Abstract
Although a number of studies have considered the neural circuitry that regulates diaphragm activity, these pathways have not been adequately discerned, particularly in animals such as cats that utilize the respiratory muscles during a variety of different behaviors and movements. The present study employed the retrograde transneuronal transport of rabies virus to identify the extended neural pathways that control diaphragm function in felines. In all animals deemed to have successful rabies virus injections into the diaphragm, large, presumed motoneurons were infected in the C4–C6 spinal segments. In addition, smaller presumed interneurons were labeled bilaterally throughout the cervical and upper thoracic spinal cord. While in short and intermediate survival cases, infected interneurons were concentrated in the vicinity of phrenic motoneurons, in late survival cases, the distribution of labeling was more expansive. Within the brain stem, the earliest infected neurons included those located in the classically defined pontine and medullary respiratory groups, the medial and lateral medullary reticular formation, the region immediately ventral to the spinal trigeminal nucleus, raphe pallidus and obscurus, and the vestibular nuclei. At longer survival times, infection appeared in the midbrain, which was concentrated in the lateral portion of the periaqueductal gray, the region of the tegmentum that contains the locomotion center, and the red nucleus. Considerable labeling was also present in the fastigial nucleus of the cerebellum, portions of the posterior and lateral hypothalamus and the adjacent fields of Forel known to contain hypocretin-containing neurons and the precruciate gyrus of cerebral cortex. These data raise the possibility that several parallel pathways participate in regulating the activity of the feline diaphragm, which underscores the multifunctional nature of the respiratory muscles in this species.
Keywords: respiration, rabies virus, motor control, reticular formation
the diaphragm, like other respiratory muscles, is multifunctional. In addition to generating force responsible for moving air into the lungs, the diaphragm participates in executing protective reflexes, such as vomiting and coughing, contributes to postural stabilization and voluntary movement, and is involved in complex behaviors, such as vocalization (37). Furthermore, the respiratory-related discharges of the diaphragm are modulated during locomotion, exercise, sleep, and emotional responses (37). As such, the neural circuitry regulating diaphragm contractions is necessarily complex. A large number of physiological studies conducted in multiple species have discerned the organization of the brain stem respiratory groups responsible for generating the respiratory rhythm and imparting that activity on phrenic motoneurons (23). There is considerable evidence, however, that additional brain stem circuitry also modulates diaphragm motoneuron firing, particularly in emetic animals such as cats. For example, bulbospinal neurons in the dorsal respiratory group (DRG) and ventral respiratory group (VRG) with inspiratory activity are inactive during vomiting, indicating that the powerful diaphragm contractions contributing to this behavior are elicited by premotor neurons in other locations (38). In addition, functional lesions that inactivate the brain stem respiratory group neurons or their axons do not eliminate changes in diaphragm activity during vestibular stimulation (60). The locations of the brain stem neurons that drive the contractions of the diaphragm during these responses, and likely others, have not been established.
A number of suprapontine regions have also been shown to participate in regulating diaphragm activity. Previous studies identified neurons in the mesencephalic tegmentum, as well as in the caudal hypothalamus, that, when stimulated, produce simultaneous increases in locomotion and respiration (25). Stimulation of specific regions of the periaqueductal gray has been shown to elicit vocalization and/or emotional responses that are accompanied by changes in the timing and magnitude of diaphragm discharges (5). Stimulation of a variety of regions of the cerebral cortex alters inspiratory activity, which is likely due to the high interconnectivity between cortical areas (19, 25). Although it is clear that a number of suprapontine regions are involved in modulating diaphragm activity in association with a multiplicity of complex behaviors, the organization of the neural circuits that synaptically link these brain centers with diaphragm motoneurons has not been well described.
Transneuronal tracing techniques using neurotropic viruses offer a powerful tool to map the polysynaptic pathways providing inputs to a particular target, as the viruses move progressively through neural circuits in a time-dependent retrograde manner (30, 55). Previous studies have employed the retrograde transneuronal transport of pseudorabies virus to determine the locations of neurons providing inputs to diaphragm motoneurons in the rat (20, 33) and ferret (10, 61). Both sets of experiments focused only on infected neurons in the spinal cord and brain stem. In particular, the ferret studies were limited to describing the locations of medullary neurons likely to provide direct inputs to phrenic motoneurons (10, 61), and the experiments in rats concentrated on the bulbospinal and spinal projections to phrenic motoneurons, as well as the direct inputs to the premotor neurons (20, 33). Furthermore, although the ferret is a common model animal employed in studies regarding emesis, there are limited physiological data regarding the neural regulation of diaphragm activity in this species (52). The cat is presumably a better animal to utilize in transneuronal tracing studies analyzing the multifunctional regulation of diaphragm activity; felines employ their diaphragm in a variety of complex behaviors, including vomiting, coughing, and postural responses, as documented in an extensive body of electrophysiological literature (37). Many of these responses are absent in rodents.
Although pseudorabies virus is a commonly employed retrograde transneuronal tracer, the agent is not effective for mapping neural projections in cats (12). However, many recent studies have utilized an alternate transneuronal tracer: rabies virus (for reviews, see Refs. 30, 55). This work demonstrated that rabies virus is transported exclusively in the retrograde direction with no evidence of uptake by fibers of passage. It was well documented in these experiments that rabies does not cause cell lysis and is effective in identifying a hierarchy of multisynaptic circuits innervating specific targets. Moreover, rabies virus is selectively uptaken by motoneuron terminals and not sympathetic or parasympathetic efferents or sensory endings (30, 54, 55). This selectivity is presumably due to the high affinity of the virus for the skeletal muscle nicotinic receptor, which concentrates rabies at the neuromuscular junction (32). As such, rabies virus is an ideal transneuronal tracer for mapping motor pathways.
The present experiments aimed to identify the extended neural pathways that regulate diaphragm function in the cat by performing transneuronal retrograde tracing with the N2C strain of rabies virus. Injecting the virus into the diaphragm and analyzing the temporal progression of labeling throughout the central nervous system established the location and hierarchy of brain regions that influence diaphragm activity. The goal of this study was to discern regions of the nervous system that may be involved in coordinating contraction of the diaphragm during behaviors other than eupnea, such that these areas can be targeted for analysis in subsequent physiological studies.
METHODS
All of the procedures used in this study conformed to the American Physiological Society's “Guiding Principles for the Care and Use of Animals” and the National Institutes of Health “Guide for the Care and Use of Laboratory Animals” and were approved by the University of Pittsburgh's Institutional Animal Care and Use Committee.
Seventeen 6- to 12-mo-old female cats (Liberty Research, Waverly, NY), weighing 2.6–3.5 kg, were used in these experiments. Fourteen of the animals were employed for the principal experiment tracing inputs to phrenic motoneurons, whereas three others were used in control studies in which rabies was injected into hindlimb muscles to determine whether sympathetic efferents transport the virus (see results). The animals were not vaccinated for rabies, and additionally serology was performed to ensure that they did not have immunity to the virus. The animals were housed individually and maintained under a 12:12-h light-dark cycle at 72°F. Before the onset of experiments, animals were moved to a facility approved for Biosafety Level 2 (BSL2) experiments. The animals were acclimated to a BSL2 housing room for a minimum of 2 days before being injected with rabies virus and were subsequently maintained and euthanized in the BSL2 facility. All procedures conformed to the Centers for Disease Control and National Institutes of Health requirements for the use of infectious pathogens (14a).
The N2C strain of rabies virus was utilized for injections into all animals. Dr. Peter Strick of the University of Pittsburgh provided the stocks of virus at a titer 1 × 108 plaque forming units/ml. The virus was stored at −80°C and thawed just before injection. All personnel handling the virus or infected animals had been vaccinated against rabies. Excess virus was inactivated with Clorox and disposed of as biohazardous waste.
Surgical Procedures
Surgical procedures were conducted under aseptic conditions within a dedicated surgical suite approved for BSL2 procedures. Animals were initially anesthetized using ketamine (15 mg/kg) and acepromazine (1 mg/kg) injected intramuscularly and subsequently intubated and maintained under deep anesthesia using 1–2% isoflurane vaporized in O2. The concentration of isoflurane delivered was titrated to maintain a stable heart rate and breathing frequency. Body temperature was maintained near 38°C using a heating pad.
In the 14 animals used for experiments, considering the neural pathways that regulate diaphragm activity, the ventral surface of the diaphragm was approached via a midline incision through the skin and musculature of the abdominal wall. The viscera were retracted to expose the diaphragm, and injections of rabies virus were made under the peritoneal lining of the muscle using a 25-μl Hamilton syringe equipped with a 22-gauge needle. Injections of 25-μl volume were made at 8 (n = 10) or 12 (n = 4) sites throughout the left costal region of the diaphragm to deliver a total of 200–300 μl of virus. At each site ∼5–10 μl of inoculum were injected at a time in several second intervals as the needle remained in the diaphragm. Additionally, the needle was maintained in the diaphragm for several seconds after completion of each injection series to minimize leakage of the virus. Upon completion of the inoculations, the peritoneal surface of the diaphragm was swabbed with cotton to absorb any leaked virus, and the abdominal musculature and skin were sutured shut. To provide analgesia, animals received intramuscular injections of ketoprofen every 24 h for 3 days (2 mg/kg initial dose and 1 mg/kg maintenance dose). The health and well-being of the animals was monitored at least every 8 h until they were anesthetized and perfused at the conclusion of the experiment.
Tissue Preparation
After survival times ranging from 61.5 to 144 h following injections of virus into the diaphragm, animals were anesthetized using ketamine (15 mg/kg) and acepromazine (1 mg/kg) injected intramuscularly, followed by pentobarbital sodium (40 mg/kg) injected intraperitoneally. After verifying the absence of nociceptive reflexes, the animals were transcardially perfused with ∼1 liter of heparinized saline followed by ∼2 liters of 4% paraformaldehyde-lysine-periodate fixative (36). The brain and spinal cord were removed, postfixed 1–2 days in 4°C paraformaldehyde-lysine-periodate, and cryoprotected in 30% sucrose in 0.1 M phosphate-buffered saline (PBS) for 2 days. Spinal cord and brain sections were cut at a thickness of 40 μm using a freezing microtome. The thoracic spinal cord was sectioned horizontally and sequentially collected in four wells of phosphate-Tris-azide buffer (PTA). In animals where injections of virus were made into the diaphragm, the cervical spinal cord was sectioned coronally and sequentially collected in four wells of PTA; the lumbar spinal cord was similarly sectioned in control experiment animals with virus injections into the hindlimb. The medulla, pons, midbrain, and diencephalon were also sectioned coronally but sequentially collected in six wells of PTA. The telencephalon from animals with virus injections into the diaphragm was either cut sagitally (n = 12) or coronally (n = 2) and collected in six wells of PTA. Tissue sections were stored at 4°C in PTA until being processed for immunohistochemical localization of rabies.
Immunohistochemistry
At least one well of tissue from each brain and spinal cord region of every animal was processed for the localization of rabies virus using avidin-biotin immunoperoxidase techniques (26). Sections were incubated in 0.3% hydrogen peroxide in distilled water for 30 min to quench endogenous peroxidase activity. After several rinses in PTA or 0.1 M PBS, sections were incubated in 1.5% normal horse serum in PTA or 10 mM PBS for 20 min with agitation. After rinsing, sections were incubated in a mouse monoclonal antibody directed against the rabies virus phosphoprotein that resides in the infective nucleocapsid core (M957, diluted 1:300 in PTA) (30) for 18–72 h at 4°C. The specificity of this antibody to rabies virus has been established previously (39). After being brought to room temperature, sections were rinsed several times in phosphate Tris (PT) or PBS buffer and incubated in biotinylated secondary antibody (1:200, biotinylated horse antimouse IgG, Vector Laboratories, Burlingame, CA) in 10 mM PBS or PT for 30 min with agitation. Following rinsing, sections were incubated in 1% ABC complex (Vector) in PT or 10 mM PBS for 1 h, rinsed again, and then incubated in a saturated solution of filtered 3,3′-diaminobenzidine in PT with 0.0002% hydrogen peroxide for 3 min or until the reaction was complete, as determined by visual inspection. Sections were then rinsed in PT or PBS, mounted on gelatin-coated slides, cleared in ascending concentrations of ethanol followed by three changes of xylene, and coverslipped with cytoseal. Most sections were counterstained with neutral red for identification of cytoarchitecture.
Analysis
Sections from animals with rabies virus injections into the diaphragm were initially inspected qualitatively to document the pattern of infection. A quantitative mapping of the distribution of infected neurons in the brain stem, midbrain, and diencephalon of six selected cases was conducted using the Stereoinvestigator image analysis system (MicroBrightField, Williston, VT), interfaced with a Nikon microscope. Neuronal cell bodies were only counted if they contained a homologous distribution of antigen within their cytoplasm, and the cell nucleus was visible. Neurons were categorized as small if the cell body was ∼10 μm in diameter, medium sized if the diameter was ∼20 μm, and large if the diameter was over 30 μm. For the quantitative analysis, sections through the medulla, pons, cerebellum, and midbrain were mapped at a frequency of every fifth section from one well of tissue. Initial qualitative analyses demonstrated that this frequency provided an accurate representation of labeling within the most heavily infected cell groups. Comparable sections were mapped in all cases considered. Similarly, the quantitative mapping of labeled neurons in the diencephalon focused upon the areas that were identified as being heavily infected in initial qualitative analyses. The telencephalon was examined qualitatively. The boundaries of specific areas were determined using the Berman cat atlas (9), digital feline brain atlases (http://BrainMaps.org), and a review of cerebral cortex connectivity (51). Quantitative maps of cervical spinal cord sections were generated for eight animals.
A subset of sections was photographed with a Zeiss Axioplan photomicroscope equipped with a Hamamatsu camera (Hamamatsu Photonics, Hamamatsu, Japan) and a Simple-32 PCI image analysis system (Compix, Lake Oswego, OR) or a Nikon Eclipse E600N photomicroscope equipped with a Spot RT monochrome digital camera (Diagnostic Instruments, Sterling Heights, MI) and MetaMorph imaging software (Universal, Downingtown, PA). Adobe Systems (San Jose, CA) Photoshop and Illustrator software were used for preparing photographs and maps of sections for publication. Brightness and contrast of images were adjusted without any other alterations.
RESULTS
Injection of rabies virus into the diaphragm produced transneuronal labeling in the nervous system of 11 animals, with survival times ranging from 61.5 to 144 h. In three additional animals, virus injections into the diaphragm were unsuccessful in producing retrograde infection of spinal or brain stem neurons after survival times ranging from 96 to 120 h. The three animals with the longest survival times (120–144 h) had infections that were too extensive to define the route through which higher cell groups were infected; these cases were not considered. The eight animals used for analysis were categorized into early, intermediate, and late infection groups based on the extensiveness of labeling (Tables 1 and 2). The early group (animals C53, C52, and C21) had infection confined to the spinal cord, medulla, and pons. The intermediate group (cases C38, C39, and C51) included cats with increased numbers of neurons in the areas labeled in early cases with the addition of new areas extending into the midbrain, diencephalons, and telencephalon. The late group (animals C36 and C37) included cats with heavier infection within areas that were labeled in intermediate cases with the addition of a few other regions. The progression of infection was not entirely correlated with the postinoculation interval, likely because factors such as the body weight of animals also affect the rate of viral transport through specific circuits (31).
Table 1.
Total numbers of labeled neurons counted bilaterally within infected areas of the medulla, pons, and cerebellum
| Brain Area | Early Group |
Intermediate Group | Late Group | |||||
|---|---|---|---|---|---|---|---|---|
| C53 | C52 | C21 | C38 | C39 | C51 | C36 | C37 | |
| Dorsal respiratory group | 9 | 154 | 294 | 371 | 747 | 488 | ✓ | ✓ |
| Medial nucleus of the solitary tract | 0 | 1 | 20 | 41 | 88 | 26 | ✓ | ✓ |
| Caudal ventral respiratory group | 1 | 25 | 85 | 101 | 181 | 81 | ✓ | ✓ |
| Rostral ventral respiratory group | 8 | 218 | 516 | 478 | 604 | 504 | ✓ | ✓ |
| Pre-Bötzinger complex | 0 | 79 | 204 | 279 | 279 | 280 | ✓ | ✓ |
| Bötzinger complex | 1 | 89 | 104 | 148 | 305 | 149 | ✓ | ✓ |
| Kölliker-Fuse nucleus | 0 | 55 | 149 | 183 | 302 | 177 | ✓ | ✓ |
| Medial parabrachial nucleus | 0 | 9 | 50 | 143 | 159 | 92 | ✓ | ✓ |
| Lateral parabrachial nucleus | 0 | 12 | 1 | 29 | 29 | 22 | ✓ | ✓ |
| Medial reticular formation | 0 | 47 | 121 | 538 | 1,570 | 1,491 | ✓ | ✓ |
| Medullary lateral reticular field | 0 | 84 | 297 | 554 | 1,405 | 919 | ✓ | ✓ |
| Pontine lateral reticular field | 0 | 22 | 39 | 133 | 650 | 340 | ✓ | ✓ |
| Ventral paratrigeminal region | 0 | 26 | 38 | 48 | 99 | 68 | ✓ | ✓ |
| Spinal trigeminal nucleus | 0 | 0 | 0 | 11 | 63 | 37 | ✓ | ✓ |
| Principal trigeminal nucleus | 0 | 0 | 0 | 0 | 9 | 20 | ✓ | ✓ |
| Medial vestibular nucleus | 0 | 0 | 0 | 10 | 35 | 21 | ✓ | ✓ |
| Inferior vestibular nucleus | 0 | 1 | 2 | 40 | 74 | 51 | ✓ | ✓ |
| Lateral vestibular nucleus | 0 | 7 | 2 | 31 | 133 | 257 | ✓ | ✓ |
| Superior vestibular nucleus | 0 | 0 | 0 | 25 | 11 | 9 | ✓ | ✓ |
| Fastigial nucleus | 0 | 0 | 1 | 6 | 41 | 30 | ✓ | ✓ |
| Dentate nucleus | 0 | 0 | 1 | 1 | 9 | 24 | ✓ | ✓ |
| Nucleus interpositus | 0 | 0 | 0 | 0 | 0 | 0 | ✓ | ✓ |
| Purkinje cell layer | 0 | 0 | 0 | 0 | 0 | 0 | ✓ | ✓ |
| Retrotrapezoid nucleus | 0 | 0 | 1 | 16 | 29 | 16 | ✓ | ✓ |
| Raphe pallidus | 0 | 2 | 1 | 15 | 43 | 16 | ✓ | ✓ |
| Raphe obscurus | 0 | 3 | 4 | 4 | 31 | 20 | ✓ | ✓ |
| Lateral reticular nucleus | 0 | 1 | 9 | 2 | 13 | 20 | ✓ | ✓ |
| Cuneate nucleus | 0 | 0 | 3 | 2 | 13 | 20 | ✓ | ✓ |
| Gracile nucleus | 0 | 0 | 0 | 0 | 0 | 2 | ✓ | ✓ |
| Dorsal cochlear nucleus | 0 | 0 | 0 | 0 | 1 | 0 | ✓ | ✓ |
| Anteroventral cochlear nucleus | 0 | 0 | 0 | 0 | 0 | 1 | ✓ | ✓ |
| Posteroventral cochlear nucleus | 0 | 0 | 0 | 0 | 0 | 0 | ✓ | ✓ |
| Nucleus of the trapezoid body | 0 | 0 | 0 | 0 | 10 | 13 | ✓ | ✓ |
Check marks (✓) indicate the presence of infection as determined by qualitative analysis.
Table 2.
Total numbers of labeled neurons counted bilaterally within infected areas of the midbrain, diencephalon, and cortex
| Brain Area | Intermediate Group |
Late Group | |||
|---|---|---|---|---|---|
| C38 | C39 | C51 | C36 | C37 | |
| Midbrain | |||||
| Lateral periaqueductal gray | 83 | 458 | 227 | ✓ | ✓ |
| Ventrolateral periaqueductal gray | 6 | 56 | 90 | ✓ | ✓ |
| Dorsal periaqueductal gray | 1 | 48 | 111 | ✓ | ✓ |
| Dorsolateral periaqueductal gray | 0 | 2 | 6 | ✓ | ✓ |
| Mesencephalic reticular nucleus | 35 | 645 | 969 | ✓ | ✓ |
| Pedunculopontine nucleus | 9 | 215 | 99 | ✓ | ✓ |
| Cuneiform nucleus | 5 | 56 | 14 | ✓ | ✓ |
| Nucleus locus coeruleus | 5 | 44 | 19 | ✓ | ✓ |
| Edinger-Westphal nucleus | 6 | 59 | 17 | ✓ | ✓ |
| Red nucleus | 1 | 51 | 204 | ✓ | ✓ |
| Intermediate layer of the superior colliculus | 0 | 29 | 20 | ✓ | ✓ |
| Deep layer of the superior colliculus | 0 | 33 | 13 | ✓ | ✓ |
| Central nucleus of the inferior colliculus | 0 | 3 | 4 | ✓ | ✓ |
| Nucleus of the posterior commissure | 0 | 13 | 14 | ✓ | ✓ |
| Dorsal nucleus of the lateral lemniscus | 0 | 0 | 9 | ✓ | ✓ |
| External nucleus of the inferior colliculus | 0 | 0 | 0 | ✓ | ✓ |
| Dorsal raphe nucleus | 0 | 0 | 0 | ✓ | ✓ |
| Substantia nigra pars compacta | 0 | 0 | 0 | ✓ | ✓ |
| Substantia nigra pars reticulata | 0 | 0 | 0 | ✓ | ✓ |
| Diencephalon and telencephalon | |||||
| Perifornical area | 0 | 108 | 148 | ✓ | ✓ |
| Paraventricular nucleus of the hypothalamus | 0 | 17 | 34 | ✓ | ✓ |
| Central lateral nucleus of the thalamus | 0 | 18 | 17 | ✓ | ✓ |
| Ventrolateral nucleus of the thalamus | 0 | 20 | 23 | ✓ | ✓ |
| Paraventricular nucleus of the thalamus | 0 | 5 | 0 | ✓ | ✓ |
| Central nucleus of the amygdala | 0 | ✓ | ✓ | ✓ | ✓ |
| Insular cortex | 0 | ✓ | ✓ | ✓ | ✓ |
| Pericruciate cortex | 0 | ✓ | ✓ | ✓ | ✓ |
| Prefrontal cortex | 0 | ✓ | ✓ | ✓ | ✓ |
Infection of Spinal Neurons Following Rabies Virus Injections Into the Diaphragm
In all animals deemed to have successful rabies inoculations into the diaphragm, large infected neurons were present in the central portion of the ventral horn of the C4–C6 spinal segments ipsilateral to the side injected with virus, between the medial and lateral columns of motoneurons. In all cases except animal C53, the large neurons formed a tight cluster, and so much reaction product was present that it was difficult to obtain an accurate count of the cells. An example of such a cell cluster in the C5 segment of animal C21 is shown in Fig. 1B. The position of this cluster of large infected neurons in the gray matter corresponds to the known location of motoneurons innervating the diaphragm in the cat (8, 48). As such, the cells were classified as putative phrenic motoneurons. In animal C53, large infected neurons were present in a similar location, but the number was much lower than in the other cases (only 1–2 cells per section). Presumably, only a fraction of phrenic motoneurons was infected in this animal, likely because the postinoculation survival time was >24 h shorter than in the other cases.
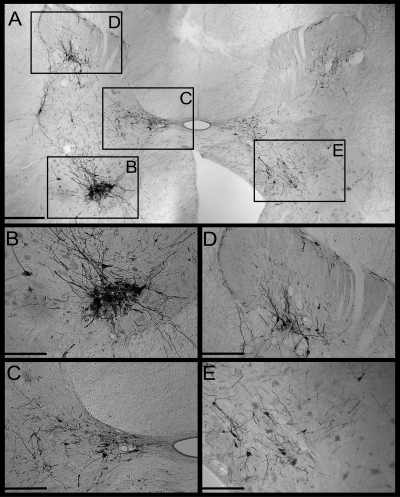
Fig. 1.
Photomicrographs of infected neurons in the C5 spinal cord of animal C21. A: photomontage of the entire C5 spinal gray matter of one section. Boxes denote regions depicted at higher magnification in subsequent panels. The left side of the section is ipsilateral to the rabies injections into the diaphragm. B: a dense cluster of presumed motoneurons in the ventral horn. C: a group of infected presumed interneurons in Rexed's lamina X and the medial portion of lamina VII. D: a group of infected presumed interneurons concentrated in Rexed's lamina V. E: infected presumed interneurons in medial lamina VII and lamina VIII contralateral to the side of injections. Bars designate 500 μm in A, and 250 μm in the other panels.
In addition to these large neurons, smaller labeled cells were distributed bilaterally in the cervical spinal cord in every animal and were deemed to be interneurons that were transneuronally infected by passage of rabies virus from phrenic motoneurons. Photomicrographs of putative interneurons in the C5 segment of animal C21 are shown in Fig. 1, C, D, and E. Figure 2A illustrates the locations of the putative interneurons in representative sections from each cervical segment of early infection case C21, whereas Fig. 2B shows the locations of putative interneurons in intermediate infection case C51. All animals except C53, the case with the shortest survival time and fewest labeled cells, exhibited infected presumed interneurons in all cervical segments. In animal C53, however, the presumed interneurons were limited to the C2 and C5–C7 segments. In the animals with the most advanced infections (C36 and C37), the relative number of labeled presumed interneurons was about the same in the upper cervical cord as in the C4–C7 segments. In contrast, in cases C21, C52, and C53, the amount of infection in C1–C2 was <15% of that in segments containing the diaphragm motoneurons. In animals C38, C51, and C39, the number of labeled C1 neurons was 30–40% of the number in C4–C6.
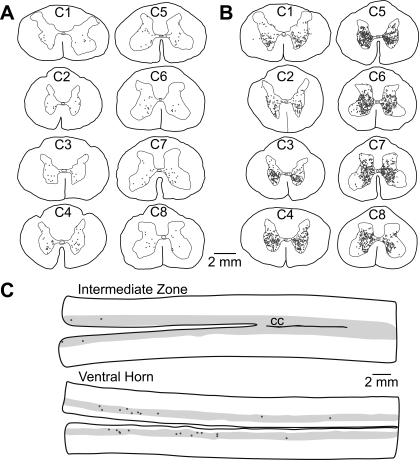
Fig. 2.
Locations of presumed interneurons labeled following the injection of rabies virus into the diaphragm. A and B: locations of labeled cervical interneurons in an early infection (animal C21; A) and an intermediate infection (animal C51; B) case. Representative transverse sections from each cervical segment are shown; the level is indicated on the section. C: locations of labeled interneurons in horizontal sections through the T5–T9 thoracic spinal cord of intermediate infection case C38. The gray matter is indicated as a shaded area, and the rostral end of the sections is toward the left side. The top section is through the intermediate zone, the portions of Rexed's lamina VII and X near the level of the central canal (CC). The bottom section is through the ventral horn. No sections that were clearly through the dorsal horn contained any infected neurons and thus are not provided.
In all of the sections shown in Fig. 2, A and B, the number of infected presumed interneurons in the dorsal horn (Rexed's laminae I-VI) is lower than in the ventral horn (laminae VII-X). The quantitative analysis revealed that, except for a few segments in the animals with the most extensive infection, the majority of labeling was confined to the ventral horn. In animals with early infection (C21, C52, C53), >70% of the presumed interneurons were located in the ventral horn in every segment. A number of other patterns were also observed with regard to the locations of the presumed interneurons. For example, in all cases, an aggregation of labeled cells was present adjacent to the central canal, in medial lamina VII and lamina X of the segments containing diaphragm motoneurons (see Fig. 1C for an example).
Horizontal sections through the thoracic spinal cord were additionally examined for all animals. Infected neurons were observed in the upper thoracic cord in all cases, mainly in sections through the ventral horn and region near the central canal (intermediate zone). However, in animals C53, C21, C52, and C38, only a few scattered neurons were present caudal to the T4 segment. For example, Fig, 2C shows the distribution of labeled neurons in sections through the intermediate zone and ventral horn of the T5–T9 segments in case C38. In these four animals, no infected cells were observed along the lateral border of the thoracic cord gray matter, particularly in the intermediolateral region that contains sympathetic preganglionic neurons. However, the thoracic infection was much more extensive in the other cases, all of which also exhibited considerable labeling in the brain that extended to the cortex. It was impossible to distinguish the intermediolateral cell column in these animals and determine whether sympathetic preganglionic neurons were infected.
Infection of Neurons in the Medulla and Pons Following Rabies Virus Injections Into the Diaphragm
Medullary respiratory groups.
The locations of the medullary respiratory groups in the cat have been documented in numerous physiological studies (for reviews, see Refs. 15, 16, 23). The DRG has been described as a focal group of neurons concentrated in the ventrolateral nucleus of the solitary tract (15, 16). Accordingly, a large number of rabies-infected neurons was concentrated bilaterally in the ventrolateral nucleus of the solitary tract; this cell group also circumscribed the solitary tract. The number of infected DRG neurons in different cases is shown in Table 1. At longer survival times, labeling increased predominantly in the region circumscribing the solitary tract and medial nucleus tractus solitarius. This is illustrated in Figs. 3 and 4, which show the pattern of infection in the DRG of animals with differing stages of infection.
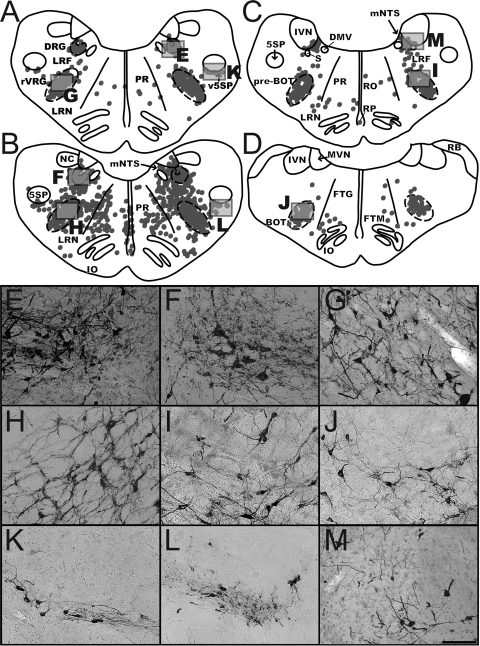
Fig. 3.
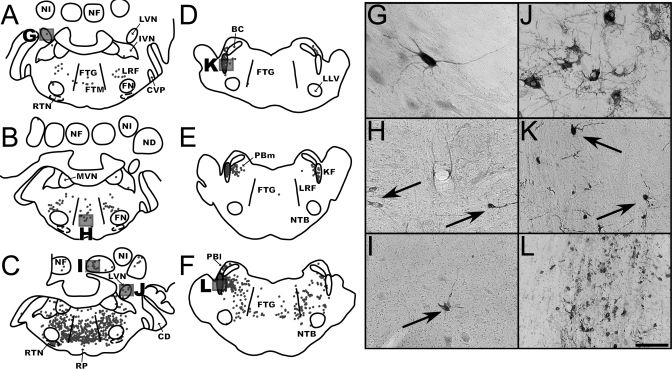
Distribution of labeled neurons in the medullary respiratory groups and adjacent regions. A and B: maps of sections from early infection (animal C21; A) and intermediate infection (animal C39; B) cases illustrating the localization of rabies-infected neurons within the dorsal respiratory group (DRG) and rostral ventral respiratory group (rVRG) at 12.1 mm posterior to the interaural plane. C: map of a section through the pre-Bötzinger (pre-BOT) complex in early infection case C21 at 11.6 mm posterior to the interaural plane. D: map of a section through the Bötzinger (BOT) complex in early infection animal C52 at 10 mm posterior to the interaural plane. Each dot in the maps represents a single labeled neuron. Boxes indicate the areas depicted in the photomicrographs at the bottom of the figure. E and F show the DRG, G and H the rVRG, I the pre-BOT, J the BOT, K and L the ventral paratrigeminal nucleus (v5SP), and M the region of nucleus tractus solitarius (NTS) adjacent to the inferior vestibular nucleus (IVN). Most of the micrographs are from the same sections used to generate the maps, although F and H are from the intermediate infection case C51 (where the contrast between the labeled neurons and background was clearer than in animal C39). Scale bars in each photomicrograph represent 250 μm. 5SP, spinal trigeminal nucleus; DMV, dorsal motor nucleus of the vagus; FTG, gigantocellular tegmental field; FTM, magnocellular tegmental field; IO, inferior olivary complex; LRF, lateral reticular field; LRN, lateral reticular nucleus; mNTS, medial nucleus of the solitary tract; MVN, medial vestibular nucleus; NC, cuneate nucleus; PR, paramedian reticular nucleus; RB, restiform body; RO, raphe obscurus; RP, raphe pallidus; S, solitary tract.
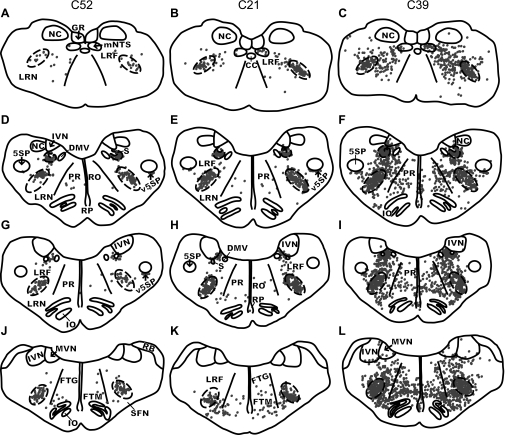
Fig. 4.
Maps of sections through the caudal and intermediate regions of the medulla in two early infection (C52, left column; C21, middle column) cases and one intermediate infection (C39, right column) case. Each dot represents a single infected neuron. The DRG and VRG respiratory columns are depicted as dashed areas on each map. A–C: the medulla at the level of the caudal VRG at 16 mm posterior from the interaural plane. D–F: the DRG and the rostral VRG at 12.1 mm posterior from the interaural plane. G–I: the pre-BOT complex at 11.6 mm posterior from the interaural plane. J–L: the BOT complex at 10 mm posterior from the interaural plane. Because of slight differences in the plane of cutting, sections at the same approximate anteroposterior level had different shapes and contained different structures. GR, gracile nucleus.
The VRG has been defined as a continuous column of neurons in the ventrolateral brain stem that extends from the first cervical roots to the middle of the pons (15, 16). This column of respiratory neurons has been functionally subdivided into the caudal VRG (cVRG), rostral VRG (rVRG), Bötzinger complex (BOT), and pre-Bötzinger complex (pre-BOT) based on the differential distribution of inspiratory and expiratory neurons (3, 17). During quantitative analyses (see Table 1), the cVRG was defined as the region of the VRG spanning from the spinomedullary junction to the level of the obex, the rVRG as the region extending to ∼2.4 mm rostral to the obex, BOT as the region from 3.1–4.7 mm rostral to the obex, and pre-BOT as the region interposed between the rVRG and BOT. In animal C53, which had the shortest survival time, a few labeled neurons were found within all of the aforementioned divisions of VRG, except for pre-BOT; most of the labeled cells were located in the rVRG. At later survival times, the number of infected neurons increased greatly in every subdivision, with the most labeled cells still found in the rVRG and the least in cVRG. The distribution of infected neurons in the VRG is indicated in Figs. 3 and 4 and Table 1.
Parabrachial nucleus.
The parabrachial nuclear complex, which is known to contain the pontine respiratory groups (15, 16, 23), comprises three major subdivisions: the Kölliker-Fuse nucleus (KF), and the medial and lateral parabrachial subfields. Infected neurons were present in all three regions of all animals, except the one with the shortest survival time. KF contained the most labeled neurons in all animals, particularly those with relatively short survival times, as indicated in Table 1 and illustrated in Fig. 5. Labeled neurons in the parabrachial regions were mostly small, round, or fusiform cells, but KF also contained some larger multipolar cells. Unlike most brain regions, infected cells in the parabrachial complex were lateralized, with 80% or more being present ipsilateral to the side of virus injections into the diaphragm.
Fig. 5.
Distribution of rabies-infected neurons in the rostral medulla at 8.5 mm posterior to the interaural plane (A–C) and the pons at 3.1 posterior to the interaural plane (D–F). A and D are from the early infection case C52, B and E are from the early infection case C21, and C and F are from the intermediate infection case C39. Each dot represents a single labeled neuron. Boxes indicate the areas depicted in the photomicrographs. G and J show the lateral vestibular nucleus (LVN), H the medial reticular formation and raphe nuclei, I the fastigial nucleus of the cerebellum (NF), and K and L the Kölliker-Fuse nucleus (KF). Arrows in the photomicrographs denote examples of infected neurons. Most of the micrographs are from the same sections used to generate the maps, although J is from the intermediate infection case C51 (where the contrast between the labeled neurons and background was clearer than in animal C39). Because of slight differences in the plane of cutting, sections at the same approximate anteroposterior level had different shapes and contained different structures. Scale bars represent 250 μm. BC, brachium conjunctivum; CD, dorsal cochlear nucleus; CVP, posteroventral cochlear nucleus; FN, facial nucleus; LLV, ventral nucleus of the lateral lemniscus; ND, dentate nucleus; NI, nucleus interpositus; NTB, nucleus of the trapezoid body; PBl, lateral parabrachial nucleus; PBm, medial parabrachial nucleus; RTN, retrotrapezoid nucleus.
Medial reticular formation.
The number of infected neurons in the medial reticular formation (MRF) of the medulla and pons, defined as the paramedian, magnocellular, and gigantocellular reticular fields (9), was also determined. MRF infection was observed in every animal, except the one with the shortest survival time. However, there was a large increase in the number of labeled MRF neurons as the brain stem infection became more advanced (see Table 1). Figures 4 and 5 illustrate the distribution of infected cells within the MRF. Labeled neurons in this region were mostly large multipolar cells that were spread bilaterally. There was a differential distribution of neurons along the rostral-caudal axis; in each animal, the largest number of infected MRF neurons was observed from 8.5 to 10 mm posterior to the interaural plane, where cells were densely packed within the ventromedial portion of the magnocellular reticular field. Neurons were more diffusely scattered in the paramedian and gigantocellular reticular fields.
Lateral reticular field.
The number of labeled neurons in the lateral reticular field (LRF, 9) was determined from sections spanning from 3.1 to 18.3 mm posterior to the interaural plane. Figures 4 and 5 illustrate the location and pattern of infection in this region. In particular, Fig. 4 shows that infected neurons in the medullary portion of the LRF were concentrated in the region surrounding the VRG and within the transitional area between the DRG and VRG. As such, and also because there was not a distinct morphological difference between neurons in the VRG and the surrounding LRF, cell counts in these regions may have been over- or underestimated. The labeled LRF neurons were mostly small to medium in size and multipolar or fusiform in shape and were distributed bilaterally. LRF infection was much heavier in the medulla (7.1–18.3 mm posterior to the interaural plane) than in the pons of all animals, as indicated in Table 1. Additionally, the number of labeled neurons increased greatly between the early and intermediate infection groups.
Trigeminal nuclei and ventral paratrigeminal nucleus.
The ventral paratrigeminal nucleus (v5SP), defined as the area just ventral to the caudal spinal trigeminal nucleus (5SP) (Ref. 44; see Fig. 3), was infected in all animals except the one with the shortest survival time (Table 1). Infected neurons in this region were mostly small fusiform cells with an occasional larger multipolar neuron present. Labeled v5SP neurons were located on both sides of the brain from 11.6–13.5 mm posterior to the interaural plane. Furthermore, the 5SP was also labeled, but only in the intermediate and late infection group animals. Similarly, the principal trigeminal nucleus contained a few labeled cells, but just in intermediate and late infection group cases. In particular, trigeminal nucleus only contained infected cells in cases that had cortical labeling (e.g., animals C39 and C51 but not C38).
Vestibular nuclei and cerebellum.
The vestibular nuclei contained infected cells bilaterally in all animals but the one with the shortest survival time, as indicated in Table 1 and Figs. 4 and 5. In early infection group animals, the labeling was confined to the inferior vestibular nucleus (IVN) and the lateral vestibular nucleus (LVN). In addition, numerous infected neurons were present at the border between IVN and NTS in these cases (see Fig. 3, C and M), which were not considered to be located in the vestibular nuclei. Thus the amount of labeling in the vestibular nucleus complex could have been underestimated. In the intermediate infection group, labeled neurons were observed in all of the vestibular nuclei, as indicated in Table 1, with the largest number of cells being located in LVN. Labeled cells in the LVN were large and multipolar, those in IVN and SVN were mostly medium-sized and multipolar, and those in MVN were small and round or fusiform.
Infected cells in the cerebellum were confined to the deep cerebellar nuclei, except in the cases with the most advanced infection that also included labeling in the Purkinje cell layer. Only a single neuron was observed in the fastigial and dentate cell groups of one early infection case (C21), but labeling increased in intermediate infection animals, as indicated in Table 1. The number of labeled cells was larger in the fastigial nucleus than in dentate, and interpositus was not labeled except in the animals with the most advanced infections. Infected cells in these nuclei were small to medium multipolar or fusiform cells. Figure 5 illustrates the locations and morphology of labeled cerebellar neurons.
Chemosensory regions.
Both the retrotrapezoid nucleus (RTN) and the medullary raphe nuclei, which comprise raphe pallidus and raphe obscurus, have been implicated in chemoreception (41, 42). The caudal extent of the RTN is located 3.2–4 mm lateral to the midline and ventral to the retrofacial nucleus; rostrally, the region spans ventral and ventromedial to the facial nucleus to the caudal pole of the superior olivary nucleus (53). The location of the RTN and the positions of infected neurons within this region are shown in Fig. 5. Only one labeled neuron was observed in the RTN of a single early infection case (C21). In the intermediate infection group, only 16–29 labeled RTN neurons were observed bilaterally (see Table 1), ventral and medial to the facial nucleus. Infected neurons in this region were mostly small, round, or fusiform cells.
The medullary raphe nuclei were infected in every animal except the one with the shortest survival time, although the number of labeled cells was only modest (see Table 1). A caveat is that the counts of labeled neurons in the rostral portions of these nuclei may have been underestimated due to the dense packing of infected neurons in the adjacent magnocellular field of the MRF. Infected cells presumed to be in the medullary raphe nuclei were small to medium in size and fusiform in shape. The distribution of raphe labeling is shown in Figs. 3 and 4.
Other medullary and pontine nuclei.
The lateral reticular nucleus, located within the caudal ventrolateral medulla (see Figs. 3 and 4), was infected in every case except for the one with the shortest survival time. However, the number of labeled cells was only moderate (see Table 1). The cuneate nucleus also was modestly labeled, but this was mainly limited to cases where infection advanced to the diencephalon and cortex. The gracile nucleus also became labeled when the brain infection was advanced, as did several nuclei that process auditory information, including the dorsal and ventral cochlear nuclei and the nucleus of the trapezoid body (see Table 1).
Midbrain Labeling Following Rabies Virus Injections Into the Diaphragm
None of the early group cases had labeling in the midbrain, but all of the intermediate infection animals did. One animal with intermediate infection (C38) had labeling in the midbrain but not the diencephalon or cerebral cortex, while infection was present in the latter two areas of the other animals belonging to this group (C51 and C39, see Table 2). In animal C38, the midbrain infection was most pronounced within a dense group of cells located in the ventral extent of the lateral periaqueductal gray matter (lPAG). In the other two intermediate group animals, considerable labeling was also observed in the lPAG, with a smaller number of infected cells present in the ventrolateral periaqueductal gray (vlPAG) and dorsal periaqueductal gray (see Table 2). The infected cells in lPAG and vlPAG were small to medium multipolar and fusiform neurons, while those in dorsal periaqueductal gray were generally small in size. The dorsolateral periaqueductal gray was the least labeled of the PAG subdivisions. In each of the areas described above, infection was bilateral. Figure 6 illustrates the location of labeled neurons within the PAG.
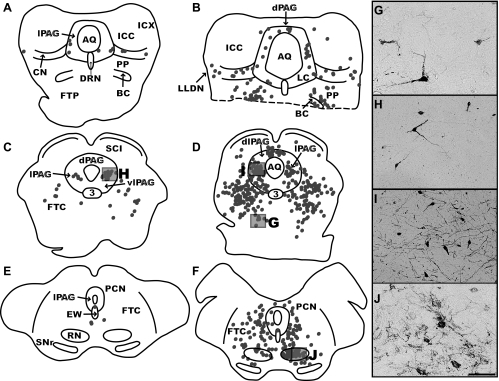
Fig. 6.
Maps of sections through the midbrain and photomicrographs from two intermediate (C38, left column; C51, middle column) cases at different stages of infection. A and B are located at ∼0.6 mm posterior to the interaural plane; C and D at 1.6 mm anterior to the interaural plane; and E and F at 5.2 mm anterior to the interaural plane. The ventral portion of B is missing, as indicated by a dashed line. Each dot represents a single labeled neuron, and boxes indicate the areas depicted in the photomicrographs. G illustrates infected neurons in the mesencephalic reticular nucleus; H and I depict infected neurons in the lateral periaqueductal gray matter (lPAG); and J shows neurons in the red nucleus (RN). Most of the micrographs are from the same cases used to generate the maps, although I is from case C39. Scale bar represents 250 μm. 3, Oculomotor nucleus; AQ, cerebral aqueduct; CN, cuneate nucleus; dlPAG, dorsolateral periaqueductal gray matter; dPAG, dorsal periaqueductal gray matter; DRN, dorsal raphe nucleus; EW, Edinger-Westphal nucleus; ICC, central nucleus of the inferior colliculus; FTC, central tegmental field; FTP, paralemniscal tegmental field; ICX, external nucleus of the inferior colliculus; LC, nucleus locus coeruleus; LLDN, dorsal nucleus of the lateral lemniscus; PCN, nucleus of the posterior commissure; PP, pedunculopontine nucleus; SCI, intermediate layer of the superior colliculus; SNr, substantia nigra pars reticulata; vlPAG, ventrolateral periaqueductal gray matter.
The mesencephalic reticular nucleus (MRN) was very heavily labeled in the two intermediate group animals with infection that advanced to cortex, although labeling was less extensive in animal C38 (see Table 2 and Fig. 6). The MRN includes the paralemniscal and central tegmental fields (9) and extends from 2.1 mm posterior to 6.4 mm anterior to the interaural plane. Infected MRN cells were distributed bilaterally; most were medium sized and either multipolar or fusiform in shape, although a few large and small neurons were noted.
The pedunculopontine (PP) and cuneiform nuclei (CN) also contained labeled cells in the intermediate group animals, as illustrated in Fig. 6. Previous studies identified the PP as a group of neurons surrounding the brachium conjunctivum in the dorsolateral part of the caudal MRN, extending from 2 mm posterior to 1 mm anterior of the interaural plane (28). Infected neurons presumed to be in the PP were not counted as part of the MRN; furthermore, due to the close proximity of these groups, it is possible that the number of labeled neurons in the MRN or PP was over- or underestimated. The CN lies at the dorsal border of PP, is bordered laterally by the dorsal nucleus of the lateral lemniscus and medially by the rostral extent of locus coeruleus (LC) and the caudal extent of PAG, and spans from 0.9 to 2 mm posterior to the interaural plane (9). In animal C38, only a few neurons were labeled in CN and PP, although infection in these regions was much more extensive in the two intermediate group cases with infection in the cerebral cortex (see Table 2). Infection in both regions was bilateral, and the labeled cells were small to medium in size and multipolar or fusiform in shape.
Three other midbrain regions were labeled in all of the intermediate group cases: nucleus LC, the Edinger-Westphal nucleus (EW), and the red nucleus (RN) (see Table 2). Infected neurons in LC were small to medium in size and multipolar or fusiform in shape and were distributed bilaterally, infected neurons in EW were mostly medium-sized multipolar cells, and infected neurons in RN were medium or large multipolar cells distributed with a strong contralateral predominance (over 80% were located on the side opposite to injections). Figure 6 illustrates the locations of the labeled cells in LC, EW, and RN.
Several other areas of the midbrain contained a limited number of infected neurons, but only in animals in which infection had advanced to the diencephalon and telencephalon. These areas included the intermediate and deep layers of the superior colliculus, the central nucleus of the inferior colliculus, and the nucleus of the posterior commissure, as indicated in Table 2. One further midbrain region, the dorsal nucleus of the lateral lemniscus, was labeled in only one of the intermediate cases (animal C51), as well as in animals with late infection. Additional midbrain regions were also labeled in the animals with late infection, including the dorsal raphe nucleus, the external nucleus of the inferior colliculus, substantia nigra pars compacta, and substantia nigra pars reticulata.
Infection of Neurons in the Diencephalon and Telencephalon Following Rabies Virus Injections into the Diaphragm
Diencephalon.
The majority of infected neurons in the diencephalon were observed in the perifornical region (PerF). This area was identified in studies that localized hypocretin-containing neurons and has been defined as a group of cells within the dorsal, posterior, and lateral hypothalamic areas concentrated dorsally and laterally from the fornix, including the region of the fields of Forel just ventral to zona incerta (1, 63). Infection in PerF comprised small to medium multipolar and fusiform cells distributed bilaterally. The number of labeled neurons in PerF increased substantially in the late infection cases. Figure 7 illustrates the pattern of infection in PerF, as well as the morphology of labeled neurons, whereas Table 2 indicates the number of cells in this region.
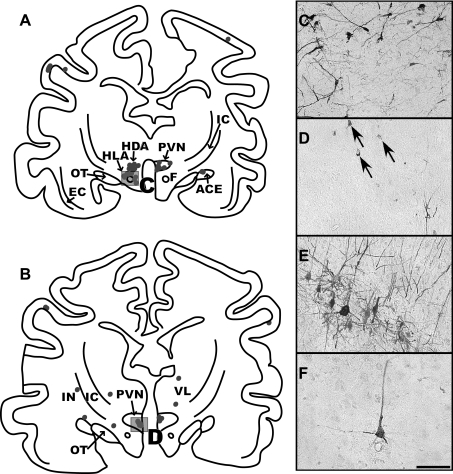
Fig. 7.
A and B: maps of sections through the diencephalon of intermediate infection animal C51. The section shown in A is located at 11 mm anterior to the interaural plane, and B is at 11.8 mm anterior to the interaural plane. Due to the plane of sectioning, the dorsal portion of sections is more rostral than the ventral part, and the right side of sections is more rostral than the left side. This is especially evident in A, where the left side contains labeled neurons in the dorsal (HDA) and lateral hypothalamic areas (HLA), consistent with infection of the perifornical region; in contrast, the right side of the section shows infection in the caudal extent of the paraventricular nucleus of the hypothalamus (PVN). The photomicrograph in C shows the morphology of labeled neurons in the perifornical area of the hypothalamus of a late infection case, animal C36; a box in A indicates the area that is depicted. The photomicrograph in D is from animal C51 and illustrates the morphology of infected neurons in the parvocellular division of the PVN, which are demarked by arrows; a box in B indicates the region illustrated. E and F: photomicrographs of infected neurons in the precruciate gyrus of a late infection (C36) and an intermediate infection (C39) animal, respectively. The cortex from both of these cases was cut in the sagittal plane. The scale bar represents 250 μm. ACE, central nucleus of the amygdala; EC, external capsule; F, fornix; IC, internal capsule; IN, insular cortex; OT, optic tract; VL, ventrolateral nucleus of the thalamus.
A few other areas of the diencephalon in intermediate infection animals also contained a modest amount of labeling (see Table 2), including the medial parvocellular division of the paraventricular nucleus of the hypothalamus, the intralaminar thalamic nuclei in the central lateral nucleus, the ventrolateral nucleus of the thalamus, and the paraventricular nucleus of the thalamus. Infected paraventricular nucleus of the hypothalamus neurons were small multipolar and fusiform cells distributed bilaterally; the location and morphology of these cells is illustrated in Fig. 7. Infected central lateral nucleus and ventrolateral nucleus of the thalamus neurons were mostly small multipolar neurons that were distributed bilaterally. Only a few paraventricular nucleus of the thalamus cells were labeled in one intermediate infection animal, although the number increased in animals with more advanced infection.
Telencephalon.
A few small multipolar neurons were labeled in the central nucleus of the amygdala of animals C39 and C51 (see Table 2). In addition, heavier bilateral labeling was noted in the cerebral cortex of these two animals, as well as in the late infection cases. The largest number of infected neurons was present within the deep lamina of the pericruciate cortex, particularly in the precruciate gyrus. These cells were mostly medium pyramidal neurons, and not large Betz neurons, as illustrated in Fig. 7. Infection was additionally present within the prefrontal and insular cortices; the labeled cells in the prefrontal cortex were small and round, whereas those in the insular cortex were small and multipolar.
Evidence That Rabies Virus was Selectively Transported by Motor Pathways Controlling Diaphragm Function
At the earliest stages of infection, viral antigen was concentrated in the cytoplasm of the soma and proximal dendrites of neurons, but was absent from the nucleus, which is consistent with the replication pattern for an RNA virus. At later stages of infection, the distribution of viral antigen extended progressively into the dendrites. Evidence of cytopathology or lysis of infected neurons was not observed. Additionally, viral antigen was not observed in glia, even those adjacent to neurons that had been replicating virus the longest (i.e., had extensive viral antigen in the dendrites). These observations are consistent with the conclusion that rabies virus was transported transneuronally through neural pathways and was not dispersed through nonspecific extracellular release.
Even in the animals with advanced infections, a variety of brain stem regions were devoid of infection. These included the dorsal motor nucleus of the vagus, which provides parasympathetic innervation of the abdominal viscera, including the organs adjacent to the diaphragm (13). Furthermore, the subretrofacial nucleus (SFN) was devoid of infection in all animals, as indicated in Fig. 4. SFN is located within 0.5 mm of the ventral surface of the brain stem, ventral to the retrofacial nucleus near the rostral tip of the inferior olive, and regulates sympathetic outflow to the vasculature (18). Infection in this area would have been expected, if the sympathetic nervous system had transported rabies virus.
To further examine whether rabies virus infects sympathetic nervous system neurons, we injected 250 μl (n = 2) or 900 μl (n = 1) of rabies virus into the gastrocnemius muscle of three animals. In two of these animals, the spinal cord had previously been severed rostral to the gastrocnemius motoneurons, at L5 or L6, such that infection rostral to the transection could only have resulted by transport of virus by the sympathetic nervous system. An analogous study performed in rats with injections of pseudorabies virus into the gastrocnemius muscle produced considerable infection of sympathetic preganglionic neurons, as well as brain areas known to control sympathetic outflow (34). The postinoculation survival times were 96 h for the animal with the intact spinal cord and 144–156 h for the cases with a severed spinal cord. In all cats, a large number of neurons in the lower lumbar spinal cord were immunopositive for rabies virus. However, no infected neurons were noted in the thoracic spinal cord or brain stem of the animals with spinal transections. Although the thoracic spinal cord did contain labeled cells in the spinal-intact animal, none were positioned in the lateral region of the intermediate zone where sympathetic preganglionic neurons are located. The findings of these control experiments are thus in accord with previous data showing that sympathetic efferents do not provide a route through which rabies virus can be transported to the central nervous system (30, 54, 55).
DISCUSSION
The main contribution of the present study was to demonstrate that a number of brain regions, in addition to the medullary and pontine respiratory groups, contribute to the regulation of diaphragm activity in felines. In the brain stem, these areas include the medial and lateral medullary reticular formation, the region immediately ventral to the 5SP (v5SP), and the raphe and vestibular nuclei. Furthermore, this study is the first to systematically employ transneuronal tracing techniques to define the areas of the cerebellum, midbrain, diencephalon, and telencephalon that contribute to respiratory control. In particular, the FN, lPAG, MRN, PP, RN, PerF, and the pericruciate cerebral cortex were relatively heavily labeled in animals with intermediate levels of infection. The present data, therefore, reveal that a multiplicity of neural pathways contribute to regulating diaphragm contractions in species that utilize their respiratory muscles during complex behaviors, such as vomiting and coordinating diaphragm activity with body movements.
Selectivity of Infection of Motor Circuits Controlling Diaphragm Activity
Following rabies injections into the diaphragm, large infected neurons were present in the area of the C4–C6 spinal gray matter known to contain phrenic motoneurons in the cat (8, 48), and heavy infection was also observed in the regions of the brain stem containing the DRG and VRG (15, 16, 23). Labeling patterns were consistent between animals, such that the areas infected following short survival times were also labeled in animals with more advanced infection. The distribution of infected neurons respected the boundaries of cell groups instead of expanding radially; the latter would be expected if the virus were released indiscriminately through lysis or other nonsynaptic mechanisms. Labeled neurons were not present in the regions of the brain stem known to be involved in regulating sympathetic and parasympathetic outflow to the vasculature or visceral organs adjacent to the diaphragm, including the dorsal motor nucleus of the vagus and the region of the rostral ventrolateral medulla (SFN) that controls blood flow (13, 34). These findings support previous data showing that rabies virus is selectively uptaken by motoneuron terminals and not sympathetic or parasympathetic efferents or sensory endings (30, 54, 55). Furthermore, additional control experiments conducted as part of the present study revealed that sympathetic preganglionic neurons were not infected following the injection of rabies virus into the gastrocnemius muscle, even in a case where a large amount of virus (900 μl) and a long survival time (156 h) were employed. Cumulatively, this evidence strongly suggests that the labeling of central nervous system neurons in the present study resulted exclusively from the transport of rabies virus by motor circuits regulating diaphragm activity.
Distribution of Infected Interneurons in the Spinal Gray Matter
In all animals, numerous infected presumed interneurons were present outside the cluster of labeled cells in C4–C6, surmised to be phrenic motoneurons. These data support electrophysiological evidence that propriospinal neurons in the upper cervical (2, 40) and thoracic (6) spinal cord, as well as local circuit neurons (7, 21), provide inputs to phrenic motoneurons. The infected interneurons were distributed bilaterally throughout the cervical and upper thoracic spinal cord, but in the early and intermediate infection cases were most heavily concentrated in the ventral horn of the segments containing diaphragm motoneurons. As such, it appears that local interneurons play a particularly important role in controlling diaphragm activity.
Connections of Brain Stem Neurons With Phrenic Motoneurons
The issues that influence the transneuronal spread of pseudorabies virus through neural circuits have been considered in detail (4) and presumably are also applicable to rabies. The speed at which virus is transported transneuronally to the cell body of a second-order neuron is affected by many factors, including the length of its axon, the density of its synaptic connections with an infected cell, and whether these connections are near the soma or are located on the distal dendrites. As such, determining the number of synapses separating a transneuronally infected neuron from the injection site can be difficult. This analysis is facilitated by the use of monosynaptic retrograde tracers and antidromic stimulation to ascertain the connectivity between two putatively linked regions.
The pattern of infection observed in the medullary and pontine respiratory groups is largely in accord with previous anatomical (43, 49) and physiological (15–17, 23) studies. In addition to the classically defined respiratory groups, an appreciable number of brain stem cells in the raphe nuclei, medial and lateral reticular formation, vestibular nuclei, and v5SP were labeled in the early infection animals. Injection of horseradish peroxidase into the vicinity of feline phrenic motoneurons also retrogradely labels cells in the raphe and vestibular nuclei and medial medullary reticular formation (43, 49), and the latter neurons can additionally be antidromically activated by microstimulation in the diaphragm motoneuron pool (57). As such, it seems likely that synaptic connections exist between cells in these regions and phrenic motoneurons. The notion that the vestibular nuclei provide direct inputs to diaphragm motoneurons is bolstered by the observation that electrical stimulation of vestibular afferents in the inner ear of cats can produce changes in phrenic nerve activity at latencies < 10 ms (59). The medullary raphe nuclei have previously been established as making direct connections with diaphragm motoneurons in multiple species (11, 20), and thus the early rabies infection of cells in raphe pallidus and obscurus was not unexpected. In contrast, the labeling of a circumscribed group of cells immediately ventral to the 5SP was unpredicted. A similar area of the muskrat defined as the v5SP receives inputs from the ethmoidal nerve, whose afferents trigger the diving reflex (44). The v5SP region was not delineated in previous studies where horseradish peroxidase was injected into the C5–C6 ventral horn of cats (43, 49), suggesting that cells located in v5SP do not make synaptic connections with phrenic motoneurons. However, the early and consistent labeling of the region suggests that it likely provides disynaptic influences on the diaphragm motor pool, perhaps via connections with bulbospinal neurons in the medullary respiratory groups. This hypothesis remains to be tested. The early infection of cells in the LRF also likely reflects the presence of substantial direct inputs of these neurons to the DRG and VRG (53).
As infection became more advanced, additional areas of the brain stem became labeled. These regions included RTN and the spinal and principal trigeminal nuclei, which provide substantial inputs to the medullary respiratory groups in the cat (53). Labeling also increased in the first-infected areas, most strikingly in the MRF. This increase in MRF infection could reflect the prevalent connections of this region with interneurons in the ventral horn (46), which also became extensively labeled at longer survival times. The MRF integrates a variety of sensory signals (45, 62), and the substantial infection of this area could provide a conduit for transneuronal passage of virus to a large variety of brain stem regions. It seems likely that late-appearing labeling in regions such as the dorsal column nuclei and structures that process auditory information was mediated, at least in part, through their connections with the MRF.
Connections of Cerebellar Neurons With Phrenic Motoneurons
Labeling was present in the fastigial nucleus of the cerebellum, and to a lesser extent the dentate nucleus, of animals with intermediate and advanced brain infection. The labeling of fastigial nucleus was not unanticipated, as this structure has extensive connections with the MRF and vestibular nuclei of the cat (14), and is known to participate in regulating diaphragm activity (58). Infection of the dentate nucleus was surprising, however, as the lateral cerebellum has not previously been postulated to participate in respiratory control. Dentate labeling might have resulted largely through its connections with the RN (47), which was also infected in the same animals. Dentate projections to RN could be involved in modulating voluntary contractions of the diaphragm, including those that are coordinated with movement of the body in space.
Connections of Midbrain Neurons With Phrenic Motoneurons
Heavy labeling of the lateral column of PAG was observed in all of the animals with intermediate and advanced brain infection. This region is complex, as stimulation of the lPAG can elicit either defensive responses that include changes in respiration or airway muscle contractions and the modulation of respiratory activity required to produce vocalization (5). The lPAG as well as the vlPAG have descending projections to both the MRF and the medullary respiratory groups (22, 35), which probably provided the route through which cells in these regions became transneuronally infected with rabies virus.
A number of midbrain regions involved in voluntary motor control were also heavily labeled in the intermediate and late infection animals. These areas included the CN and PP regions, which serve functionally as the mesencephalic locomotion center in the cat (24). Stimulation of these nuclei produces coordinated changes in breathing patterns and limb movements (25), which are largely mediated through descending connections to the medullary MRF and raphe nuclei (29). RN was also labeled in the intermediate and late infection animals. This nucleus projects heavily to the intermediate zone of the cervical spinal gray matter (47), which was infected extensively following rabies injections into the diaphragm. Since RN in the feline receives substantial inputs from motor cortex (27), this pathway provides a potential route for voluntary control of breathing.
Connections of Diencephalon and Telencephalon Neurons With Phrenic Motoneurons
The majority of infected cells in the diencephalon were observed in the PerF region; the distribution and morphology of the neurons suggested that they contain hypocretin (1, 63), although their phenotype was not established in this study. A hypothalamic region has also been identified whose stimulation triggers parallel increases in breathing and physical activity (56). Although the location of this area is not precisely defined, it is known to include portions of the posterior and lateral hypothalamus, as well as the adjacent fields of Forel (56); as such, this area overlaps the PerF region that was heavily labeled in the present study. Presumably, PerF neurons were infected through their extensive connections with the midbrain (25). Hypocretin-positive cells in the hypothalamus have been shown to play a key role in arousal (50), which raises the notion that the infected PerF neurons have a generalized role in adjusting respiratory activity in accordance with the requirements of a variety of ongoing behaviors, ranging from exercise to sleep. Further studies will be required to test this hypothesis. Infected PerF neurons could also participate in eliciting defense responses.
The most heavily infected portion of cerebral cortex was the pericruciate region, which corresponds to motor cortex in the cat (51). Pericruciate neurons could have been infected through their extensive projections to RN or the pontomedullary reticular formation, but likely not through direct projections with the spinal cord, since the large Betz cells were not infected (27). The prefrontal and insular cortices were less heavily labeled, and, unfortunately, the present data do not permit the determination of whether these areas were infected through distinct subcortical pathways or via intracortical connections with the pericruciate region. Nonetheless, the concentration of labeling in the precruciate gyrus suggests that this region plays a dominant role in the voluntary control of breathing.
Comparison of Findings With Those From Other Species
Although no previous studies have systematically identified the pattern of infection in the cerebellum, midbrain, diencephalon, or telencephalon following injections of transneuronal tracers into the diaphragm, the distribution of brain stem and spinal labeling in the present study on the cat differs from that reported previously for the rat (20, 33) or ferret (10, 61). An initial transneuronal tracing study in the rat indicated that only a very limited number of spinal interneurons provide inputs to phrenic motoneurons (20), although a recent study firmly established the existence of such spinal premotor interneurons, which are concentrated in the same segments as the infected motoneurons (33). A substantial number of spinal interneurons also appears to provide inputs to phrenic motoneurons in cats; perhaps this interneuronal network is even more extensive in felines than in rodents (33), although methodological differences between experiments prevent quantitative comparisons of the data collected in the two species. Within the brain stem, the classically defined respiratory groups appear to provide strong, direct inputs to phrenic motoneurons of all mammals that have been studied. In addition, neurons in the medullary MRF were infected early in all species following virus injections into the diaphragm (10, 20, 61). However, only a few MRF neurons were infected in the rat; in contrast, numerous labeled cells were present in this region in the ferret (10, 61) and cat. Both ferrets and felines are emetic, and one possibility is that the medullary MRF participates in driving the respiratory muscle contractions that generate emesis. This notion is supported by the observation that many MRF cells in ferrets have collateralized projections to both phrenic and abdominal motoneurons (10).
In addition, two brain stem regions were infected at short survival times following virus injections into the diaphragm in cats, but not rats or ferrets. The first of these areas is the vestibular nucleus complex. Physiological studies have demonstrated that the vestibular system contributes to modulating diaphragm activity (59), but the present data raise the possibility that vestibulo-respiratory responses in felines are elicited via a more direct reflex pathway than in some other species. The second novel area identified in the present experiments is a circumscribed region ventral to the 5SP in the caudal medulla (v5SP). One previous anatomical study conducted in muskrats described an analogous region (44), which was postulated to trigger apnea during diving reflexes. Physiological studies will be needed to ascertain whether v5SP has a similar function in cats.
Perspectives
The present study revealed that an extended network of neurons participates in regulating diaphragm activity in cats. The highest concentrations of these neurons were located in the intermediate zone of the C5–C6 spinal gray matter, the medullary and pontine respiratory groups and adjacent reticular formation, reticular formation of the medial medulla, vestibular nuclei, lateral portion of the periaqueductal gray, midbrain tegmentum, RN, the perifornical area of the hypothalamus, and the precruciate gyrus. Although other investigators have postulated that the classically defined respiratory groups have an overarching role in respiratory regulation (20), the present data suggest that many other regions are functionally relevant, particularly in large animals, such as cats that employ their respiratory muscles in a variety of different movements and behaviors. As such, further studies that compare the regulation of diaphragm activity in different species, including those conducted in nonhuman primates, will be needed to decipher the complexities involved in the neural control of breathing.
GRANTS
This work was supported by grant R01-DC-03732 from the National Institute on Deafness and Other Communication Disorders of the National Institutes of Health (NIH), as well as by grant P40-RR-018604 from NIH's National Center for Research Resources.
Acknowledgments
The authors thank Dr. J. Patrick Card for advice and encouragement throughout the course of this study, as well as Dr. Peter Strick for providing rabies virus and anti-rabies antibodies. We are also grateful to Lucy Cotter, Ronny Kalash, Amar Mehta, Ajeet Mehta, and Jen-Shew Yen for assistance with the completion of these experiments.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abrahamson EE, Moore RY. The posterior hypothalamic area: chemoarchitecture and afferent connections. Brain Res 889: 1–22, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Anker AR, Sadacca BF, Yates BJ. Vestibular inputs to propriospinal interneurons in the feline C1–C2 spinal cord projecting to the C5-C6 ventral horn. Exp Brain Res 170: 39–51, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Arita H, Kogo N, Koshiya N. Morphological and physiological properties of caudal medullary expiratory neurons of the cat. Brain Res 401: 258–266, 1987. [DOI] [PubMed] [Google Scholar]
- 4.Aston-Jones G, Card JP. Use of pseudorabies virus to delineate multisynaptic circuits in brain: opportunities and limitations. J Neurosci Methods 103: 51–61, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Bandler R, Keay KA, Floyd N, Price J. Central circuits mediating patterned autonomic activity during active vs. passive emotional coping. Brain Res Bull 53: 95–104, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Bellingham MC Synaptic inhibition of cat phrenic motoneurons by internal intercostal nerve stimulation. J Neurophysiol 82: 1224–1232, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Bellingham MC, Lipski J. Respiratory interneurons in the C5 segment of the spinal cord of the cat. Brain Res 533: 141–146, 1990. [DOI] [PubMed] [Google Scholar]
- 8.Berger AJ, Cameron WE, Averill DB, Kramis RC, Binder MD. Spatial distributions of phrenic and medial gastrocnemius motoneurons in the cat spinal cord. Exp Neurol 86: 559–575, 1984. [DOI] [PubMed] [Google Scholar]
- 9.Berman AI The Brain Stem of the Cat. Madison, WI: University of Wisconsin Press, 1968.
- 10.Billig I, Foris JM, Enquist LW, Card JP, Yates BJ. Definition of neuronal circuitry controlling the activity of phrenic and abdominal motoneurons in the ferret using recombinant strains of pseudorabies virus. J Neurosci 20: 7446–7454, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonham AC Neurotransmitters in the CNS control of breathing. Respir Physiol 101: 219–230, 1995. [DOI] [PubMed] [Google Scholar]
- 12.Card JP, Enquist LW, Miller AD, Yates BJ. Differential tropism of pseudorabies virus for sensory neurons in the cat. J Neurovirol 3: 49–61, 1997. [DOI] [PubMed] [Google Scholar]
- 13.Card JP, Rinaman L, Schwaber JS, Miselis RR, Whealy ME, Robbins AK, Enquist LW. Neurotropic properties of pseudorabies virus: uptake and transneuronal passage in the rat central nervous system. J Neurosci 10: 1974–1994, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carleton SC, Carpenter MB. Afferent and efferent connections of the medial, inferior and lateral vestibular nuclei in the cat and monkey. Brain Res 278: 29–51, 1983. [DOI] [PubMed] [Google Scholar]
- 14a.Chosewood LC, Wilson DE (editors). Biosafety in Microbiological and Biomedical Laboratories (5th Ed.). Washington, DC: US Government Printing House, 2007.
- 15.Cohen MI Neurogenesis of respiratory rhythm in the mammal. Physiol Rev 59: 1105–1160, 1979. [DOI] [PubMed] [Google Scholar]
- 16.Cohen MI Central determinants of respiratory rhythm. Ann Rev Physiol 43: 91–104, 1981. [DOI] [PubMed] [Google Scholar]
- 17.Connelly CA, Dobbins EG, Feldman JL. Pre-Bötzinger complex in cats: respiratory neuronal discharge patterns. Brain Res 590: 337–340, 1992. [DOI] [PubMed] [Google Scholar]
- 18.Dampney RAL, McAllen RM. Differential control of sympathetic fibres supplying hindlimb skin and muscle by subretrofacial neurones in the cat. J Physiol 395: 41–56, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davenport PW, Reep RL. Cerebral cortex and respiration. In: Regulation of Breathing, edited by Dempsey JA, Pack AI. New York: Dekker, 1995, p. 365–388.
- 20.Dobbins EG, Feldman JL. Brainstem network controlling descending drive to phrenic motoneurons in rat. J Comp Neurol 347: 64–86, 1994. [DOI] [PubMed] [Google Scholar]
- 21.Douse MA, Duffin J. Axonal projections and synaptic connections of C5 segment expiratory interneurones in the cat. J Physiol 470: 431–444, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ennis M, Xu SJ, Rizvi TA. Discrete subregions of the rat midbrain periaqueductal gray project to nucleus ambiguus and the periambigual region. Neuroscience 80: 829–845, 1997. [DOI] [PubMed] [Google Scholar]
- 23.Feldman JL Neurophysiology of breathing in mammals. In: Handbook of Physiology. The Nervous System. Intrinsic Regulatory Systems of the Brain. Bethesda, MD: Am. Physiol. Soc., 1986, sect. 1, vol. IV, chapt. 9, p. 463–524.
- 24.Garcia-Rill E, Skinner RD. Modulation of rhythmic function in the posterior midbrain. Neuroscience 27: 639–654, 1988. [DOI] [PubMed] [Google Scholar]
- 25.Horn EM, Waldrop TG. Suprapontine control of respiration. Respir Physiol 114: 201–211, 1998. [DOI] [PubMed] [Google Scholar]
- 26.Hsu SM, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem 29: 577–580, 1981. [DOI] [PubMed] [Google Scholar]
- 27.Ipekchyan NM Quantitative analysis of the distribution of the motor cortex representations of the fore- and hindlimbs in the red nucleus of the cat. Neurosci Behav Physiol 38: 345–347, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Jones BE, Beaudet A. Distribution of acetylcholine and catecholamine neurons in the cat brainstem: a choline acetyltransferase and tyrosine hydroxylase immunohistochemical study. J Comp Neurol 261: 15–32, 1987. [DOI] [PubMed] [Google Scholar]
- 29.Jordan LM, Liu J, Hedlund PB, Akay T, Pearson KG. Descending command systems for the initiation of locomotion in mammals. Brain Res Rev 57: 183–191, 2008. [DOI] [PubMed] [Google Scholar]
- 30.Kelly RM, Strick PL. Rabies as a transneuronal tracer of circuits in the central nervous system. J Neurosci Methods 103: 63–71, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Kerman IA, Enquist LW, Watson SJ, Yates BJ. Brainstem substrates of sympatho-motor circuitry identified using trans-synaptic tracing with pseudorabies virus recombinants. J Neurosci 23: 4657–4666, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lafon M Rabies virus receptors. J Neurovirol 11: 82–87, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Lane MA, White TE, Coutts MA, Jones AL, Sandhu M, Bloom DC, Bolser DC, Yates BJ, Fuller DD, Reier PJ. Cervical pre-phrenic interneurons in the normal and lesioned spinal cord of the adult rat. J Comp Neurol 511: 692–709, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee TK, Lois JH, Troupe JH, Wilson TD, Yates BJ. Transneuronal tracing of neural pathways that regulate hindlimb muscle blood flow. Am J Physiol Regul Integr Comp Physiol 292: R1532–R1541, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Mantyh PW Connections of the midbrain periaqueductal gray in the monkey. II. Descending efferent projections. J Neurophysiol 49: 582–594, 1983. [DOI] [PubMed] [Google Scholar]
- 36.McLean IW, Nakane PK. Periodate-lysine-paraformaldehyde for immunoelectron microscopy. J Histochem Cytochem 22: 1077–1083, 1974. [DOI] [PubMed] [Google Scholar]
- 37.Miller AD, Bianchi AL, Bishop BP (editors). Neural Control of the Respiratory Muscles. Boca Raton, FL: CRC, 1997.
- 38.Miller AD, Nonaka S, Lakos SF, Tan K. Diaphragmatic and external intercostal muscle control during vomiting: behavior of inspiratory bulbospinal neurons. J Neurophysiol 63: 31–36, 1990. [DOI] [PubMed] [Google Scholar]
- 39.Nadin-Davis SA, Sheen M, Abdel-Malik M, Elmgren I, Armstrong J, Wandeler AI. A panel of monoclonal antibodies targeting the rabies virus phophoprotein identifies a highly variable epitope of value for sensitive strain discrimination. J Clin Microbiol 38: 1397–1403, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakazono Y, Aoki M. Excitatory connections between upper cervical inspiratory neurons and phrenic motoneurons in cats. J Appl Physiol 77: 679–683, 1994. [DOI] [PubMed] [Google Scholar]
- 41.Nattie E CO2, brainstem chemoreceptors and breathing. Prog Neurobiol 59: 299–331, 1999. [DOI] [PubMed] [Google Scholar]
- 42.Nattie E, Li A. Central chemoreception 2005: a brief review. Auton Neurosci 126–127: 332–338, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Onai T, Miura M. Projections of supraspinal structures to the phrenic motor nucleus in cats studied by a horseradish peroxidase microinjection method. J Auton Nerv Syst 16: 61–77, 1986. [DOI] [PubMed] [Google Scholar]
- 44.Panneton WM, McCulloch PF, Sun W. Trigemino-autonomic connections in the muskrat: the neural substrate for the diving response. Brain Res 874: 48–65, 2000. [DOI] [PubMed] [Google Scholar]
- 45.Peterson BW, Anderson ME, Filion M. Responses of pontomedullary reticular neurons to cortical, tectal and cutaneous stimuli. Exp Brain Res 21: 19–44, 1974. [DOI] [PubMed] [Google Scholar]
- 46.Petras JM Cortical, tectal and tegmental fiber connections in the spinal cord of the cat. Brain Res 6: 275–324, 1967. [DOI] [PubMed] [Google Scholar]
- 47.Pong M, Horn KM, Gibson AR. Spinal projections of the cat parvicellular red nucleus. J Neurophysiol 87: 453–468, 2002. [DOI] [PubMed] [Google Scholar]
- 48.Rikard-Bell GC, Bystrzycka EK. Localization of phrenic motor nucleus in the cat and rabbit studied with horseradish peroxidase. Brain Res 194: 479–483, 1980. [DOI] [PubMed] [Google Scholar]
- 49.Rikard-Bell GC, Bystrzycka EK, Nail BS. Brainstem projections to the phrenic nucleus: a HRP study in the cat. Brain Res Bull 12: 469–477, 1984. [DOI] [PubMed] [Google Scholar]
- 50.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature 437: 1257–1263, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Scannell JW, Blakemore C, Young MP. Analysis of connectivity in the cat cerebral cortex. J Neurosci 15: 1463–1483, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shintani T, Mori RL, Yates BJ. Locations of neurons with respiratory-related activity in the ferret brainstem. Brain Res 974: 236–242, 2003. [DOI] [PubMed] [Google Scholar]
- 53.Smith JC, Morrison DE, Ellenberger HH, Otto MR, Feldman JL. Brainstem projections to the major respiratory neuron populations in the medulla of the cat. J Comp Neurol 281: 69–96, 1989. [DOI] [PubMed] [Google Scholar]
- 54.Tang Y, Rampin O, Giuliano F, Ugolini G. Spinal and brain circuits to motoneurons of the bulbospongiosus muscle: retrograde transneuronal tracing with rabies virus. J Comp Neurol 414: 167–192, 1999. [PubMed] [Google Scholar]
- 55.Ugolini G Use of rabies virus as a transneuronal tracer of neuronal connections: implications for the understanding of rabies pathogenesis. Dev Biol (Basel) 131: 493–506, 2008. [PubMed] [Google Scholar]
- 56.Waldrop TG, Eldridge FL, Iwamoto GA, Mitchell JH. Central neural control of respiration and circulation during exercise. In: Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems. Bethesda, MD: Am. Physiol. Soc., 1996, sect. 12, chapt. 9, p. 333–379.
- 57.Wilkinson KA, Maurer AP, Sadacca BF, Yates BJ. Responses of feline medial medullary reticular formation neurons with projections to the C5–C6 ventral horn to vestibular stimulation. Brain Res 1018: 247–256, 2004. [DOI] [PubMed] [Google Scholar]
- 58.Xu F, Frazier DT. Role of the cerebellar deep nuclei in respiratory modulation. Cerebellum 1: 35–40, 2002. [DOI] [PubMed] [Google Scholar]
- 59.Yates BJ, Jakus J, Miller AD. Vestibular effects on respiratory outflow in the decerebrate cat. Brain Res 629: 209–217, 1993. [DOI] [PubMed] [Google Scholar]
- 60.Yates BJ, Siniaia MS, Miller AD. Descending pathways necessary for vestibular influences on sympathetic and inspiratory outflow. Am J Physiol Regul Integr Comp Physiol 268: R1381–R1385, 1995. [DOI] [PubMed] [Google Scholar]
- 61.Yates BJ, Smail JA, Stocker SD, Card JP. Transneuronal tracing of neural pathways controlling activity of diaphragm motoneurons in the ferret. Neuroscience 90: 1501–1513, 1999. [DOI] [PubMed] [Google Scholar]
- 62.Yeomans JS, Frankland PW. The acoustic startle reflex: neurons and connections. Brain Res Rev 21: 301–314, 1995. [DOI] [PubMed] [Google Scholar]
- 63.Zhang J, Sampogna S, Morales FR, Chase MH. Co-localization of hypocretin-1 and hypocretin-2 in the cat hypothalamus and brainstem. Peptides 23: 1479–1483, 2002. [DOI] [PubMed] [Google Scholar]