Abstract
It is generally accepted that low levels of lipopolysaccharide (LPS)-binding protein (LBP) augment the cell's response to LPS, whereas high levels of LBP have been shown to inhibit cell responses to LPS. Clinical studies and in vitro work by our group have demonstrated that, in the setting of liver disease, increased or acute-phase levels of LBP may actually potentiate rather than inhibit an overwhelming proinflammatory response. Therefore, in the present studies we sought to determine the role of acute-phase LBP in mediating morbidity and mortality in animals challenged with LPS in the setting of biliary obstruction. Using LBP-deficient mice and LBP blockade in wild-type mice, we demonstrate that high levels of LBP are deleterious in the setting of cholestasis. Following biliary obstruction and intraperitoneal LPS challenge, hepatic injury, hepatic neutrophil infiltration, and mortality were significantly increased in animals with an intact LBP acute-phase response. Kupffer cell responses from these animals demonstrated a significant increase in several inflammatory mediators, and Kupffer cell-associated LBP appears to be responsible for these differences, at least in part. Our results indicate that the role of LBP signaling in inflammatory conditions is complex and heterogeneous, and elevated levels of LBP are not always protective. Increased LBP production in the setting of cholestatic liver disease appears to be deleterious and may represent a potential therapeutic target for preventing overwhelming inflammatory responses to LPS in this setting.
Keywords: common bile duct ligation, Kupffer cells, cytokines, chemokines
development of bacteremia, sepsis, and an overwhelming systemic inflammatory response remain major causes of morbidity and mortality in patients with cholestatic liver disease (4, 6, 27, 30, 33, 37). Numerous experimental studies have demonstrated a profound proinflammatory response following endotoxin challenge, which is marked by an increased production of tumor necrosis factor (TNF)-α, interleukin (IL)-1, and IL-6 in animals with biliary obstruction (9, 14, 17, 18, 28). This proinflammatory milieu is associated with increased markers of end organ injury [aspartate aminotransferase (AST) and creatinine] and death (14), and blockade of this response through the administration of gadolinium chloride has been shown to suppress the systemic proinflammatory response leading to improved survival (18, 24). Anti-inflammatory cytokines have also been measured in patients with biliary obstruction, with an elevation of IL-10, IL-1 receptor antagonist (IL-1ra), and soluble TNF receptors p55 and p75 in patients with obstructive jaundice associated with acute cholangitis or biliary malignancy (20, 21).
In addition to the significant elevation of pro- and anti-inflammatory mediators in the plasma of patients with cholestasis, studies have recently documented a significant increase in circulating lipopolysaccharide (LPS)-binding protein (LBP) in patients with biliary obstruction and cirrhosis from other causes (1, 2, 19). LBP is constitutively expressed at low levels, but is transcriptionally upregulated during acute-phase responses (32), increasing five- to tenfold at peak levels (8, 23). LBP appears to have a dual role in vivo, with low levels of LBP typically potentiating the cell's response to LPS by facilitating transfer of LPS to its receptor CD14 (13, 38, 42, 46), whereas high levels of LBP have been shown to inhibit cell responses to LPS by transferring the LPS to high-density lipoproteins (39, 43) or by facilitating internalization of LPS without triggering inflammatory cell stimulation (10). Lamping et al. (23) demonstrated that, in LPS-challenged and d-galactosamine-sensitized mice, acute-phase levels of LBP inhibited LPS-mediated cytokine release and prevented hepatic failure resulting in a significantly improved survival rate. Thus it has been suggested that low or constitutive levels of LBP facilitate recognition of LPS or gram-negative infection and early activation of immune cells, whereas acute-phase levels serve to neutralize LPS to prevent overstimulation of the immune system (15). However, in contrast to this proposed paradigm, cirrhotic patients with elevated LBP levels appear to have an increased risk of bacterial infection and immune and hemodynamic derangement (1, 2), rather than suppression of these responses as would be expected on the basis of the described kinetics of LBP outlined in experimental studies.
Consistent with these observations in patients with cirrhosis and biliary obstruction, we have previously observed that Kupffer cells isolated from mice following common bile duct ligation (CBDL) demonstrate a dose-dependent, exaggerated proinflammatory response to LPS when costimulated with LBP, with a loss of the expected inhibitory effects of LBP at higher doses. This is in contrast to Kupffer cells isolated from Sham animals, which demonstrate a more modest proinflammatory response to the same doses of LBP with a return to baseline levels of TNF-α and IL-6 as the LBP dose increased (26). Taken together, these observations suggest that elevated LBP levels may be deleterious in the setting of biliary obstruction compared with other inflammatory diseases in which acute-phase levels of LBP have been found to be protective. In the present studies we have explored the potential impact and mechanism by which acute-phase levels of LBP may augment the exaggerated inflammatory response, increased organ injury, and mortality observed in the setting of biliary obstruction and LPS challenge.
MATERIALS AND METHODS
Animals
LBP-deficient (LBPKO) mice were a gift from Douglas T. Golenbock (University of Massachusetts Medical School) (44). The LBPKO mice had been backcrossed into a C57BL/6 strain background at least 12 times before being acquired into our colony, and these mice were then maintained in a specific pathogen-free environment in microisolation cages and allowed to breed in our animal facility. Female LBPKO mice (age 8–12 wk) and age- and weight-matched female specific pathogen-free C57BL/6 mice from Harlan Laboratories (Indianapolis, IN) were used to complete the present studies. All mice were housed in the University of Michigan Unit for Laboratory Medicine's animal facility with unlimited chow and water for the duration of the experiments. The C57BL/6 mice were allowed to acclimate for at least 1 wk in the University of Michigan facility prior to any experimentation. All animal studies were approved by the University's Animal Care and Use Committee of the University of Michigan. The laboratory adheres to the “Guiding Principles of Laboratory Animal Care,” as promulgated by the American Physiological Society.
CBDL and LPS Challenge
On day 0 mice were anesthetized by inhalation of 2–5% isoflurane (Baxter, Deerfield, IL) in 100% oxygen via anesthesia equipment (Surgivet/Anesco, Waukesha, WI) and CBDL was performed as previously described (26) through a transverse right subcostal incision. Briefly, ligation of the common bile duct with a 4-0 silk suture was performed, with care taken not to inadvertently ligate or injure the hepatic artery or pancreatic duct. Abdominal wall closure was achieved with interrupted 4-0 silk sutures, and the skin was closed with staples. Sham animals underwent laparotomy and identical dissection without bile duct ligation.
Four days following CBDL, selected mice underwent an intraperitoneal injection of 100 ng/gram body weight (gbw), 1 μg/gbw, or 5 μg/gbw of Escherichia coli LPS (Sigma-Aldrich, St. Louis, MO) in 300 μl of sterile saline. For the survival studies, 2 and 6 h following LPS delivery 40 μl of blood was obtained via tail vein bleed for determination of liver injury and levels of systemic inflammatory mediators as outlined below. Mice were then maintained in a closed room at 23°C and survival was recorded every 6 h for 3 days. In the dose-response studies, the animals were euthanized at 6 h and terminal bleeding occurred, followed by harvest of the livers from these animals. Tail vein bleeding occurred at 2 h as well in these animals as outlined above.
In vivo blockade of LBP function in wild-type animals was achieved by intraperitoneal administration of 100 μg of the-inhibitory peptide, LBPK95A. LBPK95A was synthesized by the University of Michigan Protein Structure Facility according to the instructions provided by Arana et al. (3). The peptide is designed from the 86–99 amino acid sequence of human LBP, which is RVQGRWKVRKSFFK, with substitution of the lysine 95 (Lys95) amino acid with alanine (RVQGRWKVRASFFK). This peptide has demonstrated LBP-inhibitory activity in vivo in mice (3). The LBPK95A peptide (100 μg/500 μl of sterile saline per mouse) was delivered intraperitoneally to C57BL/6 mice 2 h prior to administration of intraperitoneal LPS (1 μg/gbw) 4 days following CBDL. Control animals underwent an intraperitoneal injection of 500 μl of sterile saline 2 h prior to LPS administration. At 6 h following LPS delivery, 40 μl of blood was obtained by tail vein bleed, and mice were followed for 5 days and evaluated at 12-h intervals for survival.
Kupffer Cell Isolation and Culture
In selected mice, Kupffer cells were isolated from mice 4 days following CBDL as previously described (35, 36) via the modified methods described by Knook and Sleyster (22). Briefly, livers were perfused retrograde through the inferior vena cava with Gey's balanced salt solution (GBSS, GIBCO-BRL, Gaithersburg, MD) followed by GBSS with 0.1% pronase E. The liver was then excised and minced prior to incubation with GBSS-pronase solution with continuous stirring at 37°C for 60 min. DNase (0.8 mg/ml) was added to prevent cell clumping. The liver slurry was filtered through gauze mesh, washed with PBS with DNAse (0.8 μg/ml), and centrifuged at 600 g for 5 min two times. Cells were then further purified by using a discontinuous Percoll gradient of 25 and 50% Percoll as described in detail by Pertoft and Smedsrod (29). Kupffer cells were enriched by differential adherence to tissue culture plates. Cells (2.0 or 4.0 × 105 cells/well) were plated on 96-well tissue culture plates at 37°C for 30 min in serum-free medium. This medium and nonadherent cells were then removed and replaced with 200 μl of serum-free medium, for incubation overnight. The following morning the cells were washed three times with serum-free medium prior to experimentation. Kupffer cell viability was assessed with Trypan blue, and their purity was confirmed by their ability to ingest latex beads. Cell viability was greater than 90% as assessed by Trypan blue.
Kupffer cells were then stimulated the following morning in the 96-well tissue culture plate in serum-free conditions with 100 ng/ml LPS and increasing doses (0, 1, and 3 μg/ml) of a monoclonal mouse LBP antibody that was specific for mouse LBP and did not inhibit binding of LPS to CD14 (Cell Sciences, Canton, MA). Supernatants were collected 6 h later for determination of inflammatory mediator production. Kupffer cells were also collected as outlined below for flow cytometry determination of cell-surface expression of LPS receptors.
Immunofluorescent Staining
Kupffer cells were isolated as outlined above from wild-type and LBPKO (negative control) mice 4 days following CBDL. Kupffer cells were attached to the chamber slide, fixed with 4% paraformaldehyde for 30 min and blocked with 5% rabbit serum plus 1% BSA for 1 h. The cells were incubated with 4 μg/ml of goat anti-mouse LBP antibody (Santa Cruz Biotechnology, Santa Cruz, CA) or 4 μg/ml of goat IgG overnight at 4°C and washed with PBS three times. The cells were then incubated with biotin-conjugated rabbit anti-goat IgG (Invitrogen, Carlsbad, CA) for 30 min at room temperature. After washing with PBS, the cells were subjected to 2 μg/ml of Alexa Fluor 488-conjugated streptavidin (Molecular Probes, Eugene, OR) for 30 min at room temperature. Lastly, nuclear staining was conducted with 300 nM 4′,6-diamidino-2-phenylindole (Molecular Probes) for 3 min. The slide was rinsed and mounted in ProLong Gold Anti-fade (Molecular Probes) and observed with a ×100 objective on a Olympus BX-51 microscope. The fluorescence distribution on the cell surface was obtained using a confocal laser-scanning microscope (Olympus FluoView FV500).
Real-Time Polymerase Chain Reaction
Liver samples were excised and snap frozen in liquid nitrogen and then stored at −80°C until RNA extraction. Total RNA was isolated and purified from each mouse liver sample using RNeasy kit and RNase free-DNase (Qiagen) according to the manufacturer's instructions. cDNA was generated from total RNA with TaqMan Reverse Transcription reagents (Applied Biosystems) with RNA (1,600 ng) as a template and oligo(dT)16 as primers. The resulting cDNA was then used for quantitative gene expression analysis on a Sequence Detection System 7,500 (Applied Biosystems). Primers and probe mix solution for IL-6 (assay ID Mm00446190_m1), TNF-α (assayed Mm00443258), IL-1ra (assay ID Mm00446185_m1), macrophage inflammatory protein (MIP)-2 (assay ID Mm004364450_m1) and β-actin (assay ID Mm006077939_s1) were purchased from Applied Biosystems. Equal amounts of cDNA were used with the TaqMan Master Mix (Applied Biosystems) and primer probe mix according to the real-time PCR protocols supplied by the manufacturer. Amplification efficiencies were validated against the housekeeping gene, β-actin. The data were normalized to β-actin mRNA level and expressed in arbitrary units. The relative quantification of target gene expression was done by the comparative cycle threshold (CT) method. The formula 2−ΔΔCT was used for each run according to the manufacturer's instructions and published methods for this system (25).
Protein Analysis
LBP measurement.
Plasma, liver homogenate, and Kupffer cell supernatant levels of LBP were determined by using a commercially available ELISA for detection of mouse LBP (Cell Sciences, Canton, MA).
Inflammatory mediator measurement.
Levels of 20 inflammatory mediators (see Table 1 for complete list) were measured in plasma and Kupffer cell supernatant by using a previously validated microarray immunoassay (MI) (5). Detailed information about the MI, including step-by-step instructions and a list of antibodies used, may be found at the following website: http://sitemaker.umich.edu/remick.lab/microimmunoassay. Use of the MI for measurement of inflammatory mediator levels allowed for determination of a large number of cytokines, chemokines, and endogenously occurring inhibitors (Table 1) from a small volume of plasma or Kupffer cell supernatant.
Table 1.
Plasma levels of inflammatory mediators are equivalent following CBDL and LPS challenge
| Inflammatory Mediator | C57BL/6 | LBPKO |
|---|---|---|
| TNF-α | 6,966±1,901 | 6,084±1,500 |
| IL-6 | 88,920±18,150 | 72,320±10,660 |
| MIP-2 | 54,050±11,510 | 44,570±8,984 |
| MCP-1 | 25,860±7,053 | 22,210±4,131 |
| IL-1β | 5,372±1,091 | 6,861±757.5 |
| IL-4 | 33,630±4,572 | 24,920±2,846 |
| IL-10 | 28,910±4,897 | 20,700±4,031 |
| IL-18 | 5,980±1,075 | 4,680±654.4 |
| Eotaxin | 12,570±2,352 | 8,696±993. |
| IL-1ra | 11,560±2,240 | 11,750±1,404 |
| IL-5 | 1,558±366.9 | 8,424±7,046 |
| IL-12 | 5,641±809.3 | 4,791±705.9 |
| IFN-γ | 7,827±1,318 | 7,850±1,171 |
| MIP-1α | 26,260±4,486 | 21,860±3,052 |
| TNFSR1 | 7,407±1,737 | 4,116±475.4 |
| TNFSR2 | 6,258±975.4 | 5,404±1,336 |
| IL-2 | 8,947±1,560 | 7,150±781.3 |
| IL-13 | 48,010±14,190 | 27,790±6,042 |
| RANTES | 1,302±208.0 | 1,882±587.7 |
| Lix | 10,240±1,460 | 8,251±430.3 |
Data are expressed as means ± SE, in pg/ml. CBDL, common bile duct ligation; LBPKO, LPS-binding protein-deficient mice. P > 0.05 for all mediators.
Markers of hepatic injury.
Plasma bilirubin and transaminase levels [AST and alanine aminotransferase (ALT)] were measured as directed using colorimetric assays purchased from Pointe Scientific (Canton, MI).
Myeloperoxidase Activity
Hepatic neutrophil sequestration was quantitated by measuring tissue levels of myeloperoxidase content as previously described (40). Snap-frozen livers (−80°C) were weighed and homogenized for 1 min in five parts volume per weight of 0.01 M KH2PO4 with 1 mM EDTA (PE buffer). Following homogenization, the resultant pellet was resuspended in 13.7 mM hexadecycltrimethylammonium bromide (H-TAB) buffer with 50 mM acetic acid, using the same volume of H-TAB as PE buffer. The resuspended pellet was then sonicated for 40 s at 60% on the sonicator (model VC 100, Sonic & Materials, Danbury, CT), and centrifuged at 10,000 rpm for 15 min. The resultant supernatant was collected and incubated in a 60°C water bath for 2 h. Myeloperoxidase activity was then measured in this solution by H2O2-dependent oxidation of 3,3′5,5′-tetramethylbenzidine, which generates a colorimetric reaction. Spectrophotometric absorbance was read at 650 nm and compared with a linear standard curve with a sensitivity of 0.03125 ELISA Units (EU).
Flow Cytometry
Cell surface expression of TLR4, CD14, and CD11b/CD18 on isolated Kupffer cells from C57BL/6 and LBPKO mice 4 days following CBDL was determined by flow cytometry. Briefly, Kupffer cells were isolated as outlined and plated overnight (following the washing away of nonadherent cells) on a 60 × 15 mm tissue culture plate at a concentration of 4 × 105 cells/100 μl of serum-free medium. The following morning, the cells were washed twice with serum-free medium and then fixed with fresh 1% paraformaldehyde. Cells were then viewed in the plate and any adherent cells were gently scraped from the plate bottom until all cells were floating. The cells were then collected and divided into tubes (1 million cells per tube) for staining. Cells were then stained with TLR4-MD-2 PE-labeled antibody (Pharmingen no. 558294) at a concentration of 1 μg/million cells, CD14 PE-labeled antibody (Pharmingen no. 553740) at a concentration of 1 μg/million cells, and with CD11b/CD18 PE-labeled antibody (Pharmingen no. 557397) at a concentration of 0.25 μg/million cells. In addition, all cells were stained with F4/80 APC-labeled antibody (Serotec no. MCA497APC), allowing for the discrimination of Kupffer cells from any potential epithelial or stellate cells that may be present in the cell suspension. Following staining, cells were again fixed in 1% paraformaldehyde and cell surface staining was analyzed and measured by the University of Michigan Flow Cytometry Core Facility on the FACSVantage SE.
Statistical Analysis
Data were analyzed by GraphPad Prism (GraphPad Software, San Diego, CA). Data are presented as means ± SE. For the in vivo studies, final n = 24 animals per group, with results pooled from triplicate experiments of n = 8 animals per group. For each experiment utilizing Kupffer cells, cells were isolated and pooled from four to five mice for each treatment group (C57BL/6 CBDL mice and LBPKO CBDL mice) and all studies were repeated in duplicate or triplicate. Analysis of variance was used to compare groups with Dunnett's post hoc analysis. Survival statistics were calculated by using a Cox-hazard ratio. Statistical significance was considered to be achieved if P < 0.05. All protein measurements and myeloperoxidase activity levels below detectable limits were set at the detection limit of the assay for the purpose of analysis.
RESULTS
LBP Mediates Increased Mortality Following CBDL
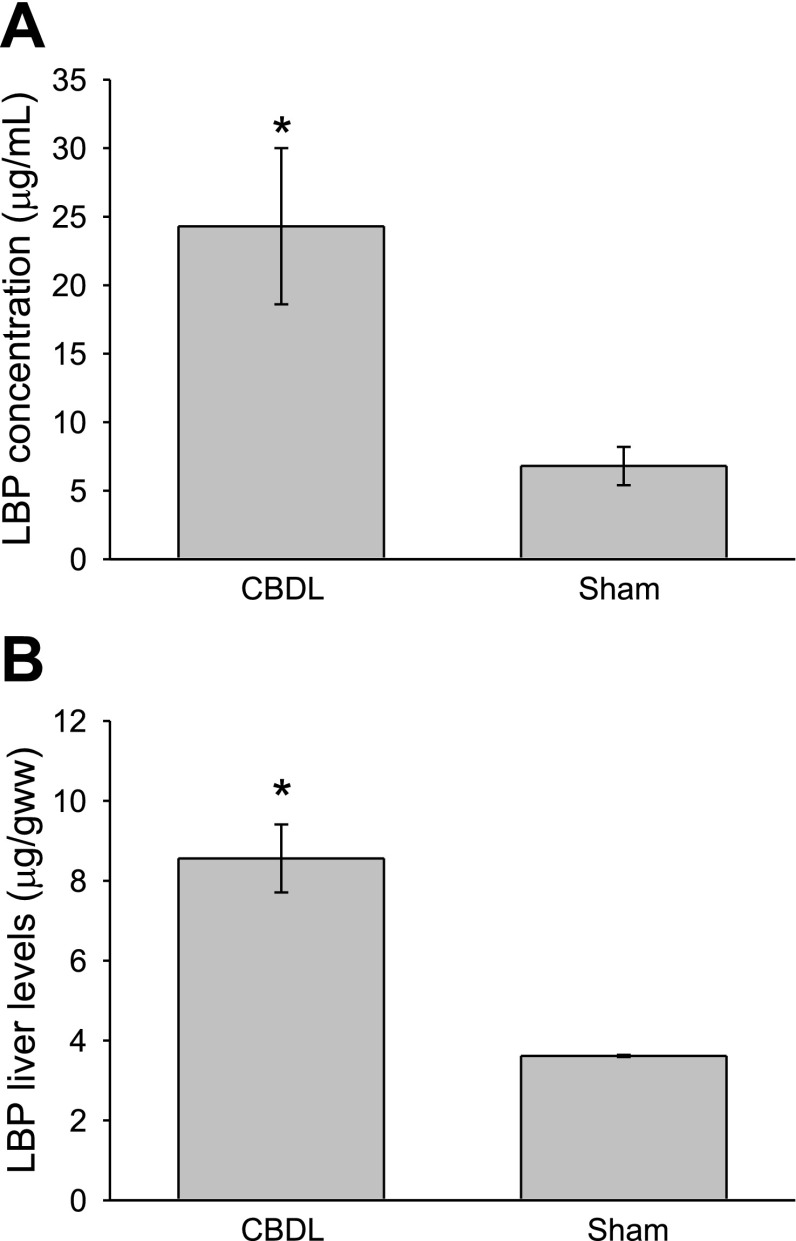
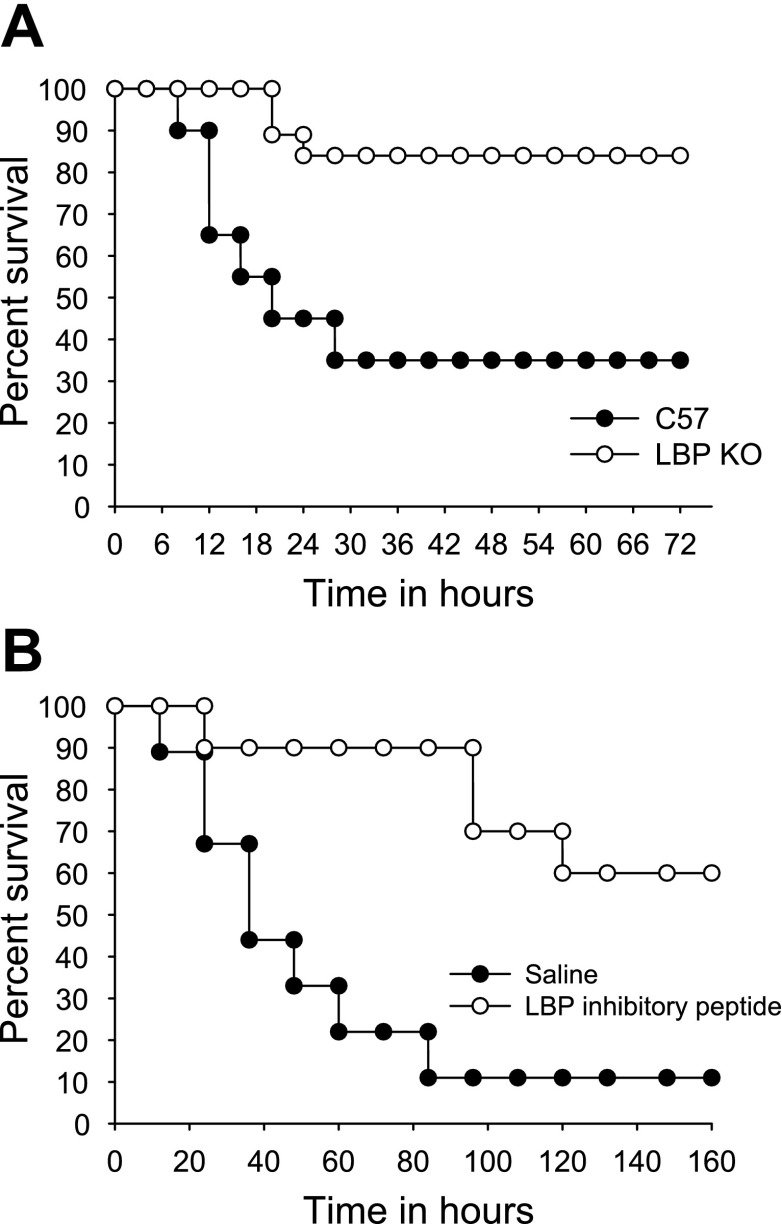
Plasma and liver LBP levels increase in an acute-phase manner following CBDL. Similar to prior reports in other liver injury models (23), plasma levels increase fivefold after CBDL (Fig. 1A) and liver protein levels double (Fig. 1B) compared with levels in Sham animals. However, in contrast to the findings of others, in which acute-phase levels of LBP were protective and improved survival, mice deficient in LBP had a significantly improved survival following CBDL and LPS challenge (1 μg/gbw) compared with wild-type controls (Fig. 2A), with 84% of the LBPKO mice surviving for 3 days following LPS delivery vs. 35% of the C57BL/6 mice, P < 0.005. A similar survival advantage was observed when wild-type mice underwent LBP blockade prior to LPS challenge, with only 11% of the saline-treated mice surviving vs. 60% of the mice treated with the synthetic inhibitory LBP peptide, LBPK95A (3), P = 0.003 (Fig. 2B). This suggests that the survival advantage observed in the LBPKO animals is due to the absence of LBP rather than an adaptive mechanism present in this genetically engineered mouse.
Fig. 1.
Plasma and liver LPS-binding protein (LBP) levels following common bile duct ligation (CBDL). Plasma (A) and liver (B) LBP levels are significantly elevated 4 days following CBDL compared with sham operation, *P < 0.001.
Fig. 2.
Survival following CBDL and LPS challenge. LBP-deficient (LBPKO) mice (n = 21) demonstrate significantly improved survival following delivery of LPS [1 μg/g body weight (gbw)] after CBDL compared with C57BL/6 controls (n = 20), P < 0.005 (A). Similarly, CBDL wild-type mice treated with an inhibitory LBP peptide (n = 10) demonstrate a survival advantage compared with controls treated with saline vehicle alone (n = 10) prior to LPS challenge (1 μg/gbw), P = 0.003 (B).
Interestingly, despite the improved survival observed in the LBPKO CBDL animals challenged with LPS compared with wild-type controls, the levels of plasma inflammatory mediators were equivalent. As shown in Table 1, 20 pro- and anti-inflammatory mediators were measured in the plasma of LBPKO and C57BL/6 CBDL animals 2 h following LPS challenge, and no significant differences were observed in the systemic inflammatory profile of LBPKO and C57BL/6 CBDL mice. These levels were also measured at 6 h following LPS challenge to determine whether the plasma levels of these inflammatory mediators possibly peaked at a later time point and were again found to be equivalent (data not shown).
A dose-response study was then carried out to determine whether higher or lower doses of LPS (100 ng/gbw and 5 μg/gbw, respectively) would yield a different response, and similar patterns were observed. Specifically, the systemic levels of inflammatory mediators as measured by serum microarray immunoassay were equivalent in C57 and LBPKO CBDL animals 2 and 6 h following challenge with 5 μg/gbw intraperitoneal LPS; following the 100 ng/gbw dose of LPS, the C57 CBDL animals had significantly higher levels of only IL-6 and monocyte chemoattractant protein (MCP)-1 at 2 h following LPS challenge compared with LBPKO CBDL animals (IL-6: 19,430 ± 5,410 pg/ml vs. 4,638 ± 2,600 pg/ml, P < 0.05; MCP-1: 21,700 ± 4,554 pg/ml vs. 4,741 ± 3,032 pg/ml, P < 0.05, respectively), whereas the levels of all other mediators were equivalent.
LBP Mediates Increased Liver Injury Following CBDL
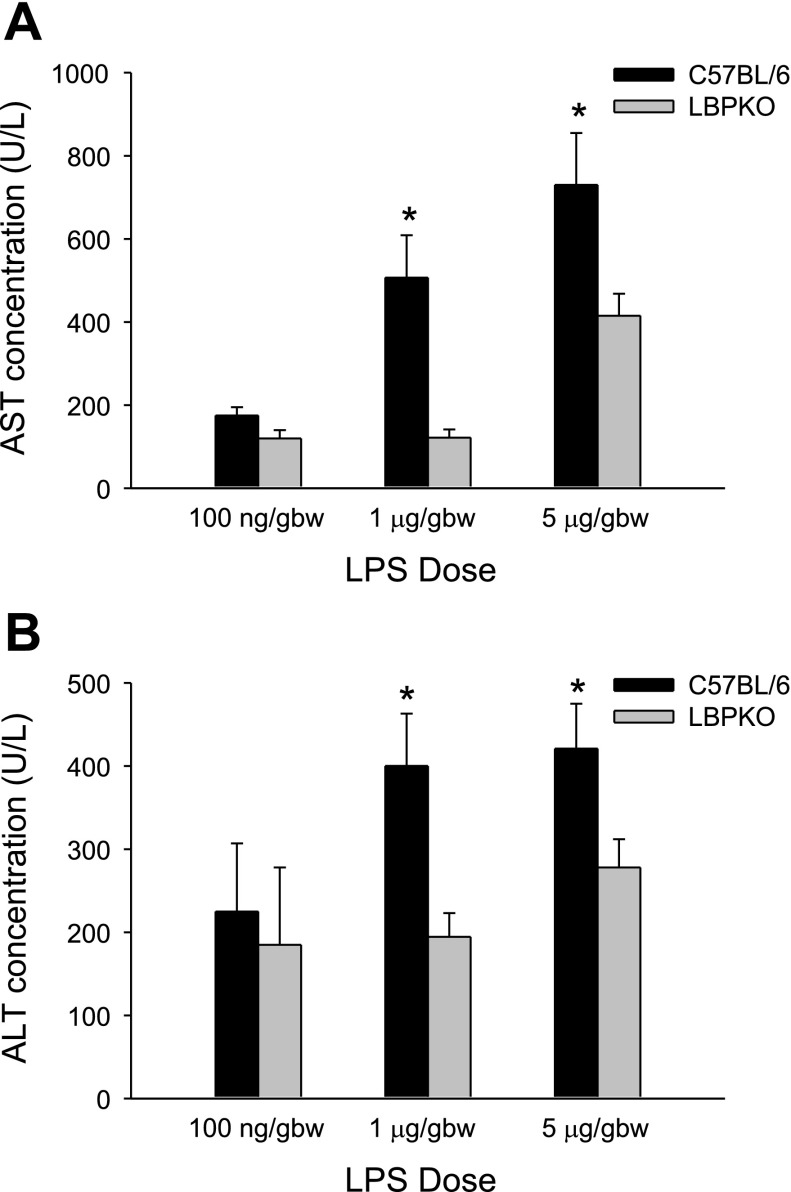
Although there were no discernible differences noted in the systemic inflammatory response between LBPKO and C57BL/6 CBDL mice challenged with LPS, a significant increase in liver injury was observed in the C57BL/6 CBDL mice compared with the LBPKO CBDL mice. Prior to LPS challenge, C57BL/6 and LBPKO mice had comparable levels of cholestasis and transaminase elevation 4 days following CBDL (Table 2). However, following LPS challenge, liver injury was significantly increased in the C57BL/6 CBDL mice compared with LBPKO CBDL mice following 1 and 5 μg/gbw doses of LPS (Fig. 3), as demonstrated by the significantly increased transaminase levels observed in the C57BL/6 mice. Following the 100 ng/gbw dose of LPS, the AST and ALT levels did not rise above the baseline levels observed following CBDL alone. A dose-dependent increase in liver injury was observed. Bilirubin levels remained equivalent following LPS challenge at all doses. Similarly, the CBDL wild-type mice receiving the LBP-inhibitory peptide had significantly lower ALT levels 6 h following LPS challenge (1 μg/gbw) than those receiving the saline vehicle: 378 ± 47 vs. 527 ± 27 IU/l, respectively, P < 0.05.
Table 2.
Hepatic transaminase and bilirubin levels following common bile duct ligation
| Bilirubin | AST | ALT | |
|---|---|---|---|
| C57BL/6 | 9.1±2.3 | 244±24 | 153±12 |
| LBPKO | 8.9±1.0 | 316±81 | 204±38 |
Data are expressed as means ± SE, in mg/dl for bilirubin and IU/l for aspartate aminotransferase (AST) and alanine aminotransferase (ALT). P > 0.05 for all comparisons.
Fig. 3.
Hepatic transaminase levels following CBDL and LPS challenge. Aspartate aminotransferase (AST; A) and alanine aminotransferase (ALT; B) levels are significantly elevated in C57BL/6 CBDL mice compared with LBPKO CBDL mice 6 h following LPS delivery (1 and 5 μg/gbw), *P < 0.001 and *P < 0.05, respectively.
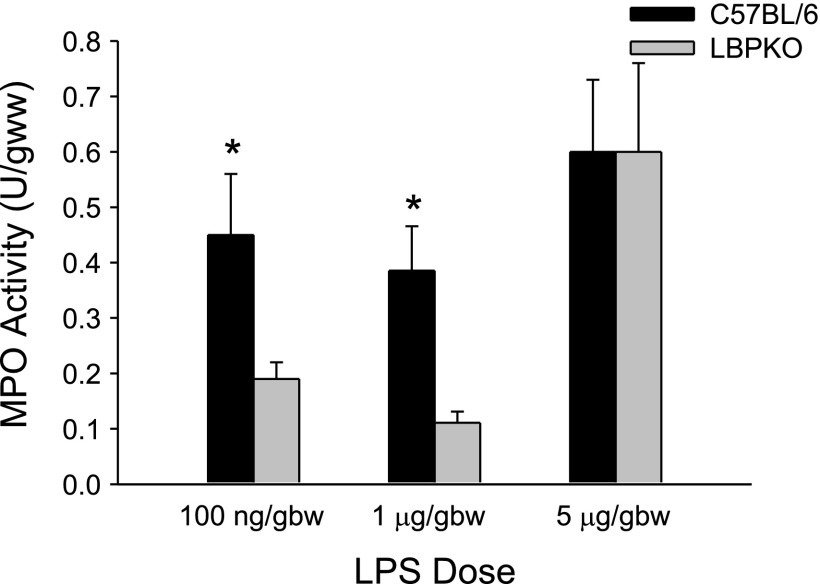
Neutrophil infiltration in the liver as measured by myeloperoxidase activity was also noted to be significantly elevated in the C57BL/6 CBDL mice compared with the LBPKO CBDL mice 6 h following LPS challenge at the 100 ng/gbw and 1 μg/gbw doses of LPS, whereas at the highest dose of LPS (5 μg/gbw) myeloperoxidase activity was significantly higher than at the lower doses of LPS, but equivalent in both the C57BL/6 and LBPKO CBDL animals (Fig. 4).
Fig. 4.
Liver myeloperoxidase (MPO) activity. Myeloperoxidase activity, a measure of neutrophil infiltration, is significantly increased in the livers of C57BL/6 CBDL mice compared with LBPKO CBDL mice following LPS delivery (100 ng/gbw and 1 μg/gbw doses), *P < 0.05.
Hepatic Levels of Inflammatory Mediators Are Increased in Presence of LBP
Given our prior observation that Kupffer cells isolated from CBDL mice demonstrate an exaggerated proinflammatory response to LPS when costimulated with LBP (26), coupled with our present observation that LBP levels are significantly elevated in the liver following CBDL (Fig. 1B) and LBPKO CBDL animals have decreased liver injury following LPS challenge (Fig. 3), we next examined Kupffer cell production of inflammatory mediators from C57BL/6 and LBPKO CBDL mice. Kupffer cells were isolated from C57BL/6 and LBPKO mice 4 days following CBDL in serum-free conditions to eliminate the potential for serum LBP or CD14 interference with LPS signaling in vitro.
In contrast to the relative equivalent production of all inflammatory mediators measured in the plasma of C57BL/6 and LBPKO CBDL mice challenged with LPS (Table 1), Kupffer cell production of four mediators was significantly increased by C57BL/6 CBDL Kupffer cells compared with LBPKO CBDL Kupffer cells following in vitro stimulation with LPS (Table 3). One of these mediators was the CXC chemokine MIP-2, which is known to be a neutrophil chemoattractant and may account for the increased myeloperoxidase activity observed in the livers of C57BL/6 CBDL mice following LPS challenge at doses of 100 ng/gbw and 1 μg/gbw (Fig. 4).
Table 3.
C57BL/6 CBDL Kupffera cell production of specific inflammatory mediators is increased following LPS stimulation
| Mediator | C57BL/6 | LBPKO | P Value |
|---|---|---|---|
| TNF-α | 1,675±152 | 47±10 | P < 0.0001 |
| IL-6 | 3,204±607 | 191±31 | P = 0.002 |
| MIP-2 | 17,256±1,851 | 1,973±85 | P < 0.0001 |
| IL1-ra | 12,138±1,199 | 1,280±335 | P < 0.0001 |
Data are expressed as means ± SE, in pg/ml. Kupffer cells stimulated with 100 ng/ml LPS.
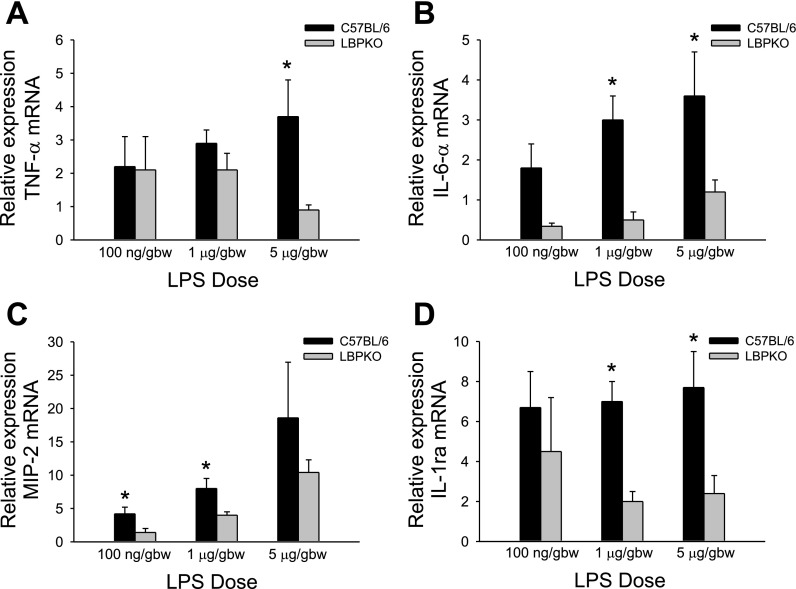
To verify these in vitro findings of increased production of TNF-α, IL-6, IL-1ra, and MIP-2 by the C57BL/6 CBDL Kupffer cells stimulated with LPS, livers from both C57BL/6 and LBPKO CBDL animals challenged with LPS in vivo at doses of 100 ng/gbw, 1 μg/gbw, and 5 μg/gbw were examined. The expression of TNF-α, IL-6, MIP-2, and IL-1ra mRNA was assessed by quantitative real-time PCR. Consistent with our findings in the Kupffer cells, mRNA levels of IL-6 and IL-1ra (Fig. 5, B and D) were significantly elevated in the livers of C57BL/6 CBDL mice challenged with LPS at doses of 1 and 5 μg/gbw compared with the LBPKO CBDL mice. MIP-2 expression (Fig. 5C) was increased in the livers of the CBDL animals challenged with 100 ng/gbw and 1 μg/gbw LPS, whereas at the highest dose of LPS (5 μg/gbw) the levels were not statistically significant. Of note, the degree of neutrophil infiltration was also equivalent at this highest dose of LPS delivered to C57BL/6 and LBPKO CBDL animals (Fig. 4). TNF-α levels did not achieve a statistically significant level of increase (Fig. 5A) in the C57BL/6 animals at 100 ng/gbw or 1 μg/gbw dose of LPS but was statistically increased at the 5 μg/gbw dose of LPS.
Fig. 5.
Expression of hepatic TNF-α, IL-6, macrophage inflammatory protein (MIP)-2, and IL-1 receptor antagonist (IL-1ra) mRNA. Hepatic levels of TNF-α (A) mRNA were significantly elevated in the C57BL/6 mice 6 h following the in vivo delivery of 5 μg/gbw LPS, whereas levels of MIP-2 (C) were significantly elevated in the C57BL/6 mice 6 h following delivery of 100 ng/gbw and 1 μg/gbw LPS, *P < 0.05. Hepatic levels of IL-6 (B) and IL-1ra (D) were significantly elevated in the C57BL/6 mice 6 h following the in vivo delivery of 1 and 5 μg/gbw LPS, *P < 0.05.
Cell Associated LBP Mediates Altered Kupffer Cell Function Following CBDL
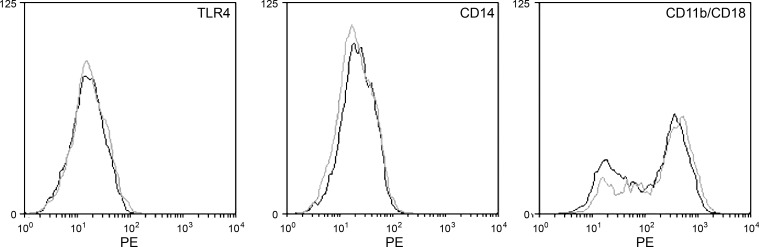
To further explore the potential mechanism by which Kupffer cells isolated from C57BL/6 mice following CBDL have altered cytokine production following LPS stimulation compared with LBPKO CBDL Kupffer cells, we next examined the cell surface expression of known LPS receptors on C57BL/6 and LBPKO CBDL Kupffer cells. Following isolation in serum-free conditions 4 days following CBDL, cell surface expression of TLR4, CD14, and CD11b/CD18 was determined by flow cytometric analysis on Kupffer cells from C57BL/6 and LBPKO CBDL mice. As shown in Fig. 6, Kupffer cell surface expression of these LPS receptors was equivalent for C57BL/6 and LBPKO CBDL mice and could not account for the differences observed in Kupffer cell inflammatory mediator production (Table 2). It has been suggested in biophysical experiments that LBP can intercalate into reconstituted phospholipid planar membranes and phospholipid liposomal complexes (11, 12, 31); therefore, we next sought to determine whether cell-associated LBP could account for the increased cytokine and chemokine production observed from Kupffer cells isolated from C57BL/6 CBDL mice.
Fig. 6.
Cell surface expression of LPS receptors. Cell surface expression of the LPS receptors TLR4, CD14, and CD11b/CD18 is equivalent on Kupffer cells isolated from C57BL/6 CBDL (gray trace) and LBPKO CBDL (black trace) mice.
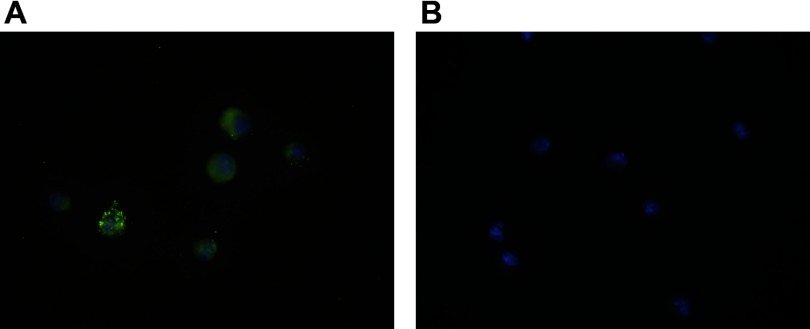
Kupffer cells from C57BL/6 and LBPKO mice were again isolated in serum-free conditions 4 days following CBDL and plated overnight as described. LBP levels were measured the following morning in the supernatants. Since Kupffer cells do not produce LBP and the isolation occurs in a completely serum-free environment and is a multistep process culminating in retrieval of the Kupffer cells from a Percoll gradient, any LBP present in the supernatant must represent LBP that was associated with the Kupffer cell at the time of isolation and later released into the supernatant. Twenty-four hours following isolation of Kupffer cells, LBP levels were significantly elevated in the supernatants collected from the C57BL/6 CBDL Kupffer cells (3,551 ± 286 pg/ml) and were undetectable (< 10 pg/ml) in the LBPKO CBDL Kupffer cell supernatants as would be expected, P < 0.001. To verify that LBP was in fact associated with the isolated C57BL/6 CBDL Kupffer cells, immunofluorescent staining was performed with LBP staining green (Alexa Fluor 488) and the nucleus staining blue (DAPI). As shown in Fig. 7, LBP is present both on the cell surface and in the cytoplasm of the C57BL/6 Kupffer cells. LBPKO CBDL Kupffer cells were also stained as a negative control and demonstrate only positive nuclear staining.
Fig. 7.
Immunofluorescent staining of LBP on C57BL/6 Kupffer cells. Four days following CBDL, Kupffer cells isolated from C57BL/6 mice demonstrate the LBP is present on the cell surface as well within the cell (A), as demonstrated by the confocal microscope images, ×100 magnification. The LBP appears green (stained with Alexa Fluor 488), whereas the nuclei appear blue (stained with DAPI). Kupffer cells isolated from LBPKO CBDL mice (negative control) demonstrate only positive nuclear staining (B).
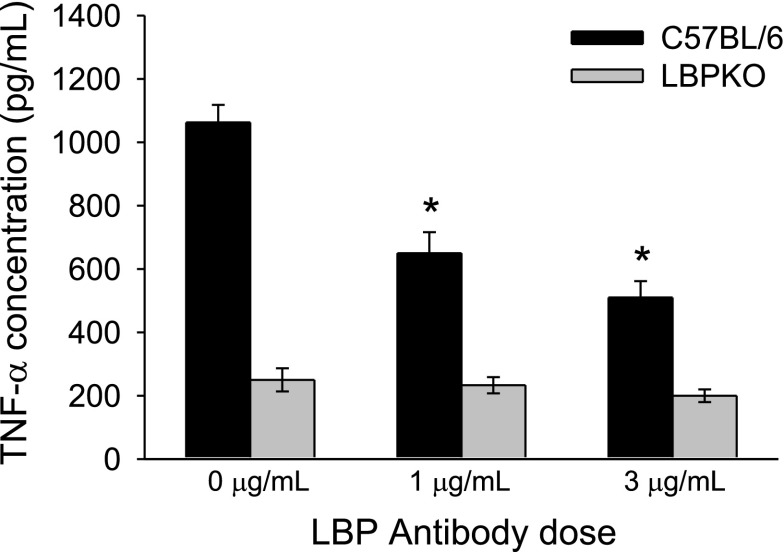
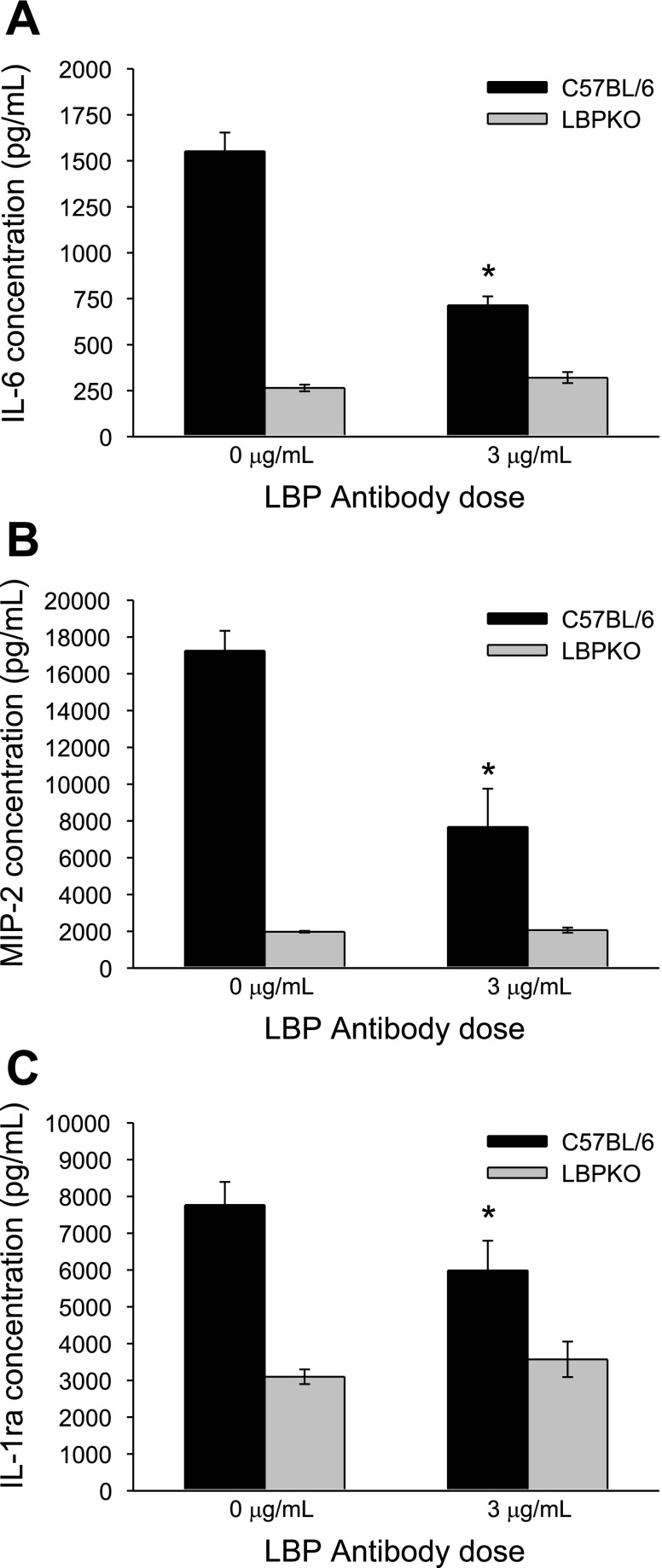
To determine whether this cell-associated LBP could account for the observed increase in TNF-α, IL-6, MIP-2, and IL-1ra production by C57BL/6 CBDL Kupffer cells compared with LBPKO CBDL Kupffer cells, Kupffer cells were isolated and stimulated with LPS (100 ng/ml) and increasing doses of a murine LBP antibody (0, 1, and 3 μg/ml). As demonstrated in Fig. 8, a dose dependent decrease was observed in TNF-α production by C57BL/6 CBDL Kupffer cells following LPS stimulation and LBP blockade, P < 0.001. These results suggest that the increased TNF-α production by C57BL/6 CBDL Kupffer cells present following LPS stimulation is at least partially attributable to cell-associated LBP. Similar to the trends observed in C57BL/6 CBDL Kupffer cell TNF-α production, IL-6, MIP-2, and IL-1ra production were also significantly reduced following LBP blockade (Fig. 9, A–C). The mediator production in response to the intermediate dose of LBP antibody (1 μg) for IL-6, MIP-2, and IL-1ra was not significantly different from the 0 μg/ml antibody dose, and thus this data is not shown. As would be expected, LBP antibody blockade did not affect LBPKO CBDL Kupffer cell production of any of these inflammatory mediators.
Fig. 8.
Effect of LBP antibody blockade on Kupffer cell production of TNF-α following LPS stimulation. LBP antibody blockade significantly reduces C57BL/6 CBDL Kupffer cell production of TNF-α in a dose-dependent manner following stimulation of cells with LPS (100 ng/ml), *P < 0.001 compared with 0-μg LBP antibody dose.
Fig. 9.
Effect of LBP antibody blockade on Kupffer cell production of IL-6, MIP-2, and IL-1ra following LPS stimulation. LBP antibody blockade significantly reduces C57BL/6 Kupffer cell production of IL-6 (A), MIP-2 (B), and IL-1ra (C) following stimulation of cells with LPS (100 ng/ml), *P < 0.001 compared with 0-μg LBP antibody dose.
DISCUSSION
The generally accepted paradigm for LBP-mediated LPS signaling in vivo has been that low levels of LBP facilitate LPS stimulatory effects, whereas high levels of LBP inhibit monocyte responses to LPS and are protective (16, 23). In the setting of murine biliary obstruction and endotoxemia, this relationship does not appear to hold, and acute-phase levels of LBP appear to be deleterious. This observation is particularly relevant given that increased circulation of endogenous LPS has been well described in the setting of biliary obstruction secondary to impaired intestinal barrier function and translocation of enteric bacteria and bacterial products to the systemic circulation (41). The present findings are also consistent with recent observations in patients with advanced cirrhosis and ascites where elevated LBP levels have been found to correlate with marked immune and hemodynamic derangement (2) and increased susceptibility to severe infectious complications (1). In addition, previous work by our group has demonstrated that LBP also contributes to increased hepatic injury and death following acetaminophen-induced liver injury (34). Taken together, this suggests that the presence of underlying liver pathology may alter the effect of LBP on LPS signaling, leading to a predominantly stimulatory rather than inhibitory immune response with acute-phase levels of LBP.
In the present studies, the survival advantage observed in the LBPKO CBDL animals does not appear to be due to marked differences in the systemic inflammatory mediator response. As shown in Table 1, both C57BL/6 and LBPKO CBDL mice challenged with LPS (1 μg/gbw) produce very high but equivalent levels of all inflammatory mediators measured. Similar findings were observed with two exceptions (IL-6 and MCP-1 2 h following 100 ng/gbw LPS dose) at multiple doses of LPS and two different time points (2 and 6 h) following LPS challenge. This is similar to the findings of Wurfel et al. (44), who also demonstrated equivalent in vivo circulating cytokine production by LBPKO mice and C57BL/6 wild-type controls following delivery of exogenous LPS. It is unclear in both their study and ours whether this represents a compensatory mechanism developed by these knockout mice or production of circulating cytokines via an LBP-independent pathway.
In contrast to the systemic inflammatory response, significant differences were found in the degree of liver injury, hepatic neutrophil infiltration, and Kupffer cell response to LPS stimulation in the LBPKO vs. C57BL/6 CBDL animals. It has been previously shown that LBP is necessary for rapid neutrophil influx in response to Salmonella infection (45) and that LBPKO mice have decreased MIP-2 and neutrophil chemotaxis following intraperitoneal S. typhimurium infection and are thus more susceptible to infection and death caused by this organism (7). Our results also suggest that local production of the neutrophil chemoattractant MIP-2 is reduced in the livers of LBPKO CBDL mice following low (100 ng/gbw) and intermediate (1 μg/gbw) doses of LPS, and this corresponds to decreased neutrophil infiltration in the liver following these doses of LPS as well. However, in the present study these decreases correlate with decreased liver injury and improved survival in these mice, in contrast to the findings of Yang et al. (45) and Fierer et al. (7). This again suggests that the effects of acute-phase LBP are dependent on the underlying disease and immune state of the animal or patient as well as the inflammatory or injurious insult. Of note, in the present study, high-dose LPS (5 μg/gbw) induced marked, but equivalent, increases of MIP-2 and myeloperoxidase levels in both C57BL/6 and LBPKO CBDL animals. We presume that this likely represents a lethal dose of LPS that stimulates the production of MIP-2 and neutrophil infiltration through LBP-independent pathways, whereas at a sublethal (100 ng/gbw) or LD50 dose of LPS (1 μg/gbw) MIP-2 production and neutrophil infiltration are significantly impacted by the presence or absence of LBP.
To elucidate the mechanism by which LBPKO CBDL mice were relatively protected from LPS challenge we chose to focus our investigations on the role of LBP on LPS signaling in the liver, since the survival benefit observed in mice deficient in LBP following CBDL and LPS challenge appears to be due at least in part to decreased liver injury and inflammation. Since the Kupffer cell is the primary producer of inflammatory mediators in the liver and we have previously observed that Kupffer cell function is altered in the setting of biliary obstruction, with Kupffer cells demonstrating an LBP-dependent exaggerated inflammatory response to LPS in the setting of biliary obstruction (26), subsequent studies focused on Kupffer cell responses to LPS following biliary obstruction. Although we initially hypothesized that cell surface expression of LPS receptors may be decreased in LBPKO CBDL mice, we did not find evidence to support this; rather, equivalent levels of CD14, TLR4, and CD11b/CD18 were found on LBPKO and C57BL/6 CBDL Kupffer cells and thus could not explain the differences observed.
Given the emerging biophysical evidence that LBP can intercalate into synthetic phospholipid membranes (11, 12, 31), we sought to determine whether perhaps LBP may be similarly associated with the Kupffer cell and could explain the altered responses we observed in the LBPKO and C57BL/6 CBDL Kupffer cells. We initially measured LBP levels in the supernatants of isolated Kupffer cells from LBPKO and C57BL/6 CBDL animals. Because Kupffer cells do not produce LBP and the final step of the isolation process involves extraction of the cells from a Percoll gradient followed by multiple washings and resuspensions in serum-free conditions, any LBP present in the supernatant is a proxy measurement for LBP that was associated with the Kupffer cells, either on the cell surface or within the cell at the time of isolation. Our results demonstrate evidence for cell-associated LBP, as well as evidence that this association is fluid, such that LBP can be released from the isolated Kupffer cell. We next verified the presence of LBP both on the cell surface and within the Kupffer cell using confocal microscopy and immunofluorescent staining. To further support our hypothesis that LBP functions to increase the production of Kupffer cell inflammatory mediators following CBDL, we demonstrated a significant LBP antibody-mediated reduction in the production of TNF-α, IL-6, MIP-2, and IL-1ra by C57BL/6 CBDL Kupffer cells. This suggests that cell-associated LBP participates in LPS stimulatory signaling in these cells and is at least in part responsible for the differences observed between the LBPKO and C57BL/6 CBDL Kupffer cells.
Elegant biophysical studies by Gutsmann and colleagues (11, 12, 31) have previously demonstrated that LBP intercalates into phospholipid liposomal complexes and reconstituted phospholipid planar membranes in a directed fashion assuming a transmembrane configuration and that this intercalated LBP is able to bind LPS and CD14 as demonstrated by fluorescence studies; however, to our knowledge, no prior studies have demonstrated clear evidence that LBP associates with Kupffer cells or monocytes. Although our antibody data is complementary to the observations of Gutsmann and colleagues in peripheral blood monocytes (12), our observation of significant levels of LBP shed into the supernatant following isolation of Kupffer cells in completely serum-free conditions provides clear evidence that LBP is able to fluidly associate with Kupffer cells, likely both intercalating into the cell's phospholipid membrane as modeled in the biophysical experiments described, but possibly also existing as an intracellular pool in the cell's cytoplasm. In the present experiments it is not possible to determine whether the intracellular or cell surface LBP visualized with immunofluorescent staining is isolated LBP or LBP which is bound to LPS. However, given the absence of LPS stimulation in these animals and the short period of time in which the animals had cholestatic liver disease (4 days), it seems unlikely that high circulating levels of LPS would have been present in these animals, making the latter possibility less likely.
Although studying isolated cell populations such as Kupffer cells provides great advantage for elucidating mechanisms and studying signaling pathways, it does not allow us to evaluate the interaction of the multiple cell types or the influences of the microenvironment that are present in vivo. Clearly further studies are needed to further evaluate the impact of cell-associated LBP vs. circulating LBP on the immune response of an animal or patient with biliary obstruction. Additionally, we currently do not know whether cell-associated LBP is signaling through CD14 and an MyD88-dependent pathway or through MyD88-independent pathways. Biophysical experiments are limited for evaluating these sorts of questions, and further investigation along these lines is clearly needed. Despite these limitations, the present study provides important observations that improve our current understanding of LBP-mediated LPS signaling as well as the importance of the underlying disease and immune state on the prevailing response to acute-phase levels of LBP. Better understanding of these processes may allow us to develop targeted therapies in the future to prevent deleterious LBP-mediated inflammatory responses.
GRANTS
This work was supported by the National Institutes of Health, NIH NIGMS K08 GM074678-01A1 (R. M. Minter), and the Society of University Surgeons Foundation.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Albillos A, de-la-Hera A, Alvarez-Mon M. Serum lipopolysaccharide-binding protein prediction of severe bacterial infection in cirrhotic patients with ascites. Lancet 363: 1608–1610, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Albillos A, de la Hera A, Gonzalez M, Moya JL, Calleja JL, Monserrat J, Ruiz-del-Arbol L, Alvarez-Mon M. Increased lipopolysaccharide binding protein in cirrhotic patients with marked immune and hemodynamic derangement. Hepatology 37: 208–217, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Arana M de J, Vallespi MG, Chinea G, Vallespi GV, Rodriguez-Alonso I, Garay HE, Buurman WA, Reyes O. Inhibition of LPS-responses by synthetic peptides derived from LBP associates with the ability of the peptides to block LBP-LPS interaction. J Endotoxin Res 9: 281–291, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Blamey SL, Fearon KC, Gilmour WH, Osborne DH, Carter DC. Prediction of risk in biliary surgery. Br J Surg 70: 535–538, 1983. [DOI] [PubMed] [Google Scholar]
- 5.Copeland S, Siddiqui J, Remick D. Direct comparison of traditional ELISAs and membrane protein arrays for detection and quantification of human cytokines. J Immunol Methods 284: 99–106, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Dixon JM, Armstrong CP, Duffy SW, Davies GC. Factors affecting morbidity and mortality after surgery for obstructive jaundice: a review of 373 patients. Gut 24: 845–852, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fierer J, Swancutt MA, Heumann D, Golenbock D. The role of lipopolysaccharide binding protein in resistance to Salmonella infections in mice. J Immunol 168: 6396–6403, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Froon AH, Dentener MA, Greve JW, Ramsay G, Buurman WA. Lipopolysaccharide toxicity-regulating proteins in bacteremia. J Infect Dis 171: 1250–1257, 1995. [DOI] [PubMed] [Google Scholar]
- 9.Fujiwara Y, Shimada M, Yamashita Y, Adachi E, Shirabe K, Takenaka K, Sugimachi K. Cytokine characteristics of jaundice in mouse liver. Cytokine 13: 188–191, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Gegner JA, Ulevitch RJ, Tobias PS. Lipopolysaccharide (LPS) signal transduction and clearance. Dual roles for LPS binding protein and membrane CD14. J Biol Chem 270: 5320–5325, 1995. [DOI] [PubMed] [Google Scholar]
- 11.Gutsmann T, Haberer N, Carroll SF, Seydel U, Wiese A. Interaction between lipopolysaccharide (LPS), LPS-binding protein (LBP), and planar membranes. Biol Chem 382: 425–434, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Gutsmann T, Muller M, Carroll SF, MacKenzie RC, Wiese A, Seydel U. Dual role of lipopolysaccharide (LPS)-binding protein in neutralization of LPS and enhancement of LPS-induced activation of mononuclear cells. Infect Immun 69: 6942–6950, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hailman E, Lichenstein HS, Wurfel MM, Miller DS, Johnson DA, Kelley M, Busse LA, Zukowski MM, Wright SD. Lipopolysaccharide (LPS)-binding protein accelerates the binding of LPS to CD14. J Exp Med 179: 269–277, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harry D, Anand R, Holt S, Davies S, Marley R, Fernando B, Goodier D, Moore K. Increased sensitivity to endotoxemia in the bile duct-ligated cirrhotic rat. Hepatology 30: 1198–1205, 1999. [DOI] [PubMed] [Google Scholar]
- 15.Heumann D, Roger T. Initial responses to endotoxins and Gram-negative bacteria. Clin Chim Acta 323: 59–72, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Jack RS, Fan X, Bernheiden M, Rune G, Ehlers M, Weber A, Kirsch G, Mentel R, Furll B, Freudenberg M, Schmitz G, Stelter F, Schutt C. Lipopolysaccharide-binding protein is required to combat a murine gram-negative bacterial infection. Nature 389: 742–745, 1997. [DOI] [PubMed] [Google Scholar]
- 17.Kennedy JA, Clements WD, Kirk SJ, McCaigue MD, Campbell GR, Erwin PJ, Halliday MI, Rowlands BJ. Characterization of the Kupffer cell response to exogenous endotoxin in a rodent model of obstructive jaundice. Br J Surg 86: 628–633, 1999. [DOI] [PubMed] [Google Scholar]
- 18.Kennedy JA, Lewis H, Clements WD, Kirk SJ, Campbell G, Halliday MI, Rowlands BJ. Kupffer cell blockade, tumour necrosis factor secretion and survival following endotoxin challenge in experimental biliary obstruction. Br J Surg 86: 1410–1414, 1999. [DOI] [PubMed] [Google Scholar]
- 19.Kimmings AN, van Deventer SJ, Obertop H, Rauws EA, Huibregtse K, Gouma DJ. Endotoxin, cytokines, and endotoxin binding proteins in obstructive jaundice and after preoperative biliary drainage. Gut 46: 725–731, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kimmings AN, van Deventer SJ, Rauws EAJ, Huibregtse K, Gouma DJ. Systemic inflammatory response in acute cholangitis and after subsequent treatment. Eur J Surg 166: 700–705, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Kimura F, Miyazaki M, Suwa T, Sugiura T, Shinoda T, Itoh H, Nagakawa K, Ambiru S, Shimizu H, Yoshitome H. Anti-inflammatory response in patients with obstructive jaundice caused by biliary malignancy. J Gastroenterol Hepatol 16: 467–472, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Knook DL, Sleyster EC. Separation of Kupffer and endothelial cells of the rat liver by centrifugal elutriation. Exp Cell Res 99: 444–449, 1976. [DOI] [PubMed] [Google Scholar]
- 23.Lamping N, Dettmer R, Schroder NW, Pfeil D, Hallatschek W, Burger R, Schumann RR. LPS-binding protein protects mice from septic shock caused by LPS or gram-negative bacteria. J Clin Invest 101: 2065–2071, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lazar G, Paszt A, Kaszaki J, Duda E, Szakacs J, Tiszlavicz L, Boros M, Balogh A, Lazar G. Kupffer cell phagocytosis blockade decreases morbidity in endotoxemic rats with obstructive jaundice. Inflamm Res 51: 511–518, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25: 402–408, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Minter RM, Fan MH, Sun J, Niederbichler A, Ipaktchi K, Arbabi S, Hemmila MR, Remick DG, Wang SC, Su GL. Altered Kupffer cell function in biliary obstruction. Surgery 138: 236–245, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Nomura T, Shirai Y, Hatakeyama K. Impact of bactibilia on the development of postoperative abdominal septic complications in patients with malignant biliary obstruction. Int Surg 84: 204–208, 1999. [PubMed] [Google Scholar]
- 28.O′Neil S, Hunt J, Filkins J, Gamelli R. Obstructive jaundice in rats results in exaggerated hepatic production of tumor necrosis factor-alpha and systemic and tissue tumor necrosis factor-alpha levels after endotoxin. Surgery 122: 281–286; discussion 286–287, 1997. [DOI] [PubMed] [Google Scholar]
- 29.Pertoft H, Smedsrod B. Separation and characterization of liver cells; In: Cell Separation: Methods and Selected Applications, edited by Pretlow TP. New York: Academic, 1987, p. 1–24.
- 30.Pitt HA, Cameron JL, Postier RG, Gadacz TR. Factors affecting mortality in biliary tract surgery. Am J Surg 141: 66–72, 1981. [DOI] [PubMed] [Google Scholar]
- 31.Roes S, Mumm F, Seydel U, Gutsmann T. Localization of the lipopolysaccharide-binding protein in phospholipid membranes by atomic force microscopy. J Biol Chem 281: 2757–2763, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Schumann RR, Kirschning CJ, Unbehaun A, Aberle HP, Knope HP, Lamping N, Ulevitch RJ, Herrmann F. The lipopolysaccharide-binding protein is a secretory class 1 acute-phase protein whose gene is transcriptionally activated by APRF/STAT/3 and other cytokine-inducible nuclear proteins. Mol Cell Biol 16: 3490–3503, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Su CH, P'eng FK, Lui WY. Factors affecting morbidity and mortality in biliary tract surgery. World J Surg 16: 536–540, 1992. [DOI] [PubMed] [Google Scholar]
- 34.Su GL, Gong KQ, Fan MH, Kelley WM, Hsieh J, Sun JM, Hemmila MR, Arbabi S, Remick DG, Wang SC. Lipopolysaccharide-binding protein modulates acetaminophen-induced liver injury in mice. Hepatology 41: 187–195, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Su GL, Goyert SM, Fan MH, Aminlari A, Gong KQ, Klein RD, Myc A, Alarcon WH, Steinstraesser L, Remick DG, Wang SC. Activation of human and mouse Kupffer cells by lipopolysaccharide is mediated by CD14. Am J Physiol Gastrointest Liver Physiol 283: G640–G645, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Su GL, Klein RD, Aminlari A, Zhang HY, Steinstraesser L, Alarcon WH, Remick DG, Wang SC. Kupffer cell activation by lipopolysaccharide in rats: role for lipopolysaccharide binding protein and toll-like receptor 4. Hepatology 31: 932–936, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Thompson JN, Edwards WH, Winearls CG, Blenkharn JI, Benjamin IS, Blumgart LH. Renal impairment following biliary tract surgery. Br J Surg 74: 843–847, 1987. [DOI] [PubMed] [Google Scholar]
- 38.Tobias PS, Soldau K, Gegner JA, Mintz D, Ulevitch RJ. Lipopolysaccharide binding protein-mediated complexation of lipopolysaccharide with soluble CD14. J Biol Chem 270: 10482–10488, 1995. [DOI] [PubMed] [Google Scholar]
- 39.Tobias PS, Soldau K, Ulevitch RJ. Isolation of a lipopolysaccharide-binding acute phase reactant from rabbit serum. J Exp Med 164: 777–793, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Welborn MB, Douglas WG, Abouhamze Z, Auffenburg T, Abouhamze AS, Baumhofer J, Seeger JM, Pruitt JH, Edwards PD, Chizzonite R, Martin D, Moldawer LL, Harward TR. Visceral ischemia-reperfusion injury promotes tumor necrosis factor (TNF) and interleukin-1 (IL-1) dependent organ injury in the mouse. Shock 6: 171–176, 1996. [PubMed] [Google Scholar]
- 41.White JS, Hoper M, Parks RW, Clements WD, Diamond T. Patterns of bacterial translocation in experimental biliary obstruction. J Surg Res 132: 80–84, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 249: 1431–1433, 1990. [DOI] [PubMed] [Google Scholar]
- 43.Wurfel MM, Kunitake ST, Lichenstein H, Kane JP, Wright SD. Lipopolysaccharide (LPS)-binding protein is carried on lipoproteins and acts as a cofactor in the neutralization of LPS. J Exp Med 180: 1025–1035, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wurfel MM, Monks BG, Ingalls RR, Dedrick RL, Delude R, Zhou D, Lamping N, Schumann RR, Thieringer R, Fenton MJ, Wright SD, Golenbock D. Targeted deletion of the lipopolysaccharide (LPS)-binding protein gene leads to profound suppression of LPS responses ex vivo, whereas in vivo responses remain intact. J Exp Med 186: 2051–2056, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang KK, Dorner BG, Merkel U, Ryffel B, Schutt C, Golenbock D, Freeman MW, Jack RS. Neutrophil influx in response to a peritoneal infection with Salmonella is delayed in lipopolysaccharide-binding protein or CD14-deficient mice. J Immunol 169: 4475–4480, 2002. [DOI] [PubMed] [Google Scholar]
- 46.Yu B, Wright SD. Catalytic properties of lipopolysaccharide (LPS) binding protein. Transfer of LPS to soluble CD14. J Biol Chem 271: 4100–4105, 1996. [DOI] [PubMed] [Google Scholar]