Abstract
Visceral hypersensitivity is the leading complaint of functional bowel disorders. Central sensitization mediated by glutamate receptor activation is implicated in pathophysiology of visceral pain. The glial glutamate transporter EAAT2 is the principal mediator of glutamate clearance to terminate glutamate-mediated responses. Transgenic mice overexpressing human EAAT2 (EAAT2 mice), which exhibited a twofold enhanced glutamate uptake, showed 39% less writhing response to intraperitoneal acetic acid than nontransgenic littermates. Moreover, EAAT2 transgenic mice showed a 53–64% reduction in visceromotor response (VMR) to colorectal distension (CRD) in assessments of the response to graded increase in pressures. Corroborating the involvement of enhanced glutamate uptake, wild-type mice treated for 1 wk with ceftriaxone, an EAAT2 expression activator, showed a 49–70% reduction in VMR to CRD. Moreover, systemic pretreatment with the selective EAAT2 transporter blocker dihydrokainate reversed the ceftriaxone-blunted nociceptive response to CRD. However, the enhanced VMR to CRD produced by intracolonic ethanol was not significantly attenuated by 1-wk ceftriaxone pretreatment. The data suggest that enhanced glutamate uptake provides protective effects against colonic distension-induced nociception and represents an exciting new mechanistic approach leading to better therapeutic options to visceral pain disorders.
Keywords: colon, pain, gastrointestinal, excitatory amino acids
functional bowel disorders represent over 20 conditions characterized by chronic or recurrent symptoms not explained by structural or biochemical abnormalities (7). The most common of these disorders, irritable bowel syndrome is the leading digestive disease diagnosis by gastroenterologists and affects 15–20% of the US population (9). The most common complaint is recurrent abdominal pain associated with disturbed defecation (8). Treatment methods have evolved toward use of integrated multicomponent pharmacological and behavioral strategies based on the severity of the patient's symptom pattern and psychosocial factors influencing it (8). Effective therapeutics is hindered by the lack of understanding of the pathophysiological mechanism(s) involved in the visceral nociception manifested by this disorder (12). Recent studies have disclosed that the mechanisms of visceral and somatic chronic pain have points in common and divergence (2). Among the similarities is the role of glutamate receptor-mediated activation of second order spinal neurons (1). Noxious stimuli evoke long-term persistent changes in the excitability of dorsal horn neurons; glutamate, acting at N-methyl-d-aspartate (NMDA) and amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) receptors, contributes to the development of this phenomenon (27). NMDA and AMPA antagonists attenuate the visceromotor response to colonic distension (5, 16, 28, 33). However, these agents have severe limitations as therapeutic agents. Approaches decreasing glutamate tone may be beneficial in the relief of visceral pain.
Glutamate is the predominant excitatory neurotransmitter in the central nervous system. Glutamate is normally rapidly cleared from the synaptic cleft by high-affinity, sodium-dependent glutamate transporters located in both neurons and glia. To date, five human excitatory amino acid transporters (EAAT1-5) have been identified (3, 10, 18, 22, 26). Within the nervous system, EAAT1 (also designated as GLAST in rodents) and EAAT2 (also designated as GLT-1 in rodents) are expressed primarily on astrocytes, whereas EAAT3 (also designated as EAAC1 in rodents), EAAT4, and EAAT5 are neuronally localized (10, 11, 23). EAAT4 and EAAT5 are largely restricted to the cerebellum and retina, respectively (3, 10). The glial transporter EAAT2 is the quantitatively dominant glutamate transporter in the mammalian central nervous system and plays a major role in terminating synaptic transmission and protecting neurons from glutamate neurotoxicity (6).
Recently, a transgenic mouse model that overexpresses normal EAAT2 has been prepared (14). The amount of EAAT2 protein and the associated Na+-dependent glutamate uptake was increased about twofold in the EAAT2 transgenic mice. In addition, a pharmacological approach, the use of the cephalosporin antibiotic ceftriaxone, to augment EAAT2 expression was revealed after high-throughput analysis of several thousand compounds (24). These approaches provide novel opportunities to explore the role of modulated glutamatergic neurotransmission affecting visceral nociceptive processes. The results of this study reveal that genomic or pharmacological enhancement of EAAT2 expression blunts the visceromotor response to colorectal distension (CRD) in mice.
METHODS
Animals.
Two- to 3-mo-old FVB/N mice (20–33 g) were used in all experiments. Mice were housed in groups of five with free access to water and food. All experiments were approved by the Institutional Animal Care and Use Committee in the Ohio State University and adhered to the guideline of the Committee for Research and Ethical Issues of the International Association for the Study of Pain. EAAT2 transgenic mice were previously generated in our laboratory (14). EAAT2 transgene expression was driven by the human glial fibrillary acidic protein (hGFAP) promoter and was restricted in astrocytes. Polymerase chain reaction (PCR) were used to determine the genotype of mice using genomic DNA extracted from tail biopsies and EAAT2 transgene-specific primers (5′-ggc aac tgg gga tgt aca-3′ and 5′-acg ctg ggg agt tta ttc aag aat-3′). PCR conditions were as follows: 95°C for 3 min, 85°C for 2 min; 95°C for 30 s, 55°C for 30 s, 72°C for 1 min for 30 cycles followed by 10-min extension at 72°C.
Writhing test.
The classical test first described by VanderWende et al. in rats (29) and subsequently validated in mice (15) was used. Briefly, mice are habituated to 4-liter plastic beakers for 30 min before acetic acid (0.6%; 1 ml/100 g ip) was administered. The animals were returned to the observation chamber and the number of characteristic writhes was counted in 5-min intervals for a total of 20 min.
CRD surgery.
The method of Kamp et al. (17) was used. Briefly, adult (20–30 g) FVB mice were used. Animals were anesthetized with ketamine (37.5 mg/kg; Hospira, Lake Forest, IL) and xylazine (10 mg/kg ip; Bayer, Shawnee Mission, KS). Electrodes (Teflon-coated stainless steel wire, 5- to 10-mm tip separation; Cooner Wire Sales, Chatsworth, CA) were sewn into the external oblique abdominal musculature, just above the inguinal ligament, for electromyographic (EMG) recording. The EMG electrodes were subcutaneously guided to the dorsum of the neck and externalized for future access to the EMG recording system. After the surgery, mice were singly housed and allowed for 4 days of recovery before CRD experiments.
CRD balloons.
Distension balloons were prepared by stretching a small square (∼3 × 3 cm) of thin (∼15 μm) polyethylene plastic over a polyvinyl chloride rod (9-mm diameter), thereby removing all compliance from the plastic and creating a balloon. These balloons, 20 mm in length, were tied with 6-0 silk to PTFE-24 thin-wall tubing (Cole-Parmer Instrument, Vernon Hills, IL) 15 mm from the tip of the tubing and 20 mm from the closed end of the balloon (5-mm allowance for inflation). Before the balloon was secured to the tubing, several holes were punched in the distal 15 mm of the tubing with a 27-gauge needle to allow the balloon to inflate even if the catheter tip was occluded by the plastic balloon. To facilitate insertion and protect the delicate balloon from damage, the balloon and tubing were covered by a 6-cm-long sheath prepared from PE-240 tubing (2-mm diameter). One wall of the sheath was cut lengthwise to accommodate the girth of the balloon and silk suture. On the day of testing, mice were briefly anesthetized with isoflurane (1–5% in 100% O2 at 2 l/min; 5 min; Halocarbon Laboratories, River Edge, NJ). The sheath was lubricated with Surgilube (E. Fougera, Melville, NY) and inserted intra-anally until the silk tie was 5 mm inside the rectum (total insertion distance, 25 mm). The sheath was removed and the tubing was taped to the base of the tail to prevent displacement. Mice were placed in restraint devices (see Restraint devices) while still sedated and were allowed to recover and acclimate for a minimum of 30 min before testing. The lack of effect of isoflurane on responses to CRD 30 min after termination of the anesthesia was determined in preliminary experiments.
Restraint devices.
Restraint devices were constructed from a plastic 60-ml syringe (Becton Dickinson, Franklin Lakes, NJ) with the plunger removed. The needle attachment port was sawed off and the remaining tube was cut at the 40-ml mark (total length, 7.5 cm). In addition, an opening (∼7 × 9 mm) was made in the top of the tube for access to the EMG recording electrodes and subcutaneous catheter. The internal diameter of these tubes is ∼25 mm, which holds a 20- to 30-g mouse. After the mouse was placed in the tube, the open end was secured with a gauze square and paper tape. The tube was then placed in a dark-colored fabric sheath (to reduce ambient light) containing a small window (∼5 × 5 mm) for access to the EMG electrodes and catheter. The behavior of mice before, during, and after distension can be easily monitored by partial retraction of the fabric.
EMG recording.
CRD-evoked contraction of the abdominal musculature, termed the visceromotor response (VMR), was the behavioral response quantified. The EMG signal were filtered, amplified, and recorded as has been described before (17).
Visceral nociceptive testing.
Stimulus-response functions to graded intensities of CRD were generated to evaluate reproducibility and differences in wild-type vs. transgenic or treated animals. Baseline recordings were first collected for 40 s for each animal. In all cases, stimulus response functions were generated at CRD pressures of 15, 30, 45, and 60 mmHg. Three distensions were performed at each pressure at 3-min intervals.
Drug administration.
Ceftriaxone, dihydrokainate, and cephalosporin C were purchased from Sigma (St. Louis, MO). Ceftriaxone (200 mg/kg) and dihydrokainate (10 mg/kg) were prepared in saline for intraperitoneal administration, and 100 mg/kg cephalosporin C was suspended in 2% Tween 80 (Fisher, Fair Lawn, NJ). Intraperitoneal administrations were given daily for 1 wk.
Effect of intracolonic ethanol.
Inflammogens such as ethanol have been characterized to augment the VMR to CRD (17). In one series of experiments, three VMR responses to 60-mmHg distensions were elicited 4 min apart, and then intracolonic ethanol (30%; 100 μl) or vehicle was administered into the distal colon, 0.5–2.5 cm proximal to the rectum. One hour later, the VMR to CRD (60 mmHg) was again elicited in triplicate and compared with the response before ethanol or vehicle.
Western blotting.
Mice were euthanized, and spinal cord tissues were dissected. Tissues were sonicated in PBS containing Complete Protease Inhibitor mixture (Roche Applied Science), assayed for protein concentration, and prepared in SDS loading buffer, and 3 μg samples were loaded and run on 10% SDS polyacrylamide gel and transferred onto nitrocellular membrane. The membrane was then immersed for 1 h in 5% milk PBST blocking buffer [rabbit anti-EAAT2 antibody, 1:2,000; goat anti-actin antibody, 1:3,000 (Santa Cruz Biotechnology, Santa Cruz, CA)] and then probed overnight at 4°C with primary antibodies in 1% milk PBST. After three 30-min washings with PBST, blots were incubated at room temperature for 1 h with horseradish peroxidase (HRP)-conjugated goat IgG (1:3,000) and rabbit IgG (1:6,000) in 1% milk PBST. After three 30-min washings with PBST, HRP signal was detected by the SuperSignal West Pico Chemiluminescent Substrate (Pierce) according to the manufacturer's directions. The further analysis was performed with Image J software on scanned images of blots. Actin was used as loading control.
Data and statistical analysis.
The VMR to CRD, performed in triplicate for each animal, was represented as the mean number of EMG spikes exceeding threshold within the 40-s data analysis window. In some cases, the response was represented as % control, in which the mean response to 60 mmHg was defined as 100%. In these cases the data were log-transformed, resulting in normally distributed data. For the experiments examining the effect of dihydrokainate, group differences were evaluated by a linear mixed model that accounted for the correlation in measures from the same animal and also for the separate variance contributions of replications, pressure levels, and experimental subjects. Since response variability differed among groups and pressure levels, the model was structured to allow nonhomogeneous error variances. The dependent variable was the VMR and the independent variables were treatment or genomic group, distension pressure, and the group-by-pressure interaction. The studies involving assessment of VMR to CRD before and after intracolonic ethanol, a paired t-test was utilized to assess group differences. Differences in the writhing study were evaluated by Student's t-test. In all cases, statistical significance was indicated when P < 0.05.
RESULTS
Genomic or pharmacological enhancement of EAAT2 expression blunts the writhing response, as well as the visceromotor response to CRD.
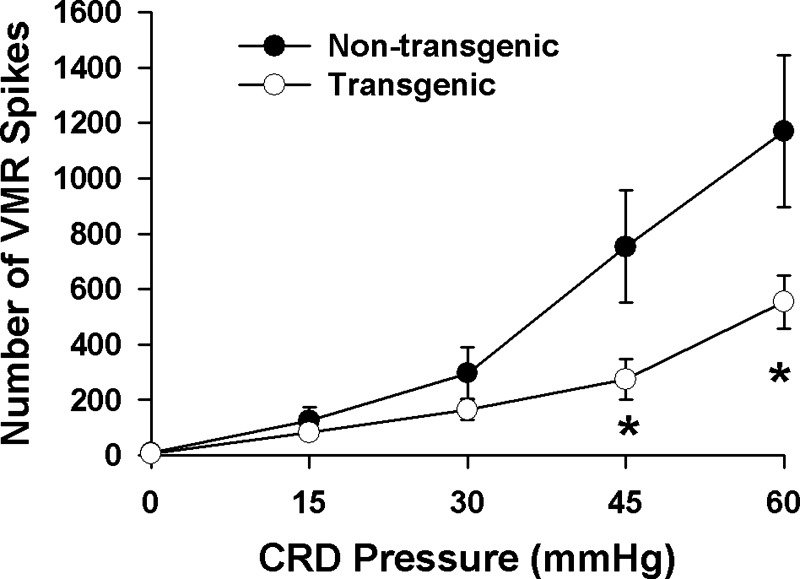
The initial studies were performed utilizing the writhing model. The EAAT2 transgenic mice used in this study expressed ∼2–2.5-fold more EAAT2 protein than the nontransgenic littermates (Fig. 1A). EAAT2 mice exhibited 39% less writhing compared with nontransgenic littermates after intraperitoneal acetic acid [writhes/20 min (n): nontransgenic mice, 27 ± 3 (35); EAAT2 mice, 16 ± 2 (33); P < 0.01] (Fig. 1B). The blunted nociceptive response in EAAT2 mice was then confirmed in the more viscero-specific model, CRD. EAAT2 mice showed a significant reduction in VMR to CRD in assessments of the response to graded distension (Fig. 2). The VMR was reduced 64% at 45 mmHg (P < 0.05) distension pressure and 53% at 60 mmHg (P < 0.05). To further assess a possible role of enhanced EAAT2 expression in mediating the visceroprotective response, wild-type mice were treated for 1 wk with ceftriaxone (200 mg·kg−1·day−1), an EAAT2 expression activator. This treatment regime enhanced EAAT2 expression ∼40–80% compared with vehicle-treated mice (Fig. 3A), similar to previous reports (24). Similar to the effects observed in EAAT2 transgenic mice, ceftriaxone pretreatment produced a 49–70% decrease (P < 0.05) in the VMR elicited from vehicle-treated cohorts throughout the 15–60 mmHg range of CRD (Fig. 3B). These results indicated that enhancement of EAAT2 expression is correlated with a blunted VMR to CRD.
Fig. 1.
Writhing test. A: representative Western blot of EAAT2 protein levels in the spinal cords of EAAT2 transgenic mice and nontransgenic littermates. EAAT2 mice exhibited a 2- 2.5-fold enhanced expression of EAAT2 protein compared with nontransgenic littermates. B: comparison of the writhing response to 0.6% acetic acid in nontransgenic (n = 35) vs. EAAT2 mice (n = 33). EAAT2 transgenic mice show 39% less writhing response to acetic acid than nontransgenic littermates. *P < 0.01.
Fig. 2.
Comparison of the EAAT2 transgenic and nontransgenic mice visceromotor response (VMR) to graded colorectal distension (CRD). EAAT2 mice (n = 12) show a 53–64% reduction in VMR to CRD, compared with nontransgenic littermate controls (n = 8). *P < 0.05.
Fig. 3.
Comparison of visceral nociception in wild-type mice receiving either ceftriaxone (CTX; 200 mg·kg−1·day−1 for 1 wk), an EAAT2 expression activator, or vehicle. A: representative Western blot of EAAT2 protein levels in the spinal cords of ceftriaxone- and vehicle (saline)-treated mice. ceftriaxone treatment enhanced EAAT2 protein expression ∼40–80%. B: effect of ceftriaxone treatment on the VMR to CRD. The 1-wk ceftriaxone-treated mice (n = 11) showed a 49–70% reduction in VMR, compared with vehicle-treated controls (n = 10). *P < 0.05; **P < 0.005.
Blocking EAAT2-mediated glutamate transport reverses ceftriaxone-blunted VMR.
To further examine whether the observed reduced visceral nociception was due to enhanced EAAT2-mediated glutamate transport function, dihydrokainate, a selective blocker of EAAT2, was intraperitoneally injected into the mice that had been treated with ceftriaxone for 1 wk 1 h before performance of CRD, thereby blocking EAAT2-mediated glutamate uptake. As shown in Fig. 4, dihydrokainate pretreatment (10 mg/kg), at an effective dose (20), significantly reversed the blunted VMR to CRD produced by ceftriaxone (Fig. 4, ceftriaxone vs. ceftriaxone+dihydrokainate; P < 0.05), whereas dihydrokainate pretreatment alone, without ceftriaxone treatment, did not significantly affect VMR to CRD (Fig. 4, vehicle vs. dihydrokainate). Consistently, there was a 52–70% decrease in the VMR in 1-wk ceftriaxone-treated mice compared with 1-wk vehicle-treated mice (Fig. 4, vehicle vs. ceftriaxone; P < 0.05). We also performed the dihydrokainate pretreatment experiments on EAAT2 transgenic mice and obtained similar results (not shown). These results suggest that the observed reduced visceral nociception was due to enhanced EAAT2-mediated glutamate transport function.
Fig. 4.
Effect of dihydrokainate (DHK; 10 mg/kg ip) on the ceftriaxone-blunted VMR to CRD. Ceftriaxone (200 mg/kg) or vehicle (veh) was administered intraperitoneally for 7 days in 2 groups each. One hour before the graded CRD response was elicited, one vehicle and ceftriaxone group was treated with either intraperitoneal (ip) saline or dihydrokainate. The 1-wk ceftriaxone + ip vehicle (n = 10) produced a significantly reduced VMR response compared with the 1-wk vehicle + ip vehicle group (n = 7) (*P < 0.05). Dihydrokainate significantly reversed the blunted VMR response produced by 1-wk ceftriaxone treatment (n = 10) (+ P < 0.05).
Lack of effect of ceftriaxone on ethanol-enhanced VMR to CRD.
To further characterize the interaction between augmented EAAT2-mediated glutamate transporter activity and blunted visceral pain response, a study was designed to see whether ethanol enhanced visceral pain response was attenuated by 1-wk ceftriaxone pretreatment. The CRD response to 60 mmHg pressure was examined both before and 1 h after intracolonic instillation of 30% ethanol (17) in both 1-wk ceftriaxone- and vehicle-treated wild-type mice.
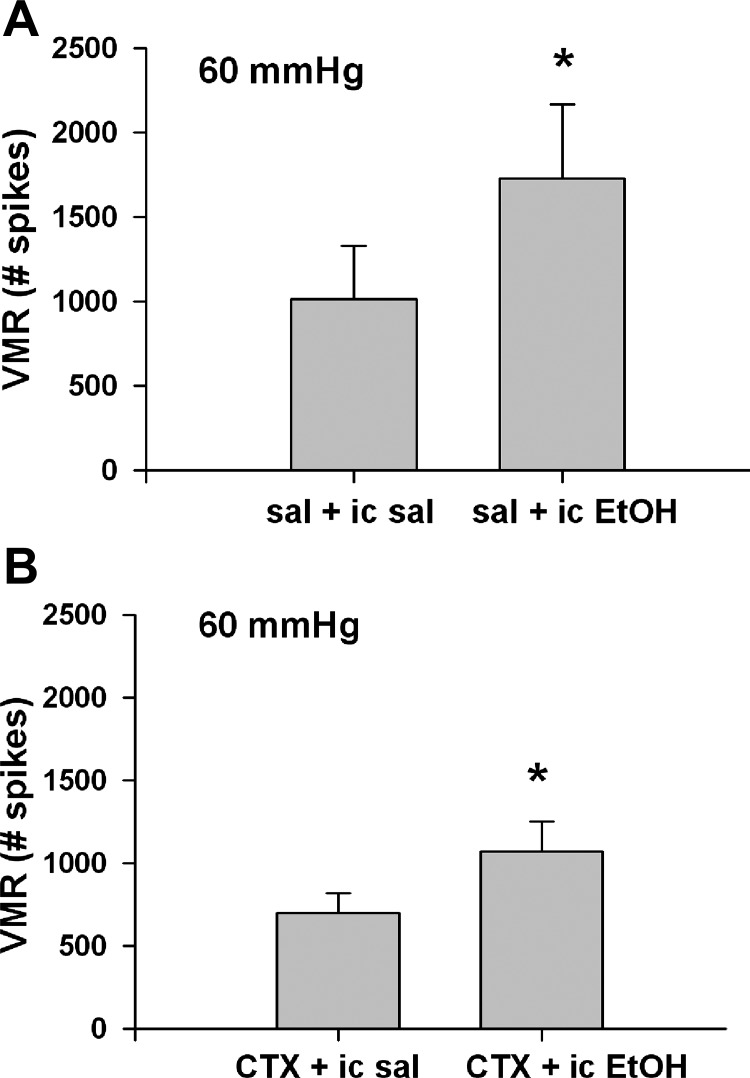
Intracolonic ethanol produced a 71% enhanced VMR in 1-wk vehicle-treated wild-type animals (Fig. 5A, P < 0.05). Similarly, in 1-wk ceftriaxone-treated wild-type animals, there was a 51% enhanced VMR response (Fig. 5B, P < 0.05). The results suggest that ceftriaxone slightly but not significantly reduced ethanol-enhanced VMR to CRD.
Fig. 5.
Comparison of the effect of 1-wk treatment with vehicle (A) or ceftriaxone (CTX) (B) on ethanol-enhanced VMR response to CRD. Mice were treated with vehicle or ceftriaxone 200 mg/kg for 7 days prior to the study. CRD at 60 mmHg was performed both before and 1 h after intracolonic (ic) 30% ethanol (EtOH) administration in each group (n = 8–9). sal, Saline. The mean responses to three 60 mmHg distensions were compared before and after treatments. *P < 0.05 with respect to the mean response before intracolonic ethanol.
DISCUSSION
The principal finding of this study was that augmented EAAT2 expression and associated glutamate transport function reduces the visceromotor response to CRD. Both transgenic and pharmacological approaches were effective. These findings represent a novel, previously unexplored approach to mitigate visceral nociception.
The effectiveness of systemic dihydrokainate to reverse the ceftriaxone-blunted VMR provides strong evidence of the involvement of glutamatergic mechanisms in mediating the enhanced EAAT2-induced blunting of the CRD response. Glutamate is a major chemical messenger mediating primary afferent input into the central nervous system. Enhanced EAAT2 glutamate transporter function leads to reduced extracellular glutamate and reduced activation of glutamate receptors mediating the visceronociceptive response. Previous work suggests that activation of lumbar NMDA receptors facilitates visceromotor and pressor responses to CRD in the rat (19). Thus reduced activation of spinal NMDA receptors is a possible mechanism of the blunted VMR response observed in the present study. Enhanced EAAT2/ceftriaxone-mediated glutamate uptake, leading to reduced intrasynaptic glutamate and decreased lumbar glutamate receptor activation, represent testable hypotheses explaining the blunted response of the present study. There may be roles of non-NMDA glutamate receptor mediating the blunted VMR response seen in transgenic or ceftriaxone-treated animals. For example, activation of mGlu5 receptors was found to be involved in the transmission of visceral pain in the spinal cord (4).
Changes induced by intracolonic ethanol appeared to interfere with the ability of ceftriaxone to blunt distension-induced VMR (Fig. 5, A and B). Thus events associated with inflammation and/or mucosal damage may mitigate ceftriaxone-induced reduction of the VMR response. The marked inflammation-mediated changes to the extracellular chemical milieu likely contribute to the mitigated effectiveness of EAAT2 overexpression. Recent work suggests that colonic distension without inflammation activates primarily lumbosacral input to the spinal cord, whereas CRD after colonic inflammation recruits enhanced thoracolumbar spinal afferent input (30). Thus differential processing of distension-induced colonic input after ethanol-induced inflammation/mucosal damage may play a role in the lack of ceftriaxone-induced blunted CRD response in this model.
Spinal or supraspinal sites of action may be involved in the EAAT2 blunted VMR to CRD. Intrathecal administration of glutamate transporter blockers augment nociception (20); thus it is plausible that enhanced glutamate transporter activity may have antinociceptive effects. With regard to putative supraspinal sites of action, descending facilitatory influences affecting visceral nociception from the rostral ventromedial medulla and cortex mediated by glutamate receptor activation has been well described (5, 31, 32). The mechanism of EAAT2/ceftriaxone-blunted visceral pain response awaits further exploration. Reduced activation of spinal glutamate receptors in EAAT2/ceftriaxone animals after CRD, leading to reduced activation of second order spinal neurons, is a leading possibility. Associated decreases in activated second messenger pathways (PKA, PKC, PIP2, NO/GC/PKC, ERK, p38) downstream from involved glutamate receptors may also be involved (25). Study of mechanisms of visceral afferent sensitization show that hollow organs are innervated by mechanosensitive receptors that have either low or high thresholds of response, with the latter representing the nociceptor innervations (13). A putative mechanism of blunted VMR response in EAAT2/ceftriaxone animals could be a lowered percentage of mechanosensitive high-threshold afferent fibers responding to distension in the noxious range.
This report reveals a new mechanistic approach to mitigate afferent input associated with visceral nociceptive neurotransmission. Illumination of this important approach may lead to improved mechanistic-based therapeutic options to visceral pain disorders.
GRANTS
This research was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-071839.
Acknowledgments
The assistance of the laboratory of Dr. Gerry Gebhart is gratefully acknowledged.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Al Chaer ED, Feng Y, Willis WD. A role for the dorsal column in nociceptive visceral input into the thalamus of primates. J Neurophysiol 79: 3143–3150, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Al Chaer ED, Traub RJ. Biological basis of visceral pain: recent developments. Pain 96: 221–225, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Arriza JL, Eliasof S, Kavanaugh MP, Amara SG. Excitatory amino acid transporter 5, a retinal glutamate transporter coupled to a chloride conductance. Proc Natl Acad Sci USA 94: 4155–4160, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bianchi R, Rezzani R, Borsani E, Rodella L. mGlu5 receptor antagonist decreases Fos expression in spinal neurons after noxious visceral stimulation. Brain Res 960: 263–266, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Coutinho SV, Urban MO, Gebhart GF. The role of CNS NMDA receptors and nitric oxide in visceral hyperalgesia. Eur J Pharmacol 429: 319–325, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Danbolt NC Glutamate uptake. Prog Neurobiol 65: 1–105, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Drossman D, Richter JE, Talley NJ, Thompson WG, Corazziari E, Whitehead WE. Functional bowel disorders and functional abdominal pain. In: The Functional Gastrointestinal Disorders: Diagnosis, Pathophysiology and Treatment: A Multinational Consensus, edited by Drossman D, Richter JE, et al. Boston, MA: Little, Brown, 1994, p. 115–173.
- 8.Drossman D, Camilleri M, Mayer E, Whitehead W. AGA technical review on irritable bowel syndrome. Gastroenterology 123: 2108–2131, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Everhart J, Renault P. Irritable bowel syndrome in office-based practice in the United States. Gastroenterology 100: 998–1005, 1991. [DOI] [PubMed] [Google Scholar]
- 10.Fairman WA, Vandenberg RJ, Arriza JL, Kavanaugh MP, Amara SG. An excitatory amino-acid transporter with properties of a ligand-gated chloride channel. Nature 375: 599–603, 1995. [DOI] [PubMed] [Google Scholar]
- 11.Furuta A, Martin LJ, Lin CL, Dykes-Hoberg M, Rothstein JD. Cellular and synaptic localization of the neuronal glutamate transporters excitatory amino acid transporter 3 and 4. Neuroscience 81: 1031–1042, 1997. [DOI] [PubMed] [Google Scholar]
- 12.Gebhart GF Pathobiology of visceral pain: molecular mechanisms and therapeutic implications. IV. Visceral afferent contributions to the pathobiology of visceral pain. Am J Physiol Gastrointest Liver Physiol 278: G834–G838, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Gebhart GF, Kuner R, Jones R, Bielefeldt K. Visceral hypersensitivity. In: Hyperalgesia: Molecular Mechanisms and Clinical Implications, edited by Brune K and Handwerker HO. Seattle, WA: IASP Press, 2004, p. 87–104.
- 14.Guo H, Lai L, Butchbach ME, Stockinger MP, Shan X, Bishop GA, Lin CL. Increased expression of the glial glutamate transporter EAAT2 modulates excitotoxicity and delays the onset but not the outcome of ALS in mice. Hum Mol Genet 12: 2519–2532, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Hendershot LC, Forsaith S, Abad F. Antagonism of the frequency of phenylquinone-induced writhing in the mouse by weak analgesics and non-analgesics. J Pharmacol Exp Ther 125: 237–240, 1959. [PubMed] [Google Scholar]
- 16.Ji Y, Traub RJ. Spinal NMDA receptors contribute to neuronal processing of acute noxious and nonnoxious colorectal stimulation in the rat. J Neurophysiol 86: 1783–1791, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Kamp EH, Jones RCW, Tillman SR, Gebhart GF. Quantitative assessment and characterization of visceral nociception and hyperalgesia in mice. Am J Physiol Gastrointest Liver Physiol 284: G434–G444, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Kanai Y, Hediger MA. Primary structure and functional characterization of a high-affinity glutamate transporter. Nature 360: 467–471, 1992. [DOI] [PubMed] [Google Scholar]
- 19.Kolhekar R, Gebhart GF. NMDA and quisqualate modulation of visceral nociception in the rat. Brain Res 651: 215–226, 1994. [DOI] [PubMed] [Google Scholar]
- 20.Liaw WJ, Stephens RL Jr, Binns BC, Chu Y, Sepkuty JP, Johns RA, Rothstein JD, Tao YX. Spinal glutamate uptake is critical for maintaining normal sensory transmission in rat spinal cord. Pain 115: 60–70, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Lin CL, Bristol LA, Jin L, Dykes-Hoberg M, Crawford T, Clawson L, Rothstein JD. Aberrant RNA processing in a neurodegenerative disease: the cause for absent EAAT2, a glutamate transporter, in amyotrophic lateral sclerosis. Neuron 20: 589–602, 1998. [DOI] [PubMed] [Google Scholar]
- 22.Pines G, Danbolt NC, Bjoras M, Zhang Y, Bendahan A, Eide L, Koepsell H, Storm-Mathisen J, Seeberg E, Kanner BI. Cloning and expression of a rat brain l-glutamate transporter. Nature 360: 464–467, 1992. [DOI] [PubMed] [Google Scholar]
- 23.Rothstein JD, Martin L, Levey AI, Dykes-Hoberg M, Jin L, Wu D, Nash N, Kuncl RW. Localization of neuronal and glial glutamate transporters. Neuron 13: 713–725, 1994. [DOI] [PubMed] [Google Scholar]
- 24.Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, Jin L, Dykes HM, Vidensky S, Chung DS, Toan SV, Bruijn LI, Su ZZ, Gupta P, Fisher PB. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature 433: 73–77, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Sakurai J, Obata K, Ozaki N, Tokunaga A, Kobayashi K, Yamanaka H, Dai Y, Kondo T, Miyoshi K, Sugiura Y, Matsumoto T, Miwa H, Noguchi K. Activation of extracellular signal-regulated protein kinase in sensory neurons after noxious gastric distention and its involvement in acute visceral pain in rats. Gastroenterology 134: 1094–1103, 2008. [DOI] [PubMed] [Google Scholar]
- 26.Storck T, Schulte S, Hofmann K, Stoffel W. Structure, expression, and functional analysis of a Na(+)-dependent glutamate/aspartate transporter from rat brain. Proc Natl Acad Sci USA 89: 10955–10959, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Traub RJ Spinal mechanisms of visceral pain and sensitization. In: Chronic Abdominal and Visceral Pain, edited by Pasricha PJ, Willis WD, Gebhart GF. New York: Informa Healthcare USA, 2007, p. 85–105.
- 28.Traub RJ, Zhai Q, Ji Y, Kovalenko M. NMDA receptor antagonists attenuate noxious and nonnoxious colorectal distention-induced Fos expression in the spinal cord and the visceromotor reflex. Neuroscience 113: 205–211, 2002. [DOI] [PubMed] [Google Scholar]
- 29.VanderWende C, Margolis S, Abad F. Analgesic tests based upon experimentally-induced acute abdominal pain in rats. Fed Proc 15: 494, 1956. [Google Scholar]
- 30.Wang G, Tang B, Traub RJ. Differential processing of noxious colonic input by thoracolumbar and lumbosacral dorsal horn neurons in the rat. J Neurophysiol 94: 3788–3794, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Wang G, Tang B, Traub RJ. Pelvic nerve input mediates descending modulation of homovisceral processing in the thoracolumbar spinal cord of the rat. Gastroenterology 133: 1544–1553, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu X, Gao J, Yan J, Fan J, Owyang C, Li Y. Role for NMDA receptors in visceral nociceptive transmission in the anterior cingulate cortex of viscerally hypersensitive rats. Am J Physiol Gastrointest Liver Physiol 294: G918–G927, 2008. [DOI] [PubMed] [Google Scholar]
- 33.Zhai QZ, Traub RJ. The NMDA receptor antagonist MK-801 attenuates c-Fos expression in the lumbosacral spinal cord following repetitive noxious and non-noxious colorectal distention. Pain 83: 321–329, 1999. [DOI] [PubMed] [Google Scholar]