2005. Oxidized-1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine induces vascular endothelial superoxide production: implication of NADPH oxidase. Free Radic. Biol. Med. 39 1512–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yeh M., N. M. Gharavi, J. Choi, X. Hsieh, E. Reed, K. P. Mouillesseaux, A. L. Cole, S. T. Reddy, and J. A. Berliner. 2004. Oxidized phospholipids increase interleukin 8 (IL-8) synthesis by activation of the c-src/signal transducers and activators of transcription (STAT)3 pathway. J. Biol. Chem. 279 30175–30181. [DOI] [PubMed] [Google Scholar]
- 37.Kronke G., A. Kadl, E. Ikonomu, S. Bluml, A. Furnkranz, I. J. Sarembock, V. N. Bochkov, M. Exner, B. R. Binder, and N. Leitinger. 2007. Expression of heme oxygenase-1 in human vascular cells is regulated by peroxisome proliferator-activated receptors. Arterioscler. Thromb. Vasc. Biol. 27 1276–1282. [DOI] [PubMed] [Google Scholar]
- 38.Leitinger N. 2003. Oxidized phospholipids as modulators of inflammation in atherosclerosis. Curr. Opin. Lipidol. 14 421–430. [DOI] [PubMed] [Google Scholar]
- 39.Yeh M., A. L. Cole, J. Choi, Y. Liu, D. Tulchinsky, J. H. Qiao, M. C. Fishbein, A. N. Dooley, T. Hovnanian, K. Mouilleseaux, et al. 2004. Role for sterol regulatory element-binding protein in activation of endothelial cells by phospholipid oxidation products. Circ. Res. 95 780–788. [DOI] [PubMed] [Google Scholar]
- 40.Hazen S. L. 2008. Oxidized phospholipids as endogenous pattern recognition ligands in innate immunity. J. Biol. Chem. 283 15527–15531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greenberg M. E., X. M. Li, B. G. Gugiu, X. Gu, J. Qin, R. G. Salomon, and S. L. Hazen. 2008. The lipid whisker model of the structure of oxidized cell membranes. J. Biol. Chem. 283 2385–2396. [DOI] [PubMed] [Google Scholar]
- 42.Jain S., M. Thomas, G. P. Kumar, and M. Laloraya. 1993. Programmed lipid peroxidation of biomembranes generating kinked phospholipids permitting local molecular mobility: a peroxidative theory of fluidity management. Biochem. Biophys. Res. Commun. 195 574–580. [DOI] [PubMed] [Google Scholar]
- 43.Kim S. M., T. Yamamoto, Y. Todokoro, Y. Takayama, T. Fujiwara, J. S. Park, and H. Akutsu. 2006. Phospholipid-dependent regulation of cytochrome c3-mediated electron transport across membranes. Biophys. J. 90 506–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dhanasekaran M., and J. Ren. 2005. The emerging role of coenzyme Q-10 in aging, neurodegeneration, cardiovascular disease, cancer and diabetes mellitus. Curr Neurovasc Res. 2 447–459. [DOI] [PubMed] [Google Scholar]