Abstract
Neutrophil dysfunction in alcoholic hepatitis is associated with endotoxemia and an increased incidence of infection, but the mechanism is unclear. We aimed to investigate the role of Toll-like-receptors (TLR)2, 4, and 9 in mediating neutrophil dysfunction in alcoholic hepatitis. Neutrophils from healthy volunteers were incubated with alcoholic hepatitis patients’ plasma (n = 12) with and without TLR2, 4, or 9 antagonists and with and without human albumin. TLR2, 4, and 9 expression, neutrophil oxidative burst, phagocytosis, and CXCR1+2 expression were measured by FACS analysis. Patients’ plasma increased oxidative burst, decreased CXCR1+2 expression, and decreased phagocytosis of normal neutrophils in association with increased expression of TLR2, 4, and 9 and depletion of ATP. Inhibition of TLR2, 4, and 9 prevented the increase in oxidative burst and the decrease in CXCR1 and CXCR2 expression but did not prevent phagocytic dysfunction. Incubation with albumin completely prevented the patient plasma induced neutrophil dysfunction. Increased expression of TLR2, 4, and 9 is associated with neutrophil dysfunction, endotoxemia, and energy depletion. TLR2, 4, and 9 inhibition does not improve phagocytosis, indicating that TLR overexpression may be the result and not the cause of neutrophil activation. Albumin, an endotoxin scavenger, prevents the deleterious effect of patients’ plasma on neutrophil phagocytosis, resting burst, and TLR expression.
Keywords: alcoholic liver disease, immune function, endotoxin, acute on chronic liver failure, chemokines
data on neutrophil function in alcoholic hepatitis is paradoxical with some studies suggesting neutrophil priming, indicating a readiness to respond to bacterial challenge (32, 34). In contrast, other studies show decreased neutrophil phagocytic capacity correlating with disease severity (24). Our recent study addressed this apparent paradox; we observed a severe functional failure of neutrophils (full activation and reduced phagocytosis) in patients with alcoholic hepatitis and showed that these defects were associated with increased risk of infection, organ failure, and mortality. Endotoxemia has been found to be important in mediating these abnormalities of neutrophil function (17). However, the exact mechanism of how endotoxin may produce neutrophil dysfunction remains unclear.
Toll-like-receptors (TLRs) are a family of receptors that are specific for the recognition of bacterial and viral components that play key roles in the early inflammatory response to pathogens. Neutrophils express all known human TLRs except TLR3 (11). TLRs are activated by bacterial lipoproteins, lipopolysaccharide (LPS), lipoteichoic acid, viral RNA, and the unmethylated CpG motifs of bacterial and viral DNA (20). In liver disease TLR2, 4, and 9 are of particular interest because they respond to gram-positive and gram-negative bacterial products and to bacterial DNA. Activation of TLR facilitates neutrophil recruitment by upregulation of adhesion molecules and alteration of neutrophil trafficking, to localize neutrophils to the site of infection. Neutrophils also respond to TLR activation with the generation of reactive oxygen species, increased phagocytosis, and secretion of cytokines, chemokines, and antimicrobial peptides (11, 20). The role of TLRs on neutrophils in patients with liver disease is not known. Since patients with alcoholic hepatitis manifest abnormalities in resting burst and phagocytosis in association with endotoxemia (17), it is possible that altered TLR expression may be important in mediating such abnormal neutrophil responses. The aims of this study were to determine the roles of neutrophil TLR2, 4, and 9 in mediating the neutrophil dysfunction in alcoholic hepatitis.
METHODS
Patient Selection
All patients gave written, informed consent, and the study was approved by the local ethics committee. Two groups of patients were studied: 1) patients admitted to the hospital with acute decompensation of alcoholic cirrhosis due to alcoholic hepatitis and 2) stable outpatients attending regular surveillance visits in the outpatient clinic without any sign of acute decompensation.
Patients were excluded if they had clinical or microbiological evidence (chest X-ray; routine cultures of urine, blood, sputum, and ascites) of infection; treatment with antibiotics within the previous 30 days; gastrointestinal bleeding within the last 7 days; evidence of organ failure (creatinine >150 μmol/l, hepatic encephalopathy > grade 2); hyponatremia; hepatic or extrahepatic malignancy; received any immunomodulatory therapy prior to study entry. All patients had histological evidence of alcoholic cirrhosis (25). Presence or absence of alcoholic hepatitis was diagnosed by using a histological grading system similar to nonalcoholic steatohepatitis (2).
Plasma samples from age- and sex-matched healthy volunteers (n = 6) with no history of liver disease served as normal controls.
Study Design
Peripheral venous blood was aseptically collected into precooled, pyrogen-free tubes (BD Vacutainer Lithium-Heparin, BD, Plymouth, UK); after centrifugation, plasma was stored at −80°C in nonpyrogenic cryotubes (Corning, Corning, NY). For experiments with cells, blood was kept at room temperature (maximum 1 h). For all experiments strict precautions were taken to avoid endotoxin contamination by working aseptically and using endotoxin-free equipment. Since we and others have previously shown that the defect in neutrophil function seen in patients can be transferred by incubation of normal neutrophils with patients’ plasma (3, 17, 21, 23, 29) we have chosen this model to test our hypothesis. We have chosen our incubation time of 90 min from previous ex vivo and in vivo studies that have shown TLR activation and effects on neutrophil function within 30–90 min (11, 17, 20, 26, 28, 35).
Follow-up data on organ failure and mortality from these patients are given in Table 1.
Table 1.
Patient characteristics
| Normal Range | Patients With Stable Alcoholic Cirrhosis And Low Neutrophil Oxidative Burst (n = 11) | Patients With Alcoholic Hepatitis Superimposed on Alcoholic Cirrhosis and High Neutrophil Oxidative Burst (n = 12) | |
|---|---|---|---|
| Age, yr | 57.0±4.3 | 55.3±2.0 | |
| Bilirubin, μmol/l | 3–17 | 42.2±15.9 | 362.7±89.0 |
| PT, s | 10–12 | 12.3±0.6 | 16.5±0.9 |
| Albumin, g/l | 35–53 | 35.7±1.7 | 28.5±2.5 |
| WBC, 109/l | 3–10 | 4.9±0.6 | 12.7±1.6 |
| CRP, mg/l | 0–5 | 6.5±1.4 | 35.6±12.2 |
| ALT, U/l | 8–63 | 36.6±6.0 | 50.3±7.9 |
| Resting oxidative burst | 2.9–13.5 | 29.3±8.9 | 69.0±7.0 |
| Maddrey's discriminant function | 37.0±7.3 | ||
| Pugh score | 6.7±0.6 | 12.7±0.6 | |
| Outcome | |||
| Death n/% | 0/0 | 4/33 | |
| Organ failure n/% | 0/0 | 7/58 |
Values are means ± SE. PT, prothrombin time; WBC, white blood cell count; CRP, c-reactive protein; ALT, alanine aminotransferase.
Neutrophils
Neutrophil isolation.
Whole blood (4 ml) from healthy volunteers was layered over 5 ml of Polymorphprep (Axis-Shield, Oslo, Norway) and spun for 30 min at 400 g at room temperature. Neutrophils were harvested from the second interface and washed with phosphate-buffered saline (PBS, Sigma Aldrich, St. Louis, MO). Neutrophils were counted and resuspended in PBS at a density of 5 × 105 in 50 μl; 50 μl of cell suspension and 50 μl of plasma were used per assay. Viability was tested by Trypan blue exclusion and was over 98%.
TLR expression.
Isolated neutrophils (50 μl), plasma (50 μl), and 50 μl of human albumin (10, 20, or 40 g/l, Zenalb, Bio Products Laboratory, Elstree, UK), or PBS were incubated for 90 min at 37°C. Expression of TLRs was determined using the following antibodies: TLR2-PE-Cy7, TLR4-APC, TLR9-PE (eBioscience, San Diego, CA) and CD16-FITC (Immunotools, Friesoythe, Germany). Cells were incubated with antibodies against TLR2 and 4 for 30 min and then permeabilized by incubation for 10 min with 500 μl of 1 × FACS Permeabilization Solution 2 (BD Biosciences, Oxford, UK) and incubated with anti-TLR9 antibodies for another 30 min, washed twice with PBS, and fixed before FACS analysis.
Endotoxin measurement.
The chromogenic limulus amoebocyte lysate kinetic assay (Charles River Laboratories, L'Arbresle, France) was used for detection of endotoxin. Heparinized plasma with a low concentration of heparin (15 IU/ml) was used (33); additionally, plasma samples (100 μl) were diluted 1:10 with endotoxin-free water and heat treated for 30 min at 75°C. Then 100 μl of sample and 100 μl of LAL reagent were mixed in a 96-well plate and analyzed at 405 nm with a spectrophotometer using the EndoscanV software.
TLR inhibition.
Isolated neutrophils were incubated with either anti-human TLR2 antibody (clone T2.5, eBioscience), anti-human TLR4 antibody (clone HTA125, eBioscience), or a TLR9 inhibitory oligodeoxynucleotide (iODN) (5′-ttagggttagggttagggttaggg-3′, Axxora, Birmingham, UK). We simultaneously incubated 50 μl of cell suspension (containing 5 × 105 cells) and 50 μl of either normal or patients’ plasma or 100 ng/ml LPS (Escherichia coli 0111:B4 lot 085K4068, Sigma, Poole, UK) with 4 μg anti-TLR2, 8 μg anti-TLR4, or 3.3 μg TLR9 iODN for 90 min at 37°C. After 60 min the respective reagents for measuring oxidative burst of phagocytosis were added to reach a total incubation time of 90 min in all experiments. The antibody concentrations were found in preliminary titration experiments (data not shown).
Neutrophil function.
The Phagoburst kit (Orpegen Pharma, Heidelberg, Germany) was used to determine the percentage of neutrophils that produce reactive oxidants as previously described (FACS Canto II, BD Bioscience) (17). The Phagotest (Orpegen Pharma) was used to measure phagocytosis by using FITC-labeled opsonized E. coli bacteria as described before (17).
CXCR1 and CXCR2 expression was measured by FACS analysis using monoclonal antibodies. After incubation of cells, plasma, and either TLR inhibitors or albumin as applicable for 90 min, they were washed with PBS and incubated with CD16-PE (3 μl) (Immunotools), CXCR1-APC (10 μl), or CXCR2-FITC (3 μl) (BD Bioscience) for 30 min. The mean fluorescence intensity of the respective antibodies on neutrophils was analyzed by FACS analysis (FACS Canto II, BD Bioscience).
Neutrophil high-energy phosphates.
High-energy phosphates were measured by HPLC as previously described (8, 9, 22). After incubation of cells with plasma for 90 min, 106 cells were centrifuged, washed with 1 ml of PBS, and then deproteinized with 200 μl of 0.4 mol/l perchloric acid. After centrifugation (12,000 g) the acid extract was neutralized with 2 mol/l potassium carbonate (4°C). The supernatant (40 μl injection volume) was used for HPLC analysis. The pellets were dissolved in 1 ml of 0.1 mol/l sodium hydroxide and further diluted 1:10 with physiological saline for protein determination (BCA Protein Assay, Pierce, THP, Vienna, Austria). The separation of the high-energy phosphates was performed on a Hypersil ODS column (5 μm, 250 mm × 4 mm ID) using a L-2200 autosampler, two L-2130 HTA pumps, and a L-2450 diode array detector (all: VWR Hitachi, Vienna, Austria). Detector signals (absorbance at 254 nm) were recorded with a personal computer. The program EZchrom Elite (VWR) was used for data requisition and analysis.
Statistics
For comparison of two groups, t-test or Mann-Whitney test were used as appropriate, whereas ANOVA test with Tukey's (equal variances) or Dunnett C (nonequal variances) post hoc analysis was used for comparison of more than two data sets (GraphPad Prism 4). A P < 0.05 was considered to be significant.
RESULTS
Patients
Plasma from 23 patients was used for this study. Twelve patients had histological evidence of alcoholic hepatitis superimposed on alcoholic cirrhosis whereas 11 had alcoholic cirrhosis alone. The 12 patients with alcoholic hepatitis superimposed on alcoholic cirrhosis had a high resting oxidative burst whereas the 11 patients with alcoholic cirrhosis had a low resting burst (Table 1). Data on neutrophil function measured in whole blood of these patients have been partly described in our previous paper (17). All patients with alcoholic hepatitis and high resting oxidative burst were hospitalized due to acute decompensation of their alcoholic cirrhosis whereas all patients with low resting oxidative burst were stable cirrhotics from the outpatient clinic. Patients were followed clinically; mortality at 90 days in the high-burst group was 33%, whereas no mortality was observed in patients in the low resting burst group. None of the patients had any clinical or microbiological evidence of infection at inclusion. Seven of the 12 patients with high resting oxidative burst and alcoholic hepatitis developed culture positive infections during their hospital admission, whereas no clinical evidence of infection was observed in patients with low resting oxidative burst and alcoholic cirrhosis alone.
Incubation of Patients’ Plasma With Normal Neutrophils Increases TLR2, 4, and 9 Expression
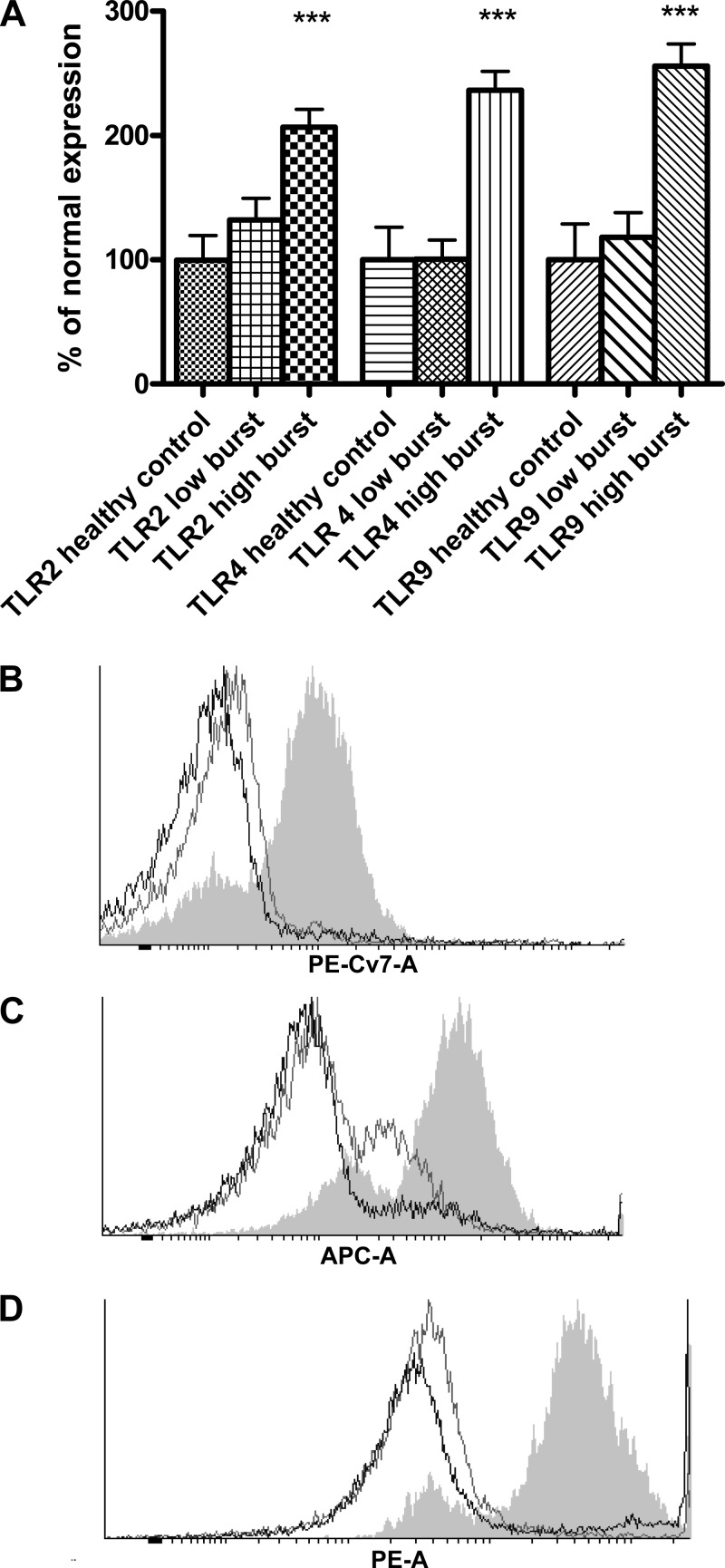
Incubation of normal neutrophils with plasma from patients with high resting burst for 90 min was associated with a significant increases in TLR2 (107%) and TLR4 (136%) surface expression and a 150% increase in TLR9 intracellular expression (all P < 0.0001), compared with normal neutrophils incubated with control plasma (Fig. 1). No significant change in TLR2, 4, and 9 surface expression was observed with plasma from the low-burst group [relative change in expression: TLR2 32%, not significant (NS); TLR4 0.7%, NS; TLR9 18%, NS].
Fig. 1.
A: FACS analysis data showing significantly increased expression of Toll-like receptor (TLR)2, 4, and 9 on neutrophils incubated for 90 min with plasma from patients with high neutrophil oxidative burst (having alcoholic hepatitis on the background of alcoholic cirrhosis, labeled as “high burst”) compared with neutrophils incubated with plasma from patients with low neutrophil oxidative burst (having alcoholic cirrhosis alone, labeled as “low burst”) and normal controls. ***P < 0.001 vs. healthy control and low-burst patients. B: representative FACS histogram for TLR2 (black line, control; gray line, low burst; gray filled area, high burst). C: representative FACS histogram for TLR 4 (black line, control; gray line, low burst; gray filled area, high burst). D: representative FACS histogram for TLR 9 (black line, control; gray line, low burst; gray filled area, high burst).
Inhibition of TLR2, 4, and 9 Is Associated With Improvement in Resting Burst but Not Phagocytosis
Oxidative burst.
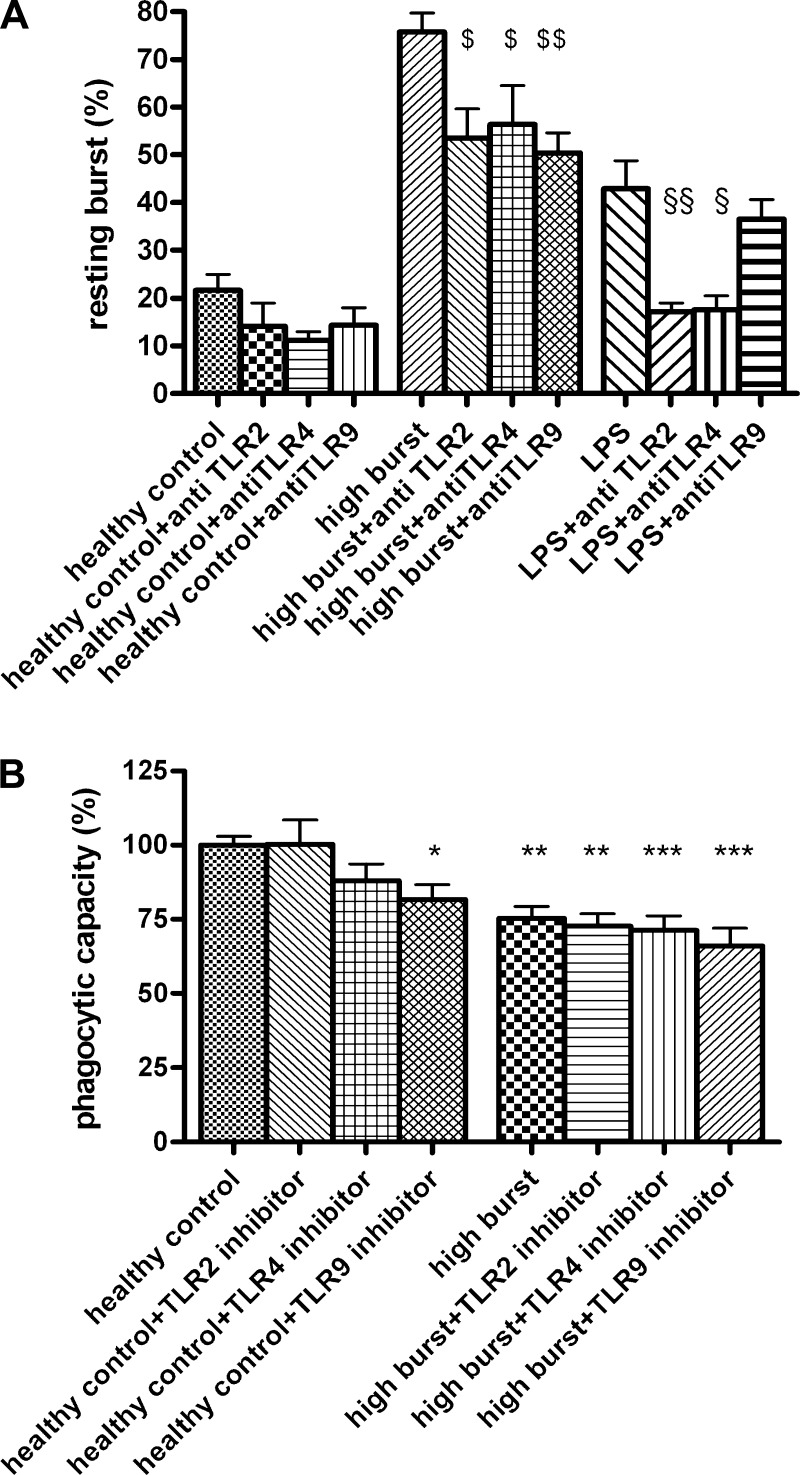
Inhibition of TLR function with the inhibitory antibodies (TLR2 and TLR4) or iODN (TLR9) decreased the oxidative burst induced by high-burst patients’ plasma significantly (total incubation time 90 min, Fig. 2A). Inhibition of TLR2, 4, or 9 did not change resting oxidative burst in neutrophils incubated with control plasma. When neutrophils were stimulated with commercial LPS (considered to stimulate TLR2 and TLR4), inhibition of TLR2 (P < 0.05) and TLR4 (P < 0.01) decreased the elevated resting oxidative burst but inhibition of TLR 9 had no effect (total incubation time 90 min, Fig. 2A).
Fig. 2.
A: incubation with TLR2, 4, and 9 antagonists significantly decrease neutrophil resting oxidative burst induced by high-burst patients’ plasma (high burst) or by incubation with LPS (100 ng/ml), as assessed by FACS analysis. Total incubation time 90 min. $P < 0.05 vs. high-burst patients, $$P < 0.01 vs. high-burst patients; §P < 0.05 vs. LPS, §§P < 0.01 vs. LPS. B: incubation with TLR2, 4, and 9 antagonists have no beneficial effect on impaired neutrophil phagocytosis, induced by high-burst patients’ plasma (high burst) (assessed by FACS analysis). Total incubation time 90 min. *P < 0.05 vs. control, **P < 0.01 vs. control, **P < 0.001 vs. control.
Phagocytosis.
Incubation of normal neutrophils with high-burst patients’ plasma decreased normal neutrophil phagocytic capacity (P < 0.01), which was not prevented by inhibition of TLR function (total incubation time 90 min), which actually further decreased phagocytic capacity (TLR2 P < 0.01, TLR4 P < 0.01, TLR9 P < 0.001; Fig. 2B). Incubation of normal neutrophils with plasma from healthy controls did not change phagocytosis. Addition of TLR2 or TLR4 inhibitors did not alter phagocytosis but TLR9 inhibition significantly decreased (P < 0.05) the phagocytic capacity of control neutrophils.
Increase in the TLR2, 4, and 9 Is Associated With Alterations in CXCR1+2 Expression and Inhibition of TLR2, 4, and 9 Prevents This Alteration
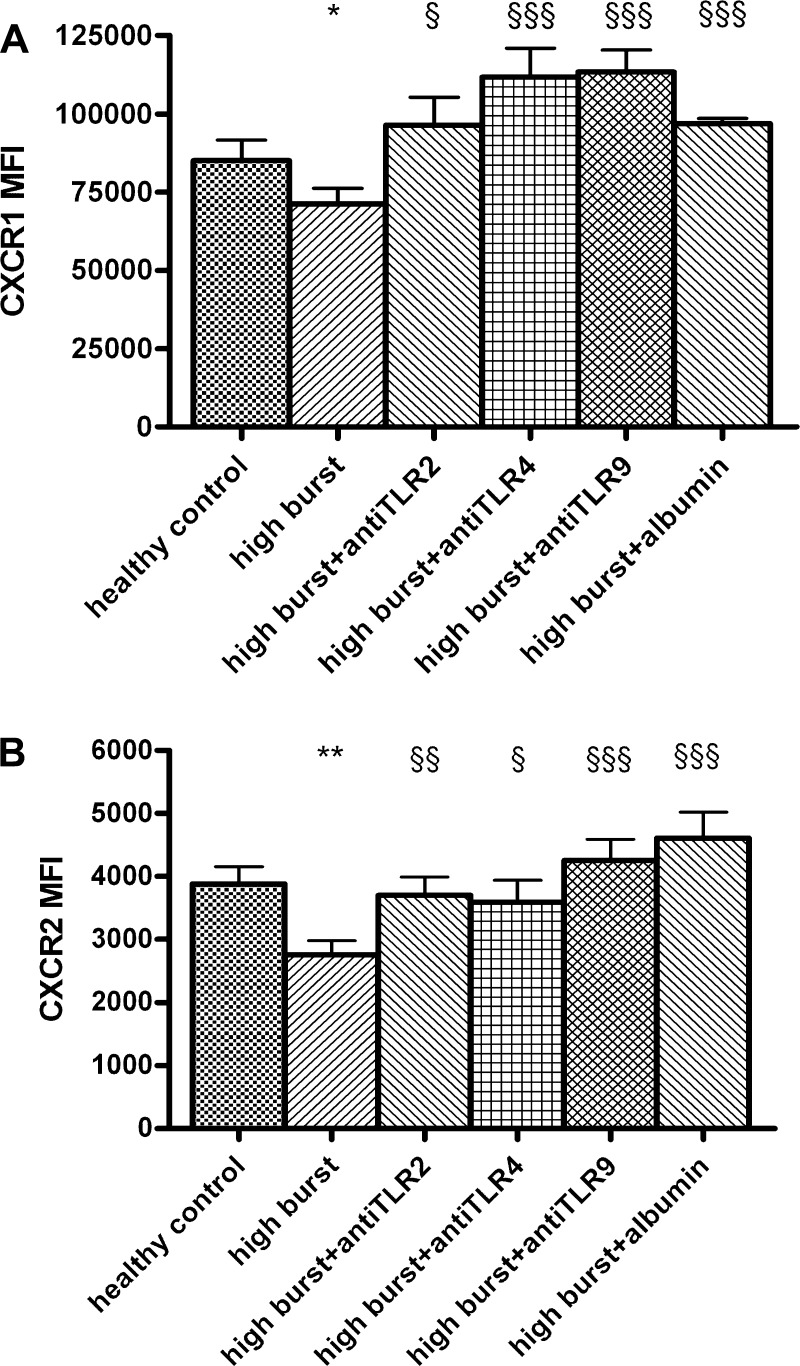
Incubation of normal neutrophils with high-burst patients’ plasma for 90 min decreased CXCR1 (P < 0.05, Fig. 3A) and CXCR2 (P < 0.01, Fig. 3B) expression. The decreased CXCR1 and CXCR2 expression (by high-burst patients’ plasma) was restored by prior inhibition of TLR2, 4, or 9 function for 90 min or by incubation with albumin for 90 min (CXCR1: TLR2 P < 0.05, TLR4 P < 0.001, TLR9 P < 0.001, albumin P < 0.001; CXCR2: TLR2 P < 0.01, TLR4 P < 0.05, TLR9 P < 0.001, albumin P < 0.001; Fig. 3).
Fig. 3.
A: high-burst patients’ plasma decreases CXCR1 expression and addition of TLR2, 4, and 9 antagonists or albumin increases CXCR1 expression (total incubation time 90 min, FACS analysis). B: high-burst patients’ plasma decreases CXCR2 expression and addition of TLR2, 4, and 9 antagonists or albumin increases CXCR2 expression (total incubation time 90 min, FACS analysis). *P < 0.05 vs. control; $P < 0.05 vs. high burst; $$P < 0.01 vs. high burst; $$$P < 0.001 vs. high burst. MFI, mean fluorescence intensity.
Albumin Reduces TLR Expression and Prevents Neutrophil Dysfunction
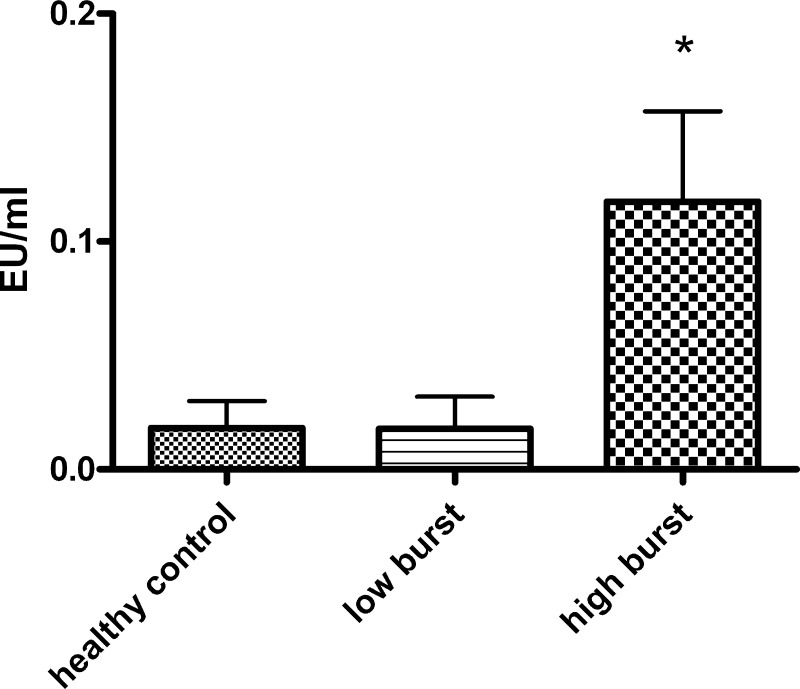
Endotoxin was measurable in peripheral venous blood in 50% of the patients having high-burst but only in 2 of 11 patients with low burst and in 1 of 7 healthy controls. Endotoxin levels were significantly higher in patients with high-burst (P < 0.05; Fig. 4).
Fig. 4.
Endotoxin levels are increased in plasma from patients with high resting oxidative burst but not in plasma from low-burst patients or healthy controls (Limulus Amoebocyte Lysate assay). EU, endotoxin units; *P < 0.05 vs. control.
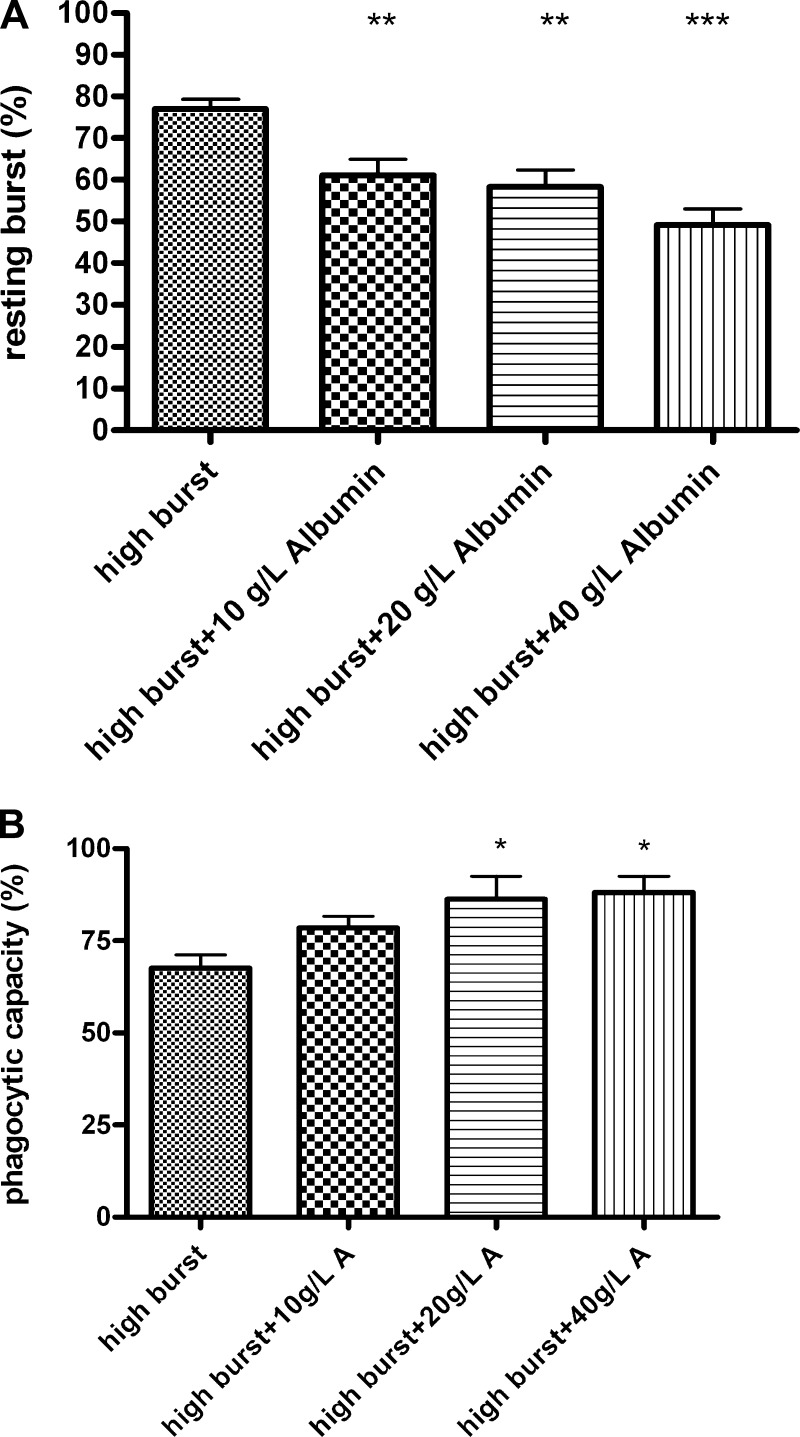
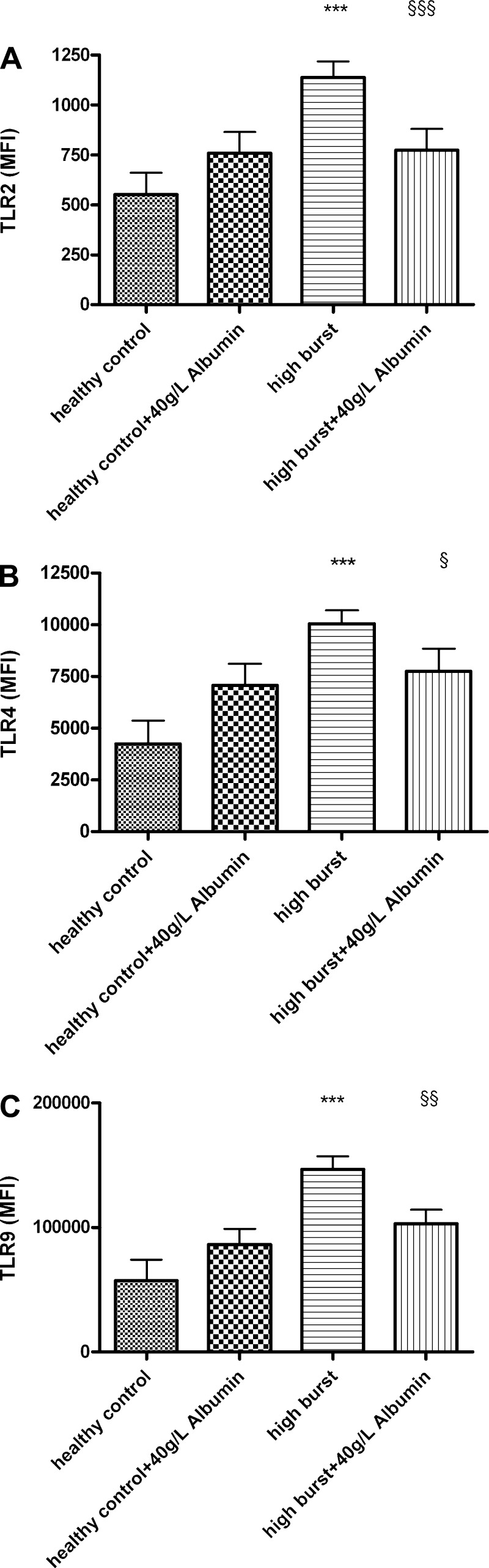
Simultaneous incubation of normal neutrophils with high-burst patients’ plasma and with 10 g/l (P < 0.01), 20 g/l (P < 0.01), or 40 g/l (P < 0.001) human albumin for 90 min decreased the high burst induced by patients’ plasma in a dose-dependent manner (Fig. 5A). Simultaneous incubation of normal neutrophils with high-burst patients’ plasma and with 20 g/l (P < 0.05) and 40 g/l (P < 0.05) human albumin for 90 min also prevented the reduction in phagocytic capacity induced by high-burst patients’ plasma (Fig. 5B). Using plasma from healthy controls in this experiment did not change resting burst or phagocytic capacity (data not shown). Incubation of isolated neutrophils from healthy volunteers with 200 ng/ml of LPS for 90 min increased resting oxidative burst (78.6 ± 2.2%) and simultaneous incubation with 20 or 40 g/l albumin significantly reduced oxidative burst (59.1 ± 6.1 and 51.8 ± 6.6%, respectively, P < 0.01). Incubation of normal neutrophils with high-burst patients’ plasma and 40 g/l human albumin for 90 min also reduced TLR2 (P < 0.001), TLR4 (P < 0.05), and TLR9 (P < 0.01) expression to values not different from control (Fig. 6).
Fig. 5.
A: incubation for 90 min with rising concentrations of human albumin decreases the increased neutrophil resting oxidative burst caused by high-burst patients’ plasma (FACS analysis). B: incubation with human albumin for 90 min increases the decreased neutrophil phagocytosis caused by high-burst patients’ plasma (FACS analysis). *P < 0.05 vs. control, **P < 0.01 vs. control, ***P < 0.001 vs. control.
Fig. 6.
A: incubation with human albumin for 90 min decreases the increased TLR2 surface expression caused by high-burst patients’ plasma. B: incubation with human albumin for 90 min decreases the increased TLR4 surface expression caused by high-burst patients’ plasma. C: incubation with human albumin for 90 min decreases the increased intracellular TLR9 expression caused by high-burst patients’ plasma. ***P < 0.001 vs. healthy control, §P < 0.05 vs. high-burst patients, §§P < 0.01 vs. high-burst patients, §§§P < 0.001 vs. high-burst patients.
ATP Levels Are Reduced in Neutrophils Incubated With High-Burst Patients’ Plasma
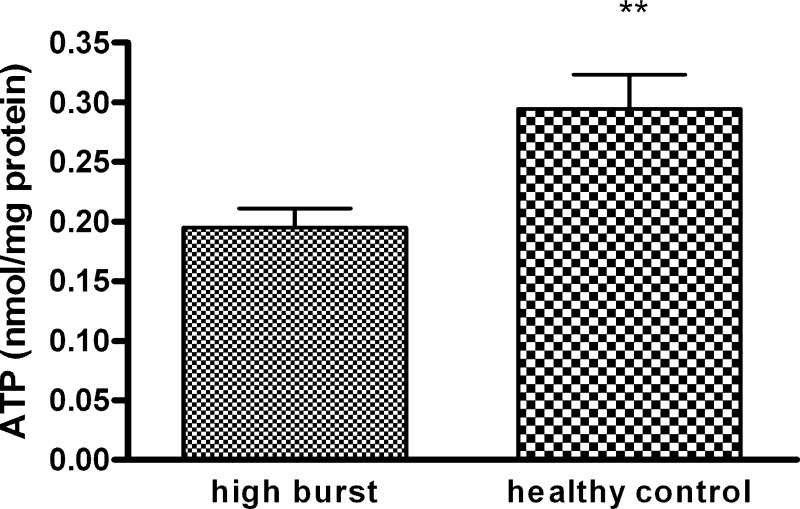
To determine the reason for decreased neutrophil phagocytosis in relation to increased resting oxidative burst, we measured the high-energy phosphates in neutrophils incubated for 90 min with high-burst plasma and in neutrophils incubated with plasma from healthy controls. ATP levels were significantly lower (P < 0.01, Fig. 7) in neutrophils incubated with high-burst plasma compared with neutrophils incubated with plasma from healthy controls, whereas ADP and AMP levels were not significantly different from controls.
Fig. 7.
ATP concentration in neutrophils after 90 min of incubation with high-burst patients’ plasma or plasma of healthy controls. **P < 0.02 vs. control.
DISCUSSION
This study explored the role of TLRs 2, 4, and 9 in mediating neutrophil dysfunction in patients with alcoholic hepatitis, which is known to be associated with increased risk of infection, organ failure, and mortality. The most important, novel observation made in the present study was that the induction of the functional defect in the neutrophils following incubation with high-burst plasma was associated with an increase in their expression of TLRs 2, 4, and 9. The inhibition of these TLRs by using specific antagonists prevented an increase in neutrophil resting burst and the expression of chemokine receptors but did not prevent phagocytic dysfunction. However, addition of human albumin, which is known to scavenge endotoxin, in pathophysiologically relevant concentrations, prevented an increase in resting burst, normalized chemokine receptor expression, and phagocytic dysfunction. This was associated with prevention of increases in TLR2, 4, and 9 expressions, providing support for the concept of using albumin and/or other endotoxin removal strategies to prevent neutrophil dysfunction in severe alcoholic hepatitis.
TLRs play a pivotal role in orchestrating immune responses (11). Since bacterial infections are common in patients with alcoholic cirrhosis with superimposed alcoholic hepatitis (6, 17), we studied TLR2, 4, and 9, which are known to respond to bacterial products. TLR4 is the designated LPS receptor, TLR2 is activated by peptidoglycans and also commercial LPS, and TLR9 (which is found intracellularly) is activated by bacterial DNA (1). Incubation of normal neutrophils with plasma from alcoholic hepatitis patients with high resting burst resulted in an increase in surface expression of TLR2 and 4 and of intracellular TLR 9 expression.
To determine the role of TLR2, 4, and 9 in mediating the neutrophil functional defect observed in patients with alcoholic hepatitis, we investigated the effect of inhibiting TLRs on neutrophil function (as measured by oxidative burst, chemokine receptor expression, and phagocytosis). Inhibition of TLR2, 4, and 9 prevented an increased resting oxidative burst and normalized chemokine receptor expression induced by patients’ plasma but this did not prevent phagocytic dysfunction. When cells were stimulated with commercial LPS [known to stimulate both TLR2 and TLR4 activity (27)], only inhibition of TLR2 and 4 were able to decrease resting oxidative burst, whereas TLR9 inhibition had no effect on resting oxidative burst. This observation suggests that TLR2, 4, and 9 overexpression and activation is likely to be the result of stimulation of the cells by a variety of bacterial products such as endotoxin, peptidoglycans, or bacterial DNA and that inhibition of TLRs can be a two-edged sword.
On the one hand, although neutrophil oxidative burst is crucial in defying microbial intruders and in mounting an inflammatory response when necessary, prevention of a high resting oxidative burst of neutrophils might be beneficial because this inadequate production of free oxygen radicals can cause oxidative damage to tissue and induce apoptosis of other immune cells (5). Furthermore, normal neutrophil function is critically dependent on the capacity of the cell to undergo directed migration from the blood to local tissue sites in which the CXC chemokines, interleukin-8, and the expression of the receptors CXCR1 and CXCR2 are crucial factors (18). During neutrophil activation, CXCR1 and CXCR2 are rapidly downregulated (14). Incubation of neutrophils by the high-burst plasma resulted in a reduction in the expression of CXCR1 and CXCR2, which was preventable by inhibition of TLR2, 4, and 9. Additionally, albumin also prevented this downregulation, suggesting that endotoxin, acting via a TLR-related mechanism, is likely to be responsible for this phenomenon. Cleavage of CXCR1 has been shown to disable bacterial killing of neutrophils (10), and CXCR2 has been shown to be downregulated in patients with sepsis, resulting in markedly reduced neutrophil migration (4). Therefore, the prevention of receptor downregulation is likely to be beneficial in preventing neutrophil dysfunction.
On the other hand, the role of TLRs in mediating phagocytic function is unclear and it is likely that other mechanisms, such as energy depletion of the cells due to increase oxidative burst, are responsible for the decreased phagocytic capacity. This notion is supported by our finding of decreased ATP levels in neutrophils incubated with high-burst patients’ plasma compared with healthy control plasma. Since NADPH oxidase-derived free radicals are key oxidants in alcohol-induced liver disease (16) and inhibition of NADPH oxidase prevents early alcohol-induced liver injury (15), it would be also possible that changes in expression of NADPH oxidase on neutrophils could play a pathophysiologically important role in this process.
Our findings imply that inhibition of TLR function as a novel therapeutic strategy might be beneficial when considering the increase in neutrophil oxidative burst and the systemic consequences of oxidative damage. When considering the effects of decreased phagocytic capacity, however, TLR inhibition is unlikely to be beneficial and may even be deleterious since TLR9 inhibition decreased phagocytic capacity in control cells.
In our previous study we showed that endotoxin may be one of the factors that induce functional defects in neutrophils in patients with alcoholic hepatitis (17). Endotoxin levels were significantly increased in the patients with high resting burst, but endotoxin was not measurable in most healthy controls and patients with low resting burst. Albumin, the major plasma protein in humans, has the ability to bind endotoxin; one molecule of albumin can bind up to 10 molecules of endotoxin (13) but the relevance of this function of albumin is not clear. Incubating neutrophils with physiological concentrations of albumin completely prevented neutrophil dysfunction (both resting oxidative burst and also phagocytic dysfunction) in a dose-dependent manner. Albumin also prevented the increase in TLR2, 4, and 9 expression induced by patients’ plasma. These ex vivo data reveal an important biological function of albumin and describe a novel therapeutic role for albumin to prevent or treat endotoxin-mediated neutrophil dysfunction in patients with high neutrophil resting burst. This assertion is supported by our recent preliminary observation of improved neutrophil function in patients with severe alcoholic hepatitis treated with albumin dialysis (31).
However, it has to be emphasized that endotoxin is not the only substance causing dysfunction of neutrophils, since endotoxin alone does not cause the same degree of increase in oxidative burst compared with patient's plasma (Fig. 3A). Furthermore, the LPS-related increase in oxidative burst is inhibited to a higher degree by anti-TLR strategies compared with the oxidative burst induced by patients’ plasma. From recent studies in patients with cirrhosis and noninfected ascites, it is known that 34% have detectable levels of bacterial DNA, with a close correlation between the level of bacterial DNA and the inflammatory response, supporting the concept that the presence of bacterial products (endotoxin, bacterial DNA, and possibly other substances) is related to the inflammatory response seen in these patients (7).
In conclusion, our study provides novel evidence about the important role of TLR2, 4, and 9 in contributing to the neutrophil dysfunction in alcoholic hepatitis. TLR2, 4, and 9 are overexpressed, most likely as a consequence of the presence of endotoxin and possibly other bacterial products. The beneficial effect of human albumin (acting as an endotoxin scavenger) on preventing the effect of patients’ plasma on normal neutrophils may define the mechanism through which albumin reduces inflammatory response and improves clinical outcome (12, 19, 30).
GRANTS
V. Stadlbauer was supported by an Erwin-Schrödinger fellowship (J2547) from the Austrian Science Foundation. Part of this work was funded by a grant from Plasma Proteins Therapeutic Association. G. A. K. Wright was supported by the European Association for the Study of the Liver Sheila Sherlock Fellowship. The measurements of neutrophil energy status were supported by a grant of the Franz-Lanyar-Stiftung. This work was undertaken at University College London Hospitals/University College London, which received a proportion of funding from the Department of Health's National Institute of Health Research Biomedical Research Centres funding scheme.
Acknowledgments
The authors gratefully acknowledge Dr. F Andreola and Dr. S Hodges for technical help and support and G. Kager for performing the protein analysis.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Broad A, Jones DE, Kirby JA. Toll-like receptor (TLR) response tolerance: a key physiological “damage limitation” effect and an important potential opportunity for therapy. Curr Med Chem 13: 2487–2502, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol 94: 2467–2474, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Bugajski P, Kalawski R, Balinski M, Wysocki H, Olszewski R, Szczepanik A, Siminiak T. Plasma-mediated stimulation of neutrophil superoxide anion production during coronary artery bypass grafting: role of endothelin-1. Thorac Cardiovasc Surg 47: 144–147, 1999. [DOI] [PubMed] [Google Scholar]
- 4.Cummings CJ, Martin TR, Frevert CW, Quan JM, Wong VA, Mongovin SM, Hagen TR, Steinberg KP, Goodman RB. Expression and function of the chemokine receptors CXCR1 and CXCR2 in sepsis. J Immunol 162: 2341–2346, 1999. [PubMed] [Google Scholar]
- 5.Dahlgren C, Karlsson A. Respiratory burst in human neutrophils. J Immunol Methods 232: 3–14, 1999. [DOI] [PubMed] [Google Scholar]
- 6.Fernandez J, Navasa M, Gomez J, Colmenero J, Vila J, Arroyo V, Rodes J. Bacterial infections in cirrhosis: epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology 35: 140–148, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Frances R, Zapater P, Gonzalez-Navajas JM, Munoz C, Cano R, Moreu R, Pascual S, Bellot P, Perez-Mateo M, Such J. Bacterial DNA in patients with cirrhosis and noninfected ascites mimics the soluble immune response established in patients with spontaneous bacterial peritonitis. Hepatology 47: 978–985, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Furst W, Hallstrom S. Simultaneous determination of myocardial nucleotides, nucleosides, purine bases and creatine phosphate by ion-pair high-performance liquid chromatography. J Chromatogr 578: 39–44, 1992. [DOI] [PubMed] [Google Scholar]
- 9.Hallstrom S, Gasser H, Neumayer C, Fugl A, Nanobashvili J, Jakubowski A, Huk I, Schlag G, Malinski T. S-nitroso human serum albumin treatment reduces ischemia/reperfusion injury in skeletal muscle via nitric oxide release. Circulation 105: 3032–3038, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Hartl D, Latzin P, Hordijk P, Marcos V, Rudolph C, Woischnik M, Krauss-Etschmann S, Koller B, Reinhardt D, Roscher AA, Roos D, Griese M. Cleavage of CXCR1 on neutrophils disables bacterial killing in cystic fibrosis lung disease. Nat Med 13: 1423–1430, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi F, Means TK, Luster AD. Toll-like receptors stimulate human neutrophil function. Blood 102: 2660–2669, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Jalan R, Kapoor D. Reversal of diuretic-induced hepatic encephalopathy with infusion of albumin but not colloid. Clin Sci (Lond) 106: 467–474, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Jurgens G, Muller M, Garidel P, Koch MH, Nakakubo H, Blume A, Brandenburg K. Investigation into the interaction of recombinant human serum albumin with Re-lipopolysaccharide and lipid A. J Endotoxin Res 8: 115–126, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Khandaker MH, Xu L, Rahimpour R, Mitchell G, DeVries ME, Pickering JG, Singhal SK, Feldman RD, Kelvin DJ. CXCR1 and CXCR2 are rapidly down-modulated by bacterial endotoxin through a unique agonist-independent, tyrosine kinase-dependent mechanism. J Immunol 161: 1930–1938, 1998. [PubMed] [Google Scholar]
- 15.Kono H, Rusyn I, Uesugi T, Yamashina S, Connor HD, Dikalova A, Mason RP, Thurman RG. Diphenyleneiodonium sulfate, an NADPH oxidase inhibitor, prevents early alcohol-induced liver injury in the rat. Am J Physiol Gastrointest Liver Physiol 280: G1005–G1012, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Kono H, Rusyn I, Yin M, Gabele E, Yamashina S, Dikalova A, Kadiiska MB, Connor HD, Mason RP, Segal BH, Bradford BU, Holland SM, Thurman RG. NADPH oxidase-derived free radicals are key oxidants in alcohol-induced liver disease. J Clin Invest 106: 867–872, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mookerjee RP, Stadlbauer V, Lidder S, Wright GA, Hodges SJ, Davies NA, Jalan R. Neutrophil dysfunction in alcoholic hepatitis superimposed on cirrhosis is reversible and predicts the outcome. Hepatology 46: 831–840, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Murphy PM Neutrophil receptors for interleukin-8 and related CXC chemokines. Semin Hematol 34: 311–318, 1997. [PubMed] [Google Scholar]
- 19.Ortega R, Gines P, Uriz J, Cardenas A, Calahorra B, De Las Heras D, Guevara M, Bataller R, Jimenez W, Arroyo V, Rodes J. Terlipressin therapy with and without albumin for patients with hepatorenal syndrome: results of a prospective, nonrandomized study. Hepatology 36: 941–948, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Parker LC, Whyte MK, Dower SK, Sabroe I. The expression and roles of Toll-like receptors in the biology of the human neutrophil. J Leukoc Biol 77: 886–892, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Pascual C, Bredle D, Karzai W, Meier-Hellmann A, Oberhoffer M, Reinhart K. Effect of plasma and LPS on respiratory burst of neutrophils in septic patients. Intensive Care Med 24: 1181–1186, 1998. [DOI] [PubMed] [Google Scholar]
- 22.Pelzmann B, Hallstrom S, Schaffer P, Lang P, Nadlinger K, Birkmayer GD, Vrecko K, Reibnegger G, Koidl B. NADH supplementation decreases pinacidil-primed I K ATP in ventricular cardiomyocytes by increasing intracellular ATP. Br J Pharmacol 139: 749–754, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piccolo MT, Sannomiya P. Inhibition of neutrophil chemotaxis by plasma of burned patients: effect of blood transfusion practice. Burns 21: 569–574, 1995. [DOI] [PubMed] [Google Scholar]
- 24.Rajkovic IA, Williams R. Mechanisms of abnormalities in host defences against bacterial infection in liver disease. Clin Sci (Lond) 68: 247–253, 1985. [DOI] [PubMed] [Google Scholar]
- 25.Runyon BA Management of adult patients with ascites due to cirrhosis. Hepatology 39: 841–856, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Sabroe I, Dower SK, Whyte MK. The role of Toll-like receptors in the regulation of neutrophil migration, activation, and apoptosis. Clin Infect Dis 41, Suppl 7: S421–S426, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Sabroe I, Jones EC, Usher LR, Whyte MK, Dower SK. Toll-like receptor (TLR)2 and TLR4 in human peripheral blood granulocytes: a critical role for monocytes in leukocyte lipopolysaccharide responses. J Immunol 168: 4701–4710, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Sabroe I, Jones EC, Whyte MK, Dower SK. Regulation of human neutrophil chemokine receptor expression and function by activation of Toll-like receptors 2 and 4. Immunology 115: 90–98, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siminiak T, Egdell RM, O'Gorman DJ, Dye JF, Sheridan DJ. Plasma-mediated neutrophil activation during acute myocardial infarction: role of platelet-activating factor. Clin Sci (Lond) 89: 171–176, 1995. [DOI] [PubMed] [Google Scholar]
- 30.Sort P, Navasa M, Arroyo V, Aldeguer X, Planas R, Ruiz-del-Arbol L, Castells L, Vargas V, Soriano G, Guevara M, Gines P, Rodes J. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med 341: 403–409, 1999. [DOI] [PubMed] [Google Scholar]
- 31.Stadlbauer V, Davies NA, Sen S, Jalan R. Artificial liver support systems in the management of complications of cirrhosis. Semin Liver Dis 28: 96–109, 2008. [DOI] [PubMed] [Google Scholar]
- 32.Stanley AJ, MacGregor IR, Dillon JF, Bouchier IA, Hayes PC. Neutrophil activation in chronic liver disease. Eur J Gastroenterol Hepatol 8: 135–138, 1996. [DOI] [PubMed] [Google Scholar]
- 33.Sturk A, Joop K, ten Cate JW, Thomas LL. Optimalization of a chromogenic assay for endotoxin in blood. Prog Clin Biol Res 189: 117–137, 1985. [PubMed] [Google Scholar]
- 34.Taieb J, Mathurin P, Elbim C, Cluzel P, Arce-Vicioso M, Bernard B, Opolon P, Gougerot-Pocidalo MA, Poynard T, Chollet-Martin S. Blood neutrophil functions and cytokine release in severe alcoholic hepatitis: effect of corticosteroids. J Hepatol 32: 579–586, 2000. [DOI] [PubMed] [Google Scholar]
- 35.Van Deventer SJ, Buller HR, ten Cate JW, Aarden LA, Hack CE, Sturk A. Experimental endotoxemia in humans: analysis of cytokine release and coagulation, fibrinolytic, and complement pathways. Blood 76: 2520–2526, 1990. [PubMed] [Google Scholar]