Abstract
The immune system is highly sensitive to stressors present during spaceflight. The major emphasis of this study was on the T lymphocytes in C57BL/6NTac mice after return from a 13-day space shuttle mission (STS-118). Spleens and thymuses from flight animals (FLT) and ground controls similarly housed in animal enclosure modules (AEM) were evaluated within 3–6 h after landing. Phytohemagglutinin-induced splenocyte DNA synthesis was significantly reduced in FLT mice when based on both counts per minute and stimulation indexes (P < 0.05). Flow cytometry showed that CD3+ T and CD19+ B cell counts were low in spleens from the FLT group, whereas the number of NK1.1+ natural killer (NK) cells was increased (P < 0.01 for all three populations vs. AEM). The numerical changes resulted in a low percentage of T cells and high percentage of NK cells in FLT animals (P < 0.05). After activation of spleen cells with anti-CD3 monoclonal antibody, interleukin-2 (IL-2) was decreased, but IL-10, interferon-γ, and macrophage inflammatory protein-1α were increased in FLT mice (P < 0.05). Analysis of cancer-related genes in the thymus showed that the expression of 30 of 84 genes was significantly affected by flight (P < 0.05). Genes that differed from AEM controls by at least 1.5-fold were Birc5, Figf, Grb2, and Tert (upregulated) and Fos, Ifnb1, Itgb3, Mmp9, Myc, Pdgfb, S100a4, Thbs, and Tnf (downregulated). Collectively, the data show that T cell distribution, function, and gene expression are significantly modified shortly after return from the spaceflight environment.
Keywords: cytokines, cancer, immune system, leukocytes
as human presence expands into low Earth orbit and beyond, there is a critical need for a better understanding of health consequences inherent to the spaceflight environment. A major concern is the impact that spaceflight stressors may have on the risk for cancer. The T lymphocytes are especially important in this respect; CD4+ T helpers (Th) secrete cytokines that regulate both innate and adaptive immunity against aberrant cell populations and the CD8+ T cytotoxic (Tc) cells can directly kill cells that are recognized as being different from normal self. Furthermore, if spaceflight stressors result in higher mutation rates in the human body, as has been observed in bacteria (37), the possibility for malignant transformation may be increased. This possibility together with radiation-induced immune dysfunction and reactivation of endogenous viruses with oncogenic potential may further increase the risk for cancer (65).
It has been known for more than three decades that spaceflight can have significant effects on the immune system (69). Some reports suggest that the observed alterations may increase the possibility for neoplastic growth. For example, data from astronauts and rodents flown in space have shown lymphocytopenia (8, 9, 40), low numbers of CD4+ and CD8+ T cells (2, 40), compromised lymphocyte response to stimulating agents (5, 53, 68, 77), and increased aberrations in lymphocyte DNA (18, 55). In contrast, other studies have found increased numbers of CD4+ T cells after flight (72), no difference in either intrachromosomal or complex-type exchanges between pre- and postflight samples (38), and no correlation between chromosome damage and the number of flights, duration in space, and length of extravehicular activity (24). The interferons (IFN) were among the first cytokines to be evaluated in the context of the space environment. In the 1980s, Talas et al. (76) reported results of experiments conducted aboard space laboratory Solyut-6. Lymphocytes isolated from healthy human donors were kept under spaceflight conditions for 1 wk and activated by use of IFN inducers, including a virus. Production of IFN by the activated cells kept in the space laboratory was increased compared with ground controls. In contrast, decreased IFN secretion and low natural killer (NK) cell activity were observed for lymphocytes isolated from peripheral blood of cosmonauts during the first day after return from a 7-day spaceflight (76). In another study, splenocytes from rats flown for 1 wk on Space Shuttle SL-3 exhibited decreased IFN-γ production in response to concanavalin-A (ConA), a T cell mitogen (23). The samples were obtained 12 h after landing and IFN-γ level was determined by plaque or microplaque reduction of vesicular stomatitis virus on mouse L-929 cells. Thus the modulatory effects of spaceflight on the immune system need further clarification. Many factors could account for the observed inconsistencies, including time of assessment postlanding, cell phenotype, and specific assays used. In the case of rodents and humans, genetic background, age, and gender are of course also potential causes for variability.
The spleen and thymus samples evaluated in the present study were from mice that were part of the Commercial Biomedical Test Module-2 payload experiment, only the second time that immune parameters have been characterized in mice flown in space. Spleen cells were evaluated for DNA synthesis in response to a T cell mitogen, composition of the major lymphocyte populations including the CD4+ and CD8+ T cell subsets, and ability to secrete cytokines after activation with anti-CD3 monoclonal antibody. The four cytokines selected for quantification [IL-2, IL-10, IFN-γ, and macrophage inflammatory protein-1α (MIP-1α)] are among the most important regulators not only of T cell activities, but also adaptive and innate immune mechanisms that include protection against potentially oncogenic viruses. As mentioned earlier, the development of cancer is a major long-term concern as human exploration increases beyond the Earth's radioprotective magnetosphere, and predictions of cancer risk in flight personnel are currently based on minimal data with large uncertainties (15). The thymus was used to evaluate the expression of cancer-related genes because thymic lymphoma in mice is a classical model for studying normal cell transformation to malignancy, especially after radiation exposure (44). Indeed, lymphomas have been noted in many sites in mice exposed to radiation types and doses that would be expected in space. A good example of this is a recent report on the efficacy of dietary antioxidants to ameliorate the carcinogenic effects of proton and iron ion radiation (43). In addition, the thymus can serve as a source of T cell reconstitution under conditions of great T cell loss (22, 30, 48), which could occur after exposure to relatively high doses of radiation during a solar particle event. The increased proliferation of sublethally damaged thymocytes during reconstitution would increase the risk for cell transformation to the malignant stage.
The overall hypothesis of the present study was that significant T cell abnormalities would be readily measurable in both lymphoid organs shortly after return from a flight on the space shuttle. We further proposed that at least some of the genes necessary for normal cell survival, as well as frequently associated with malignant cells, would be upregulated.
MATERIALS AND METHODS
Animals, housing, and sample collection.
C57BL/6NTac female mice (n = 36; Taconic Farms, Germantown, NY) were shipped directly to the National Aeronautics and Space Administration (NASA) Space Life Sciences Laboratory (SLSL) at Kennedy Space Center at ∼7 wk of age. Animal enclosure modules (AEM) with food bars and water were used to house flight mice (FLT, n = 12) and ground controls (AEM, n = 12). Mice were adapted to the food bars, Lixit water system units, and raised mesh floor 1 wk before the start of the investigation, at which time the mice were 9 wk of age. Additional control mice (n = 12) were housed under conventional vivarium (Viv) conditions. The FLT mice flew onboard the Space Shuttle Endeavour (STS-118) for 13 days. Muscle strength testing and nuclear magnetic resonance body composition measurements were performed at Kennedy Space Center by Amgen investigators prior to euthanasia in 100% CO2, all of which took place within 3–5 h after landing. Collection of organs, including spleen and thymus, at the SLSL and conditions of shipment to Loma Linda University (LLU) have been described elsewhere (Baqai AP, Gridley DS, Slater JM, Luo-Owen X, Stodieck LS, Ferguson VL, Chapes SK, Pecaut MJ, unpublished observations). Since all mice used here were part of a larger study, the FLT and AEM (but not Viv) animals were given a placebo treatment (subcutaneous injection of PBS) 24 h prior to launch. The NASA, Amgen, University of Colorado, and LLU Institutional Animal Care and Use Committees approved this study. Approval was also obtained for the transfer of mouse tissues.
Phytohemagglutinin (PHA)-induced blastogenesis.
This procedure has been previously described in detail (42). Briefly, spleen leukocytes were first adjusted to 2 × 106 cells/ml in complete RPMI 1640 medium (Irvine Scientific, Santa Ana, CA) and then dispensed into 96-well microtiter plates (1 × 105 cells/well), both with and without PHA (Sigma Chemical, St. Louis, MO). The cells were incubated for 48 h. [3H]thymidine (3H-TdR; specific activity = 46 Ci/μmol; ICN Biochemicals, Costa Mesa, CA) was added at 1 μCi/50 μl/well during the last 4 h and the cells were harvested with a multiple-sample harvester (Harvester 96 Mack III-m; Tomtec, Hamden, CT). The amount of 3H-TdR incorporated into cell DNA was counted in a liquid beta-scintillation counter (EG&G-Wallac, Turku, Finland). Leukocyte counts obtained with an ABC Vet Hematology Analyzer (Heska, Waukesha, WI) and volume of each sample were used to convert the counts per minute (cpm) into cpm/106 leukocytes. A stimulation index (SI) was also calculated: SI = (cpm with mitogen − cpm without mitogen)/cpm without mitogen.
Lymphocyte populations.
Immunophenotyping of splenic lymphocytes was carried out by using a two-tube custom mixture of fluorescence-labeled monoclonal antibodies and a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA) as previously reported (27, 58). All antibodies were purchased from Pharmingen (San Diego, CA). Five thousand to 10,000 events/tube were acquired and analyzed via CellQuest software (v3.1, Becton Dickinson) to identify and obtain percentages for CD3+ T, CD4+ Th, CD8+ Tc, CD19+ B, and NK1.1+ NK cells; the antibody clones were 145-2C11, RM4-5, 53-6.7, ID3, and PK136, respectively. To obtain the number of cells for each lymphocyte population, the following formula was used: number of cells in population/ml = number of leukocytes/ml × percentage of population. The number of leukocytes per milliliter, as well as a three-part differential (lymphocytes, monocyte-macrophages, and granulocytes), was obtained with an automated hematology analyzer and these data are reported separately (Baqai AP, Gridley DS, et al., unpublished observations).
Activation with anti-CD3 antibody.
Spleen leukocytes were diluted to 4 × 106/ml in RPMI 1640 medium (Irvine Scientific) supplemented as described above. Cell activation was triggered by dispensing 0.2 ml/well into 96-well plates containing immobilized monoclonal antibody against mouse CD3 (BioCoat anti-CD3 T Cell Activation Plates, BD Biosciences/BD Pharmingen, San Diego, CA). After a 48-h incubation at 37°C in 5% CO2, supernatants were aspirated and centrifuged to remove floating cells and debris. The supernatants were then frozen for cytokine analysis.
Quantification of cytokines.
Frozen supernatants from splenocytes activated with anti-CD3 were thawed immediately before cytokine analyses. IL-2, IL-10, IFN-γ, and macrophage inflammatory protein-1α (MIP-1α) were quantified by using ELISA kits (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. These cytokines were selected because they play major roles in regulation of both innate and adaptive immune responses.
Analysis of gene expression in thymus by quantitative RT-PCR.
The thymus was quick-frozen at −70°C and shipped overnight to LLU. The frozen tissue was homogenized in Trizol and RNA was extracted by a standard protocol (Invitrogen, Carlsbad, CA). RNA integrity was assessed spectrophotometrically; all samples had 260/280 ratios above 2.0 and 250/230 ratios above 1.7. Reverse transcription (RT) was done using Reaction Ready First Strand cDNA Synthesis Kit (SuperArray Biosciences, Frederick, MD). Polymerase chain reaction (PCR) was performed using RT2 Profiler PCR Array PAMM-033, Mouse Cancer Pathway Finder (SuperArray) on a Bio-Rad cycler using RT2 Real-Time SYBR Green PCR Master Mix PA-011. Five house-keeping genes, RT controls, and PCR controls were included. A more detailed description of these procedures has been published (25). The array evaluated the expression of 84 genes involved in cell transformation and tumorigenesis. Genes of FLT mice that differed by >1.5-fold compared with AEM controls and for which a P < 0.05 was obtained are emphasized. Additional genes for which fold change was 1.1 to 1.5, but for which P value was also <0.05, are briefly noted.
Statistical analysis.
The data were evaluated by Student's t-test (Systat, Systat Software, Richmond, CA). However, since there was a 48-h delay between euthanasia of FLT and AEM groups, and one-half of the Viv controls were euthanized on each day of assessment, the Viv data were used to normalize the means (NOM) prior to statistical analysis: NOM = [AEM/average of VivAEM or FLT] × average of Vivall. P < 0.05 and P < 0.1 indicated significance difference and a trend, respectively.
RESULTS
PHA-induced DNA synthesis.
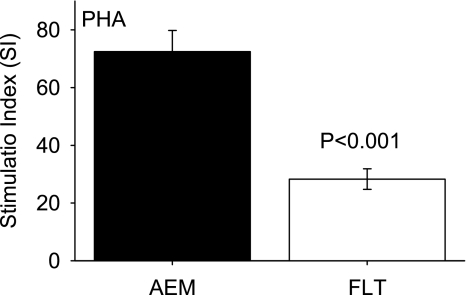
DNA synthesis in splenocytes from FLT mice was low in response to PHA compared with AEM controls (one-way ANOVA, P < 0.001). Figure 1 shows that the SI for FLT mice was less than 40% of that for the AEM animals. The mean cpm values with PHA were 219,793 ± 28,045 (AEM) and 121,390 ± 13,549 (FLT) (P < 0.001); cpm values without PHA were 2,881 ± 190 (AEM) and 3,774 ± 315 (FLT) (P < 0.05).
Fig. 1.
Stimulation index (SI) for splenocytes after activation with phytohemagglutinin (PHA). Data were obtained using [3H]thymidine incorporation. AEM, animal enclosure modules; FLT, flight animals. SI = (cpm with mitogen − cpm without mitogen)/cpm without mitogen, where cpm is counts per minute. Bars represent means ± SE (n = 12 mice/group).
Lymphocyte populations.
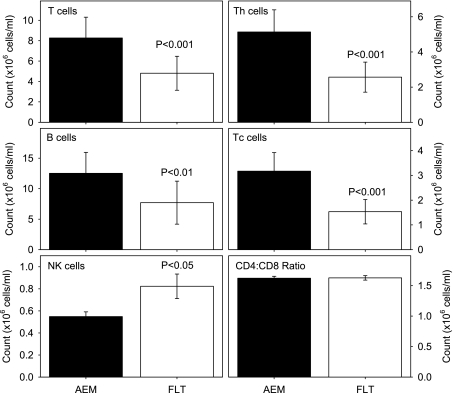
Figure 2 presents the numbers obtained for the different lymphocyte phenotypes in the spleen. The CD3+ T and CD19+ B cell counts were lower in the FLT compared with the AEM mice, whereas the NK1.1+ NK cell population was increased (P < 0.01 for all three cell types). These numerical changes translated into a lower percentage of T cells and higher percentage of NK cells in the FLT animals (P < 0.05), but the percentage of B cells was similar to AEM controls (Table 1). Further analysis showed that the CD4+ and CD8+ T cell counts were greatly reduced in the FLT mice (P < 0.001), but the CD4-to-CD8 T cell ratio did not differ significantly from the AEM mice (Fig. 2).
Fig. 2.
Lymphocyte populations in the spleen. Data were obtained using fluorescence-labeled monoclonal antibodies and flow cytometry. Th, T helper cells; Tc, T cytotoxic cells; NK, natural killer cells. Bars represent means ± SE (n = 12 mice/group).
Table 1.
Percentage of lymphocyte populations in spleen
| Cell Type | Group |
|
|---|---|---|
| AEM | FLT | |
| CD3+ T cells | 38.9±0.7 | 36.6±0.9* |
| CD19+ B cells | 58.5±0.6 | 57.4±0.9 |
| NK1.1+ NK cells | 2.6±0.1 | 6.0±0.2† |
Numbers represent means ± SE of total lymphocytes (n = 12/group). Data were obtained using fluorescence-labeled monoclonal antibodies and flow cytometry. AEM, ground controls housed in animal enclosure modules; FLT, mice flown for 13 days on space shuttle; NK, natural killer.
P < 0.05 vs. AEM;
P < 0.001 vs. AEM.
Secreted cytokines.
As shown in Fig. 3, secretion capacity for all four quantified cytokines in response to activation with anti-CD3 monoclonal antibody was significantly different in the FLT mice compared with the AEM controls. Levels of IL-10, IFN-γ, and MIP-1α were increased, whereas IL-2 was decreased (P < 0.05 vs. AEM).
Fig. 3.
Cytokine levels after splenocyte activation with anti-CD3 monoclonal antibody. Cytokines in spleen cell supernatants were quantified by ELISA. MIP-1α, macrophage inflammatory protein-1α. Bars represent means ± SE (n = 12 mice/group).
Gene expression in thymus.
Gene expression profiles in the thymus differed greatly between the FLT and AEM groups. Table 2 shows that fold change for 13 genes in FLT mice was greater than ± 1.5 (P < 0.05); there were four upregulated genes (Birc5, Figf, Grb2, and Tert) and nine downregulated genes (Fos, Ifnb1, Itgb3, Mmp9, Myc, Pdgfb, S100a4, Thbs, and Tnf) compared with the AEM group. Statistical significance (P < 0.05) was obtained for an additional 17 genes (8 upregulated and 9 downregulated) as shown in Table 3, although fold change for these was only 1.1 to 1.5 vs. the AEM group.
Table 2.
Up- and downregulated genes with >1.5-fold change in thymus from FLT mice
| Gene | Fold Change | Official Name |
|---|---|---|
| Upregulated | ||
| Birc5 | 1.51 | Baculoviral IAP repeat-containing 5 |
| Figf | 1.78 | C-fos induced growth factor |
| Grb2 | 1.60 | Growth factor receptor bound protein 2 |
| Tert | 1.61 | Telomerase reverse transcriptase |
| Downregulated | ||
| Fos | −2.41 | FBJ osteosarcoma oncogene |
| Ifnb1 | −2.18 | Interferon beta 1, fibroblast |
| Itgb3 | −1.52 | Integrin beta 3 |
| Mmp9 | −2.62 | Matrix metallopeptidase 9 |
| Myc | −1.74 | Myelocytomatosis oncogene |
| Pdgfb | −1.54 | Platelet derived growth factor, B polypeptide |
| S100a4 | −1.61 | S100 calcium binding protein A4 |
| Thbs1 | −1.87 | Thrombospondin 1 |
| Tnf | −1.63 | Tumor necrosis factor |
Expression of the listed genes had P < 0.05 compared to AEM ground controls. Data were obtained using quantitative RT-PCR (n = 6 FLT mice; n = 6 AEM mice).
Table 3.
Up- and downregulated genes with 1.1- to 1.5-fold change in thymus from FLT mice
| Gene | Fold Change | Official Name |
|---|---|---|
| Upregulated | ||
| Akt1 | 1.17 | Thymoma viral proto-oncogene 1 |
| Bax | 1.12 | BclII-associated X protein |
| Casp8 | 1.24 | Caspase 8 |
| Fgfr2 | 1.48 | Fibroblast growth factor receptor 2 |
| Itga2 | 1.24 | Integrin alpha 2 |
| Itga4 | 1.46 | Integrin alpha 4 |
| Mta2 | 1.22 | Metastasis-associated gene family, member 2 |
| Nme4 | 1.27 | Nonmetastatic cells 4, protein expressed in |
| Downregulated | ||
| Ccnd1 | −1.38 | Cyclin D1 |
| Cdc25a | −1.26 | Cell division cycle 25 homolog A |
| E2f1 | −1.30 | E2f transcription factor 1 |
| Hsp90ab1 | −1.26 | Heat shock protein 90 kDa alpha, class B member 1 |
| Jun | −1.31 | Jun oncogene |
| Mdm2 | −1.46 | Transformed mouse 3T3 cell double minute 2 |
| Serpinb2 | −1.47 | Serine peptidase inhibitor, clade B, member 2 |
| Tgfb1 | −1.20 | Transforming growth factor, beta 1 |
| Tnfrsf10b | −1.33 | Tumor necrosis factor receptor superfamily, member 10b |
Expression of the listed genes had P < 0.05 compared to AEM ground controls. Data were obtained using quantitative RT-PCR (n = 6 FLT mice; n = 6 AEM mice).
DISCUSSION
PHA and 3H-TdR were used to assess the ability of splenocytes to synthesize DNA. This approach was selected because PHA is a potent T cell mitogen that has been extensively used in previous studies to quantify blastogenesis and the incorporation of a radiolabeled base is a highly sensitive measure of DNA synthesis. The data show that this response was compromised in the FLT mice based on raw cpm, as well as on calculated SI that took into account the cpm values obtained without PHA. However, the background cpm without mitogen was significantly higher in the FLT mice than in the AEM animals. This is consistent with the results of a spontaneous blastogenesis assay on splenocytes that was also performed on these mice, i.e., no mitogen or antigen was used (Baqai AP, Gridley DS, et al., unpublished observations). Low response to T cell mitogens has been previously reported in rats (5, 53, 68), as well as astronauts (77), after spaceflight. These data suggest that the T cell response to immunogenic agents may be poor owing to preoccupation with regaining a normal balance of T cells. This possibility is supported by the low response to lipopolysaccharide (also known as endotoxin) derived from Escherichia coli that was observed in the STS-118 mice (Baqai AP, Gridley DS, et al., unpublished observations).
Quantification of lymphocyte populations in the spleen showed that T and B cell counts in the FLT mice were low, whereas NK cell counts were high. In our previous study of spleens from C57BL/6 mice flown on Space Shuttle Endeavour (STS-108), there was a similar shift away from T cell proportions within hours after landing (59). However, in that previous flight, there was a corresponding shift toward B cells. In STS-108, B220 was the marker used to identify B cells, whereas here we used CD19. Since B220 is also found on NK cells, the increase in B220+ cells noted previously may simply correspond to the increase in NK cells noted here.
CD4+ and CD8+ T cell counts were both reduced by approximately the same degree and thus a normal CD4-CD8 T cell ratio was maintained. This contrasts with results from our previous flight (STS-108) in which we found a decreased ratio (59). However, rats flown on Space Shuttles Endeavour (STS-77) and Columbia (SLS-1) also had low percentage and number of CD4+ T cells, respectively (2, 60). After the Columbia mission, low counts for total white blood cells, CD8+ T cells, and B cells were also noted. Variable results for lymphocyte populations have also been reported for crewmembers after spaceflight (13, 14, 49, 72). Collectively, the data support the high sensitivity of lymphocytes to differences in spaceflight conditions that are difficult to control, as well as postflight procedures, environment, and experimental design.
In the present study, cytokines were quantified in spleen cell supernatants after activation with anti-CD3 antibody, a technique that activates the same signal transduction pathways that are activated during antigen presentation in vivo. Although anti-CD3 has been used in ground-based studies simulating microgravity (31, 57), this is one of very few studies in which such an approach has been utilized after return of either rodents or human crew from space. Of the four cytokines evaluated, only IL-2 concentration was decreased. IL-2, a product of the Th1 subset, is a major cytokine that stimulates proliferation of Th and Tc lymphocytes; it also increases activities of NK cells and cells of the monocyte-macrophage lineage. Low IL-2 production capacity could compromise immune defenses not only against infectious agents but also against neoplastic cells during extended space missions. There are, indeed, numerous studies that include IL-2 in efforts to improve immune responses against cancer (1, 17, 39, 57). Furthermore, reports that IL-2 protects memory T cells from apoptotic death (52) implies that insufficient amounts of the factor could endanger responsiveness to recall antigens, e.g., vaccines, including those recently approved for cervical cancer due to human papillomavirus types 16 and 18. Although the mechanisms remain unclear, ground-based investigations simulating microgravity by clinorotation suggest that the decreased production of IL-2 in the present study may be related to alterations in molecules needed for signal transduction, e.g., CD25 (31).
The low IL-2 level seen in the FLT mice can be at least partly explained by the enhanced production of IL-10, a Th2 cell-derived cytokine with potent immunosuppressive properties (10). However, IFN-γ was also increased in the FLT group. IFN-γ is a highly multifunctional cytokine that promotes both adaptive and innate immune responses. The cytokine enhances major histocompatibility class II expression, antigen presentation by macrophages, leukocyte migration to sites of tissue damage, and cytotoxic activity of NK cells. Studies have demonstrated that IFN-γ can prevent the development of primary and transplanted tumors (41). IFN-γ is a major product of Th1 cells but can also be secreted by CD8+ Tc, NK, and dendritic cells. The enhanced production of IFN-γ in our study, despite low T cell numbers, suggests that the stress of landing, readaptation, and postflight procedures prior to sample collection facilitated T cell differentiation into a phenotype with greater capacity to produce IFN-γ. This possibility may also be true for the NK and dendritic cells present in the splenocyte mixture that was analyzed. Alternatively, the increase in NK cell numbers may be responsible for the difference.
It has been reported that IFN-γ depresses Th2 cell activity (63, 83) and that a low IFN-γ-to-IL-10 ratio indicates a shift toward the Th2 subset that, in turn, can facilitate development of allergic inflammatory diseases and asthma (84). In the present study, FLT mice had a significantly higher IFN-γ-IL-10 ratio compared with the AEM ground controls (FLT 0.68 vs. AEM 0.27, P < 0.05), thereby suggesting a shift away from Th2 cells. However, it should be noted that cells other than Th1 and Th2 in the splenocyte mixture could have contributed to the levels obtained for both cytokines. Interesting cytokine data were recently reported by Crucian and colleagues (14) for crewmembers on short-duration shuttle missions (including STS-118, same as our mice) and long-duration missions on the International Space Station. Although some differences were noted in T cell subsets and other leukocyte populations between shuttle and space station occupants, both groups had a low secreted IFN-γ-IL-10 ratio on the day of landing after activation of peripheral blood T cells with antibody against CD3 and CD28. There is no simple explanation for the discrepancy in the ratio found for the mice and that obtained for the crewmembers, except to emphasize major differences between the two studies: human vs. mouse, source of T cells (peripheral blood vs. spleen), activating agent (anti-CD3/CD28 combination vs. anti-CD3 alone), and postflight procedures. In addition, the difference between the long-term space station inhabitants and our mice may be at least partly related to length of mission duration (∼6 mo vs. 13 days, respectively).
The essential role of IFN-γ in controlling viral infections is well known. In a recent study, Shearer and colleagues (65) found that production of IFN-γ in polyomavirus (PyV)-infected BALB/c mice was impaired by whole-body exposure to radiation. The data were based on response of splenocytes after activation with ConA. PyV is a potentially oncogenic herpesvirus that stays latent in the body after infection and immunodepression can result in reactivation. Thus the murine model mimicked the scenario in humans infected with the Epstein-Barr virus, a member of the Herpesviridae family that is extremely common in the general population. The investigators concluded that space radiation may lead to chronic virus replication and increase the risk for malignancy in astronauts.
We found that MIP-1α (also known as CCL3) was increased in the FLT mice. MIP-1α is among the strongest chemotactic cytokines produced by activated macrophages (67). This factor enhances inflammation by activating granulocytes (neutrophils, eosinophils, and basophils) and by inducing production of other proinflammatory cytokines. Inflammation, especially when chronic, has long been known to increase the risk for cancer (61). In the FLT mice, the high production of MIP-1α may assist hematopoietic progenitor recruitment and differentiation into granulocytes to compensate for the observed decrease in these cells, as reported elsewhere (Baqai AP, Gridley DS, et al., unpublished observations).
Cytokine production has been previously evaluated by use of cells cultured in vitro on spacecraft and leukocytes obtained from astronauts, cosmonauts, nonhuman primates, and rodents (6–8, 14, 29, 45, 53, 70). Studies of flight crew have generally shown diminished capacity to secrete cytokines, especially IL-2 and IFN-γ, although there has been variability. The cytokine findings in the present study are partially consistent with our previous studies of same-strain mice flown aboard Space Shuttle Endeavour (STS-108) in which cytokines were quantified after spleen cell stimulation with PHA (26). In our previous study, both IL-2 and IFN-γ were significantly lower after flight compared with AEM controls (IL-4 was also low; IL-5 and TNF-α were unaffected). The difference in the two studies may be at least partly explained by the use of agents that activate different signal transduction pathways. The PHA used in STS-108 cross-links surface carbohydrates and induces T cell activation independently of the T cell antigen receptor (TCR), whereas the immobilized anti-CD3 used in STS-118 binds to a CD3 subunit of the TCR that, together with accessory molecules, relays interactive signaling pathways to the nucleus (71).
Our interest in the thymus stems from the fact that thymic lymphoma is a common finding after radiation exposure in mouse models of human cancer (4). Solar particle events (SPE) during space missions could result in relatively large radiation doses that deplete T cell populations (27, 28, 42, 58). Loss of CD4+, CD8+, and CD4+CD8+ precursor T cells has been reported in fetal thymus organ cultures after spaceflight (81). Although the thymus is most important early in life, thymopoiesis can occur following demise of T cells, as seen in patients with acquired immunodeficiency syndrome (22) and after chemotherapy (48) or bone marrow transplantation (30). Thymic regeneration has also been noted in both mice and humans after androgen blockade (75). Although a number of studies have analyzed various aspects of thymuses from rodents flown in space (5, 11, 26, 51, 74), none have focused specifically on genes associated with carcinogenesis.
In the present study, expression of 30 of 84 evaluated thymic genes associated with cancer was significantly altered within hours postflight. Although we cannot separate the landing effects from the prolonged effects of space flight, the FLT mice had low thymus mass compared with AEM ground controls (Baqai AP, Gridley DS, et al., unpublished observations). Since the thymic atrophy is likely to reflect chronic stress in the mice during the flight and not the acute stress of landing, at least some of the observed changes could very well have been induced during the flight. Here we present a few highlights on the genes that were most highly affected. The data show that Birc5, also known as Survivin, was one of four upregulated genes. Birc5 encodes a protein that inhibits apoptosis and whose expression is greatly increased in most human tumors (82). Figf was also increased after flight. The protein encoded by this gene belongs in the PDGF/VEGF family and functions similarly to VEGF-C (56). Its activity promotes angiogenesis and endothelial cell growth and thus can facilitate tumor progression like other proangiogenic factors (20). Grb2 was another upregulated gene after flight. The protein derived from this gene serves as a docking site and participates in the EGF signal transduction pathway. Agents that block Greb2 protein and disrupt this pathway may have anti-tumor effects (19, 79). The upregulated Tert encodes a catalytic subunit of telomerase, a polymerase that is expressed in cancer cells, but not in normal postnatal cells (12).
Fos, encoding the c-Fos protein, was one of the nine thymic genes for which downregulation was most pronounced. Under normal conditions, c-Fos facilitates development of double-negative (CD4−CD8−) T cells into the mature single-positive (CD4+CD8−) T cells, a process known as positive selection (54). The roles of this proto-oncogene as part of an osteoclastogenic transcription factor (Fos/AP-1) (80) and overexpression in cancer cells (50) are well known. A significantly low level of c-Fos protein expression was found on granular bone marrow cells from these same mice after flight (Ortega MT, Pecaut MJ, Gridley DS, Stodieck LS, Ferguson VL, Chapes SK, unpublished observations). Other investigators have reported reduced EGF- and 12-O-tetradecanoyl-phorbol-13-acetate (TPA)-induced fos expression in human A431 epidermoid carcinoma cells under microgravity conditions (16). Spaceflight-related studies of the human U937 monocyte cell line and peripheral blood T cells indicate that microgravity induces changes in protein kinase C isoforms that may account at least partially for decreased expression of fos (32, 33).
Similarly to fos, high levels and mutated forms of c-Myc protein from the downregulated myc gene are closely linked to carcinogenesis; the 8:14 translocation in lymphoma is especially well characterized (36). IFNb1 belongs to the type I interferons that facilitate immune recognition of abnormal cells, either directly or indirectly, and possess significant antitumor activity (78). The downregulated Itgb3 gene encodes an integrin that is often highly expressed in angiogenic pathologies (34). The matrix metalloproteinase produced by Mmp9 is associated with invasion and metastasis of leukemic cells (73). Pdgfb, a homolog of the simian sarcoma virus oncogene (v-sis), belongs to the PDGF family. Expression of the PDGF-B protein has been implicated in malignant transformation of cells (35) and fibrosis (66). The downregulated S100a4 encodes a calcium-binding protein that is elevated in a wide range of tumors and appears to be directly involved in generating a metastatic phenotype (21). Thrombospondin-1 derived from the Thbs gene is involved in both angiogenesis and metastatic spread (62). The Tnf gene encodes TNF-α, a cytokine implicated in inflammation, cancer, and many other diseases (64).
Analysis of gene expression has been performed by Boonyaratanakornkit and colleagues (3) to identify gravity-dependent genes and pathways. In that study, CD3+ T cells from three human blood donors were isolated, activated with ConA and anti-CD28 antibody, and incubated for 4 h at 1 g or rotated in an apparatus that simulated a freefall environment; microarrays and nearly 8,800 probe sets were utilized to evaluate gene expression patterns in stimulated and unstimulated cells. The affected genes were primarily those that were regulated by early transcription factors such as NF-κB, CREB, ELK, AP-1, and STAT. The two key cytokine genes that were greatly downregulated in mitogen-stimulated T cells subjected to altered gravity were IL2 and CSF2 (encoding GM-CSF); six chemokine genes and five genes in the TNF superfamily were also suppressed. Thus there is some overlap with the data reported here. However, extensive comparisons between the results obtained with the isolated human T cells in culture that were mitogen/antibody-activated and our mouse data on constitutive gene expression are difficult because of the obviously great differences in study conditions.
In conclusion, the results demonstrate that splenic T lymphocytes are greatly affected in this mouse strain shortly after return from a 13-day flight in space. T cell numbers, percentages, responsiveness to a potent mitogen, and secretion of cytokines critical for optimal immune defense and homeostasis were all significantly affected. There was also a significant impact on expression of many cancer-related genes in the thymus. The changes observed in some genes suggest that the possibility for carcinogenesis may be increased, whereas others suggest decreased risk. Additional points that must be taken into account are that T cells in the thymus are likely to be in different stages of maturation and that cells other than T cell reside in this organ (e.g., epithelial reticular cells, granular cells, and cells of blood vessels). Furthermore, it remains to be determined whether the quantified changes are brief and due primarily to the tremendous physiological stress of landing and readaptation or have an enduring effect on risk for infection and/or cancer. These as yet unanswered questions are especially important in the context of Lunar and Mars missions during which relatively high radiation exposure is likely (e.g., during SPE) and may have an additive, or perhaps even synergistic, effect when it occurs together with other spaceflight stressors. To establish firm conclusions regarding the combined effects of all factors associated with space missions, sampling should be conducted before, during, and after flight. As science utilization increases on the International Space Station with a six-member crew, opportunities to perform sequential analyses such as this will hopefully become available in the not too distant future.
GRANTS
Funding for all assessments reported in this paper was provided by the Loma Linda University Department of Radiation Medicine, Molecular Radiation Biology Laboratories. The project was also partly supported by NASA grant NAG2-1274, NASA space grant consortium, and National Institutes of Health Grants AI052206 and RR16475. This is Kansas Agriculture Experiment Station publication 09-050-J.
Acknowledgments
The authors are very grateful for Amgen, for sponsoring the flight investigation and generously providing the tissues required to conduct this study. In particular, we thank H. Q. Han and David Lacey, the principal investigators at Amgen. Furthermore, we thank Gregory A. Nelson, Lora M. Green, Xiao Wen Mao, Adeola Y. Makinde, Melba L. Andres, Erben J. M. Bayeta, and Brandon Bianski at Loma Linda University for valuable assistance in various aspects of this study. We also thank Ramona Bober and the rest of the staff at NASA SLSL at Kennedy Space Center for their support and the students and technicians from the University of Colorado for assistance with tissue collection.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Acquavella N, Kluger H, Rhee J, Farber L, Tara H, Ariyan S, Narayan D, Kelly W, Sznol M. Toxicity and activity of a twice daily high-dose bolus interleukin 2 regimen in patients with metastatic melanoma and metastatic renal cell cancer. J Immunother 31: 569–576, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Allebban Z, Ichiki A, Gibson L, Jones J, Congdon C, Lange R. Effects of spaceflight on the number of rat peripheral blood leukocytes and lymphocyte subsets. J Leukoc Biol 55: 209–213, 1994. [DOI] [PubMed] [Google Scholar]
- 3.Boonyaratanakornkit JB, Cogoli A, Li CF, Schopper T, Pippia P, Galleri G, Meloni MA, Hughes-Fulford M. Key gravity-sensitive signaling pathways drive T cell activation. FASEB J 19: 2020–2022, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Boulton E, Cleary H, Plumb M. Myeloid, B and T lymphoid and mixed lineage thymic lymphomas in the irradiated mouse. Carcinogenesis 23: 1079–1085, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Chapes SK Lessons from Immune 1-3: what did we learn and what do we need to do in the future? J Gravit Physiol 11: P45–P48, 2004. [PubMed] [Google Scholar]
- 6.Chapes SK, Morrison DR, Guikema JA, Lewis ML, Spooner BS. Cytokine secretion by immune cells in space. J Leukoc Biol 52: 104–110, 1992. [DOI] [PubMed] [Google Scholar]
- 7.Chapes SK, Morrison DR, Guikema JA, Lewis ML, Spooner BS. Production and action of cytokines in space. Adv Space Res 14: 5–9, 1994. [DOI] [PubMed] [Google Scholar]
- 8.Chapes SK, Simske SJ, Forsman AD, Bateman TA, Zimmerman RJ. Effects of space flight and IGF-1 on immune function. Adv Space Res 23: 1955–1964, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Chapes SK, Simske SJ, Sonnenfeld G, Miller ES, Zimmerman RJ. Effects of spaceflight and PEG-IL-2 on rat physiological and immunological responses. J Appl Physiol 86: 2065–2076, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Commins S, Steinke JW, Borish L. The extended IL-10 superfamily: IL-10, IL-19, IL-20, IL-22, IL-24, IL-26, IL-28, and IL-29. J Allergy Clin Immunol 121: 1108–1111, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Congdon CC, Allebban Z, Gibson LA, Kaplansky A, Strickland KM, Jago TL, Johnson DL, Lange RD, Ichiki AT. Lymphatic tissue changes in rats flown on Spacelab Life Sciences-2. J Appl Physiol 81: 172–177, 1996. [DOI] [PubMed] [Google Scholar]
- 12.Cong Y, Shay JW. Actions of human telomerase beyond telomeres. Cell Res 18: 725–732, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Crucian BE, Cubbage ML, Sams CF. Altered cytokine production by specific human peripheral blood cell subsets immediately following space flight. J Interferon Cytokine Res 20: 547–556, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Crucian BE, Stowe RP, Pierson DL, Sams CF. Immune system dysregulation following short- vs long-duration spaceflight. Aviat Space Environ Med 79: 835–843, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Cucinotta FA, Durante M. Cancer risk from exposure to galactic cosmic rays: implications for space exploration by human beings. Lancet Oncol 7: 431–435, 2006. [DOI] [PubMed] [Google Scholar]
- 16.De Groot RP, Rijken PJ, den Hertog J, Boonstra J, Verkleij AJ, de Laat SW, Kruijer W. Nuclear responses to protein kinase C signal transduction are sensitive to gravity changes. Exp Cell Res 197: 87–90, 1991. [DOI] [PubMed] [Google Scholar]
- 17.Den Otter W, Jacobs JJ, Battermann JJ, Hordijk GJ, Krastev Z, Moiseeva EV, Stewart RJ, Ziekman PG, Koten JW. Local therapy of cancer with free IL-2. Cancer Immunol Immunother 57: 931–950, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fedorenko B, Druzhinin S, Yudaeva L, Petrov V, Akatov Y, Snigiryova G, Novitskaya N, Shevchenko V, Rubanovich A. Cytogenetic studies of blood lymphocytes from cosmonauts after long-term space flights on Mir station. Adv Space Res 27: 355–359, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Feller SM, Lewitzky M. Potential disease targets for drugs that disrupt protein—protein interactions of Grb2 and Crk family adaptors. Curr Pharm Des 12: 529–548, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Funaki H, Nishimura G, Harada S, Ninomiya I, Terada I, Fushida S, Tani T, Fujimura T, Kayahara M, Shimizu K, Ohta T, Miwa K. Expression of vascular endothelial growth factor D is associated with lymph node metastasis in human colorectal carcinoma. Oncology 64: 416–422, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Garrett SC, Varney KM, Weber DJ, Bresnick AR. S100A4, a mediator of metastasis. J Biol Chem 281: 677–680, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Gautier D, Beq S, Cortesão CS, Sousa AE, Cheynier R. Efficient thymopoiesis contributes to the maintenance of peripheral CD4 T cells during chronic human immunodeficiency virus type 2 infection. J Virol 81: 12685–12688, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gould CL, Lyte M, Williams J, Mandel AD, Sonnenfeld G. Inhibited interferon-gamma but normal interleukin-3 production from rats flown on the space shuttle. Aviat Space Environ Med 58: 983–986, 1987. [PubMed] [Google Scholar]
- 24.Greco O, Durante M, Gialanella G, Grossi G, Pugliese M, Scampoli P, Snigiryova G, Obe G. Biological dosimetry in Russian and Italian astronauts. Adv Space Res 31: 1495–1503, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Gridley DS, Coutrakon GB, Rizvi A, Bayeta EJM, Luo-Owen X, Makinde AY, Baqai F, Koss P, Slater JM, Pecaut MJ. Low-dose photons modify liver response to simulated solar particle event protons. Radiat Res 169: 280–287, 2008. [DOI] [PubMed] [Google Scholar]
- 26.Gridley DS, Nelson GA, Peters LL, Kostenuik PJ, Bateman TA, Morony S, Stodieck LS, Lacey DL, Simske SJ, Pecaut MJ. Genetic models in applied physiology: selected contribution: effects of spaceflight on immunity in the C57BL/6 mouse. II. Activation, cytokines, erythrocytes, and platelets. J Appl Physiol 94: 2095–2103, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Gridley DS, Pecaut MJ, Dutta-Roy R, Nelson GA. Dose and dose rate effects of whole-body proton irradiation on leukocyte populations and lymphoid organs: part I. Immunol Lett 80: 55–66, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Gridley DS, Rizvi A, Luo-Owen X, Makinde AY, Coutrakon GB, Koss P, Slater JM, Pecaut MJ. Variable hematopoietic responses to acute photons, protons and simulated solar particle event protons. In Vivo 22: 159–170, 2008. [PubMed] [Google Scholar]
- 29.Grove DS, Pishak SA, Mastro AM. The effect of a 10-day space flight on the function, phenotype, and adhesion molecule expression of splenocytes and lymph node lymphocytes. Exp Cell Res 219: 102–109, 1995. [DOI] [PubMed] [Google Scholar]
- 30.Hakim FT, Memon SA, Cepeda R, Jones EC, Chow CK, Kasten-Sportes C, Odom J, Vance BA, Christensen BL, Mackall CL, Gress RE. Age-dependent incidence, time course, and consequences of thymic renewal in adults. J Clin Invest 115: 930–939, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hashemi BB, Penkala JE, Vens C, Huls H, Cubbage M, Sams CF. T cell activation responses are differentially regulated during clinorotation and in spaceflight. FASEB J 13: 2071–2082, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Hatton JP, Gaubert F, Cazenave JP, Schmitt D. Microgravity modifies protein kinase C isoform translocation in the human monocytic cell line U937 and human peripheral blood T-cells. J Cell Biochem 87: 39–50, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Hatton JP, Gaubert F, Lewis ML, Darsel Y, Ohlmann P, Cazenave JP, Schmitt D. The kinetics of translocation and cellular quantity of protein kinase C in human leukocytes are modified during spaceflight. FASEB J 13 Suppl: S23–S33, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Hayashi H, Sano H, Seo S, Kume T. The Foxc2 transcription factor regulates angiogenesis via induction of integrin beta 3 expression. J Biol Chem 283: 23791–23800, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ho CL, Hsu LF, Phyliky RL, Li CY. Autocrine expression of platelet-derived growth factor B in B cell chronic lymphocytic leukemia. Acta Haematol 114: 133–140, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Hoffman B, Amanullah A, Shafarenko M, Liebermann DA. The proto-oncogene c-myc in hematopoietic development and leukemogenesis. Oncogene 21: 3414–3421, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Horneck G Impact of microgravity on radiobiological processes and efficiency of DNA repair. Mutat Res 430: 221–228, 1999. [DOI] [PubMed] [Google Scholar]
- 38.Horstmann M, Durante M, Johannes C, Pieper R, Obe G. Space radiation does not induce a significant increase of intrachromosomal exchanges in astronauts’ lymphocytes. Radiat Environ Biophys 44: 219–224, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Hotte S, Waldron T, Canil C, Winquist E. Interleukin-2 in the treatment of unresectable or metastatic renal cell cancer: a systematic review and practice guideline. Can Urol Assoc J 1: 27–38, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ichiki A, Gibson L, Jago T, Strickland K, Johnson D, Lange R, Allebban Z. Effects of spaceflight on rat peripheral blood leukocytes and bone marrow progenitor cells. J Leukoc Biol 60: 37–43, 1996. [DOI] [PubMed] [Google Scholar]
- 41.Ikeda H, Old LJ, Schreiber RD. The roles of IFN gamma in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev 13: 95–109, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Kajioka EH, Andres ML, Li J, Mao XW, Moyers MF, Nelson GA, Slater JM, Gridley DS. Acute effects of whole-body proton irradiation on the immune system of the mouse. Radiat Res 153: 587–594, 2000. [DOI] [PubMed] [Google Scholar]
- 43.Kennedy AR, Davis JG, Carlton W, Ware JH. Effects of dietary antioxidant supplementation on the development of malignant lymphoma and other neoplastic lesions in mice exposed to proton or iron-ion radiation. Radiat Res 169: 615–625, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kominami R, Niwa O. Radiation carcinogenesis in mouse thymic lymphomas. Cancer Sci 97: 575–581, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Konstantinova IV, Rykova MP, Lesnyak AT, Antropova EA. Immune changes during long-duration missions. J Leukoc Biol 54: 189–201, 1993. [DOI] [PubMed] [Google Scholar]
- 46.Lesnyak A, Sonnenfeld G, Avery L, Konstantinova I, Rykova M, Meshkov D, Orlova T. Effect of SLS-2 spaceflight on immunologic parameters of rats. J Appl Physiol 81: 178–182, 1996. [DOI] [PubMed] [Google Scholar]
- 47.Limouse M, Manié S, Konstantinova I, Ferrua B, Schaffar L. Inhibition of phorbol ester-induced cell activation in microgravity. Exp Cell Res 197: 82–86, 1991. [DOI] [PubMed] [Google Scholar]
- 48.Mackall CL, Fleisher TA, Brown MR, Andrich MP, Chen CC, Feuerstein IM, Horowitz ME, Magrath IT, Shad AT, Steinberg SM, Wexler LH, Gress RE. Age, thymopoiesis, and CD4+ T-lymphocyte regeneration after intensive chemotherapy. N Engl J Med 332: 143–149, 1995. [DOI] [PubMed] [Google Scholar]
- 49.Mehta SK, Kaur I, Grimm EA, Smid C, Feeback DL, Pierson DL. Decreased non-MHC-restricted (CD56+) killer cell cytotoxicity after spaceflight. J Appl Physiol 91: 1814–1818, 2001. [DOI] [PubMed] [Google Scholar]
- 50.Milde-Langosch K The Fos family of transcription factors and their role in tumourigenesis. Eur J Cancer 41: 2449–2461, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Misurova E, Tigranyan RA, Praslicka M. Changes of deoxyribonucleoprotein in the spleen, thymus and liver of rats exposed to weightlessness and artificial gravity aboard the Cosmos biosatellites. Adv Space Res 1: 225–230, 1981. [DOI] [PubMed] [Google Scholar]
- 52.Mor F, Cohen IR. IL-2 rescues antigen-specific T cells from radiation or dexamethasone-induced apoptosis. Correlation with induction of Bcl-2. J Immunol 156: 515–522, 1996. [PubMed] [Google Scholar]
- 53.Nash PV, Mastro AM. Variable lymphocyte responses in rats after space flight. Exp Cell Res 202: 125–131, 1992. [DOI] [PubMed] [Google Scholar]
- 54.Nunomura S, Sato T, Habu S. Molecular basis for functional maturation of thymocytes: increase in c-fos translation with positive selection. J Immunol 164: 5590–5595, 2000. [DOI] [PubMed] [Google Scholar]
- 55.Obe G, Johannes I, Johannes C, Hallman K, Reitz G, Facius R. Chromosomal aberrations in blood lymphocytes of astronauts after long-term space flights. Int J Radiat Biol 72: 727–734, 1997. [DOI] [PubMed] [Google Scholar]
- 56.Orlandini M, Marconcini L, Ferruzzi R, Oliviero S. Identification of a c-fos-induced gene that is related to the platelet-derived growth factor/vascular endothelial growth factor family. Proc Natl Acad Sci USA 93: 11675–1180, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ortiz-Sánchez E, Helguera G, Daniels TR, Penichet ML. Antibody-cytokine fusion proteins: applications in cancer therapy. Expert Opin Biol Ther 8: 609–632, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pecaut MJ, Nelson GA, Gridley DS. Dose and dose rate effects of whole-body γ-irradiation. I. Lymphocytes and lymphoid organs. In Vivo 15: 195–208, 2001. [PubMed] [Google Scholar]
- 59.Pecaut MJ, Nelson GA, Peters LL, Kostenuik PJ, Bateman TA, Morony S, Stodieck LS, Lacey DL, Simske SJ, Gridley DS. Effects of spaceflight on immunity in the C57BL/6 mouse. I. Immune population distributions. J Appl Physiol 94: 2085–2094, 2003. [DOI] [PubMed] [Google Scholar]
- 60.Pecaut MJ, Simske SJ, Fleshner M. Spaceflight induces changes in splenocyte subpopulations: effectiveness of ground-based models. Am J Physiol Regul Integr Comp Physiol 279: R2072–R2078, 2000. [DOI] [PubMed] [Google Scholar]
- 61.Robak P, Smolewski P, Robak T. The role of non-steroidal anti-inflammatory drugs in the risk of development and treatment of hematologic malignancies. Leuk Lymphoma 49: 1452–1462, 2008. [DOI] [PubMed] [Google Scholar]
- 62.Sargiannidou I, Qiu C, Tuszynski GP. Mechanisms of thrombospondin-1-mediated metastasis and angiogenesis. Semin Thromb Hemost 30: 127–136, 2004. [DOI] [PubMed] [Google Scholar]
- 63.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-γ: an overview of signals, mechanisms and functions. J Leukoc Biol 75: 163–189, 2004. [DOI] [PubMed] [Google Scholar]
- 64.Sethi G, Sung B, Aggarwal BB. TNF: a master switch for inflammation to cancer. Front Biosci 13: 5094–5107, 2008. [DOI] [PubMed] [Google Scholar]
- 65.Shearer WT, Zhang S, Reuben JM, Lee BN, Butel JS. Effects of radiation and latent virus on immune responses in a space flight model. J Allergy Clin Immunol 115: 1297–1303, 2005. [DOI] [PubMed] [Google Scholar]
- 66.Shen YC, Chiu CF, Chow KC, Chen CL, Liaw YC, Yeh SP. Fatal pulmonary fibrosis associated with BCNU: the relative role of platelet-derived growth factor-B, insulin-like growth factor I, transforming growth factor-beta1 and cyclooxygenase-2. Bone Marrow Transplant 34: 609–614, 2004. [DOI] [PubMed] [Google Scholar]
- 67.Sherry B, Tekamp-Olson P, Gallegos C, Bauer D, Davatelis G, Wolpe SD, Masiarz F, Coit D, Cerami A. Resolution of the two components of macrophage inflammatory protein 1, and cloning and characterization of one of those components, macrophage inflammatory protein 1-beta. J Exp Med 168: 2251–2259, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sonnenfeld G, Foster M, Morton D, Bailliard F, Fowler NA, Hakenewerth AM, Bates R, Miller ES. Spaceflight and development of immune responses. J Appl Physiol 85: 1429–1433, 1998. [DOI] [PubMed] [Google Scholar]
- 69.Sonnenfeld G, Mandel A, Konstantinova I, Berry W, Taylor G, Lesnyak A, Fuchs B, Rakhmilevich A. Space flight alters immune cell function and distribution. J Appl Physiol 73: 191S-195S, 1992. [DOI] [PubMed] [Google Scholar]
- 70.Sonnenfeld G, Miller ES. The role of cytokines in immune changes induced by spaceflight. J Leukoc Biol 54: 253–258, 1993. [DOI] [PubMed] [Google Scholar]
- 71.Stefanová I, Hemmer B, Vergelli M, Martin R, Biddison WE, Germain RN. TCR ligand discrimination is enforced by competing ERK positive and SHP-1 negative feedback pathways. Nat Immunol 4: 248–54, 2003. [DOI] [PubMed] [Google Scholar]
- 72.Stowe RP, Sams CF, Pierson DL. Effects of mission duration on neuroimmune responses in astronauts. Aviat Space Environ Med 74: 1281–1284, 2003. [PubMed] [Google Scholar]
- 73.Sun Y, Dong LJ. Role of matrix metalloproteinases in the pathogenesis and therapy of leukemia [in Chinese]. Zhongguo Shi Yan Xue Ye Xue Za Zhi 11: 316–320, 2003. [PubMed] [Google Scholar]
- 74.Sushkov FV, Rudneva SB, Durnova GN, Ponomareva TF. Results of a quantitative cytological analysis of the thymus of rats exposed on biosatellites [in Russian]. Kosm Biol Aviakosm Med 17: 56–61, 1983. [PubMed] [Google Scholar]
- 75.Sutherland JS Activation of thymic regeneration in mice and humans following androgen blockade. J Immunol 175: 2741–2753, 2005. [DOI] [PubMed] [Google Scholar]
- 76.Talas M, Batkai L, Stoger I, Nagy K, Hiros L, Konstantinova I, Rykova M, Mozgovaya I, Guseva O, Kozharinov V. Results of space experiment program “Interferon.” Acta Astronaut 11: 379–386, 1984. [DOI] [PubMed] [Google Scholar]
- 77.Taylor GR, Neale LS, Dardano JR. Immunological analysis of U. S. space shuttle crewmembers. Aviat Space Environ Med 57: 213–217, 1986. [PubMed] [Google Scholar]
- 78.Vannucchi S, Chiantore MV, Mangino G, Percario ZA, Affabris E, Fiorucci G, Romeo G. Perspectives in biomolecular therapeutic intervention in cancer: from the early to the new strategies with type I interferons. Curr Med Chem 14: 667–679, 2007. [DOI] [PubMed] [Google Scholar]
- 79.Vidal M, Liu WQ, Gril B, Assayag F, Poupon MF, Garbay C. Design of new anti-tumor agents interrupting deregulated signaling pathways induced by tyrosine kinase proteins. Inhibition of protein-protein interaction involving Grb2 [in French]. J Soc Biol 198: 133–137, 2004. [PubMed] [Google Scholar]
- 80.Wagner EF, Eferl R. Fos/AP-1 proteins in bone and the immune system. Immunol Rev 208: 126–140, 2005. [DOI] [PubMed] [Google Scholar]
- 81.Woods CC, Banks KE, Gruener R, DeLuca D. Loss of T cell precursors after spaceflight and exposure to vector-averaged gravity. FASEB J 17: 1526–1528, 2003. [DOI] [PubMed] [Google Scholar]
- 82.Yamamoto H, Ngan CY, Monden M. Cancer cells survive with survivin. Cancer Sci 99: 1709–1714, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Young HA, Bream JH. IFN-gamma: recent advances in understanding regulation of expression, biological functions, and clinical applications. Curr Top Microbiol Immunol 316: 97–117, 2007. [DOI] [PubMed] [Google Scholar]
- 84.Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood 112: 1557–1569, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]