Abstract
Most mitotically competent mammalian cell types can react to stress by undergoing a phenotypically distinctive and permanent form of growth arrest called “cellular senescence.” This response has been extensively characterized in cell culture and more recently it has been found to occur also in vivo in a number of tissues. In this review I will present the case for the occurrence of senescence in the vascular endothelium. I will also discuss the mechanisms and factors that modulate endothelial cell replicative capacity and the onset of senescence. Finally, I will examine the senescent phenotype and its possible consequences for the development and progression of vascular diseases.
Keywords: endothelium, oxidative stress, telomere
diseases of the vascular system have long been considered to be age related in terms of their onset and progression (45). Ageing is associated with endothelial dysfunction (9, 87, 94), arterial stiffening and remodeling (49), impaired angiogenesis (72), defective vascular repair (94), and with an increasing prevalence of atherosclerosis (18, 81, 93). The reasons for these associations are still unclear, but it is plausible that organismal ageing and vascular disease may share common cellular mechanisms. One process that has been increasingly linked to both ageing and the development of vascular pathologies is cellular senescence (19, 27, 56).
Senescence is a stress and damage response phenomenon that locks up mitotically competent diploid cells in a permanent form growth arrest. Senescent cells undergo distinct changes in gene expression that may cause an impairment of cellular function. In endothelial cells these changes result in a phenotype that is pro-inflammatory, pro-atherosclerotic, and prothrombotic. Endothelial cell senescence can be induced by a number of factors implicated in vascular pathologies, particularly by sustained cell replication and oxidative stress. In this review I will examine the occurrence, mechanisms, and pathophysiological implications of this process as they emerge from cell culture and in vivo studies.
MECHANISMS OF SENESCENCE—AN OVERVIEW
Senescence was initially considered to reflect the finite capacity for division that normal diploid cells exhibit when propagated in culture (reviewed in Ref. 35), hence the term “replicative senescence.” At the molecular level senescence resulting from successive rounds of cell division has been linked to the progressive shortening and eventual dysfunction of telomeres, the physical ends of chromosomes (reviewed in Refs. 28, 77). In mammalian cells telomeres consist of a repeated DNA sequence (TTAGGG) that extends over a length of several thousand base pairs and associates to an array of specialized telomere binding proteins. Synthesis of telomeric DNA requires the presence of telomerase, a ribonucleoprotein complex that catalyses the addition of TTAGGG repeats to the 3′-end of the DNA chain, using as a template a complementary sequence within the RNA portion of the complex (50). The majority of human adult somatic cells either lack or have very low levels of telomerase (25). Under these conditions, and due to the inability of conventional DNA polymerases to replicate the end of the lagging strand, DNA synthesis during cell division results in a gradual loss of telomeric DNA (68). In addition, due to its high GGG content, telomeric DNA is particularly susceptible to oxidative damage and the generation of single strand breaks. Accordingly, the rate of telomere erosion is also greatly affected by the oxidative burden of the cell (91). Telomere erosion eventually compromises its functional integrity and leads to the induction of a DNA damage checkpoint response that halts the cell-cycle permanently (15).
Senescence can also be triggered by telomere-independent events including non-telomeric DNA damage and persistent mitogenic stimulation (76). In cell culture these events induce an acute form of senescence, sometimes termed “stress-induced premature senescence” (82), which does not require extensive cell proliferation but which otherwise resembles that induced by damaged telomeres. Ultimately, the majority of senescence-inducing signals engage either or both the p53/p21 and p16/retinoblastoma protein tumor suppressor pathways, as the final effectors of the senescence program (reviewed in Ref. 8). The involvement of these mechanisms highlights the notion that senescence, although harmful in later life, might have evolved as barrier to cell transformation, promoting survival of the young to reach the stage of reproductive maturity (7).
EVIDENCE THAT ENDOTHELIAL CELL SENESCENCE OCCURS IN VIVO
While the occurrence of senescence in cell culture has been extensively documented, it is only recently that the importance of this phenomenon in vivo has begun to be appreciated. Senescent cells have been found in a number of mammalian tissues in association with ageing, age-related pathologies, hyperplastic lesions, and cellular stress (reviewed in Refs. 8, 19). In the case of the endothelium, firm evidence of its occurrence was obtained by several laboratories using senescence-associated β-galactosidase (SA-β-gal) as a histochemical marker. Using this method, senescent endothelial cells were found to accumulate after repeated balloon endothelial denudation of the rabbit carotid artery, an injury model that provokes endothelial and smooth muscle cell proliferation (24). Similarly, other laboratories demonstrated the presence of senescent endothelial cells overlying atherosclerotic plaques of human aorta and coronary arteries (57, 90) and in the aortae of diabetic rats (12). In addition, the occurrence of stress-induced endothelial cell senescence has recently been demonstrated in a murine model of oxidative stress (70).
The occurrence of endothelial cell senescence in vivo has also been inferred from examination of telomere length in the vasculature. A number of independent studies have shown that telomeres in the endothelium shorten with age and that this erosion is more pronounced in atherosclerosis-prone areas (2, 10, 67). Furthermore, a study examining the relationship between telomere length and coronary artery disease found that telomeres in endothelial cells derived from diseased portions of arteries were shorter than those from non-diseased regions (66).
FACTORS AFFECTING ENDOTHELIAL CELL SENESCENCE
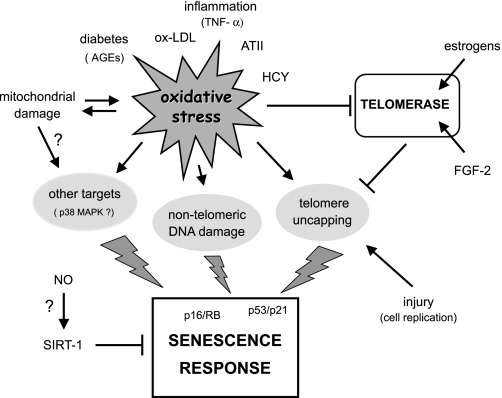
Studies on cultured endothelial cells have shown that the onset of senescence can be modulated by a plethora of factors affecting vascular function. These include mitogens (48), inflammatory molecules (5), angiotensin II (40), oxidants and antioxidants (26, 47), nitric oxide (89), high glucose (96), advanced glycation end-products (AGEs) (12), and mitochondria (74). Most of these factors influence senescence via two main processes: by altering the intracellular levels of cellular oxidative stress and/or by modulating telomerase activity (Fig. 1).
Fig. 1.
Factors that induce endothelial cell senescence. ATII, angiotensin II; HCY, homocysteine; ox-LDL, oxidized LDL.
Oxidative Stress
Oxidative stress is a major stimulus for the induction of senescence (62). A substantial body of evidence indicates that in endothelial cells reactive oxygen species (ROS) generated from either intracellular or extracellular sources can induce or accelerate the development of senescence by acting at multiple subcellular levels (20). As indicated above, telomeres are particularly susceptible to oxidative damage. In addition, besides affecting telomeres directly, or indirectly via inhibition of telomerase (see below), ROS can induce senescence by telomere-independent mechanisms. The latter include direct damage to genomic DNA, mitochondrial damage, and activation of cytosolic stress response kinases or other redox-sensitive signaling proteins that have been implicated in senescence responses (Fig. 1).
Oxidative stress and telomere damage.
In endothelial cells, an association between oxidative stress, accelerated telomere shortening, and senescence has been suggested by studies in which the intracellular redox environment was manipulated by incubation with a vitamin C analog (26), by exposure to homocysteine (95), or by interference with the glutathione (GSH) redox cycle (47). In contrast, telomere dysfunction was not apparent when oxidative stress-induced senescence was caused by exposure to an AGE (12).
ROS as mediators of sustained mitogenic stimulation.
Excessive mitogenic stimulation caused by forced expression of activated oncogenes is known to induce senescence (76). In endothelial cells this phenomenon has been observed when active forms of Akt (58), Ras (80), or Rac1 (16) were overexpressed. Promotion of senescence under these conditions is thought to result from a dysregulation of the cellular redox-balance leading to an increase in ROS production which in turn may stimulate p53 activity (reviewed in Ref. 20). The pathophysiological significance of these findings may lie in the fact that pro-atherogenic conditions, such as hyperinsulinaemia, chronic inflammation, and hypercholesterolaemia, are known to activate Akt- and Ras-mediated signaling in endothelial cells.
The role of mitochondria.
ROS can damage mitochondrial DNA and other redox-sensitive components of this organelle, thus impairing mitochondrial function. Mitochondria by themselves generate ROS during normal respiration, and some studies suggest that when the normal function of the electron transport chain is affected ROS output may be augmented, thus increasing the oxidative burden of the cell (reviewed in Refs. 3, 52). Recently, the importance of mitochondria-derived ROS in the induction of endothelial cell senescence has been highlighted by a study examining the role of prohibitin-1 (PHB1) in this process (74). PHB1 is a constituent of the inner mitochondrial membrane thought to be important for the maintenance of mitochondrial functional integrity (65). It has now been demonstrated that knockdown of PHB1 in endothelial cells increases mitochondrial ROS generation, which causes cellular senescence (74). In this study the induction of senescence was considered to be the consequence of a sustained ROS-dependent Akt activation. However, other ROS-induced signaling mechanisms or direct oxidative damage cannot be discounted at this stage. Aside from the precise identity of the downstream effectors of mitochondrial ROS, the significance of this finding may have implications beyond understanding the function of PHB1 in senescence. In this regard, the complex interplay between levels of NO, oxygen availability, and the redox environment may have an important role (21).
Modulation of Telomerase Activity
In endothelial cells levels of telomerase activity are substantially lower than those found in a typical cancer cell line or in cells from other renewable tissues (48). Nevertheless, expression in these cells could be physiologically relevant since its overall activity is growth regulated (39, 48) and inhibition by genetic means is associated with a reduction in the replicative capacity of the cells (22).
Studies on the regulation of this enzyme in endothelial cells have implicated endothelial cell mitogens (17, 48), nitric oxide (34, 89), inflammatory mediators (5), and oxidative stress (30, 47). Regulation has been reported to occur at multiple levels, including transcription (17, 48), post-translational Akt-mediated phosphorylation of the telomerase catalytic subunit (TERT) (5, 17), redox-controlled changes in intracellular localization (30), and oxidative modification of TERT (4).
The role of endothelial cell mitogens.
A number of growth factors known to be important for endothelial homeostasis and angiogenesis have been investigated for their effects on telomerase activity and senescence (41, 48). Among these, FGF-2 was shown to enhance telomerase activity and to maintain cellular replicative life span, in association with an increase in TERT transcripts. In contrast, VEGF-A, at concentrations that had the same mitogenic effect as FGF-2 did, had no effect on TERT levels or activity and was not able to support long-term endothelial cell replication (48, 83). Another study, however, investigating the role of telomerase in angiogenesis, concluded that VEGF-A can activate telomerase via NO signaling (97). Other studies have shown that estrogens also increase telomerase activity in endothelial cells via transcriptional and post-translational mechanisms, the latter involving engagement of the PI3K-AKT pathway and subsequent phosphorylation of TERT (17, 41). Also in these cases the effects were accompanied by retardation of the onset of senescence.
The role of NO.
It is generally assumed that NO counteracts endothelial cell senescence by stimulating telomerase activity and reducing telomere erosion (34, 89). However, experiments using a combination of pharmacological tools and silencing RNA technology suggest that this might not be the case (38). Indeed, manipulation of NO levels by either NO donors, NO synthase (NOS) inhibitors, or downregulation of endothelial NOS (eNOS) by RNA interference, had no effect on telomerase activity, cellular replicative capacity, or the accumulation of senescent cells (38). Similarly, eNOS inhibition or NO donors failed to affect telomerase activity or endothelial progenitor cell senescence in other studies (1, 5), in disagreement with initial claims from the same laboratory (89).
Recent findings examining the role of SIRT1 in the regulation of endothelial cell senescence (70) could shed some light on the above conflicting results. SIRT1 is a NAD+-dependent protein deacetylase involved in the regulation of energy metabolism, stress responses, and cell survival (31). In endothelial cells, overexpression of SIRT1 prevents oxidative stress-induced premature senescence (69), most probably by promoting the deacetylation and consequent inactivation of p53 (51). Importantly, NO donors or a phosphodiesterase (PDE) III inhibitor known to increase eNOS activity (32) have been recently shown to upregulate SIRT1 expression and to inhibit the onset of senescence in cells subjected to oxidative stress, with the effects of the PDEIII inhibitor on senescence being abrogated by SIRT-1 downregulation. Notably, non-stressed cells exhibited high constitutive levels of SIRT-1 that were not affected by the putative increase in NO production (70). While the above study also showed an increase in telomerase activity upon inhibition of senescence, this effect may be attributed to the maintenance of the cells in a proliferative state (48) rather than to a direct effect of SIRT-1 or NO on telomerase. Taken together these findings suggest that NO may counteract senescence in the context of cellular stress, including inadvertent cell culture stress, through upregulation of SIRT1. It should be mentioned, however, that in cells with higher levels of telomerase such as immortalized cell lines, fibroblasts expressing telomerase ectopically and hematopoietic stem cells, SIRT-1 acts a negative regulator of growth and this behavior is associated with a decrease in telomerase activity (64). The notion that activation of SIRT-1 may have opposing effects in different cells and organisms is not new (43), thus suggesting that its role in counteracting endothelial cell senescence may be specific to this cell type.
The role of oxidative stress in the regulation of telomerase activity.
Substances that induce oxidative stress and have pro-atherogenic properties such as TNF-α and oxidized LDL were reported to reduce telomerase activity in endothelial cells in association with the inhibition of the PI3K/AKT pathway (5). In addition, increased generation of ROS has been shown to promote the translocation of TERT from the nucleus to the cytoplasm (30), thus preventing the enzyme from accessing the telomere. Furthermore, evidence from other cell types (4, 33) suggests that oxidation of a TERT cysteine residue that is sensitive to the intracellular levels of GSH may account, at least in part, for the decrease in activity that occurs in endothelial cells upon inhibition GSH synthesis (47) or nitrosative stress (22). While it is not clear what is the relative contribution of each of these mechanisms to the inactivation of telomerase in vivo, it is likely that their importance may ultimately depend on the nature and duration of the stress.
THE ENDOTHELIAL SENESCENT PHENOTYPE AND ITS PATHOPHYSIOLOGICAL CONSEQUENCES
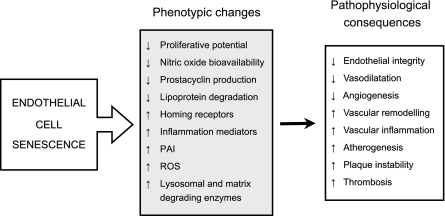
Apart from the alterations related to the block in cell replication, senescent endothelial cells show characteristic changes in gene expression, morphology, and function (20). Some of these changes may be important in affecting the regenerative and angiogenic capacity of the endothelium, its reactivity and the progression of atherosclerosis, and its clinical sequelae (Fig. 2).
Fig. 2.
The endothelial senescent phenotype and its pathophysiological consequences.
Changes Affecting Regeneration and Angiogenic Potential
As a direct consequence of the permanent growth arrest, endothelial cell senescence may impair the repair capacity of the endothelial lining (24). In this context the contribution of oxidative stress-induced senescence of circulating endothelial progenitor cells and in particular the involvement of angiotensin II in promoting this phenomenon, could also play an important role (36, 40, 42).
Endothelial senescence also results in changes in the expression levels of proteins associated with cellular architecture and cytoskeletal function (11, 44, 78, 90). These changes can affect the motility of the cells and these, in conjunction with the loss of replicative capacity may also reduce their angiogenic capacity. Indeed, a link between senescence and impaired angiogenesis has been established by several lines of evidence, including the demonstration that inhibition of telomerase reduces angiogenesis in tumor and therapeutic neovascularization models (23, 97), and that silencing of SIRT1 or PHB1 in endothelial cells, in addition to inducing senescence (69, 74), also abolishes their angiogenic properties (71, 74).
Pro-Atherogenic and Pro-Thrombotic Changes
Upon replicative senescence in culture, endothelial cells overexpress several proteins that typify the pro-inflammatory/pro-thrombotic phenotype of the endothelium in human atherosclerosis (61, 75, 88), including interleukin-1α (54), the intercellular adhesion molecule ICAM-1 (53), and plasminogen activator inhibitor-1 (PAI-1) (14). ICAM-1 has been shown to increase during senescence induced by dysfunctional telomeres (57), whereas overexpression of PAI-1 has been demonstrated to occur both in replicative and stress-induced senescence (69, 95, 96).
A common feature of senescent endothelial cells is the presence of senescence-associated β-galactosidase (SA-β-gal) (86). This activity is a manifestation of an increase in lysosomal mass (46) and probably reflects the accumulation of autophagic vacuoles in the senescent cell containing non-degradable intracellular macromolecules and organelles. The increase in lysosomal mass has an uncertain underlying cause and while its pathophysiological significance in the arterial wall ought to be investigated, the possibility that the release of the vacuolar content could contribute to the inflammatory or pro-thrombotic phenotype, or even to the remodeling of the arterial wall, should be considered.
Senescent endothelial cells also undergo changes in the expression of proteins associated with the remodeling of the extracellular matrix (11, 29, 44, 78) and degrade less atherogenic lipoproteins than their younger counterparts, suggesting that they have a reduced capacity to metabolize atherogenic lipids (90).
Changes Affecting Endothelium-Dependent Vasodilatation
Nitric oxide bioavailability is critical to normal endothelial function (60). Advanced age leads to impairment of endothelial NO production (84) and to increased inactivation of NO by superoxide (87), which contribute to age-related endothelial dysfunction (9, 98). A number of studies have investigated whether endothelial senescence may be involved in these phenomena. These studies have established that senescent endothelial cells have lower levels of eNOS activity and produce decreased levels of NO (37, 55, 73). This phenotypic change was also brought about after senescence was induced in aortic endothelial cells by introducing the dominant negative form of the telomere-binding protein TRF-2 (57). In contrast, endothelial cells bypassing senescence by ectopically expressing TERT displayed no decline in eNOS expression or NO production (55, 57). One of the most potent inducers of eNOS expression in endothelial cells is laminar shear stress. The effect of shear stress on eNOS expression was also markedly blunted in senescent endothelial cells, but again could be rescued by TERT overexpression. The same was true of the NO-mediated inhibition of monocyte adhesion to the endothelial monolayer (55). Levels of another important vasodilator, prostacyclin, were also found to be reduced in endothelial cultures undergoing senescence in vitro (63).
In contrast to endothelial cells that became senescent after successive rounds of replication, endothelial cells undergoing premature senescence due to AGE-induced oxidative stress actually increased eNOS expression, despite the fact that their ability to produce NO was reduced (12). Similarly, in the aortic wall of ageing rats in vivo, while NO production was impaired, eNOS was upregulated about sevenfold (87). This reduction in NO bioavailability was attributed to an increase in mitochondrial-derived ROS and concomitant production of peroxynitrite (87). Evidence of peroxynitrite formation and nitrosative stress, which could be prevented by anti-oxidant treatment, was also observed in association with senescence induced by AGEs in vitro (12) and by diabetes in vivo (6). While on the one hand these findings would be consistent with the occurrence of oxidative stress-induced senescence in the vasculature, it is important to point out that senescent endothelial cells produce higher levels of ROS than their younger counterparts (85). Hence, oxidative stress is not only a stimulus for senescence but also an outcome of this process, which can also impinge on NO bioavailability.
Senescence and Apoptosis
The final fate of senescent cells may also have pathophysiological consequences because, given their altered phenotype, effective removal could be more beneficial for the maintenance of endothelial homeostasis. At present it is not clear whether in vivo senescent endothelial cells are removed by an active physiological mechanism, whether they accumulate indefinitely or simply succumb to the forces of flow, eventually shedding from the endothelial surface.
Studies examining the relationship between senescence and apoptosis in endothelial cells have yielded conflicting results. One study indicated that, unlike fibroblasts, the final fate of senescent endothelial cells is to undergo apoptosis (92). Other studies suggest that endothelial cell senescence by itself does not result in apoptosis, but rather it increases the sensitivity of these cells to apoptotic stimuli such as TNF-α and oxidized LDL (37, 80). In one case this effect has been attributed to the reduced levels of NO present in senescent cells (37) and this is consistent with the notion that physiological concentrations of NO promote cell survival (59).
Studies in HUVEC examining the effects of p53, p21, and p16 expression on the induction of apoptosis or senescence indicate that the p53/p21 pathway is involved in the induction of both phenomena, whereas the p16 pathway is only involved in the induction of senescence (13). In addition, these studies showed that the primary role of the p53/p21 pathway is in the control of apoptosis. Thus these findings suggest that in endothelial cells whether apoptosis occurs in association with senescence may also depend in part on the tendency of different types of stresses to engage one or both intracellular effector pathways.
SUMMARY AND FUTURE DIRECTIONS
The occurrence of endothelial cell senescence in the vasculature is gaining increasing recognition. Cell culture studies indicate that both cell turnover and oxidative stress may contribute to this phenomenon by inducing telomere shortening. Although the evidence for the occurrence of this process in the vessel wall in vivo is compelling, the existence of a causal relationship between telomere dysfunction and endothelial senescence awaits direct demonstration. In particular, while cell culture studies clearly show that endothelial cells express telomerase, that this activity is growth regulated and sensitive to the redox environment, and that telomerase promotes angiogenesis in models of disease, the relevance of these laboratory findings to human adult vascular homeostasis remains to be elucidated. In addition, oxidative stress may induce senescence by telomere-independent mechanisms and these may be more relevant in the context of vascular pathophysiology. Animal studies have confirmed that oxidative stress indeed induces senescence in vivo and that this may be particularly relevant in the context of type II diabetes (6). However, extending these findings to humans is currently hampered by the unavailability of non-invasive techniques to assess endothelial cell senescence in vivo. Development of new markers of endothelial cell senescence amenable to clinical investigation will help to ascertain whether pharmacological interventions, such as statins and peroxynitrite scavengers that have demonstrable ability to reduce senescence in laboratory models of disease (6, 79), can also be effective in the human setting.
GRANTS
The studies from the author's laboratory have been supported by the British Heart Foundation.
Acknowledgments
The author thanks Rachel Lovering for help in the preparation of this manuscript.
REFERENCES
- 1.Assmus B, Urbich C, Aicher A, Hofmann WK, Haendeler J, Rossig L, Spyridopoulos I, Zeiher AM, Dimmeler S. HMG-CoA reductase inhibitors reduce senescence and increase proliferation of endothelial progenitor cells via regulation of cell cycle regulatory genes. Circ Res 92: 1049–1055, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Aviv H, Khan MY, Skurnick J, Okuda K, Kimura M, Gardner J, Priolo L, Aviv A. Age dependent aneuploidy and telomere length of the human vascular endothelium. Atherosclerosis 159: 281–287, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, aging. Cell 120: 483–495, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Borras C, Esteve JM, Vina JR, Sastre J, Vina J, Pallardo FV. Glutathione regulates telomerase activity in 3T3 fibroblasts. J Biol Chem 279: 34332–34335, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Breitschopf K, Zeiher AM, Dimmeler S. Pro-atherogenic factors induce telomerase inactivation in endothelial cells through an Akt-dependent mechanism. FEBS Lett 493: 21–25, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Brodsky SV, Gealekman O, Chen J, Zhang F, Togashi N, Crabtree M, Gross SS, Nasjletti A, Goligorsky MS. Prevention and reversal of premature endothelial cell senescence and vasculopathy in obesity-induced diabetes by ebselen. Circ Res 94: 377–384, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Campisi J Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell 120: 513–522, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Campisi J, d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol 8: 729–740, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol 24: 471–476, 1994. [DOI] [PubMed] [Google Scholar]
- 10.Chang E, Harley CB. Telomere length and replicative aging in human vascular tissues. Proc Natl Acad Sci USA 92: 11190–11194, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang MWF, Grillari J, Mayrhofer C, Fortschegger K, Allmaier G, Marzban G, Katinger H, Voglauer R. Comparison of early passage, senescent and hTERT immortalized endothelial cells. Exp Cell Res 309: 121–136, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Chen J, Brodsky SV, Goligorsky DM, Hampel DJ, Li H, Gross SS, Goligorsky MS. Glycated collagen I induces premature senescence-like phenotypic changes in endothelial cells. Circ Res 90: 1290–1298, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Chen J, Huang X, Halicka D, Brodsky S, Avram A, Eskander J, Bloomgarden NA, Darzynkiewicz Z, Goligorsky MS. Contribution of p16INK4a and p21CIP1 pathways to induction of premature senescence of human endothelial cells: permissive role of p53. Am J Physiol Heart Circ Physiol 290: H1575–H1586, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Comi P, Chiaramonte R, Maier JA. Senescence-dependent regulation of type 1 plasminogen activator inhibitor in human vascular endothelial cells. Exp Cell Res 219: 304–308, 1995. [DOI] [PubMed] [Google Scholar]
- 15.d'Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, von Zglinicki T, Saretzki G, Carter NP, Jackson SP. A DNA damage checkpoint response in telomere-initiated senescence. Nature 426: 194–198, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Deshpande SS, Qi B, Park YC, Irani K. Constitutive activation of rac1 results in mitochondrial oxidative stress and induces premature endothelial cell senescence. Arterioscler Thromb Vasc Biol 23: e1–e6, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Doshida M, Ohmichi M, Tsutsumi S, Kawagoe J, Takahashi T, Du B, Mori-Abe A, Ohta T, Saitoh-Sekiguchi M, Takahashi K, Kurachi H. Raloxifene increases proliferation and up-regulates telomerase activity in human umbilical vein endothelial cells. J Biol Chem 281: 24270–24278, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Eggen DA, Solberg LA. Variation of atherosclerosis with age. Lab Invest 18: 571–579, 1968. [PubMed] [Google Scholar]
- 19.Erusalimsky JD, Kurz DJ. Cellular senescence in vivo: its relevance in ageing and cardiovascular disease. Exp Gerontol 40: 634–642, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Erusalimsky JD, Kurz DJ. Endothelial cell senescence. Handb Exp Pharmacol 176: 213–248, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Erusalimsky JD, Moncada S. Nitric oxide and mitochondrial signaling: from physiology to pathophysiology. Arterioscler Thromb Vasc Biol 27: 2524–2531, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Erusalimsky JD, Skene C. Mechanisms of endothelial senescence. Exp Physiol; DOI: 10.1113/expphysiol.2008.043133. In press. [DOI] [PubMed]
- 23.Falchetti ML, Mongiardi MP, Fiorenzo P, Petrucci G, Pierconti F, D'Agnano I, D'Alessandris G, Alessandri G, Gelati M, Ricci-Vitiani L, Maira G, Larocca LM, Levi A, Pallini R. Inhibition of telomerase in the endothelial cells disrupts tumor angiogenesis in glioblastoma xenografts. Int J Cancer 122: 1236–1242, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Fenton M, Barker S, Kurz DJ, Erusalimsky JD. Cellular senescence after single and repeated balloon catheter denudations of rabbit carotid arteries. Arterioscler Thromb Vasc Biol 21: 220–226, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Forsyth NR, Wright WE, Shay JW. Telomerase and differentiation in multicellular organisms: turn it off, turn it on, and turn it off again. Differentiation 69: 188–197, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Furumoto K, Inoue E, Nagao N, Hiyama E, Miwa N. Age-dependent telomere shortening is slowed down by enrichment of intracellular vitamin C via suppression of oxidative stress. Life Sci 63: 935–948, 1998. [DOI] [PubMed] [Google Scholar]
- 27.Fuster JJ, Andres V. Telomere biology and cardiovascular disease. Circ Res 99: 1167–1180, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Greider CW Telomeres and senescence: the history, the experiment, the future. Curr Biol 8: R178–R181, 1998. [DOI] [PubMed] [Google Scholar]
- 29.Grillari J, Hohenwarter O, Grabherr RM, Katinger H. Subtractive hybridization of mRNA from early passage and senescent endothelial cells. Exp Gerontol 35: 187–197, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Haendeler J, Hoffmann J, Brandes RP, Zeiher AM, Dimmeler S. Hydrogen peroxide triggers nuclear export of telomerase reverse transcriptase via Src kinase family-dependent phosphorylation of tyrosine 707. Mol Cell Biol 23: 4598–4610, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haigis MC, Guarente LP. Mammalian sirtuins–emerging roles in physiology, aging, and calorie restriction. Genes Dev 20: 2913–2921, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Hashimoto A, Miyakoda G, Hirose Y, Mori T. Activation of endothelial nitric oxide synthase by cilostazol via a cAMP/protein kinase A- and phosphatidylinositol 3-kinase/Akt-dependent mechanism. Atherosclerosis 189: 350–357, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Hayakawa N, Nozawa K, Ogawa A, Kato N, Yoshida K, Akamatsu K, Tsuchiya M, Nagasaka A, Yoshida S. Isothiazolone derivatives selectively inhibit telomerase from human and rat cancer cells in vitro. Biochemistry (Mosc) 38: 11501–11507, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Hayashi T, Matsui-Hirai H, Miyazaki-Akita A, Fukatsu A, Funami J, Ding QF, Kamalanathan S, Hattori Y, Ignarro LJ, Iguchi A. Endothelial cellular senescence is inhibited by nitric oxide: Implications in atherosclerosis associated with menopause and diabetes. Proc Natl Acad Sci USA 103: 17018–17023, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayflick L Living forever and dying in the attempt. Exp Gerontol 38: 1231–1241, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med 348: 593–600, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Hoffmann J, Haendeler J, Aicher A, Rossig L, Vasa M, Zeiher AM, Dimmeler S. Aging enhances the sensitivity of endothelial cells toward apoptotic stimuli: important role of nitric oxide. Circ Res 89: 709–715, 2001. [DOI] [PubMed] [Google Scholar]
- 38.Hong Y, Quintero M, Frakich NM, Trivier E, Erusalimsky JD. Evidence against the involvement of nitric oxide in the modulation of telomerase activity or replicative capacity of human endothelial cells. Exp Gerontol 42: 904–910, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Hsiao R, Sharma HW, Ramakrishnan S, Keith E, Narayanan R. Telomerase activity in normal human endothelial cells. Anticancer Res 17: 827–832, 1997. [PubMed] [Google Scholar]
- 40.Imanishi T, Hano T, Nishio I. Angiotensin II accelerates endothelial progenitor cell senescence through induction of oxidative stress. J Hypertens 23: 97–104, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Imanishi T, Hano T, Nishio I. Estrogen reduces endothelial progenitor cell senescence through augmentation of telomerase activity. J Hypertens 23: 1699–1706, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Imanishi T, Moriwaki C, Hano T, Nishio I. Endothelial progenitor cell senescence is accelerated in both experimental hypertensive rats and patients with essential hypertension. J Hypertens 23: 1831–1837, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Kaeberlein M The Ongoing saga of sirtuins and aging. Cell Metabolism 8: 4–5, 2008. [DOI] [PubMed] [Google Scholar]
- 44.Kamino H, Hiratsuka M, Toda T, Nishigaki R, Osaki M, Ito H, Inoue T, Oshimura M. Searching for genes involved in arteriosclerosis: proteomic analysis of cultured human umbilical vein endothelial cells undergoing replicative senescence. Cell Struct Funct 28: 495–503, 2003. [DOI] [PubMed] [Google Scholar]
- 45.Kohn RR Heart and cardiovascular system. In: Handbook of the Biology of Aging, edited by Finch CE and Hayflick L. New York: Van Nostrand Reinhold, 1977, p. 281–317.
- 46.Kurz DJ, Decary S, Hong Y, Erusalimsky JD. Senescence-associated (beta)-galactosidase reflects an increase in lysosomal mass during replicative ageing of human endothelial cells. J Cell Sci 113: 3613–3622, 2000. [DOI] [PubMed] [Google Scholar]
- 47.Kurz DJ, Decary S, Hong Y, Trivier E, Akhmedov A, Erusalimsky JD. Chronic oxidative stress compromises telomere integrity and accelerates the onset of senescence in human endothelial cells. J Cell Sci 117: 2417–2426, 2004. [DOI] [PubMed] [Google Scholar]
- 48.Kurz DJ, Hong Y, Trivier E, Huang HL, Decary S, Zang GH, Luscher TF, Erusalimsky JD. Fibroblast growth factor-2, but not vascular endothelial growth factor, upregulates telomerase activity in human endothelial cells. Arterioscler Thromb Vasc Biol 23: 748–754, 2003. [DOI] [PubMed] [Google Scholar]
- 49.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation 107: 139–146, 2003. [DOI] [PubMed] [Google Scholar]
- 50.Lingner J, Hughes TR, Shevchenko A, Mann M, Lundblad V, Cech TR. Reverse transcriptase motifs in the catalytic subunit of telomerase. Science 276: 561–567, 1997. [DOI] [PubMed] [Google Scholar]
- 51.Luo J, Nikolaev AY, Imai S, Chen D, Su F, Shiloh A, Guarente L, Gu W. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell 107: 137–148, 2001. [DOI] [PubMed] [Google Scholar]
- 52.Madamanchi NR, Runge MS. Mitochondrial dysfunction in atherosclerosis. Circ Res 100: 460–473, 2007. [DOI] [PubMed] [Google Scholar]
- 53.Maier JA, Statuto M, Ragnotti G. Senescence stimulates U937-endothelial cell interactions. Exp Cell Res 208: 270–274, 1993. [DOI] [PubMed] [Google Scholar]
- 54.Maier JA, Voulalas P, Roeder D, Maciag T. Extension of the life-span of human endothelial cells by an interleukin-1 alpha antisense oligomer. Science 249: 1570–1574, 1990. [DOI] [PubMed] [Google Scholar]
- 55.Matsushita H, Chang E, Glassford AJ, Cooke JP, Chiu CP, Tsao PS. eNOS activity is reduced in senescent human endothelial cells: Preservation by hTERT immortalization. Circ Res 89: 793–798, 2001. [DOI] [PubMed] [Google Scholar]
- 56.Minamino T, Komuro I. Role of telomere in endothelial dysfunction in atherosclerosis. Curr Opin Lipidol 13: 537–543, 2002. [DOI] [PubMed] [Google Scholar]
- 57.Minamino T, Miyauchi H, Yoshida T, Ishida Y, Yoshida H, Komuro I. Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation 105: 1541–1544, 2002. [DOI] [PubMed] [Google Scholar]
- 58.Miyauchi H, Minamino T, Tateno K, Kunieda T, Toko H, Komuro I. Akt negatively regulates the in vitro lifespan of human endothelial cells via a p53/p21-dependent pathway. EMBO J 23: 212–220, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moncada S, Erusalimsky JD. Does nitric oxide modulate mitochondrial energy generation and apoptosis? Nat Rev Mol Cell Biol 3: 214–220, 2002. [DOI] [PubMed] [Google Scholar]
- 60.Moncada S, Higgs EA. Nitric oxide and the vascular endothelium. Handb Exp Pharmacol 213–254, 2006. [DOI] [PubMed]
- 61.Moyer CF, Sajuthi D, Tulli H, Williams JK. Synthesis of IL-1 alpha and IL-1 beta by arterial cells in atherosclerosis. Am J Pathol 138: 951–960, 1991. [PMC free article] [PubMed] [Google Scholar]
- 62.Muller FL, Lustgarten MS, Jang Y, Richardson A, Van Remmen H. Trends in oxidative aging theories. Free Radic Biol Med 43: 477–503, 2007. [DOI] [PubMed] [Google Scholar]
- 63.Nakajima M, Hashimoto M, Wang F, Yamanaga K, Nakamura N, Uchida T, Yamanouchi K. Aging decreases the production of PGI2 in rat aortic endothelial cells. Exp Gerontol 32: 685–693, 1997. [DOI] [PubMed] [Google Scholar]
- 64.Narala SR, Allsopp RC, Wells TB, Zhang G, Prasad P, Coussens MJ, Rossi DJ, Weissman IL, Vaziri H. SIRT1 acts as a nutrient-sensitive growth suppressor and its loss is associated with increased AMPK and telomerase activity. Mol Biol Cell 19: 1210–1219, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nijtmans LG, de JL, Artal SM, Coates PJ, Berden JA, Back JW, Muijsers AO, van der SH, Grivell LA. Prohibitins act as a membrane-bound chaperone for the stabilization of mitochondrial proteins. EMBO J 19: 2444–2451, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ogami M, Ikura Y, Ohsawa M, Matsuo T, Kayo S, Yoshimi N, Hai E, Shirai N, Ehara S, Komatsu R, Naruko T, Ueda M. Telomere shortening in human coronary artery diseases. Arterioscler Thromb Vasc Biol 24: 546–550, 2004. [DOI] [PubMed] [Google Scholar]
- 67.Okuda K, Khan MY, Skurnick J, Kimura M, Aviv H, Aviv A. Telomere attrition of the human abdominal aorta: relationships with age and atherosclerosis. Atherosclerosis 152: 391–398, 2000. [DOI] [PubMed] [Google Scholar]
- 68.Olovnikov AM Telomeres, telomerase, and aging: origin of the theory. Exp Gerontol 31: 443–448, 1996. [DOI] [PubMed] [Google Scholar]
- 69.Ota H, Akishita M, Eto M, Iijima K, Kaneki M, Ouchi Y. Sirt1 modulates premature senescence-like phenotype in human endothelial cells. J Mol Cell Cardiol 43: 571–579, 2007. [DOI] [PubMed] [Google Scholar]
- 70.Ota H, Eto M, Kano MR, Ogawa S, Iijima K, Akishita M, Ouchi Y. Cilostazol inhibits oxidative stress-induced premature senescence via upregulation of Sirt1 in human endothelial cells. Arterioscler Thromb Vasc Biol 28: 1634–1639, 2008. [DOI] [PubMed] [Google Scholar]
- 71.Potente M, Ghaeni L, Baldessari D, Mostoslavsky R, Rossig L, Dequiedt F, Haendeler J, Mione M, Dejana E, Alt FW, Zeiher AM, Dimmeler S. SIRT1 controls endothelial angiogenic functions during vascular growth. Genes Dev 21: 2644–2658, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rivard A, Fabre JE, Silver M, Chen D, Murohara T, Kearney M, Magner M, Asahara T, Isner JM. Age-dependent impairment of angiogenesis. Circulation 99: 111–120, 1999. [DOI] [PubMed] [Google Scholar]
- 73.Sato I, Morita I, Kaji K, Ikeda M, Nagao M, Murota S. Reduction of nitric oxide producing activity associated with in vitro aging in cultured human umbilical vein endothelial cell. Biochem Biophys Res Commun 195: 1070–1076, 1993. [DOI] [PubMed] [Google Scholar]
- 74.Schleicher M, Shepherd BR, Suarez Y, Fernandez-Hernando C, Yu J, Pan Y, Acevedo LM, Shadel GS, Sessa WC. Prohibitin-1 maintains the angiogenic capacity of endothelial cells by regulating mitochondrial function and senescence. J Cell Biol 180: 101–112, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schneiderman J, Sawdey MS, Keeton MR, Bordin GM, Bernstein EF, Dilley RB, Loskutoff DJ. Increased type 1 plasminogen activator inhibitor gene expression in atherosclerotic human arteries. Proc Natl Acad Sci USA 89: 6998–7002, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Serrano M, Blasco MA. Putting the stress on senescence. Curr Opin Cell Biol 13: 748–753, 2001. [DOI] [PubMed] [Google Scholar]
- 77.Shay JW, Wright WE. Hayflick, his limit, and cellular ageing. Nat Rev Mol Cell Biol 1: 72–76, 2000. [DOI] [PubMed] [Google Scholar]
- 78.Shelton DN, Chang E, Whittier PS, Choi D, Funk WD. Microarray analysis of replicative senescence. Curr Biol 9: 939–945, 1999. [DOI] [PubMed] [Google Scholar]
- 79.Shimada T, Takeshita Y, Murohara T, Sasaki K, Egami K, Shintani S, Katsuda Y, Ikeda H, Nabeshima Y, Imaizumi T. Angiogenesis and vasculogenesis are impaired in the precocious-aging klotho mouse. Circulation 110: 1148–1155, 2004. [DOI] [PubMed] [Google Scholar]
- 80.Spyridopoulos I, Isner JM, Losordo DW. Oncogenic ras induces premature senescence in endothelial cells: role of p21(Cip1/Waf1). Basic Res Cardiol 97: 117–124, 2002. [DOI] [PubMed] [Google Scholar]
- 81.Strong JP, McGill HC Jr. The natural history of coronary atherosclerosis. Am J Pathol 40: 37–49, 1962. [PMC free article] [PubMed] [Google Scholar]
- 82.Toussaint O, Medrano EE, von Zglinicki T. Cellular and molecular mechanisms of stress-induced premature senescence (SIPS) of human diploid fibroblasts and melanocytes. Exp Gerontol 35: 927–945, 2000. [DOI] [PubMed] [Google Scholar]
- 83.Trivier E, Kurz DJ, Hong Y, Huang HL, Erusalimsky JD. Differential regulation of telomerase in endothelial cells by fibroblast growth factor-2 and vascular endothelial growth factor-A: association with replicative life span. Ann NY Acad Sci 1019: 111–115, 2004. [DOI] [PubMed] [Google Scholar]
- 84.Tschudi MR, Barton M, Bersinger NA, Moreau P, Cosentino F, Noll G, Malinski T, Luscher TF. Effect of age on kinetics of nitric oxide release in rat aorta and pulmonary artery. J Clin Invest 98: 899–905, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Unterluggauer H, Hampel B, Zwerschke W, Jansen-Durr P. Senescence-associated cell death of human endothelial cells: the role of oxidative stress. Exp Gerontol 38: 1149–1160, 2003. [DOI] [PubMed] [Google Scholar]
- 86.Van der Loo B, Fenton MJ, Erusalimsky JD. Cytochemical detection of a senescence-associated beta-galactosidase in endothelial and smooth muscle cells from human and rabbit blood vessels. Exp Cell Res 241: 309–315, 1998. [DOI] [PubMed] [Google Scholar]
- 87.Van der Loo B, Labugger R, Skepper JN, Bachschmid M, Kilo J, Powell JM, Palacios-Callender M, Erusalimsky JD, Quaschning T, Malinski T, Gygi D, Ullrich V, Luscher TF. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med 192: 1731–1744, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Van der Wal AC, Das PK, Tigges AJ, Becker AE. Adhesion molecules on the endothelium and mononuclear cells in human atherosclerotic lesions. Am J Pathol 141: 1427–1433, 1992. [PMC free article] [PubMed] [Google Scholar]
- 89.Vasa M, Breitschopf K, Zeiher AM, Dimmeler S. Nitric oxide activates telomerase and delays endothelial cell senescence. Circ Res 87: 540–542, 2000. [DOI] [PubMed] [Google Scholar]
- 90.Vasile E, Tomita Y, Brown LF, Kocher O, Dvorak HF. Differential expression of thymosin beta-10 by early passage and senescent vascular endothelium is modulated by VPF/VEGF: evidence for senescent endothelial cells in vivo at sites of atherosclerosis. FASEB J 15: 458–466, 2001. [DOI] [PubMed] [Google Scholar]
- 91.Von Zglinicki T Oxidative stress shortens telomeres. Trends Biochem Sci 27: 339–344, 2002. [DOI] [PubMed] [Google Scholar]
- 92.Wagner M, Hampel B, Bernhard D, Hala M, Zwerschke W, Jansen-Durr P. Replicative senescence of human endothelial cells in vitro involves G1 arrest, polyploidization and senescence-associated apoptosis. Exp Gerontol 36: 1327–1347, 2001. [DOI] [PubMed] [Google Scholar]
- 93.Weingand KW, Clarkson TB, Adams MR, Bostrom AD. Effects of age and/or puberty on coronary artery atherosclerosis in cynomolgus monkeys. Atherosclerosis 62: 137–144, 1986. [DOI] [PubMed] [Google Scholar]
- 94.Weinsaft JW, Edelberg JM. Aging-associated changes in vascular activity: a potential link to geriatric cardiovascular disease. Am J Geriatr Cardiol 10: 348–354, 2001. [DOI] [PubMed] [Google Scholar]
- 95.Xu D, Neville R, Finkel T. Homocysteine accelerates endothelial cell senescence. FEBS Lett 470: 20–24, 2000. [DOI] [PubMed] [Google Scholar]
- 96.Yokoi T, Fukuo K, Yasuda O, Hotta M, Miyazaki J, Takemura Y, Kawamoto H, Ichijo H, Ogihara T. Apoptosis signal-regulating kinase 1 mediates cellular senescence induced by high glucose in endothelial cells. Diabetes 55: 1660–1665, 2006. [DOI] [PubMed] [Google Scholar]
- 97.Zaccagnini G, Gaetano C, Della PL, Nanni S, Grasselli A, Mangoni A, Benvenuto R, Fabrizi M, Truffa S, Germani A, Moretti F, Pontecorvi A, Sacchi A, Bacchetti S, Capogrossi MC, Farsetti A. Telomerase mediates vascular endothelial growth factor-dependent responsiveness in a rat model of hind limb ischemia. J Biol Chem 280: 14790–14798, 2005. [DOI] [PubMed] [Google Scholar]
- 98.Zeiher AM, Drexler H, Saurbier B, Just H. Endothelium-mediated coronary blood flow modulation in humans. Effects of age, atherosclerosis, hypercholesterolemia, and hypertension. J Clin Invest 92: 652–662, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]