Abstract
Shiga toxin 1 and 2 production is a cardinal virulence trait of enterohemorrhagic Escherichia coli infection that causes a spectrum of intestinal and systemic pathology. However, intestinal sites of enterohemorrhagic E. coli colonization during the human infection and how the Shiga toxins are taken up and cross the globotriaosylceramide (Gb3) receptor-negative intestinal epithelial cells remain largely uncharacterized. We used samples of human intestinal tissue from patients with E. coli O157:H7 infection to detect the intestinal sites of bacterial colonization and characterize the distribution of Shiga toxins. We further used a model of largely Gb3-negative T84 intestinal epithelial monolayers treated with B-subunit of Shiga toxin 1 to determine the mechanisms of non-receptor-mediated toxin uptake. We now report that E. coli O157:H7 were found at the apical surface of epithelial cells only in the ileocecal valve area and that both toxins were present in large amounts inside surface and crypt epithelial cells in all tested intestinal samples. Our in vitro data suggest that macropinocytosis mediated through Src activation significantly increases toxin endocytosis by intestinal epithelial cells and also stimulates toxin transcellular transcytosis. We conclude that Shiga toxin is taken up by human intestinal epithelial cells during E. coli O157:H7 infection regardless of the presence of bacterial colonies. Macropinocytosis might be responsible for toxin uptake by Gb3-free intestinal epithelial cells and transcytosis. These observations provide new insights into the understanding of Shiga toxin contribution to enterohemorrhagic E. coli-related intestinal and systemic diseases.
Keywords: O157:H7 colonization, toxin endocytosis, transport across the monolayer
shiga toxins (Stx) 1 and 2 are established virulence factors (3, 17, 64, 75) in the spectrum of illnesses caused by noninvasive enterohemorrhagic Escherichia coli (EHEC). These infections are currently untreatable, since antibiotics are associated with a higher risk of sequelae, including the hemolytic uremic syndrome (79). Therefore, it is critical to understand the pathophysiology underlying these illnesses. Particularly poorly understood are the mechanisms of Stx uptake and transcytosis through the intestinal epithelial monolayer, although these processes are necessary for the systemic complications of EHEC to occur. Whereas the intestinal sites and frequency of EHEC colonization are largely unknown, it is well established that Stx1 and Stx2 are produced by these bacteria and released into the intestinal lumen (12, 25, 68). Interactions of Stxs with intestinal epithelial cells precede the systemic aspects of the disease. Ever since the discovery of the Stx1 receptor, a glycosphingolipid globotriaosylceramide (Gb3) (17), Stx1 interaction with intestinal epithelial cells has been assumed to be mostly mediated through the Gb3 on the apical cell surface. However, the recently confirmed finding (18, 29, 41, 51, 66) that normal human colonic and ileal epithelia cells, which are the major intestinal sites damaged by EHEC infection, do not express Gb3, has caused rethinking of the EHEC-induced intestinal pathogenesis.
It has been shown by use of an in vitro organ culture (IVOC) system and the polarized human intestinal epithelial T84 cell line that, despite the absence of Gb3 receptors, Stx1 and Stx2 enter intestinal epithelial cells (4, 24, 66, 69). Additionally, both toxins translocate across the T84 monolayers via a transcellular pathway (4, 32, 69). However, in vivo evidence of the presence and the distribution of Stx1 and Stx2 in human intestinal tissue, particularly inside epithelial cells, in the course of EHEC infection had been lacking. Moreover, the endocytic mechanisms of Stx1 and Stx2 uptake and transcytosis by Gb3 receptor-free cells in vitro and in vivo are largely unknown.
Here, using previously clinically characterized (52) intestinal samples from EHEC-infected patients, we show the presence of bacteria at the apical surface of epithelial cells. Importantly, we detected both Stx1 and Stx2 throughout the tissue, particularly inside both surface and crypt epithelial cells, and this occurred regardless of the identified presence of EHEC on the intestinal epithelial cells. Additionally, using T84 cells, we examined the possible molecular mechanisms of Stx1 uptake by Gb3-free intestinal epithelial cells. Recently several receptor-independent mechanisms of endocytosis have been described (8, 36, 42, 49, 52, 62, 63). This classification was based on the different requirements for dynamin, caveolin, clathrin, and small GTPases. Moreover, internalization of a single cargo can employ several different endocytic pathways (11, 26, 43). One of the most studied examples of a receptor-independent endocytotic mechanism is macropinocytosis, a stimulated fluid-phase uptake pathway that uses actin turnover. Our previous studies of the mechanism of Stx uptake by intestinal cells revealed that the process had characteristics similar to those described for macropinocytosis (73), including that 1) internalized Stx1 was present within actin-coated vesicles in T84 cells, 2) the vesicles were large, and 3) Stx1 uptake and transcytosis were actin cytoskeleton dependent (46, 50). Here, we studied the interaction between intestinal epithelial T84 cells and recombinant B-subunit of Stx1 (Stx1B) and tested the hypothesis that macropinocytosis might be responsible for Stx1 endocytosis into receptor-free human intestinal epithelial cells and subsequent toxin transcellular transcytosis.
MATERIALS AND METHODS
Reagents and antibodies.
Purified Stx1 and recombinant Stx1B were prepared as previously described (1, 2). The quality of the Stx1 and Stx1B was verified by 15% SDS-PAGE followed by GelCode Blue staining (Pierce, Rockford, IL). Two bands from Stx1 (∼32 kDa for A-subunit and <10 kDa for B-subunit) and one band from the Stx1B (<10 kDa for B-subunit) were detected, as previously reported (1, 2). Pirl-1 (8-cyclohexil-5,6-dihidro-4H-pyrazino[3,2,1-jk]carbazole) was purchased from Chembridge (San Diego, CA); chlorpromazine (CPZ), phorbol 12-myristate 13-acetate (TPA), N-ethylmaleimide (NEM), polybrene, horseradish peroxidase (HRP), lucifer yellow, saponin, and propidium iodide were from Sigma (St. Louis, MO). Monoclonal antibodies were obtained as follows: against actin, total Src, and GAPDH were from Sigma, against Cdc42 from Santa Cruz Biotechnology (Santa Cruz, CA), against Stx2 (STX2–11E10) from Toxin Technology (Sarasota, FL); and CD77 antibody against Gb3 from Immunotech (Marseille, France). Polyclonal antibodies against active phospho-Src (p-Src) were from Upstate (Temecula, CA) and against Stx1B from Dr. E. Boedeker. Fluorescent secondary antibodies, phalloidin conjugated to Alexa 488 fluorescent dye, Alexa 488, Alexa 568, and Alexa 680 reactive fluorescent dyes, Hoechst 33342, 3- or 10-kDa dextrans conjugated to Alexa 680, and 40-kDa dextran conjugated to Alexa 488 were from Molecular Probes-Invitrogen (Carlsbad, CA). FITC-labeled goat anti-E. coli O157:H7 antibody was from Kirkegaard & Perry Laboratories (Gaithersburg, MD).
Human tissue specimens.
The human tissue samples of EHEC-infected patients collected during the 1993 E. coli O157:H7 epidemic in the Western United States were obtained from the tissue bank of Seattle Children's Hospital via a Johns Hopkins-sponsored material transfer agreement. The samples were coded, so no patient identifiers were linked to the specimens. These studies were approved by the Institutional Review Boards (or equivalent committees) of the Children's Hospital and Regional Medical Center, Seattle, Washington.
Cell culture.
Human colonic epithelial T84 cells (ATCC, Manassas, VA) were grown and maintained in culture in DMEM-Ham's F-12 medium (1:1) supplemented with 10% fetal bovine serum, 100 units/ml penicillin, and 100 μg/ml streptomycin. All medium ingredients were obtained from Invitrogen. Monolayers (passage 27–45) were grown on polycarbonate inserts with 0.4-μm pore size (Costar, Cambridge, MA) or on glass coverslips for 7–14 days. The experiments were performed on confluent monolayers with transepithelial electrical resistance (TER) > 1,500 Ω·cm2.
Detection of intracellular Stx1B in the in vitro model.
Confluent T84 cells were incubated in control and experimental conditions with 0.8 μg/ml of Stx1B conjugated to Alexa 680 fluorescent dye (Stx1B-680) for the times specified. Cells then were washed three times with cold PBS to remove extracellular toxin and then lysed in RIPA buffer (1% Triton X-100, 0.5% deoxycholic acid, 0.1% SDS, 50 mM Tris·HCl, pH 7.4, 150 mM NaCl) containing 0.5 mM Na3VO4 and protease inhibitor cocktail 1:1,000 (Sigma P8340). Lysates were centrifuged at 20,000 g at 4°C for 15 min, and equal amounts of collected proteins were loaded into the 96-well plates in triplicate. The relative fluorescence intensity of Stx1B-680 in 150 μg of protein in total cell lysates, which corresponds to the relative amount of intracellular Stx1B, was measured in triplicate by the Odyssey Fluorescence Imaging System (Li-Cor Biosciences, Lincoln, NE) and normalized by the autofluorescence of an equal amount of protein from cells not exposed to Stx1B. Similar experiments were done to measure the endocytosis of fluorescently labeled HRP-Alexa 680 (apical concentration 10 μg/ml).
Detection of Stx1B transcytosis.
Cells grown on polycarbonate inserts were incubated with 0.8 μg/ml of fluorescently labeled Stx1B in the absence (basal transcytosis) or presence of specified pharmacological agents (stimulated transcytosis) over the indicated times. At the end of incubation, the inserts were removed, 150-μl samples of the medium from the lower chamber were collected, and the Stx1B relative fluorescence intensity was measured in triplicate by the Odyssey Fluorescence Imaging System and normalized by the autofluorescence of an equal volume of conditioned medium without Stx1B. The measurements of transcytosis of 3- and 10-kDa dextrans and HRP were done similarly (apical concentration of each tracer was 10 μg/ml).
Detection of lucifer yellow transcytosis.
Lucifer yellow (200 μM) was added to the apical chamber of cells growing on polycarbonate inserts. Four hours later the fluorescence in lower chamber was measured in 100 μl of basal medium using fluorometer and excitation wavelength 430 nm. The emission fluorescence was collected at maximum (528 nm) and was normalized by the emission fluorescence of the conditioned medium without lucifer yellow. The basolateral application of tannic acid did not affect the emission spectra of lucifer yellow.
Rabbit model of EHEC-induced colitis.
The well-established rabbit cecal model of EHEC-induced colitis was used as described (66). Briefly, in this model the enteroadherent rabbit E. coli pathogen RDEC-1, which forms attaching/effacing lesions similar to those in EHEC-infected humans, was transduced with Stx1-converting phage ΦH19A to express Stx1 and given by gavage. In our experiments, to discriminate between Stx1-specific intestinal epithelial changes and the changes related to enteric colonization, male New Zealand White rabbits (weighing 2.5–5 kg, from Myrtle's Rabbitry, Thompsons Station, TN) were infected with 1010 RDEC-1 (n = 2) or 1010 Stx1-producing RDEC-H19A (n = 3) or treated with PBS (n = 2). Experiments with animals were performed using protocols approved by the Animal Use Committee of the University of New Mexico School of Medicine.
Five days after challenge, cecal tissues from control and experimental rabbits were collected, rinsed with ice-cold PBS, opened along the mesenteric border, snap frozen in liquid nitrogen, and stored at −80°C until further use.
Immunofluorescence.
Formalin-fixed paraffin-embedded 10-μm-thick slices of human tissue were deparaffinized by a cascade of 5-min incubation with xylene (2 times), 100% ethanol, 95% ethanol, and 70% ethanol, followed by tissue rehydration in PBS. Samples were permeabilized with 0.1% saponin and blocked with 2% BSA and 15% FBS for 45 min. For optimal detection of Stx1 and Stx2 and EHEC, nonspecific binding of secondary antibody was avoided by direct conjugation of primary antibodies against Stx1 and Stx2 with Alexa 568 and Alexa 488 fluorescent dyes, respectively, and the use of Hoechst 33342 for DNA labeling in human cells and bacteria. After incubation for 1 h at room temperature with antibodies and Hoechst, samples were washed extensively in PBS and mounted by using gel mount on glass slides for confocal microscopy.
To confirm that bacteria detected in human tissue represent EHEC, the tissue samples were incubated with FITC-labeled anti-E. coli O157:H7 antibody (isolated from a serum pool of goats immunized with heat-killed whole cells of E. coli serotype O157) for 1 h at room temperature, as has been previously described (47, 55).
For p-Src immunostaining, 10-μm sections of frozen cecal tissues from rabbits infected either with RDEC-1 or RDEC-H19A bacteria were fixed in 4% formaldehyde in PBS for 30 min, washed extensively in PBS, permeabilized with 0.1% saponin, and blocked with 2% BSA and 15% FBS for 45 min. Sections were then incubated with primary antibodies at room temperature for 1 h, washed three times in PBS, and incubated with secondary fluorescent antibodies, phalloidin-Alexa 488 for F-actin and Hoechst for nuclear staining, for an additional 1 h, washed again, immersed in gel mount, and mounted on glass slides for confocal microscopy.
For cell immunofluorescence experiments, confluent T84 monolayers grown on filters were fixed with 3% formaldehyde in PBS for 10 min, washed extensively in PBS, permeabilized with 0.1% saponin, blocked with 2% BSA and 15% FBS for 30 min, incubated with primary nonfluorescent antibody to the protein of interest for 1 h at room temperature, washed extensively, and then exposed for 1 h to the corresponding secondary fluorescent antibody, phalloidin-Alexa 488 for F-actin and Hoechst for nuclear staining. Filters with cells were then washed with PBS, immersed in gel mount, and mounted on glass slides for microscopy.
Fluorescent imaging of tissue and cells was performed using Zeiss 410 and Zeiss 510 LSM imaging systems. Eight or 12-bit fluorescence images of confocal optical 0.5-μm sections were collected for further qualitative and quantitative analysis using MetaMorph software (Roper Industries, Marlow, UK) as we previously described (35).
Quantification of G-actin and F-actin.
Fractionation of globular G-actin and fibrillar F-actin was performed by Triton X-100 extraction of intracellular actin as described (34). Briefly, T84 cells were washed with HBSS and G-actin was extracted by gentle shaking for 5 min at room temperature in HBSS containing 1% Triton X-100, proteinase inhibitor cocktail, and 1 μg/ml phalloidin to prevent filament disassembly. The Triton X-100-soluble G-actin fraction was mixed with an equal volume of 2× SDS sample buffer and boiled. Cells were then briefly washed with HBSS, and the Triton X-100-insoluble F-actin fraction was collected by scraping cells in two volumes of SDS sample buffer, and then boiled. The amount of actin in each fraction was determined by gel electrophoresis, Western blotting, and Odyssey Fluorescence Imaging System as described above.
Lentiviral transduction of T84 cell with shRNA constructs against Cdc42.
Three sequence-verified short hairpin RNA (shRNA) lentiviral plasmids in a hairpin-pLKO.1-puromycin vector for cell division cycle 42 (Cdc42) gene silencing in human cells were obtained through the Johns Hopkins High Throughput Biology Center from Open Biosystems (Huntsville, AL) and were used to generate lentiviral transduction particles. These shRNA constructs from The RNAi Consortium (TRC) library (http://www.broad.mit.edu/rnai/trc/lib) were shCdc42-1 CCGGCCCTCTACTATTGAGAAACTTCTCGAGAAGTTTCTCAATAG TAGAGGGTTTTTG (TRCN0000047628); shCdc42-2 CCGGCCTGATATCCTACACAACAAACTCGAGTTT GTTGTGTAGGATATCAGGTTTTTG (TRCN0000047630), and shCdc42-3 CCGGCAGATGTATTTCTAGTCTGTTCTCGAGAACAGACTAGAAAT ACATCTGTTTTTG (TRCN0000047632).
For production of lentiviral particles, three components were transfected into HEK 293T cells: 1) pLKO.1 vector containing shRNA; 2) a packaging vector pCMV-dR8.91 containing gag, pol, and rev genes; and 3) envelope vector pCMV-VSVG. The plasmids were prepared by using an EndoFree Plasmid Maxi kit (Qiagen, Valencia, CA). Twenty-four hours before transfection, 20 × 106 HEK 293T cells were plated on a 10-cm petri dish, and 1 h before transfection the medium was changed to OPTI-MEM serum-free medium. The Lipofectamine 2000 method of transfection (Invitrogen) was used according to the manufacturer's protocol. For the transfection, the mixture of 10 μg of packaging plasmid + 6 μg of envelope coding plasmid +10 μg shRNA coding plasmid in 500 μl OPTI-MEM solution was combined with Lipofectamine 2000 solution and added to the cells. Before the plates were returned to the incubator they were rocked to achieve a uniform distribution of the reagent. Production of lentiviruses was enhanced by replacement of the cell culture medium at 16 h posttransfection with 5 ml of fresh medium containing 10 mM sodium butyrate (23, 61) for 8 h. The sodium butyrate was then replaced with 5 ml of fresh medium for another 16 h before virus harvesting. The lentivirus supernatants were passed through 0.45-μm-pore polyvinylidene difluoride Durapore filters (Millipore, Bedford, MA) and were either used immediately to transduce T84 cells with shRNA of interest or stored at −80°C for future use.
For lentiviral transduction, T84 cells were plated on six-well plates 24 h before lentiviral transduction to achieve 30–40% confluency. Harvested viral particles were mixed with an equal volume of complete T84 cell medium, incubated in the presence of 90 μg/ml polybrene for 30 min at 37°C, and then added to the cells. Twenty-four hours later, medium was replaced with complete T84 cell medium containing 5 μg/ml puromycin. For the experiments, cells were split into six-well polycarbonate inserts. Cells transduced with the shRNACdc42 constructs grew slower compared with the cells transduced with lentiviral particles containing GFP, achieving confluency in 14–20 days. The cells with shCdc42-3 did not form confluent monolayers at this time. Thus we limited our studies to cells transduced with shCdc42-1 and shCdc42-2 constructs. This delay in cell proliferation was probably related to Cdc42 downregulation, since Cdc42 modulates cell polarity and cell-cycle control. For instance, the mice totally deficient in Cdc42 function die during embryogenesis (81).
Bacterial infection of T84 cells.
Following a previously published protocol (24), T84 cells grown on filters were inoculated apically with varied concentrations of EHEC strain EDL933 (10) modified to be Stx1-negative (a generous gift from Dr. J. M. Leong, Harvard University) and incubated at 37°C in 5% CO2 for 16 h in antibiotic-free medium. Cells then were washed three times with cold PBS and lysed in RIPA buffer for further immunoblotting, as described above.
Statistical analysis.
Values are presented as means ± SE and the number (n) of independent preparations. The data sets were compared by ANOVA. A level of statistically significant difference of P value <0.05 was considered significant.
RESULTS
Stx1 and Stx2 are taken up by receptor-negative human intestinal epithelial cells during E. coli O157:H7 infection.
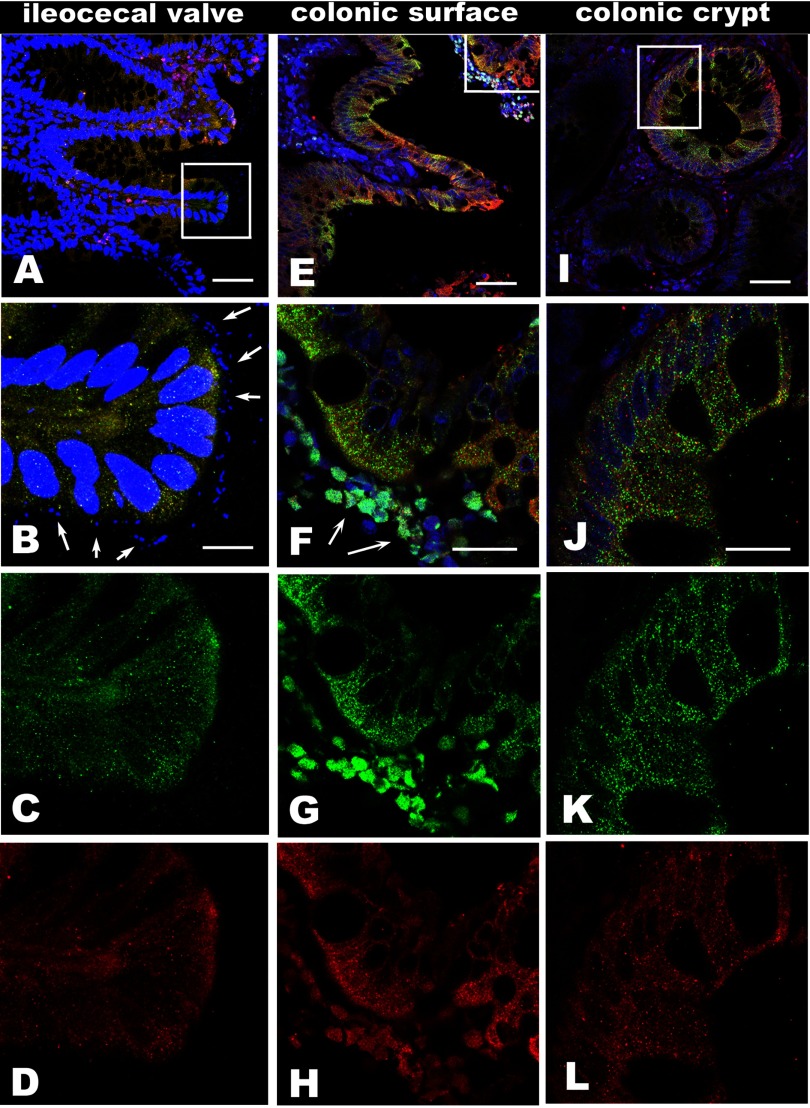
In human EHEC infection, both Stx1 and Stx2 are secreted by bacteria into the gut lumen. Both toxins, together with the bacteria, have been detected in stool samples. However, the presence of attached/effaced bacteria in the course of human disease has never been convincingly observed (68). Also, it is not clear whether Gb3-negative human enterocytes are able to endocytose luminal toxin in disease and whether the presence of attached bacteria is necessary for toxin endocytosis by intestinal epithelial cells. To address these questions, we examined intestinal tissue samples from EHEC-infected patients collected during the 1993 E. coli O157:H7 epidemic in the Western United States for the presence and the distributions of bacteria and toxins. The clinical courses and observed colonic pathologies in these specimens, which had been obtained at surgery or at the time of autopsy, have been described and discussed in detail (52). We analyzed representative tissue samples from colon (4 samples) and ileocecal valve (1 sample) by immunofluorescence confocal microscopy. We detected the bacteria at the apical surface of the epithelial cells in the ileocecal valve sample only (Fig. 1, A and B). The bacteria detected by Hoechst were also identified with antibody against E. coli O157:H7 (Supplemental Fig. S1, C–F; the online version of this article contains supplemental data). This specimen was obtained at surgery. In contrast, Stx1 and Stx2 were present in ileal and colonic epithelial cells (Fig. 1). Stx were inside both surface (Fig. 1, E–H) and crypt (Fig. 1, I–L) epithelial cells in all colonic samples, whether or not surface bacteria were detected. Additionally, Stx1 and Stx2 were present in the lamina propria and in the dead cells expelled from the tissue into the lumen (Fig. 1, F–H). Inside the epithelial cells, the toxins have a punctuate pattern, which represents association with a vesicular compartment.
Fig. 1.
Both Shiga toxin (Stx) 1 and Stx2 are present inside the intestinal epithelial cells in samples from O157:H7-infected patients regardless of bacterial presence. Representative single 3D projection from a stack of 10 1-μm confocal optical sections obtained by ×40 water immersion lens from ileocecal valve (A–D), surface region of colon (E–H), and crypt region of colon (I–L), which were immunostained with antibody against Stx2 (green), Stx1 (red), and nuclei and bacteria labeled by Hoechst 33342 (blue). A, B, C, and D (zoomed region from A) demonstrate the O157:H7 bacteria at the apical surface of epithelial cells (arrows) with the ribbon of bacteria that line the mucosa. Both Stx2 (C) and Stx1 (D) are present inside the epithelial cells and in lamina propria. E: representative 3D projection of colonic surface region without the presence of enterohemorrhagic Escherichia coli (EHEC). F–H: confocal optical section of magnified area from (E) shows the vesicular distribution of toxins inside the colonocytes, similar to these in B–D. The Stx2 (G) and Stx1 (H) are taken up by surface epithelial cells. Both toxins are also present in the dead cells expelled into the lumen (arrows in F). I: representative 3D projection of the base of crypt region. Both toxins are present inside the crypt epithelial cells and in lamina propria. J–L: confocal optical section of magnified area from I shows the punctate distribution of toxins inside the crypt epithelial cells similar to these in surface cells. Both Stx2 (K) and Stx1 (L) are taken up by crypt epithelial cells. All fluorescent labeling as in A. Bars in A, E, and I, 50 μm; bar in B, 10 μm; bars in F and J, 20 μm. The specificity of antibodies against Stx1 and Stx2 were checked by using normal human colon tissue samples, and no immunostaining was observed (Supplemental Fig. S1, A and B).
We also tested all tissue samples for the presence of Gb3. No Gb3 was detected in intestinal epithelial cells either by immunostaining with fluorescently labeled Stx1B (Supplemental Fig. S1, E and F) or by anti-Gb3 antibody (data not shown). These data show that, in the absence of Gb3 receptor, Stxs are taken up into human colonic epithelial cells. Moreover, the presence of toxins in lamina propria indicates that toxins are crossing the epithelial monolayer in disease (Fig. 1).
We calculated from 12-bit images the relative fluorescence intensity that reflects the relative amount of Stx1 and Stx2 (means ± SE) separately in all five analyzed samples (60 cells in each sample). There was variability in the relative amount of Stx1 and Stx2 between samples. However, the relative amount of either toxin in sample from the ileocecal valve (the only sample with EHEC associated with epithelial cells) was not the highest. The relative amount of Stx2 varied between samples from 2,876 ± 376 gray levels to 812 ± 135 with ileocecal sample (1,798 ± 319). The relative amount of Stx1 in all samples was not significantly different from each other (∼1,098 ± 196), except for the one with the highest (1,589 ± 164) relative amount.
These data raise questions about the mechanism of Stx1 and Stx2 uptake and transcytosis across the epithelium. Previous studies of toxin uptake in vitro using Gb3-negative T84 cells showed that after 6 h of incubation, only 7.5% of T84 cells were positive for Stx1 and only 0.4% cells were positive for Stx2 (66). The previously measured amount of transcytosed Stx1 through T84 cells, a key step in systemic toxin spread, was also relatively low, and only ∼2% of the apical amount of Stx1 was transferred across the monolayer in 24 h (4). In contrast, in the tissue samples from EHEC-infected patients (Fig. 1) that we examined, virtually all intestinal epithelial cells had high concentration of both toxins.
This finding prompted us to look for a model of efficient non-Gb3-receptor-mediated Stx endocytosis by intestinal epithelial cells and toxin transcytosis. We chose T84 cells as a model because this is the only well-established polarized intestinal epithelial cell line that is virtually Gb3 free, as has been reported (24, 41, 46, 51, 66; in our hands only ∼5% of T84 cells express Gb3 receptors). In this T84 cell model we tested the ability to stimulate baseline Stx1 uptake and transcytosis and characterized the molecular mechanisms of Stx1-stimulated endocytosis, using fluorescently labeled Stx1B as a toxin surrogate.
Inhibition of clathrin-dependent endocytic pathway increases Stx1B uptake.
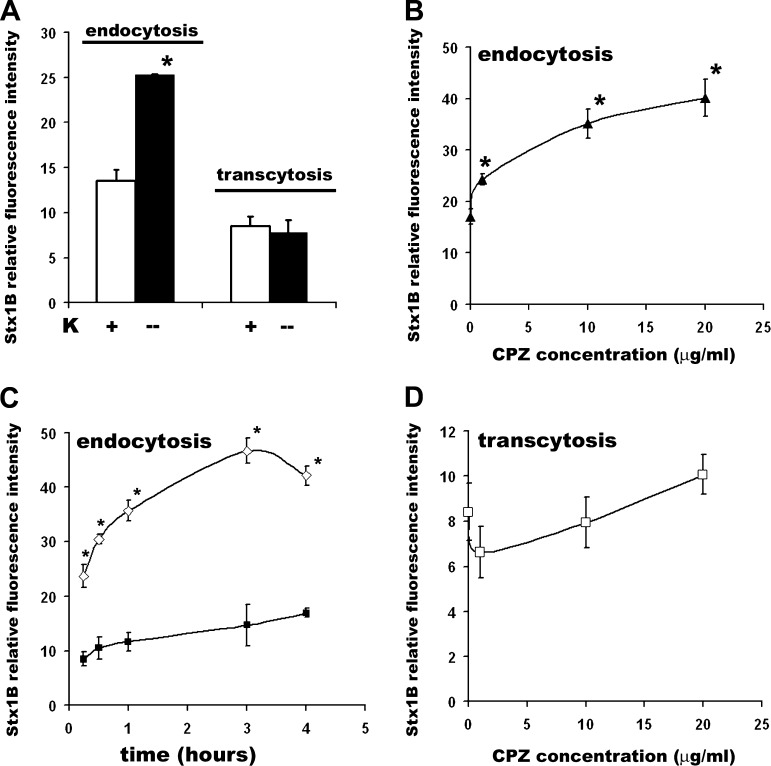
Clathrin-dependent uptake is the one of the major endocytic pathways in epithelial cells (8, 77). We tested the contribution of clathrin-dependent endocytosis to the Stx1B uptake by T84 cells using two established methods to inhibit the clathrin-dependent pathway. Inhibition of clathrin-dependent uptake either by low potassium (5, 27) or by the amphiphilic drug CPZ, which inhibits assembly of the clathrin adapter protein AP2 on clathrin-coated pits (45, 21), resulted in a significant increase in Stx1B uptake (Fig. 2, A–C). Similar results were obtained in an endocytic assay of HRP, a fluid-phase marker the molecular weight of which closely resembles that of the Stx1B pentamer (Supplemental Fig. S2). This suggests that Stx1B endocytosis is clathrin independent.
Fig. 2.
Inhibition of clathrin-dependent endocytosis significantly stimulates Stx1B uptake by intestinal epithelial cells. A: potassium depletion significantly increases the Stx1B uptake (1) by T84 monolayers exposed to Stx1B for 4 h but does not affect Stx1B transcytosis (2). Open bars, basal Stx1B uptake and transcytosis; solid bars, Stx1B endocytosis and transcytosis in low-potassium conditions. B: chlorpromazine (CPZ) stimulates Stx1B uptake at 4 h in a concentration-dependent manner and 1 μg/ml CPZ significantly increases Stx1B endocytosis in T84 cells. C: time dependency of Stx1B uptake in the presence (◊) of CPZ 10 μg/ml or in control conditions (▪). CPZ effect on stimulation of Stx1B uptake was already significant at 15 min compared with control cells. D: CPZ does not affect Stx1B transcytosis at 4 h of incubation at any tested concentration. Data were obtained from at least 5 independent experiments. *P < 0.05 compared with the controls.
Next, we examined the impact of the clathrin-dependent pathway on toxin transcytosis. The increase in Stx1B uptake in the presence of CPZ or in the absence of potassium did not affect Stx1B transcytosis over the 4 h of experimental time (Fig. 2, A and D). Similarly to the Stx1B transcytosis, the transcytosis of HRP was not affected by CPZ in 4 h (Supplemental Fig. S2).
These findings, together with our previous observation (46) that during basal endocytosis the Stx1B resides inside variably sized F-actin-coated vesicles at the apical surface of T84 cells, allowed us to hypothesize that a clathrin-independent actin-driven endocytic mechanism is the pathway for Stx1B endocytosis by intestinal epithelial cells and toxin transcytosis. These characteristics are necessary features of macropinocytosis, a form of non-receptor-mediated fluid-phase endocytosis that provides an efficient route for nonselective uptake of extracellular solute macromolecules (73).
Stx1 uptake increases by treatment with NEM or TPA, two stimulators of macropinocytosis.
The rate of macropinocytosis in nonstimulated epithelial cells is generally low but increases following application of various stimuli, including activation of growth factor receptors, protein kinase C, phorbol esters, and small G proteins (20, 36, 38, 65, 73). For example, NEM and TPA have been shown to stimulate the macropinocytosis of ricin in polarized Madin-Darby canine kidney (MDCK) cells (30, 62).
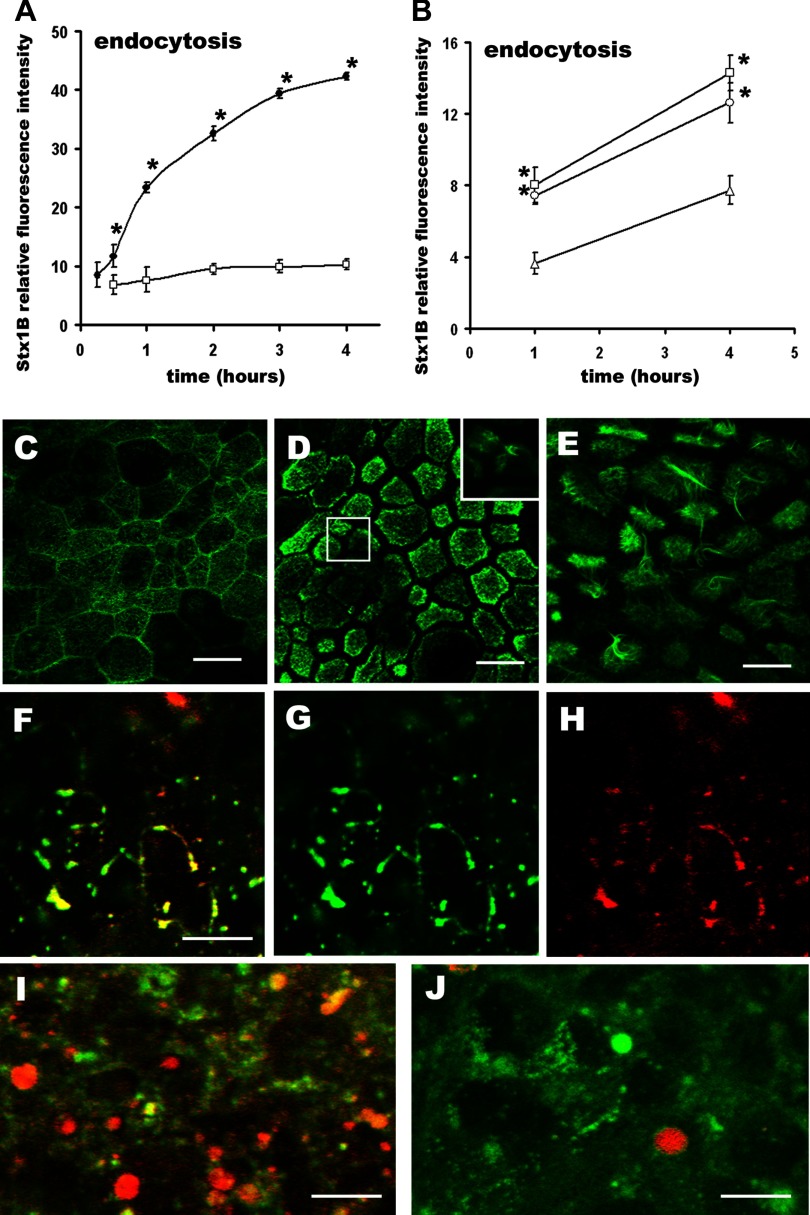
Consequently, we tested NEM and TPA effects on Stx1B endocytosis by T84 cells. Following previously described experimental conditions (62), we pretreated T84 cells either with NEM for 30 min or TPA for 10 min before the Stx1B-680 introduction and monitored toxin endocytosis over time. In NEM (200 μM)-treated cells (Fig. 3A), a significant increase in Stx1B uptake compared with control cells was seen as early as 15 min after toxin introduction. By 60 min, NEM increased the Stx1B uptake approximately threefold; this effect continued to increase over 4 h.
Fig. 3.
Both N-ethylmaleimide (NEM) and phorbol 12-myristate 13-acetate (TPA) significantly stimulate Stx1B endocytosis by T84 colonic epithelial cells. A: time dependency of NEM-stimulated Stx1B endocytosis. NEM rapidly and significantly stimulates Stx1B endocytosis over time, starting after 15 min of incubation with Stx1B with effect lasting at least for 4 h (•) compared with control cells not exposed to NEM (□). Each point represents the means from 4 independent experiments done in duplicate. *P < 0.05 compared with the time controls. B: time dependency of TPA on Stx1B uptake at different concentrations. ▴, Controls (no TPA); ○, 100 nM TPA; □, 1 μM TPA. TPA in concentration 100 nM slightly but significantly increased the relative amount of Stx1B inside the cells measured after 1 h and 4 h of incubation with Stx1B. Each point represents the means from 4 independent experiments done in duplicate. *P < 0.05 compared with the controls. C–E: representative confocal images of the apical region of control T84 cells labeled with phalloidin conjugated to Alexa 488 (green) to visualize F-actin. C: in control cells apical F-actin is mostly organized into the perijunctional rings and apical dotted structures. D: in cells pretreated for 30 min with 200 μM NEM F-actin is redistributed from the perijunctional ring into the apical region and apical F-actin ruffles (magnified inset). Ruffling progresses over time and (E) at 60 min of the NEM treatment virtually each cell has ruffles, a necessary feature of macropinocytosis. F–H: representative confocal optical section through the apical region of T84 cells pretreated for 30 min with 200 μM NEM and coincubated with Stx1B-568 (red, H) for additional 30 min. Cells were fixed and counterstained for F-actin (green, G). Virtually each apical actin ruffle (green) is filled with Stx1B (red). Representative confocal optical section through the subapical region of cells exposed for 30 min to Stx1B (red) in the presence of 200 μM NEM (I) or control T84 cells exposed for 30 min to Stx1B (red) without NEM and counterstained against F-actin (green) (J). NEM substantially increases the number of Stx1B vesicles inside the cells. Bars in C, D, and E, 30 μm; bars in F, I, and J, 10 μm.
TPA in concentration of 100 nM almost doubled the amount of endocytosed toxin at 60 min (Fig. 3B) but did not affect Stx1B uptake at early times (data not shown). Neither higher TPA concentrations (1 μM) nor longer incubation time (up to 4 h) significantly increased Stx1B endocytosis compared with 100 nM TPA at 60 min (Fig. 3B). TPA was more effective in stimulating the endocytosis of small 3-kDa molecular mass dextran at 4 h. But its dose dependency (Supplemental Fig. S3) at 4 h on the uptake of HRP was similar to the TPA effects on Stx1B uptake.
NEM induces membrane ruffling.
Preincubation with NEM was required to increase Stx1B uptake; simultaneous addition of NEM and toxin did not result in increased toxin uptake by 15 min (data not shown). This concurs with the previous observation that the NEM effect on ricin endocytosis required NEM preincubation (62), suggesting that a series of NEM-induced cellular events has to occur prior to the endocytosis.
A necessary feature of macropinocytosis is formation of F-actin-based cell surface ruffles followed by formation of discrete macropinosomes that are accumulated inside the cells (20, 73). NEM has been shown to cause membrane ruffling and macropinosome formation in MDCK cells (62). To investigate whether F-actin-based membrane ruffling also occurs in the NEM-treated T84 cells and whether the appearance of ruffles correlates with the increase in Stx1B uptake, we studied the NEM effects on cell morphology over time using immunofluorescence microscopy. Control T84 cells do not have apical F-actin ruffles (Fig. 3C). Exposure of T84 cells to NEM for 60 min changed the F-actin apical distribution and caused massive ruffle formation (Fig. 3E). Thus the 30-min preincubation with NEM was necessary to initiate the apical actin ruffle formation. Shorter preincubation times (less than 30 min) resulted in only random ruffles (Fig. 3D). The appearance of NEM-induced F-actin ruffles correlated with the timing of increased Stx1B endocytosis and might account for the requirement for NEM preincubation. NEM-induced ruffles were filled with Stx1B (Fig. 3, F–H), and the amount of Stx1B-positive vesicles significantly increased in subapical regions of cells with F-actin ruffles compared with the control cells (Fig. 3, I–J). Monitoring of the NEM-induced endocytosis of 40-kDa dextran (Supplemental Fig. S4) indicates that NEM similarly increases the uptake of this fluid-phase marker. We conclude that F-actin ruffles followed by formation of macropinosomes contribute to the increase in Stx1B endocytosis.
Stx1B macropinocytosis induced by NEM precedes significant increase in Stx1B transcytosis.
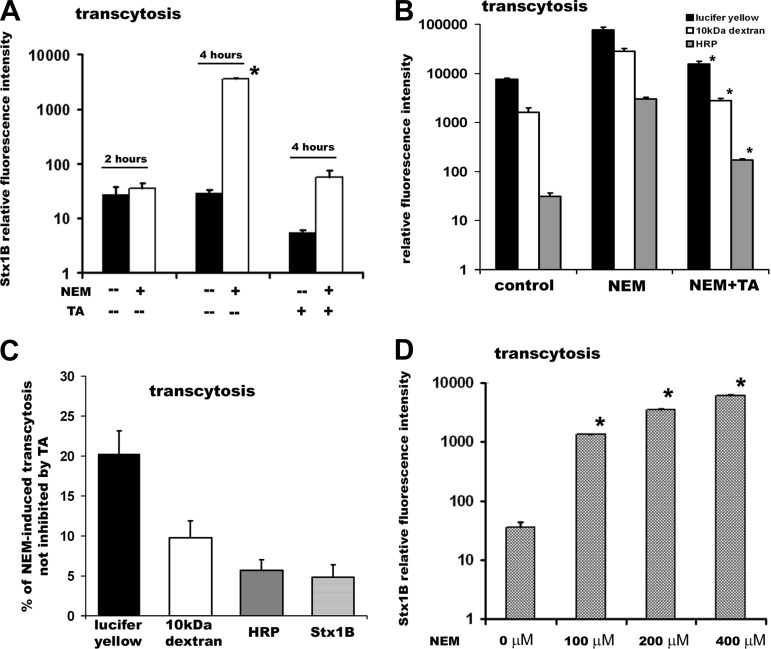
To spread systemically, the intact Stxs have to cross the epithelial cells. Consequently, we tested the relationship between Stx1B macropinocytosis and toxin transcytosis. Preincubation of T84 monolayers with 200 μM NEM for 30 min did not affect Stx1B transcytosis up to 60 min after toxin introduction. However, at later time points (4 h shown), NEM significantly increased apical-to-basolateral Stx1B transcytosis (Fig. 4A). These data suggest that apical macropinocytosis is necessary for the increase in Stx1B transcytosis by Gb3-receptor-free intestinal epithelial cells.
Fig. 4.
NEM-induced macropinocytosis significantly increases the transcytosis of toxin mostly through the T84 cells. A: Stx1B relative fluorescence intensity, which reflects the amount of transcytosed toxin in the absence (solid bars) and presence (open bars) of NEM after: 2 or 4 h of incubation with Stx1B and 4 h of incubation with Stx1B in the presence of the 0.1% basolateral tannic acid (TA) for 4 h. NEM significantly increases Stx1B transcytosis in 4 h, but not in 2 h. Tannic acid significantly inhibits basal and NEM-induced Stx1B transcytosis, indicating that toxin crosses the monolayer mostly transcellularly. B: relative fluorescence intensity, which reflects the amount of transcytosed molecules in 4 h: lucifer yellow (solid bars), 10-kDa dextran (open bars), and horseradish peroxidase (HRP; shaded bars) in control conditions, in NEM-treated cells, and in NEM-treated cells in the presence of the 0.1% basolateral tannic acid for 4 h. NEM-induced increase in transcytosis of each molecule was significantly (*P < 0.05) inhibited by tannic acid. C: the % of NEM-induced transcytosis for each studied molecule that was not inhibited by tannic acid. This was calculated based on assumption that the transcytosis of each tested molecule in the presence of NEM corresponds to 100%. The % of paracellular transcytosis substantially decrease with the increase in the molecular weight, and paracellular transcytosis for HRP and Stx1B was ∼5%. D: NEM-stimulated Stx1B transcytosis at 4 h of exposure to the toxin in a concentration-dependent manner. Stx1B relative fluorescence intensity, which reflects the amount of transcytosed toxin in 1) control cells or cells pretreated for 30 min with NEM in concentrations, 2) 100 μM; 3) 200 μM; 4) 400 μM. Data in A and D were collected from at least 6 independent experiments done in duplicate. *P < 0.05 compared with control. Each bar in B represents at least 4 independent experiments done in duplicate.
Treatment with NEM significantly decreased the TER of T84 cells from 2,645 ± 210 to 279 ± 11 Ω·cm2 (P > 0.05; n = 86 monolayers) after 15 min of incubation. Consequently, we tested the contribution of transcellular vs. paracellular pathway to the NEM-induced transcytosis of Stx1B and compared it with several fluid-phase markers. The NEM-stimulated Stx1B transcytosis was greatly inhibited by basolateral application of 0.1% tannic acid, a cell-impermeable cross-linker of cell surface carbohydrates (56, 59, 78) that selectively fixes the plasma membrane but does not diffuse across the tight junctions (TJ) (Fig. 4A, logarithmic scale). We estimated the percentage of NEM-induced paracellular transcytosis that was not inhibited by basolateral tannic acid by assuming that total NEM-induced transcytosis corresponds to 100% and found that only ∼5% of NEM-induced toxin movement across the T84 monolayers was through the TJ (Fig. 4C). Similarly, the tannic acid inhibited ∼95% of NEM-stimulated transcytosis of HRP, which molecular weight closely resembles the molecular weight of Stx1B pentamer (Fig. 4, B, logarithmic scale, and C). The paracellular component of NEM-stimulated transcytosis was higher (∼20%) in case of the small fluid-phase molecule lucifer yellow (Fig. 4, B and C), reflecting the size selectivity of TJ. But the transcytosis of 10-kDa dextran was ∼90% transcellular, indicating that even at low TER the TJ are virtually sealed for large molecules. Thus we concluded that NEM-induced transcytosis of Stx1B occurred mostly by a transcellular pathway.
NEM-stimulated Stx1B transcytosis at 4 h occurred in a concentration-dependent manner (Fig. 4D). We concluded that macropinocytosis significantly increased both Stx1B uptake and transcytosis by intestinal epithelial cells. However, the time delay between Stx1B macropinocytosis and transcytosis indicates that additional cascades of cell signaling events were necessary to link these two processes.
TPA (100 nM) treatment for 4 h also increased Stx1B transcytosis approximately three times compared with the control (data not shown). Similarly to the Stx1B uptake, higher TPA concentrations did not further stimulate toxin transcytosis. Immunostaining of TPA-treated T84 cells grown either on glass or on filters did not exhibit the F-actin ruffles on the apical surface (data not shown), indicating that compared with the NEM-treated cells these events are very rare or absent.
Cdc42 is involved in Stx1B macropinocytosis and transcytosis.
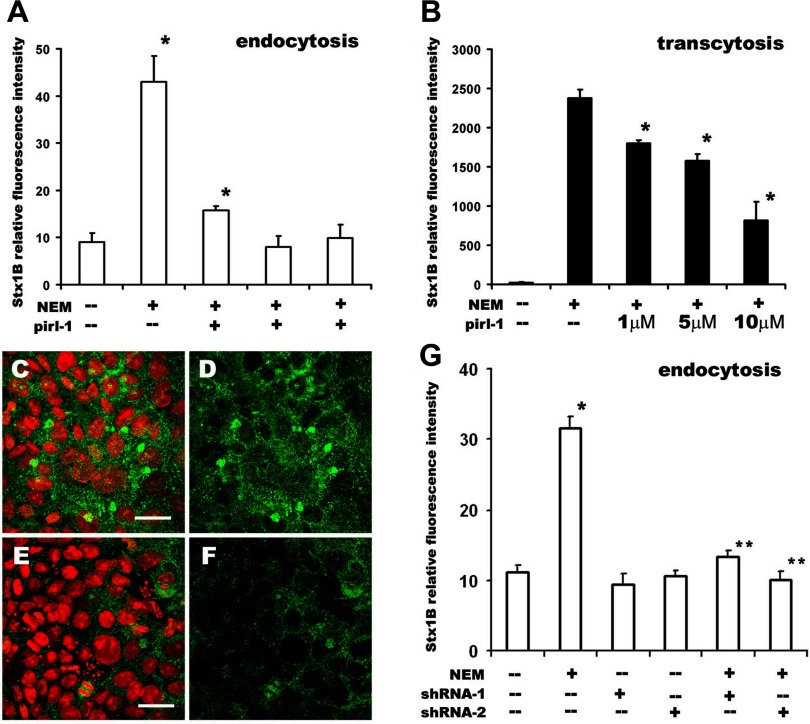
Having shown that the Stx uptake occurs by clathrin-independent endocytosis, we further characterized the process making use of the demonstration that such endocytic processes in other systems involve the small GTPases Cdc42, RhoA, or Arf6. We tested the involvement of Cdc42 on the basis of the fact that it was reported to be necessary for macropinocytosis in dendritic cells (54). To test the possible role of Cdc42 in the NEM-stimulated Stx1B uptake and transcytosis, we treated cells with pirl-1, a specific blocker of nucleotide exchange on Cdc42 (57, 58). Pirl-1 significantly inhibited both Stx1B endocytosis and transcytosis in a dose-dependent manner (Fig. 5, A–B).
Fig. 5.
Cdc42 inhibition significantly decreases Stx1B endocytosis and transcytosis in NEM-stimulated cells. A: pirl-1 inhibits NEM-stimulated Stx1B uptake at 4 h in a concentration-dependent manner. Relative fluorescence intensity from endocytosed Stx1B in the absence (control cells) or presence of 200 μM NEM, or cells exposed to the combination of 200 μM NEM and increasing pirl-1 concentrations: 5, 10, or 20 μM. Each bar represents data from at least 4 independent measurements. *P < 0.05 compared with basal uptake in control cells. B: pirl-1 inhibits NEM-stimulated Stx1B transcytosis at 4 h in a concentration-dependent manner. Relative fluorescence intensity from transcytosed Stx1B in the absence (control cells) or presence of 200 μM NEM or cells exposed to the combination of 200 μM NEM and increasing pirl-1 concentrations: 1, 5, or 10 μM. Each bar represents data from at least 3 independent measurements. *P < 0.05 compared with NEM-treated cells. Although pirl-1 downregulated basal Stx1B endocytosis and transcytosis, the effects were not significant (data not shown). C–F: Cdc42 is downregulated in T84 monolayers by lentiviral short hairpin RNA (shRNA) transduction. Representative images of T84 cells immunostained against Cdc42 from (C and D) control monolayer or (E and F) monolayer transduced with shRNA1 lentivirus. Cdc42 (green) was detected by primary monoclonal antibody and secondary goat anti-mouse antibody conjugated to Alexa 488 fluorescent dye. Nuclei (red) was labeled by propidium iodide. The Cdc42 fluorescence intensity (green) is lower in cells affected by shRNA than in control cells. Bars in C and E, 20 μm. G: decrease in Cdc42 expression by either of 2 shRNAs significantly inhibits NEM-stimulated Stx1B endocytosis. Relative fluorescence intensity from endocytosed Stx1B for 4 h in control cells or cells with decreased amounts of Cdc42 due to shRNA1 or shRNA2, in the absence or presence of 200 μM NEM. Cdc42 downregulation did not affect the Stx1B basal uptake. However, this downregulation significantly inhibited NEM-stimulated endocytosis of Stx1B. *P < 0.05 compared with control. **P < 0.05 compared with NEM treatment. Each bar represents data from at least 3 independent measurements.
To further implicate Cdc42 in Stx1B macropinocytosis, we transduced T84 cells with lentiviruses containing shRNA constructs against Cdc42 to decrease Cdc42 expression (Fig. 5, C–F). Quantification of relative fluorescence intensity from 12-bit images obtained from control, shRNACdc42-1, and shRNACdc42-2-transduced cells after immunostaining for Cdc42 showed significant decrease in Cdc42 expression in shRNA-containing cells. Thus the shRNACdc42-1 and shRNACdc42-2 constructs decreased the relative amount of Cdc42 to 48 ± 7 and 38 ± 10%, respectively, of control cells. The Cdc42 depletion did not compromise the integrity of T84 monolayers, and TER in case of both shRNA constructs was similar to this in control cells infected with GFP or noninfected. Thus in 10- to 14-day-old control cells the TER was 2,645 ± 287 Ω·cm2 (n = 86 monolayers) whereas in Cdc42-depleted cells it was 2,391 ± 310 Ω·cm2 (n = 24 monolayers). Additionally, the transcytosis of lucifer yellow at 4 h in Cdc42-depleted cells was not different from this in control monolayers (data not shown). We concluded that Cdc42 downregulation did not increase the TJ permeability. However, the decrease in Cdc42 expression resulted in inhibition of NEM-stimulated Stx1B uptake (Fig. 5G), suggesting that NEM-stimulated macropinocytosis of Stx1B is Cdc42 dependent.
NEM-induced apical macropinocytosis is not mediated by NSF.
The target of NEM resulting in stimulation of macropinocytosis is unknown. NEM has been widely used as an inhibitor of NEM-sensitive factor (NSF), because it alkylates cysteine residues on NSF molecules (44). However, NEM is an artificial compound, and alkylation of cysteines is not physiological. Instead, nitric oxide (NO), a physiological messenger, modifies cysteines by S-nitrosylation and inhibits NSF activity (48). NSF is a key component of the exocytic machinery, and its inhibition decreases granule trafficking from Golgi to the plasma membrane (80). However, the role of NSF in endocytosis is not well established. We speculated that if NSF inhibition is important for macropinocytosis stimulation, NO could duplicate the effect of NEM and stimulate Stx1B uptake and transcytosis. To test this hypothesis we preincubated the T84 monolayers with the NO donors, SNAP (the half-life is ∼6 h) for 1 h and DETA NONOate (the half-life is ∼20 h) for 12 h in concentrations up to 500 μM and then treated with Stx1B for an additional 4 h. Neither Stx1B endocytosis nor transcytosis was changed in the presence of NO donors (data not shown). We conclude that NEM-induced macropinocytosis does not depend on NSF inhibition.
NEM induces changes in F-actin and causes Src activation.
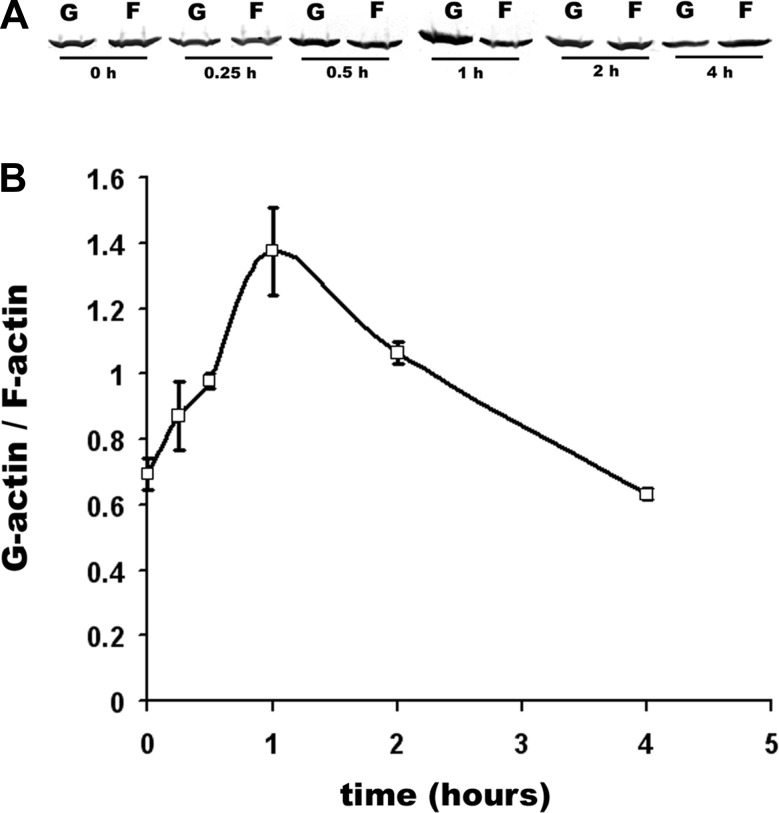
It has been shown that NEM significantly decreased the amount of cytoskeletal F-actin in several cell types (28, 35). We speculated that NEM-induced actin polymerization-depolymerization might be necessary for T84 cell ruffle formation. As a way to assess an actin rearrangement, we tested whether changes in the ratio between polymerized F and monomeric G-actin occur in NEM-treated T84 cells. NEM significantly increased F-actin depolymerization with the maximal effect 1 h after application (Fig. 6, A–B). However, the effect was transient and at 4 h the G-to-F ratio did not differ from that in control cells not exposed to NEM. These data suggest that NEM-induced transient changes in actin polymerization might be important for the initiation of ruffling.
Fig. 6.
NEM transiently changes the ratio between F- and G-actin. A: representative Western blot of the amount of G- and F-actin expressed in control cells or cells treated with 200 μM NEM over time. B: quantification of NEM-induced G-actin/F-actin changes over time from 3 Western blots. *P < 0.05 compared with time 0 (control).
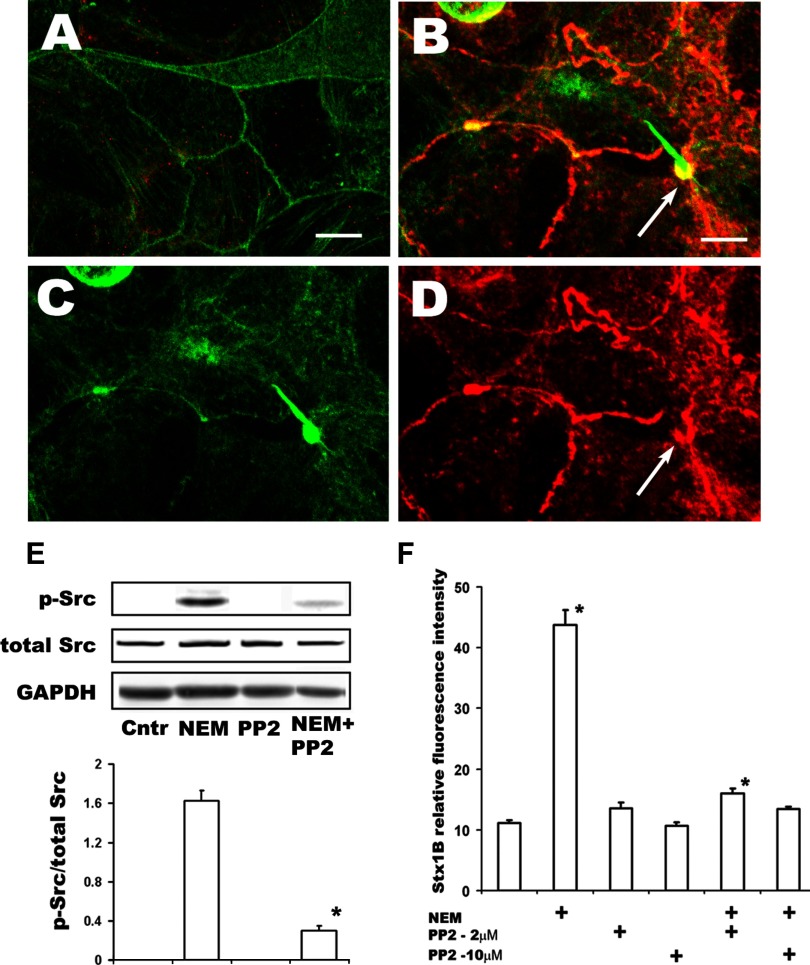
How NEM depolymerizes F-actin is not known. However, it has been shown that in polarized epithelial cells Src activation is sufficient to selectively trigger actin ruffling and macropinocytosis at the apical membrane (50), and this requires fast actin turnover/treadmilling. We hypothesized that NEM might induce Src activation, given that activated Src is known to cause actin ruffle formation through dynamic changes in actin polymerization. Src contains a SH1 kinase domain, the autophosphorylation site (p-Src-Tyr416) of which is necessary for full activation (33, 82). Inactive Src is localized at perinuclear sites, but activated Src is ultimately translocated to the cell periphery, where it attaches to the plasma membrane inner surface by myristoylation. We used Western blot with an antibody that recognized p-Src-Y416 in T84 monolayers treated with NEM and immunofluorescence microscopy to detect the localization of active p-Src. The amount of p-Src was significantly increased in NEM-treated cells compared with controls. This could be demonstrated by immunofluorescence (Fig. 7, A–D) or by Western blot (Fig. 7E). Moreover, virtually all p-Src was localized at the apical surface and was concentrated in the base of F-actin ruffles (Fig. 7, B–D), indicating association with rearranged actin. Incubation of cells with NEM in the presence of the specific Src family inhibitor PP2 significantly decreased both the NEM-induced Src phosphorylation (Fig. 7E) and the amount of endocytosed Stx1B (Fig. 7F). We conclude that NEM-induced macropinocytosis probably involves Src activation and this active Src may play an important role in Stx1B macropinocytosis.
Fig. 7.
NEM activates Src in T84 cells. A: representative single 3D xy projection from 0.5-μm confocal optical sections of control cells not treated with NEM. Small amount of activated p-Src (red) is present all through the cells. F-actin green (by phalloidin) in all windows. B–D: representative single 3D xy projection from 0.5-μm confocal optical sections of apical region of cells incubated 1 h with 100 μM of NEM. Bars in A and B, 10 μm. Active p-Src (red), which appears in the apical region of cells, follows the pattern of perijunctional F-actin ring (green), displaces the F-actin in it, and concentrates in the base of F-actin ruffles (arrows in B and D). E: immunoblot shows increase in active Src (p-Src Y416) in T84 cells treated with 200 μM NEM for 4 h, whereas in control cells the amount of p-Src was below detection level. Treatment with 10 μM PP2 significantly inhibited Src activation by NEM (*P < 0.05 compared with NEM-stimulated conditions, n = 4 independent experiments done in duplicate). GAPDH loading control. F: the Src family inhibitor PP2 significantly decreased the NEM-stimulated Stx endocytosis but did not affect the basal uptake of toxin by T84 cells. Thus simultaneous pretreatment of cells with 200 μM NEM and 10 μM PP2 for 30 min completely prevents NEM-stimulated Stx1B uptake measured 4 h later. *P < 0.05 compared with control. Each bar represents at least 4 independent experiments done in duplicate.
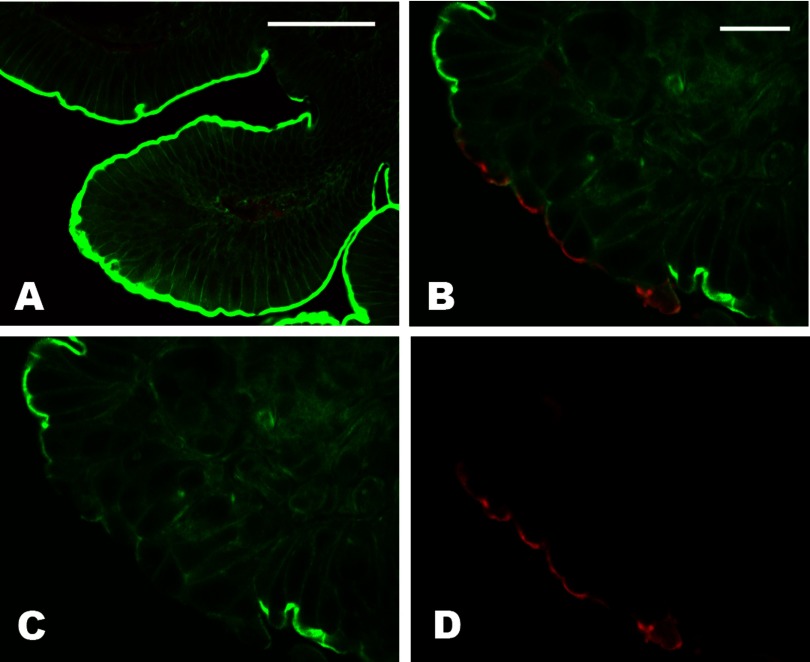
To test whether activation of Src might be relevant to the Stx-induced intestinal pathophysiology in vivo, we used a well-established rabbit model of EHEC-caused colitis (71). Five days after infection, two of the three experimental rabbits challenged with Stx1-producing RDEC-H19A strain developed diarrhea with mild edema of the cecum. In contrast, the two rabbits infected with RDEC-1 bacteria or rabbits exposed to PBS only (controls) did not have diarrhea. Additionally, all three RDEC-H19A-infected animals lost more weight than the control rabbits infected with RDEC-1 bacteria. We first analyzed the cecal tissue samples from experimental and control rabbits for the expression of Gb3 receptors and found no Gb3 in cecal epithelial cells (Supplemental Fig. S5). We conclude that rabbit cecal model matches the human intestinal tissue in terms of absence of Gb3 in epithelial cells. Next we determined the presence of activated Src (p-Src) in epithelial cells by immunofluorescence confocal microscopy. The p-Src was not detectable in epithelial cells from rabbits treated with PBS only (Fig. 8A). However, p-Src appears in patches of epithelial cells from rabbits infected with either Stx1-producing RDEC-H19A or with RDEC-1 bacteria (Fig. 8, B–D). The p-Src was localized to the apical surface and was accompanied by a decrease in apical F-actin compared with the neighboring cells without p-Src immunostaining. The appearance of cells with p-Src did not correlate with sites of bacterial attachment. Also, Stx1 production by RDEC-H19A bacteria was not necessary for Src activation in rabbit cecal epithelial cells. We conclude that Src is activated in intestinal epithelial cells by infection with enteropathogenic bacteria in a manner independent of Stx1 presence. This active Src is involved in actin rearrangement and may potentially stimulate the macropinocytosis in intestinal epithelial cells in vivo.
Fig. 8.
Enteropathogenic bacteria in vivo activate Src in rabbit cecal epithelial cells. A: representative single 3D xy projection from 0.5 μm confocal optical sections of cecal tissue from PBS-treated animals; bar, 50 μm. There is virtually no active p-Src (red) present in epithelial cells, whereas some red signal is detected in lamina propria. B–D: representative single 3D xy projection from 0.5-μm confocal optical sections of cecal tissue from RDEC-H19A-infected animal; bar in H, 20 μm. Active p-Src (red) appears at the apical surface of epithelial cells, follows the pattern of perijunctional F-actin ring (green), and displaces the F-actin compared with the neighboring cells without p-Src apical signal and with prominent F-actin apical staining. Similar images were obtained from cecal tissue from RDEC-1-infected rabbits, indicating that Stx1 itself is not involved in Src activation.
To test whether EHEC colonization can activate Src in vitro, we infected T84 cells apically with EHEC strain EDL933 modified to be Stx1 negative in concentrations ∼104 or ∼106 bacteria/ml for 16 h. The ratio between the p-Src and total Src was calculated in total cell lysates separated on SDS-PAGE and immunoblotted. Both bacterial concentrations significantly increased active Src amount. The p-Src/total-Src ratio in the presence of bacteria were 1.8 ± 0.4 and 2.1 ± 0.3, respectively, whereas in control cells the amount of p-Src was below detection level.
DISCUSSION
In the present studies we found EHEC at the apical surface of intestinal epithelial cells in a sample from the ileocecal valve region of a patient with severe EHEC illness but not in association with the colon of several additional similarly ill patients. These studies are the first to demonstrate association of EHEC with the apical surface of human intestine in samples from EHEC foodborne illness. These initial results in a small number of archival samples do not allow us the conclusions that bacterial association with epithelial cells is a common feature of EHEC-induced disease. However, future observations in larger numbers of samples studied soon after excision are necessary to better define the true prevalence of these findings in normal and pathologically involved intestinal regions and to define the intestinal segment in which attachment occurs to determine whether there is specificity in the area of attachment.
Importantly, we found Stx1 and Stx2 inside human intestinal epithelial cells in all analyzed samples regardless of the presence of bacterial attachment. Toxins were present in large amounts inside virtually each ileal and colonic epithelial cell in both surface and crypt regions. The possible artifact related to the bacterial disattachment does not allow conclusions about the relationship between EHEC adherence to particular cells and Stx1 and Stx2 uptake by these cells. However, the presence of both toxins in crypt epithelial cells makes it unlikely that bacterial attachment is necessary for toxin uptake.
Systemic spread and systemic complications of EHEC depend on lamina propria toxin that has been transcytosed in small intestine, colon, or both. Recent studies in an IVOC model have indicated that EHEC demonstrate a tropism for follicular human epithelium of distal ileal Peyer's patches without colonization of either small or large intestine (12, 67). Additionally, Paneth cells of duodenum were shown to specifically express the Stx receptor Gb3 and to have the ability to bind toxin in vitro. These authors suggested that systemic spread of toxins may occur specifically through the regions of Paneth cells and Peyer's patches and that severe damage of colonic epithelial cells due to EHEC infection may be secondary to the endothelial damage. However, the presence of Stx1 and Stx2 inside virtually all intestinal epithelial cells in all distal intestinal samples from patients with EHEC infection and the presence of both toxins in ileal and colonic lamina propria strongly suggests that the Stx released from intestinal luminal bacteria crosses the epithelial cell layers in vivo at many sites. Also, all expelled cells in colonic lumen were filled with both toxins, indicating the possibility of direct damage of these cells by toxins, although the latter could also have been due to endothelial damage.
In contrast to our tissue observations, previous studies using a T84 cell model exposed to Stx1 in concentrations up to 10 μg/ml for up to 24 h did not show any significant cell damage (4, 69), despite the presence of the furin-cleaved (activated) toxin inside the cells (66). Moreover, the amount of Stx that was transcytosed from apical into basolateral medium after 24 h of incubation was relatively small (66). A possible explanation proposed for T84 cell resistance to toxin and the low efficiency of transcytosis was that only a very small percent of T84 cells were able to endocytose either Stx1 or Stx2 (66). Our findings allow us to speculate that in the course of EHEC infection the Stx endocytosis and transcytosis might be facilitated by some bacterial factors. Indeed, when T84 cells were infected for 6 h with Stx2- or Stx1-producing EHEC strains, virtually all Stxs produced into the culture medium were internalized by T84 cells. Under these conditions, Stx2 was detected inside virtually each cell in the T84 monolayer (24).
Our studies began characterizing the mechanisms of toxin uptake and transcytosis by intestinal epithelial cells. Stx1 uptake by T84 cells is not clathrin dependent and is rather similar to HRP uptake. The fact that inhibiting clathrin- dependent endocytosis increased Stx uptake would not have been predicted but appears to be a characteristic of the uptake mechanism. This phenomenon was observed in the past and it was suggested that cells may compensate for loss of one endocytic pathway by upregulating others (63). Previous observations of this phenomenon include the following: 1) Clathrin-independent pinocytosis was induced in HeLa cells overexpressing a defective dynamin that prevented clathrin-coated vesicle formation (13). 2) In polarized MDCK cells ricin uptake (receptor-mediated process) and fluid-phase uptake at the apical side both used clathrin-independent but RhoA-dependent endocytic mechanism (22). 3) Inhibition of clathrin-dependent endocytosis increased Cdc42-dependent uptake, which operates independently of dynamin and has been reported to be involved in uptake of glycosylphosphatidylinositol-anchored proteins as well as being responsible for a major fraction of fluid-phase endocytosis (37). 4) Inactivation of clathrin heavy chain increased bulk membrane uptake by synaptic vesicles further supports that non-receptor-mediated uptake of Stx1 increases upon inhibition of the clathrin-dependent pathway (39). Whereas the explanation for this augmentation of non-clathrin-dependent endocytosis when the clathrin dependent process is inhibited is unknown, we speculate that this might occur due to the overlap in molecules involved in both the clathrin-dependent and -independent endocytic pathways (e.g., actin). Inhibition of clathrin function possibly allows the rest of the endocytic machinery originally belonging to the clathrin-dependent pathway to be available and operate in clathrin-independent processes that share some of the endocytic machinery (63).
On the basis of these data, together with our previous observations that actin turnover significantly facilitated Stx1 uptake and transcytosis in T84 cells (46), we tested the hypothesis that macropinocytosis, a form of actin-driven stimulated bulk uptake of fluid and solid cargo (20, 36, 73), might represent the mechanism of the efficient toxin uptake. Thus NEM-stimulated Stx1B uptake by T84 cells fulfils the major characteristics of macropinocytosis: apical actin ruffle formation and appearance of cargo-containing macropinosomes with size larger than 1 μm. NEM has been previously shown to trigger the macropinocytosis of ricin, a plant toxin with enzymatic activity identical to Stx, by epithelial cells in vitro (62). As in case of ricin, NEM stimulated Stx1B macropinocytosis in T84 cells through the formation of F-actin ruffles, which increased over time. The time course of stimulated Stx1B uptake correlated with the increase in the number of ruffles, and these NEM-induced ruffles were filled with Stx1B. Concurrently, the amount of Stx1B-bearing subapical vesicles also significantly increased. There are several other publications that have documented concentration of fluid-phase cargo in actin ruffles during macropinocytosis. Thus, in MDCK cells expressing constitutively active v-Src, which caused macropinocytosis, apical actin patches and circular ruffles were filled with dextran (50). Additionally, active c-Src was colocalized with dextran in macropinocytic ruffles in Cos-1 cells (16). We believe that additional studies have to be done, particularly using polarized epithelial cells, to determine how macropinocytic actin ruffles are able to concentrate fluid-phase cargo.
Subtypes of macropinocytosis most likely exist. Thus TPA has been shown to stimulate macropinocytosis in many nonpolarized cell types including fibroblasts and neutrophils via activation of protein kinase C (30, 73). In contrast, the role of TPA in macropinocytosis in polarized cells is controversial. Despite TPA stimulation of apical uptake of HRP and ricin in polarized MDCK and Caco-2 cells (30) in a clathrin-independent manner, it was also reported that phorbol ester (PMA) significantly stimulated clathrin-dependent uptake of ricin in Caco-2 cells by increasing the formation and pinching off of clathrin-coated pits at the apical surface (70). Additionally, both PMA and TPA failed to induce apical F-actin ruffling and formation of macropinosomes in polarized epithelial cells either with ricin uptake (30) or with PMA-stimulated transcytosis of a polymeric immunoglobulin receptor (9). These published results are in good agreement with our data that TPA stimulates Stx1B or fluid-phase markers uptake in T84 cells without ruffle formation. The relatively small TPA effects on Stx1B and HRP uptake and the failure to produce apical actin ruffles, a characteristic feature of macropinocytosis, indicate that, in contrast to nonpolarized cells, the TPA-induced endocytosis in polarized cells does not match the full definition of macropinocytosis.
Importantly, Stx1B macropinocytosis led to a significant increase in toxin transcytosis. This process was mostly transcellular for Stx1B, as well as for HRP, because it was substantially (∼95%) blocked by basolateral application of tannic acid. The selectivity of tannic acid (apical vs. basolateral) has been previously tested in T84 cells (56). The application of tannic acid from the basolateral side has been shown to inhibit the apical to basolateral transcellular transcytosis of adeno-associated virus (15). Tannic acid had a much less effect on decreasing of NEM-induced transcytosis of lucifer yellow, indicating that NEM increased permeability of the paracellular pathway for small molecules. However, even for this small tracer the paracellular component comprised only ∼20% of apical to basolateral movement, indicating that NEM-induced cellular trafficking effects are much greater than paracellular effects. Moreover, the selectivity of NEM-modified changes in TJ permeability for higher molecular weight tracers, such as 10-kDa dextran, sharply increases and only allowed ∼10% paracellular transport. Our data that NEM-induced transcytosis of toxin and other high molecular weight fluid-phase markers is mostly cellular, rather than paracellular process, are in good agreement with previously published studies (4, 69), including those in which Stx1 transcytosis was measured in the presence of several adherent EHEC strains. The EHEC infection caused a significant drop in TER of the T84 monolayer, as NEM did. However, toxin was detected exclusively inside the epithelial cells, but not in TJ regions, by immunogold electron microscopy (69). However, artificially induced macropinocytosis (by NEM in our experiments) might not reflect its impact on paracellular transport. Thus additional studies remain to be done to estimate the possible contribution of macropinocytosis to the TJ permeability.
Our data also demonstrated that the specific inhibition of Cdc42, the key player in regulation of macropinocytosis (14, 19, 60), by pharmacological and molecular approaches significantly inhibited the NEM-stimulated Stx1B uptake by T84 cells. Moreover, inhibition of Cdc42 also significantly decreased Stx1B transcytosis, demonstrating a direct link between macropinocytosis and transcytosis.
The direct apical target for NEM in macropinocytosis has not been identified. Our data linked NEM-induced macropinocytosis to Src activation. A number of studies in different cell types have shown that activation of Src promoted macropinocytosis and its kinase activity led to constitutive formation of macropinosomes in the absence of external growth factors (36, 38). Active Src has been shown sufficient to trigger macropinocytosis in polarized MDCK cells (50). Although the mechanism by which transcytosis allows exit from the basolateral membrane in epithelial cells is largely unknown, as a partial contributor, Src family protein tyrosine kinases have been implicated in upstream signaling pathways leading to an increase in intracellular transendothelial permeability to proteins (31).
Importantly, our data indicate that Src-induced macropinocytosis of Stx might take place in EHEC-induced intestinal disease. Thus our rabbit model of Stx1 and enteropathogenic bacterial cecitis showed a significant increase in active p-Src at the apical surface of cecal epithelial cells independent of attachment of RDEC-H19A or RDEC-1. We also showed here that EHEC infection of T84 cells significantly increased the p-Src pool. This suggests that Src activation may occur independently of the locus for enterocyte effacement pathogenicity island and the presence of Stx. It suggests that both types of Stx-producing bacteria, the O157:H7, which express intimin, and non-O157:H7, which do not express a functional type III secretion system, may equally utilize the Src activation pathway for effective transport of Stx inside the enterocyte and into the bloodstream. These data highlight a need to better understand the difference in EHEC serotypes and how they affect enterocytes.
Macropinocytosis is well recognized in intestinal microbiology as a significant factor in host-pathogen interaction. Thus Salmonella has been shown to induce macropinocytosis in the host cells and use its phosphatase SigD to promote ruffling (7, 76). Both Shigella and enteropathogenic E. coli have been shown to mimic the activity of many RAS-like GTPases, including Cdc42 (6). Formation of macropinocytic pockets triggered by Shigella effectors requires Src activity, which amplifies bacteria-induced actin remodeling and ruffle formation (7, 53).
In summary, these results are the first demonstration of the presence of O157:H7 bacteria at the apical surface of human intestinal epithelial cells as part of EHEC foodborne illness. Both Stx1 and Stx2 were found in large amounts inside surface and crypt epithelial cells in human intestine during EHEC infection. In vitro studies show that macropinocytosis might be responsible for toxin uptake by receptor-free intestinal epithelial cells. The toxin macropinocytosis leads to significant increase in its transcytosis, a necessary step in EHEC-related Stx systemic dissemination and multiple organ targeting. Moreover, enteropathogenic bacteria-dependent activation of Src in intestinal epithelial cells may have a significant role in the process of Stx1 uptake.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants RO1DK58928 and R24DK064388 (The Hopkins Basic Research Digestive Disease Development Core Center).
Supplementary Material
Acknowledgments
We acknowledge the Ross Confocal Facility and the confocal microscopy assistance of John Gibas, Mouse Physiology Core, and the tissue cutting assistance of Jennifer Sipes.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Acheson DW, Calderwood SB, Boyko SA, Lincicome LL, Kane AV, Donohue-Rolfe A, Keusch GT. Comparison of Shiga-like toxin I B-subunit expression and localization in Escherichia coli and Vibrio cholerae by using trc or iron-regulated promoter systems. Infect Immun 61: 1098–1104, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acheson DW, Jacewicz M, Kane AV, Donohue-Rolfe A, Keusch GT. One step high yield affinity purification of Shiga-like toxin II variants and quantitation using enzyme linked immunosorbent assays. Microb Pathog 14: 57–66, 1993. [DOI] [PubMed] [Google Scholar]
- 3.Acheson DW, Kane AV, Keusch GT. Shiga toxins. Methods Mol Biol 145: 41–63, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Acheson DW, Moore R, De Breucker S, Lincicome L, Jacewicz M, Skutelsky E, Keusch GT. Translocation of Shiga toxin across polarized intestinal cells in tissue culture. Infect Immun 64: 3294–3300, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Altankov G, Grinnell F. Depletion of intracellular potassium disrupts coated pits and reversibly inhibits cell polarization during fibroblast spreading. J Cell Biol 120: 1449–1459, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alto NM, Shao F, Lazar CS, Brost RL, Chua G, Mattoo S, McMahon SA, Ghosh P, Hughes TR, Boone C, Dixon JE. Identification of a bacterial type III effector family with G protein mimicry functions. Cell 124: 15–17, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Amyere M, Mettlen M, Van Der Smissen P, Platek A, Payrastre B, Veithen A, Courtoy PJ. Origin, originality, functions, subversions and molecular signalling of macropinocytosis. Int J Med Microbiol 291: 487–494, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Benmerah A, Lamaze C. Clathrin-coated pits: vive la différence? Traffic 8: 970–982, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Cardone MH, Smith BL, Song W, Mochly-Rosen D, Mostov KE. Phorbol myristate acetate-mediated stimulation of transcytosis and apical recycling in MDCK cells. J Cell Biol 124: 717–727, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campellone KG, Roe AJ, Løbner-Olesen A, Murphy KC, Magoun L, Brady MJ, Donohue-Rolfe A, Tzipori S, Gally DL, Leong JM, Marinus MG. Increased adherence and actin pedestal formation by dam-deficient enterohaemorrhagic Escherichia coli O157:H7. Mol Microbiol 63: 1468–1481, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Chinnapen DJ, Chinnapen H, Saslowsky D, Lencer WI. Rafting with cholera toxin: endocytosis and trafficking from plasma membrane to ER. FEMS Microbiol Lett 266: 129–137, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chong Y, Fitzhenry R, Heuschkel R, Torrente F, Frankel G, Phillips AD. Human intestinal tissue tropism in Escherichia coli O157:H7 initial colonization of terminal ileum and Peyer's patches and minimal colonic adhesion ex vivo. Microbiology 153: 794–802, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Damke H, Baba T, van der Bliek AM, Schmid SL. Clathrin-independent pinocytosis is induced in cells overexpressing a temperature-sensitive mutant of dynamin. J Cell Biol 131: 69–80, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dharmawardhane S, Schürmann A, Sells MA, Chernoff J, Schmid SL, Bokoch GM. Regulation of macropinocytosis by p21-activated kinase-1. Mol Biol Cell 11: 3341–3352, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Pasquale G, Chiorini JA. AAV transcytosis through barrier epithelia and endothelium. Mol Ther 13: 506–516, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Donepudi M, Resh MD. c-Src trafficking and co-localization with the EGF receptor promotes EGF ligand-independent EGF receptor activation and signaling. Cell Signal 20: 1359–67, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donohue-Rolfe A, Jacewicz M, Keusch GT. Isolation and characterization of functional Shiga toxin subunits and renatured holotoxin. Mol Microbiol 3: 1231–1236, 1989. [DOI] [PubMed] [Google Scholar]
- 18.Ergonul Z, Clayton F, Fogo AB, Kohan DE. Shigatoxin-1 binding and receptor expression in human kidneys do not change with age. Pediatr Nephrol 18: 246–253, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Fabbri A, Falzano L, Travaglione S, Stringaro A, Malorni W, Fais S, Fiorentini C. Rho-activating Escherichia coli cytotoxic necrotizing factor 1: macropinocytosis of apoptotic bodies in human epithelial cells. Int J Med Microbiol 291: 551–554, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Falcone S, Cocucci E, Podini P, Kirchhausen T, Clementi E, Meldolesi J. Macropinocytosis: regulated coordination of endocytic and exocytic membrane traffic events. J Cell Sci 119: 4758–4769, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Feugaing DD, Tammi R, Echtermeyer FG, Stenmark H, Kresse H, Smollich M, Schönherr E, Kiesel L, Götte M. Endocytosis of the dermatan sulfate proteoglycan decorin utilizes multiple pathways and is modulated by epidermal growth factor receptor signaling. Biochimie 89: 637–657, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Garred O, Rodal SK, van Deurs B, Sandvig K. Reconstitution of clathrin-independent endocytosis at the apical domain of permeabilized MDCK II cells: requirement for a Rho-family GTPase. Traffic 2: 26–36, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Gasmi M, Glynn J, Jin MJ, Jolly DJ, Yee JK, Chen ST. Requirements for efficient production and transduction of human immunodeficiency virus type 1-based vectors. J Virol 73: 1828–1834, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gobert AP, Vareille M, Glasser AL, Hindré T, de Sablet T, Martin C. Shiga toxin produced by enterohemorrhagic Escherichia coli inhibits PI3K/NF-kappaB signaling pathway in globotriaosylceramide-3-negative human intestinal epithelial cells. J Immunol 178: 8168–8174, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Griffin PM, Olmstead LC, Petras RE. Escherichia coli O157:H7-associated colitis. A clinical and histological study of 11 cases. Gastroenterology 99: 142–149, 1990. [DOI] [PubMed] [Google Scholar]
- 26.Hansen GH, Dalskov SM, Rasmussen CR, Immerdal L, Niels-Christiansen LL, Danielsen EM. Cholera toxin entry into pig enterocytes occurs via a lipid raft- and clathrin-dependent mechanism. Biochemistry 44: 873–882, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Hansen SH, Sandvig K, van Deurs B. The preendosomal compartment comprises distinct coated and noncoated endocytic vesicle populations. J Cell Biol 113: 731–741, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haviv L, Gillo D, Backouche F, Bernheim-Groswasser A. A cytoskeletal demolition worker: myosin II acts as an actin depolymerization agent. J Mol Biol 375: 325–330, 2008. [DOI] [PubMed] [Google Scholar]
- 29.Holgersson J, Jovell PA, Breimer ME. Glycosphingolipids of the human large intestine: detailed structural characterization with special reference to blood group compounds and bacterial receptor structures. J Biochem (Tokyo) 110: 120–131, 1991. [DOI] [PubMed] [Google Scholar]
- 30.Holm PK, Eker P, Sandvig K, van Deurs B. Phorbol myristate acetate selectively stimulates apical endocytosis via protein kinase C in polarized MDCK cells. Exp Cell Res 217: 157–168, 1995. [DOI] [PubMed] [Google Scholar]
- 31.Hu G, Place AT, Minshall RD. Regulation of endothelial permeability by Src kinase signaling: vascular leakage versus transcellular transport of drugs and macromolecules. Chem Biol Interact 171: 177–189, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hurley BP, Jacewicz M, Thorpe CM, Lincicome LL, King AJ, Keusch GT, Acheson DW. Shiga toxins 1 and 2 translocate differently across polarized intestinal epithelial cells. Infect Immun 67: 6670–6677, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ingley E Src family kinases: regulation of their activities, levels and identification of new pathways. Biochim Biophys Acta 1784: 56–65, 2008. [DOI] [PubMed] [Google Scholar]
- 34.Ivanov AI, Hunt D, Utech M, Nusrat A, Parkos CA. Differential roles for actin polymerization and a myosin II motor in assembly of the epithelial apical junctional complex. Mol Biol Cell 16: 2636–2650, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iwamoto Y, Tamura M, Nakatsuka K, Yamanouchi U. Influence of sulfhydryl agents on cytoskeleton in cultured human trabecular cells. Jpn J Ophthalmol 33: 318–326, 1989. [PubMed] [Google Scholar]
- 36.Jones AT Macropinocytosis: searching for an endocytic identity and role in the uptake of cell penetrating peptides. J Cell Mol Med 11: 670–684, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kalia M, Kumari S, Chadda R, Hill MM, Parton RG, Mayor S. Arf6-independent GPI-anchored protein-enriched early endosomal compartments fuse with sorting endosomes via a Rab5/phosphatidylinositol-3′-kinase-dependent machinery. Mol Biol Cell 17: 3689–3704, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kasahara K, Nakayama Y, Sato I, Ikeda K, Hoshino M, Endo T, Yamaguchi N. Role of Src-family kinases in formation and trafficking of macropinosomes. J Cell Physiol 211: 220–232, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Kasprowicz J, Kuenen S, Miskiewicz K, Habets RLP, Smitz L, Verstreken P. Inactivation of clathrin heavy chain inhibits synaptic recycling but allows bulk membrane uptake. J Cell Biol 182: 1007–1016, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kovbasnjuk O, Edidin M, Donowitz M. Role of lipid rafts in Shiga toxin 1 interaction with the apical surface of Caco-2 cells. J Cell Sci 114: 4025–4031, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Kovbasnjuk O, Mourtazina R, Baibakov B, Wang T, Elowsky C, Choti MA, Kane A, Donowitz M. The glycosphingolipid globotriaosylceramide in the metastatic transformation of colon cancer. Proc Natl Acad Sci USA 102: 19087–19092, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lajoie P, Nabi IR. Regulation of raft-dependent endocytosis. J Cell Mol Med 11: 644–653, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lei JT, Martinez-Moczygemba M. Separate endocytic pathways regulate IL-5 receptor internalization and signaling. J Leukoc Biol 84: 499–509, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lowenstein CJ Nitric oxide regulation of protein trafficking in the cardiovascular system. Cardiovasc Res 75: 240–246, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu L, Khan S, Lencer W, Walker WA. Endocytosis of cholera toxin by human enterocytes is developmentally regulated. Am J Physiol Gastrointest Liver Physiol 289: G332–G341, 2005. [DOI] [PubMed] [Google Scholar]
- 46.Malyukova I, Gutsal O, Laiko M, Kane A, Donowitz M, Kovbasnjuk O. Latrunculin B facilitates Shiga toxin 1 transcellular transcytosis across T84 intestinal epithelial cells. Biochim Biophys Acta 1782: 370–377, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maruyama F, Kenzaka T, Yamaguchi N, Tani K, Nasu M. Detection of bacteria carrying the stx2 gene by in situ loop-mediated isothermal amplification. Appl Environ Microbiol 69: 5023–5028, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matsushita K, Morrell CN, Cambien B, Yang SX, Yamakuchi M, Bao C, Hara MR, Quick RA, Cao W, O'Rourke B, Lowenstein JM, Pevsner J, Wagner DD, Lowenstein CJ. Nitric oxide regulates exocytosis by S-nitrosylation of N-ethylmaleimide-sensitive factor. Cell 115: 139–150, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mayor S, Pagano RE. Pathways of clathrin-independent endocytosis. Nat Rev Mol Cell Biol 8: 603–612, 2007. [DOI] [PubMed] [Google Scholar]
- 50.Mettlen M, Platek A, Van Der Smissen P, Carpentier S, Amyere M, Lanzetti L, de Diesbach P, Tyteca D, Courtoy PJ. Src triggers circular ruffling and macropinocytosis at the apical surface of polarized MDCK cells. Traffic 7: 589–603, 2006. [DOI] [PubMed] [Google Scholar]
- 51.Miyamoto Y, Iimura M, Kaper JB, Torres AG, Kagnoff MF. Role of Shiga toxin versus H7 flagellin in enterohaemorrhagic Escherichia coli signalling of human colon epithelium in vivo. Cell Microbiol 8: 69–79, 2006. [DOI] [PubMed] [Google Scholar]
- 52.Murray KF, Patterson K. Escherichia coli 0157:H7-induced hemolytic-uremic syndrome: histopathologic changes in the colon over time. Pediatr Dev Pathol 3: 232–239, 2000. [DOI] [PubMed] [Google Scholar]
- 53.Nhieu GT, Enninga J, Sansonetti P, Grompone G. Tyrosine kinase signaling and type III effectors orchestrating Shigella invasion. Curr Opin Microbiol 8: 16–20, 2005. [DOI] [PubMed] [Google Scholar]
- 54.Nobes C, Marsh M. Dendritic cells: new roles for Cdc42 and Rac in antigen uptake? Curr Biol 10: R739–R741, 2000. [DOI] [PubMed] [Google Scholar]
- 55.Orth RN, Clark TG, Craighead HG. Avidin-biotin micropatterning methods for biosensor applications. Biomed Microdevices 5: 29–34, 2003. [Google Scholar]
- 56.Paladino S, Pocard T, Catino MA, Zurzolo C. GPI-anchored proteins are directly targeted to the apical surface in fully polarized MDCK cells. J Cell Biol 172: 1023–1034, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pelish HE, Ciesla W, Tanaka N, Reddy K, Shair MD, Kirchhausen T, Lencer WI. The Cdc42 inhibitor secramine B prevents cAMP-induced K+ conductance in intestinal epithelial cells. Biochem Pharmacol 71: 1720–1726, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peterson JR, Lebensohn AM, Pelish HE, Kirschner MW. Biochemical suppression of small-molecule inhibitors: a strategy to identify inhibitor targets and signaling pathway components. Chem Biol 13: 443–452, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Polishchuk R, Di Pentima A, Lippincott-Schwartz J. Delivery of raft-associated, GPI-anchored proteins to the apical surface of polarized MDCK cells by a transcytotic pathway. Nat Cell Biol 6: 297–307, 2004. [DOI] [PubMed] [Google Scholar]
- 60.Sabharanjak S, Sharma P, Parton RG, Mayor S. GPI-anchored proteins are delivered to recycling endosomes via a distinct cdc42-regulated, clathrin-independent pinocytic pathway. Dev Cell 2: 411–423, 2002. [DOI] [PubMed] [Google Scholar]
- 61.Sakoda T, Kasahara N, Hamamori Y, Kedes L. A high-titer lentiviral production system mediates efficient transduction of differentiated cells including beating cardiac myocytes. J Mol Cell Cardiol 31: 2037–2047, 1999. [DOI] [PubMed] [Google Scholar]
- 62.Sandvig K, Llorente A, Rodal SK, Eker P, Garred O, Stahlhut M, van Deurs B. Apical macropinocytosis in polarized MDCK cells: regulation by N-ethylmaleimide-sensitive proteins. Eur J Cell Biol 79: 447–457, 2000. [DOI] [PubMed] [Google Scholar]
- 63.Sandvig K, Torgersen ML, Raa HA, van Deurs B. Clathrin-independent endocytosis: from nonexisting to an extreme degree of complexity. Histochem Cell Biol 129: 267–276, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sandvig K, van Deurs B. Endocytosis, intracellular transport, and cytotoxic action of Shiga toxin and ricin. Physiol Rev 76: 949–966, 1996. [DOI] [PubMed] [Google Scholar]
- 65.Schneider B, Schueller C, Utermoehlen O, Haas A. Lipid microdomain-dependent macropinocytosis determines compartmentation of Afipia felis. Traffic 8: 226–240, 2007. [DOI] [PubMed] [Google Scholar]
- 66.Schüller S, Frankel G, Phillips AD. Interaction of Shiga toxin from Escherichia coli with human intestinal epithelial cell lines and explants: Stx2 induces epithelial damage in organ culture. Cell Microbiol 6: 289–301, 2004. [DOI] [PubMed] [Google Scholar]
- 67.Schüller S, Heuschkel R, Torrente F, Kaper JB, Phillips AD. Shiga toxin binding in normal and inflamed human intestinal mucosa. Microbes Infect 9: 35–39, 2007. [DOI] [PubMed] [Google Scholar]
- 68.Serna A, Boedeker EC. Pathogenesis and treatment of Shiga toxin-producing Escherichia coli infections. Curr Opin Gastroenterol 24: 38–47, 2008. [DOI] [PubMed] [Google Scholar]
- 69.Sherman A, Philpott DJ, Ackerley CA, Kiliaan AJ, Karmali MA, Perdue MH, Sherman PM. Translocation of verotoxin-1 across T84 monolayers: mechanism of bacterial toxin penetration of epithelium. Am J Physiol Gastrointest Liver Physiol 273: G1349–G1358, 1997. [DOI] [PubMed] [Google Scholar]
- 70.Shurety W, Bright NA, Luzio JP. The effects of cytochalasin D and phorbol myristate acetate on the apical endocytosis of ricin in polarised Caco-2 cells. J Cell Sci 109: 2927–2935, 1996. [DOI] [PubMed] [Google Scholar]
- 71.Sjogren R, Neill R, Rachmilewitz D, Fritz D, Newland J, Sharpnack D, Colleton C, Fondacaro J, Gemski P, Boedeker E. Role of Shiga-like toxin I in bacterial enteritis: comparison between isogenic Escherichia coli strains induced in rabbits. Gastroenterology 106: 306–317, 1994. [DOI] [PubMed] [Google Scholar]
- 72.Swanson JA Shaping cups into phagosomes and macropinosomes. Nat Rev Mol Cell Biol 9: 639–649, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Swanson JA, Watts C. Macropinocytosis. Trends Cell Biol 5: 424–428, 1995. [DOI] [PubMed] [Google Scholar]
- 74.Swidsinski A, Weber J, Loening-Baucke V, Hale LP, Lochs H. Spatial organization and composition of the mucosal flora in patients with inflammatory bowel disease. J Clin Microbiol 43: 3380–3389, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tarr PI, Gordon CA, Chandler WL. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet 365: 1073–1086, 2005. [DOI] [PubMed] [Google Scholar]
- 76.Terebiznik MR, Vieira OV, Marcus SL, Slade A, Yip CM, Trimble WS, Meyer T, Finlay BB, Grinstein S. Elimination of host cell PtdIns(4,5)P(2) by bacterial SigD promotes membrane fission during invasion by Salmonella. Nat Cell Biol 4: 766–773, 2002. [DOI] [PubMed] [Google Scholar]
- 77.Ungewickell EJ, Hinrichsen L. Endocytosis: clathrin-mediated membrane budding. Curr Opin Cell Biol 19: 417–425, 2007. [DOI] [PubMed] [Google Scholar]
- 78.Utech M, Ivanov AI, Samarin SN, Bruewer M, Turner JR, Mrsny RJ, Parkos CA, Nusrat A. Mechanism of IFN-γ-induced endocytosis of tight junction proteins: myosin II-dependent vacuolarization of the apical plasma membrane. Mol Biol Cell 16: 5040–5052, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wong CS, Jelacic S, Habeeb RL, Watkins SL, Tarr PI. The risk of the hemolytic-uremic syndrome after antibiotic treatment of Escherichia coli O157:H7 infections. N Engl J Med 342: 1930–1936, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yamakuchi M, Ferlito M, Morrell CN, Matsushita K, Fletcher CA, Cao W, Lowenstein CJ. Exocytosis of endothelial cells is regulated by N-ethylmaleimide-sensitive factor. Methods Mol Biol 440: 203–215, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang L, Wang L, Zheng Y. Gene targeting of Cdc42 and Cdc42GAP affirms the critical involvement of Cdc42 in filopodia induction, directed migration, and proliferation in primary mouse embryonic fibroblasts. Mol Biol Cell 17: 4675–4685, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yeatman TJ A renaissance for SRC. Nat Rev Cancer 4: 470–480, 2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.