Abstract
Physical inactivity is associated with the increased risk of developing chronic metabolic diseases. To understand early alterations caused by physical inactivity, we utilize an animal model in which rats are transitioned from daily voluntary wheel running to a sedentary condition. In the hours and days following this transition, adipose tissue mass rapidly increases, due in part to increased lipogenesis. However, whether a concurrent decrease in fatty acid oxidative capacity (FAO) in skeletal muscle, liver, and adipose tissue occurs during this period is unknown. Following 6 wk of access to voluntary running wheels (average distance of ∼6 km a night), rats were rapidly transitioned to a sedentary state by locking the wheels for 5 h (WL5) or 173 h (WL173). Complete ([14C]palmitate oxidation to 14CO2) and incomplete ([14C]palmitate oxidation to 14C-labeled acid soluble metabolites) was determined in isolated mitochondrial and whole homogenate preparations from skeletal muscle and liver and in isolated adipocytes. Strikingly, the elevated complete FAO in the red gastrocnemius at WL5 fell to that of rats that never ran (SED) by WL173. In contrast, hepatic FAO was elevated at WL173 above both WL5 and SED groups, while in isolated adipocytes, FAO remained higher in both running groups (WL5 and WL173) compared with the SED group. The alterations in muscle and liver fat oxidation were associated with changes in carnitine palmitoyl transferase-1 activity and inhibition, but not significant changes in other mitochondrial enzyme activities. In addition, peroxisome proliferator-activated receptor coactivator-1α mRNA levels that were higher in both skeletal muscle and liver at WL5 fell to SED levels at WL173. This study is the first to demonstrate that the transition from high to low daily physical activity causes rapid, tissue-specific changes in FAO.
Keywords: mitochondria, metabolism
alterations in our current environment, including physical inactivity and the excessive consumption of high-calorie/fat foods, are root causes for the rapid rise of obesity and diabetes in humans (1, 6, 15, 41). Although many investigations have detailed the role of overnutrition following changes in substrate utilization and storage, the effects of a transition from high to low physical activity remain largely understudied and underappreciated.
Our research group utilizes “the rodent wheel lock model” to study acute transitions from high to low levels of daily physical activity (26, 27, 30, 34, 38). After days or weeks of daily voluntary wheel running (5–15 km/night), wheels are locked for progressive periods of time (from 5 to 173 h), providing an acute window of time to study the metabolic alterations that ensue. While resulting in only modest increases in mitochondrial enzymes compared with forced treadmill training (11, 28), voluntary wheel running (5–15 km/night) can increase energy expenditure far greater than treadmill training (∼2 km/60 min treadmill bout). Thus it is a good model to study the transition from high to low daily physical activity, but an inappropriate model to study detraining. In our laboratory's previous studies, the sudden cessation of daily running caused rapid and robust increases in the weight of retroperitoneal and omental fat pads after 53 and 173 h of wheel lock, whose causes are beginning to be explained by intrinsic changes in substrate partitioning and not simply a positive energy balance due to decreased daily energy expenditure (26, 30). For example, rates of key enzymes in adipose tissue de novo lipogenesis and palmitate incorporation into adipose triglycerides remain significantly higher in the hours and days immediately following wheel lock (26, 27) and after the cessation of forced treadmill running (9). Although, enhanced de novo lipogenesis could partially explain the rapid increases in fat pad weight after the cessation of activity, we also speculated that rapid decreases in fatty acid oxidative capacity (FAO) of skeletal muscle, liver, and adipose tissue also may play a significant role.
Decreased FAO in metabolic tissues has been strongly associated with chronic disease(s) (5, 13, 14, 16, 20, 21, 42). Although findings are not universal (12, 25), extremely obese and Type 2 diabetic subjects display reduced systemic and skeletal muscle FAO, which has been linked to increased adiposity, increased intramuscular lipid storage, and skeletal muscle insulin resistance (5, 14, 21, 44). Decreased hepatic FAO directly causes or increases susceptibility for fatty liver and steatosis (17, 39) and also has been linked to increased susceptibility to diet-induced obesity (18). In addition to skeletal muscle and liver, FAO in adipocytes has become an important area of study. FAO is decreased in the adipose tissue of db/db mice (7), and mitochondrial markers are also reduced in the adipocytes of several animal models of Type 2 diabetes and obesity (8, 13, 16, 20, 42, 46). However, while it is well known that increased physical activity decreases the risks of Type 2 diabetes and obesity, it remains unknown whether physical activity modifies adipocyte FAO.
The aim of this study was to determine if the cessation of voluntary wheel running leads to concurrent alterations in tissue fatty acid catabolism, carnitine palmitoyl transferase-1 (CPT-1) activity and regulation, and mitochondrial enzyme activity. Since the transition from high to low physical activity is likely to cause systemic changes, we assessed FAO and mitochondrial function in adipose tissue, liver, and mixed skeletal muscle in hopes of better understanding the integrative response(s) of an organism to reductions in energy expenditure and rapid gains in adiposity. We hypothesized that the transition from high to low physical activity would lead to a coincident reduction in FAO and mitochondrial enzyme activity in skeletal muscle, liver, and adipocytes.
METHODS
Animal protocol.
The animal protocol was approved by the Institutional Animal Care and Use Committee at the University of Missouri. Thirty-six male Fischer 344 × Brown Norway F1 hybrid rats (Harlan) were obtained at 21 days of age. Animals were randomly separated into those with access to running wheels [wheel lock (WL)], and those without access to the wheels [sedentary (SED)]. Rats assigned to running groups were immediately housed (at the age of 21 days) in cages equipped with a voluntary running wheel outfitted with a Sigma Sport BC 800 bicycle computer (Cherry Creek Cyclery, Foster Falls, VA) for measuring daily running activity. Voluntary running was selected to approximate the more natural physical activity level of the animal. Rats were maintained on a 12:12-h light-dark cycle that ran from 0600 to 1800. Running activity was obtained every day of running between 0800 and 1000, and rats in the running group had access to wheels and food and water ad libitum until ∼9 wk of age, at which time (0600) wheels were locked. Rats were anesthetized [ketamine (80 mg/kg), xylazine (10 mg/kg), and acepromazine (4 mg/kg)] and killed by exsanguination by removal of the heart either 5 h (WL5), or 173 h (WL173) after locking of wheels; the SED rats were killed at the same time. All animals were fasted for 5 h before death.
Dual-energy X-ray absorptiometry.
One week before and the day of death, animals were anesthetized with isoflurane (1 wk prior) or ketamine (80 mg/kg), xylazine (10 mg/kg), and acepromazine (4 mg/kg) (day of death), and whole body composition was measured using a Hologic QDR-1000/w dual-energy X-ray absorptiometry machine calibrated for rats.
Serum measures.
Plasma glucose (Sigma), triglycerides (Sigma), and free fatty acids (FFAs; Wako Chemicals, Richmond, VA) were measured with commercially available kits, according to the manufacturer's instructions.
Adipocyte isolation.
Adipocytes from epididymal fat pads were isolated by a modification of the Rodbell method (26, 40). Fresh triplicate 30-μl aliquots of packed adipocytes were used for FAO, and a single 30-μl aliquot was frozen at −80°C for DNA content, with the remainder lysed in Cell Lytic (Sigma) for enzyme activity assays.
Adipocyte DNA content.
DNA content in isolated adipocytes was determined by the flurometric assay described by Labarca and Paigen (29). Briefly, packed adipocytes were lysed in 2 M NaCl and 50 mM NaPO4 (pH 7.4), and 20 μl were added to 80 μl of working solution (0.04 μg/ml of Hoeschst no. 33258, 10 mM Tris·HCl, 1 mM EDTA, 200 mM NaCl, pH 7.4) in a 96-well plate. Samples were excited at 356 nm, resulting in an emission at 458 nm. Calf thymus DNA (Sigma) was used to generate a standard curve.
Tissue homogenization procedure.
Red gastrocnemius and livers were quickly excised from anesthetized rats and placed in ice-cold isolation buffer (100 mM KCl, 40 mM Tris·HCl, 10 mM Tris base, 5 mM MgCl2·6H2O, 1 mM EDTA, and 1 mM ATP; pH 7.4). For fresh fatty acid oxidation assays, 50–100 mg of tissue were thoroughly minced with scissors in 200 μl SET buffer (250 mM sucrose, 1 mM EDTA, 10 mM Tris·HCl, and 2 mM ATP; pH 7.4), and then the buffer volume was brought up to yield a 20-fold (wt/vol) diluted sample. This was transferred to a 3-ml Potter-Elvehjem glass homogenization vessel. Tissue suspensions were mechanically homogenized on ice with a Teflon pestle at 10 passes over the course of 30 s at 1,200 rpm. Homogenates were kept on ice until oxidation experiments were performed.
Mitochondrial isolation from red gastrocnemius skeletal muscle and liver.
Mitochondrial suspensions were prepared according to modified methods of Koves et al. (23, 24). A portion of the red gastrocnemius and liver was quickly excised from unconscious rats and placed in 5 ml of fresh buffer A (100 mM KCl, 50 mM MOPS, 5 mM MgSO4, 1 mM EGTA, 1 mM ATP, pH 7.4). The tissue was then minced and suspended sevenfold (wt/vol) in buffer A and homogenized for 15 s. The homogenate was then centrifuged at 800 g for 10 min at 4°C, and the supernatant was filtered through gauze and set aside. The remaining pellet was resuspended in buffer A and homogenized, and the homogenate was then centrifuged at 800 g for 10 min at 4°C. The remaining supernatant was added to the previously set aside supernatant after filtering through gauze. The pooled supernatants were centrifuged at 9,000 g to pellet the mitochondria. The pellet was resuspended in buffer B (buffer A, 0.2% BSA, pH 7.4), and centrifuged at 8,000 g before the pellet was washed and resuspended in buffer C (100 mM KCl, 50 mM MOPS, 0.5 mM EGTA, pH 7.4) and centrifuged for 10 min at 7,000 g. The final pellet was resuspended in 0.5 ml of SET buffer, and protein content was determined.
Fatty acid oxidation.
FAO was measured with radiolabeled [1-14C]palmitate (American Radiochemicals) in fresh red gastrocnemius skeletal muscle and liver homogenates and isolated mitochondria using modified methods of Dohm et al. (10) and Rector et al. (39). The reaction buffer used for whole homogenates utilized a cold palmitate concentration of 200 μM, while the reaction buffer used for isolated mitochondria utilized a concentration of 50 μM. For isolated adipocytes, 30 μl of freshly isolated adipocytes/reaction were added to 120-μl reaction buffer in a trapping device, with an adjacent chamber containing 100 μl of NaOH that captured the 14CO2, representing the complete oxidation radiolabeled palmitate, when 100 μl of perchloric acid were added to the reaction following a 2-h incubation at 37°C. The 14C-labeled acid soluble metabolites (ASM), representing incomplete FAO, were also collected.
Citrate synthase, β-hydroxyacyl-CoA dehydrogenase, and cytochrome-c oxidase activity.
Citrate synthase and β-hydroxyacyl-CoA dehydrogenase (β-HAD) activities were determined in red gastrocnemius, liver, and adipocyte homogenates using the methods of Srere (43) and Bass et al. (4), respectively, and as previously described by our group (39). β-HAD activity was undetectable in the adipocyte fraction. Cytochrome-c oxidase activity was determined by a commercially available kit (Sigma, St. Louis, MO) in all three tissues, as previously described by our group (38). All assays were performed at 37°C.
CPT-1 assay.
CPT-1 activity and inhibition by malonyl-CoA using modified methods of Koves et al. (25). Ninety microliters of reaction buffer (117 mM Tris·HCl, 0.28 mM reduced glutathione, 4.4 mM ATP, 50 μM palmitoyl-CoA, 4 mg/ml rotenone, 0.1% BSA) containing 0.2 mM [3H]carnitine and either 0 or 10 μM malonyl-CoA was added to 10 μl of isolated mitochondria of red gastrocnemius skeletal muscle and liver homogenate for 6 min at 37°C. [3H]palmitoyl-carnitine formed was extracted with 350 μl of water-saturated butanol, quantified by liquid-scintillation counting, and normalized to protein content.
Statistics.
Each outcome measure was examined in six to eight animals. For each outcome measure, a one-way analysis of variance was performed (Sigma Stat). However, if normality of the data set failed, a Kruskal-Wallis ANOVA on ranks was performed, with Dunn's method comparison (control group = WL5) as the post hoc test. A significant main effect (P < 0.05) was followed up with Student-Newman-Keuls post hoc comparisons. Values are reported as means ± SE, and a P < 0.05 denotes a statistically significant difference.
RESULTS
Body composition.
Dual-energy X-ray absorptiometry measures were taken 7 days before and on the day of death to capture any changes leading to death. In the 173-h wheel lock period, the WL173 group increased percent body fat and total body fat mass by 45 and 46%, respectively (P < 0.05), essentially returning body fat levels to those measured in the SED group (Table 1). No changes in percent or total body fat mass occurred in the 7 days before death in the WL5 or SED groups. Intriguingly, total lean mass showed a different pattern than total fat mass (Table 1). Total lean mass increased in WL5 and SED in the 7 days before death, suggesting a positive protein balance (P < 0.05). In contrast, the WL173 group did not show a net increase in lean body mass during the 7 days of wheel lock before death, suggesting different rates of lean mass catabolism and/or anabolism during the 7 days following the cessation of running. All groups gained equal body mass in the 7 days before death.
Table 1.
Body composition and food intakes
| DEXA Measurements | WL5 | WL173 | SED |
|---|---|---|---|
| Body fat, % | |||
| 7 Days before death | 4.1±0.5a | 4.7±0.6a | 6.6±0.4b |
| Day of death | 4.5±0.4a | 6.8±0.4b | 6.8±0.7b |
| Δ | 0.4±0.4 | 2.1±0.8* | 0.2±0.6 |
| Total body fat, g | |||
| 7 Days before death | 9.1±1.0a | 10.7±1.7a,b | 13.8±1.1b |
| Day of death | 10.6±0.8a | 15.6±1.2b | 15.6±2.1b |
| Δ | 1.5±0.8 | 4.9±1.7* | 1.8±1.3 |
| Total lean mass, g | |||
| 7 Days before death | 214.7±4.6 | 211.1±8.5 | 193.4±8.4 |
| Day of death | 223.6±4.1 | 212.1±7.4 | 208.3±7.1 |
| Δ | 8.9±2.8* | 0.9±3.1 | 14.9±1.5* |
| Total body mass, g | |||
| 7 Days before death | 223.9±4.4 | 221.8±9.5 | 207.2±9.2 |
| Day of death | 234.1±4.0 | 227.7±8.0 | 223.8±8.6 |
| Δ | 10.3±2.4* | 5.9±2.0* | 16.6±1.5* |
| Average daily food intake, g | |||
| Week −2 to −1 | 18.3±0.8a | 18.8±0.6a | 13.7±0.3b |
| Week −1 to 0 | 19.3±0.3a | 16.6±0.3*b | 15.2±0.6*b |
Values are means ± SE; n = 7–8/group. WL5, wheel lock for 5 h; WL173, wheel lock for 173 h; SED, sedentary; DEXA, dual-energy X-ray absorptiometry. Body anthropometric measurements were taken 7 days before death and on the day of death by small-animal DEXA; Δ is the difference between the two measures. Weekly food intakes were made for the 2 wk preceding death.
Different letters indicate that values are significantly different between groups at 7 days before or on the day of death (P < 0.05).
Significant increase during the 7-day period within the same group (P < 0.05). Daily food intake was averaged for the week before death (week −1 to 0) in WL5 and SED, and from 2 wk before death to 1 wk before death (week −2 to −1) in WL173, allowing comparison of pre-wheel lock food intake to post-wheel lock in the WL173 group.
Food intake and running distances.
Average daily food intakes during the last week of life for WL5 and WL173 were 27 and 37%, respectively, higher than SED (P < 0.05) (Table 1). This is consistent with our previous findings that voluntary wheel-running rats have greater food intakes than SED counterparts in this strain, age, and sex of rat (26, 30). During the 7 days of wheel lock, the WL173 group decreased their average daily food intake by 12% (P < 0.05) compared with the previous week; at the same time, food intake by the SED group increased 11% (P < 0.05). Therefore, any differences detected were not due to overeating by the WL173 group, since they ate a similar level as the SED during the 173 h of WL.
Neither total 6-wk nor average daily running distances (6.12 vs. 5.43 km/day, P = 0.42) differed between WL5 and WL173 groups. The average daily running distance in the last week of running was 8.87 and 9.73 km in WL5 and WL173 groups, respectively (P = 0.68).
Fat pad and serum characteristics.
Absolute epididymal fat masses did not differ among groups (P = 0.086), but, when normalized to total mass, WL5 were lower than WL173 by 25% (P < 0.05). Serum triglycerides (P = 0.146), FFAs (P = 0.188), and glucose (P = 0.147) did not statistically differ among WL5, WL173, and SED groups (Table 2).
Table 2.
Epididymal adipose and serum measurements
| WL5 | WL173 | SED | |
|---|---|---|---|
| Epididymal fat pad characteristics | |||
| Epididymal fat pad, g/g body wt ×100 | 5.13±0.24a | 6.84±0.38b | 6.63±0.42b |
| Epididymal fat pad, g | 1.20±0.06 | 1.57±0.13 | 1.50±0.15 |
| Serum measurements | |||
| Triacylglycerol, mg/ml | 34.3±2.4 | 44.8±3.3 | 38.3±4.2 |
| Free fatty acids, mmol/l | 146.2±9.7 | 168.6±9.4 | 176.3±13.7 |
| Glucose, mg/dl | 470.9±10.6 | 462.8±19.7 | 429.9±13.7 |
Values are means ± SE; n = 6–8/group.
Different letters indicate that values are significantly different between groups (P < 0.05).
Fatty acid oxidation and mitochondrial enzyme activities.
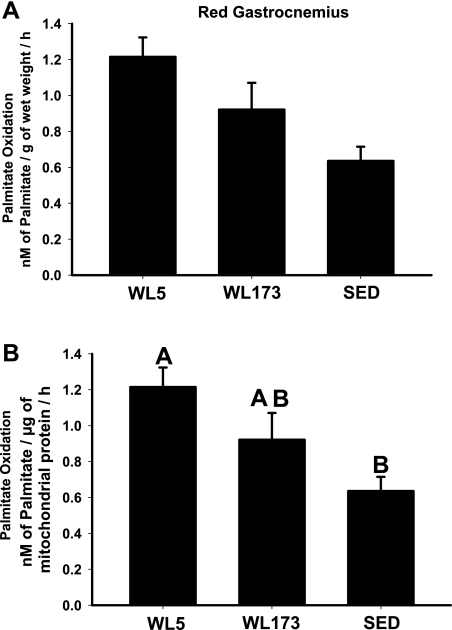
Complete [14CO2] and incomplete [14C-labeled ASM] capacity for palmitate oxidation was determined in isolated mitochondria or whole tissue homogenates from red gastrocnemius muscle and liver. In addition, complete and incomplete FAO was also measured in isolated adipocytes from epididymal fat pads. For whole red gastrocnemius homogenate, palmitate oxidation at WL5 was 33 and 50% higher than WL173 and SED, respectively (P = 0.059; Fig. 1A). For isolated mitochondria from the red gastrocnemius muscle, complete palmitate oxidation to CO2 was 91% higher at WL5 compared with SED (P < 0.05, Fig. 1B) but did not differ from WL173 (P = 0.24).
Fig. 1.
Complete oxidation of palmitate to CO2 in whole red gastrocnemius homogenate (A) and isolated mitochondria from red gastrocnemius (B). WL5, wheel lock for 5 h group; WL173, wheel lock for 173 h group; SED, sedentary. In A, there is a main effect P value of 0.059. Values are means ± SE; n = 6–8/group. Values with different letters are significantly different (P < 0.05).
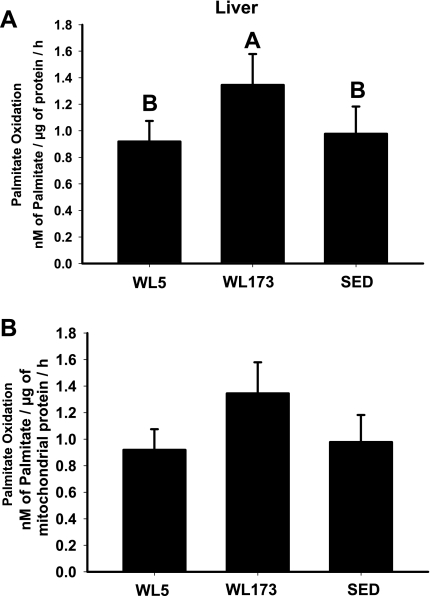
In a contrast to what was found in the red gastrocnemius skeletal muscle, complete hepatic FAO increased after the transition to physical inactivity. Whole liver homogenates from the WL173 group completely oxidized 65 and 46% more palmitate than WL5 and SED groups, respectively (P < 0.05; Fig. 2A). Total palmitate oxidation (CO2 + ASMs) in liver did not differ among groups, an effect potentially in part due to the contribution of extramitochondrial peroxisomal activity. In addition, complete oxidation in the isolated liver mitochondria was not statistically different among groups, but showed a similar trend as the whole homogenate (Fig. 2B).
Fig. 2.
Complete oxidation of palmitate to CO2 in whole liver homogenate (A) and isolated mitochondria from liver (B). Values are means ± SE; n = 6–8/group. Values with different letters are significantly different (P < 0.05).
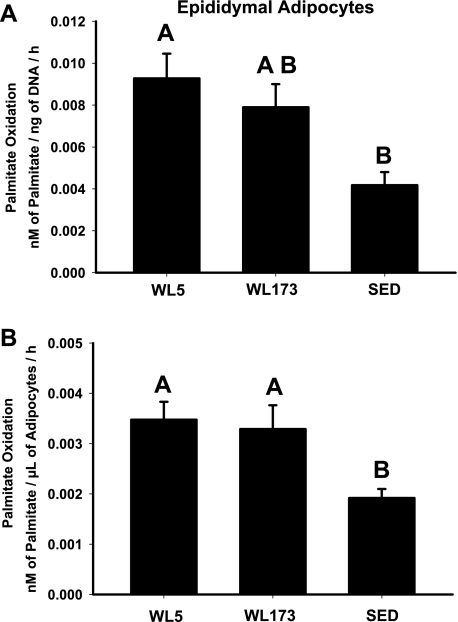
In isolated epididymal adipocytes, complete palmitate oxidation was 81% higher in the WL5 compared with SED (P < 0.05) and 71% higher in the WL173 compared with SED (P < 0.05), but not different between WL5 and WL173 when normalized to the volume of packed adipocytes (Fig. 3A). However, when complete palmitate oxidation was normalized to DNA content (ng of DNA/30 μl of packed adipocytes), WL173 no longer remained significantly higher than SED group (Fig. 3B). Total palmitate oxidation (CO2 + ASMs) in the adipocytes did not differ among groups.
Fig. 3.
Complete oxidation of palmitate to CO2 in isolated adipocytes from the epididymal fat pad normalized to nanograms of DNA per 30 μl of packed adipocytes (A) or to volume (μl) of packed adipocytes (B). Values are means ± SE; n = 6–8/group. Values with different letters are significantly different (P < 0.05).
No statistically significant differences in β-hydroxy-acetyl-CoA dehydrogenase, citrate synthase, or cytochrome-c oxidase activities in red gastrocnemius, liver, or isolated adipocytes were detected among groups (Table 3).
Table 3.
Enzyme activities
| WL5 | WL173 | SED | |
|---|---|---|---|
| Red gastrocnemius | |||
| β-HAD, nmol·min−1·μg−1 | 26.5±3.7 | 26.6±2.6 | 23.1±2.0 |
| Citrate synthase, nmol·min−1·μg−1 | 1,786±184 | 1,908±270 | 1,342±141 |
| Cytochrome-c oxidase, U·min−1·g−1 | 75.1±6.9 | 78.8±8.5 | 65.5±10.3 |
| Liver | |||
| β-HAD, nmol·min−1·μg−1 | 16.6±1.2 | 18.3±1.7 | 16.7±0.7 |
| Citrate synthase, nmol·min−1·μg−1 | 408±25 | 376±32 | 374±15 |
| Cytochrome-c oxidase, U·min−1·g−1 | 46.6±1.9 | 42.7±2.6 | 43.7±1.4 |
| Epididymal adipose | |||
| β-HAD, nmol·min−1·μg−1 | UD | UD | UD |
| Citrate synthase, nmol·min−1·μg−1 | 21±7 | 20±4 | 21±6 |
| Cytochrome-c oxidase, U·min−1·g−1 | 0.7±0.1 | 1.0±0.2 | 0.9±0.2 |
Values are means ± SE; n = 6–8/group. Enzyme activities are shown in red gastrocnemius, liver, and epididymal adipocytes. No significant differences between groups were detected. β-HAD, β-hydroxyacyl-CoA dehydrogenase; UD, undetectable.
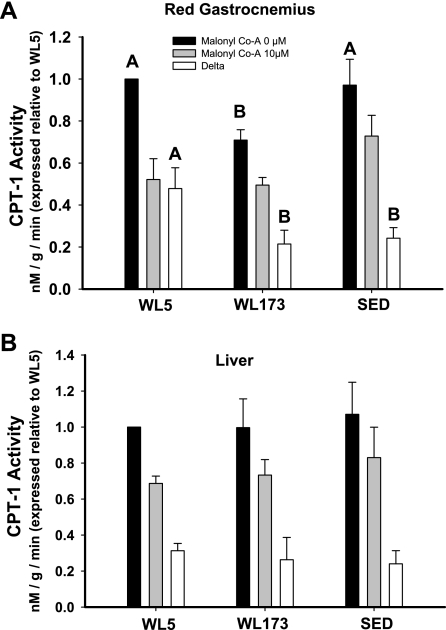
CPT-1 activity.
In isolated mitochondria from red gastrocnemius muscle, the WL5 group had 41% higher baseline CPT-1 activity than the WL173 group (P < 0.05), but WL5 did not differ from SED. As expected, the addition of 10 μM malonyl-CoA caused decreases in CPT-1 activity in all three groups (Fig. 4). Unexpectedly, the change in CPT-1 activity from baseline to 10 μM malonyl-CoA was nearly twice as much (delta) in the WL5 group compared with the WL173 and SED groups (P < 0.05), suggesting a greater CPT-1 sensitivity to malonyl-CoA in a physically active condition (WL5) that was lost after 7 days of lowered physical activity (WL173). No differences in the CPT-1 activity of isolated mitochondria from liver existed among groups for 0 or 10 μM malonyl-CoA concentrations.
Fig. 4.
Carnitine palmitoyltransferase-1 (CPT-1) activities in isolated mitochondria from red gastrocnemius muscle (A) and liver (B). CPT-1 activity was measured in the absence (solid bars) or presence of an inhibitory amount of malonyl-CoA (10 μM, shaded bars), and expressed as the difference between the malonyl-CoA doses (delta, open bars). Data are normalized to 1 in WL5 group and then expressed relative to WL5. Values are means ± SE; n = 6–8/group. Values with different letters are significantly different (P < 0.05).
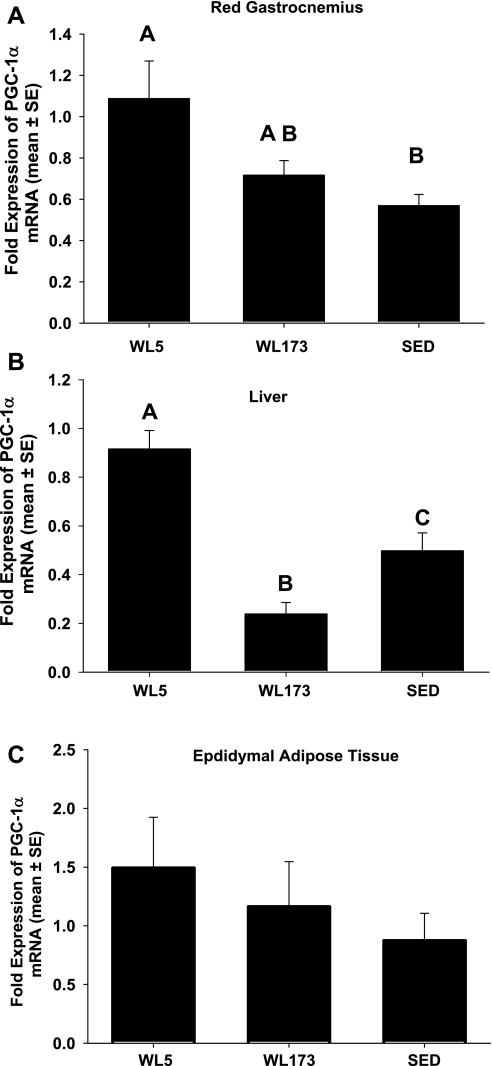
PGC-1α mRNA levels.
PGC-1α mRNA levels were significantly higher at WL5 compared with the SED group in both red gastrocnemius and liver (Fig. 5, A and B). The transition from WL5 to WL173 led to a 35 and 68% decrease in red gastrocnemius (P = 0.05) and liver (P < 0.05) PGC-1α mRNA levels, respectively. Consequently, WL173 and SED groups PGC-1α mRNA did not differ in red gastrocnemius (Fig. 5A). Remarkably, in the liver, WL173 PGC-1α mRNA fell to levels significantly less than those in the SED group (Fig. 5B). In epididymal adipose tissue, PGC-1α mRNA did not differ among groups (Fig. 5C).
Fig. 5.
Peroxisome proliferator-activated receptor coactivator-1α (PGC-1α) mRNA in red gastrocnemius (A), liver (B), and epididymal adipose tissue (C). Data are normalized to 18S expression and expressed in fold difference. Values are means ± SE; n = 6–8/group. Values with different letters are significantly different (P < 0.05).
DISCUSSION
The transition from high to low physical activity in our rat model caused the following: 1) dissimilar responses for FAO in skeletal muscle (decreasing from its elevated value in daily running), liver (increasing from its unchanged value in daily running), and adipose tissue (maintenance of its increased value in daily running); 2) decreased CPT-1 activity and increased CPT-1 sensitivity to malonyl-CoA inhibition in muscle; 3) reduced PGC-1α mRNA in both skeletal muscle and liver; and 4) impaired growth of lean body mass compared with animals that sustain wheel running or the SED condition.
The transition from high to low physical activity likely decreases FAO in skeletal muscle by decreasing cellular energy requirements and decreasing PGC-1α expression, a critical transcriptional coactivator that coordinates increases in FAO enzyme, mitochondrial biogenesis, and a general increase in oxidative capacity. Observing similar trends in both homogenate and mitochondrial fraction leads us to speculate that the changes in FAO are not entirely due to a mass action effect, but also, in part, to an inherent change in mitochondrial “quality,” which may be related to PGC-1α expression.
PGC-1α, as a coactivator, putatively increases FAO by increasing the promoter activity and thus expression of pyruvate dehydrogenase kinase-4 and CPT-1 (31, 47). This results in more acetyl-CoA being derived from fatty acids and increased fatty acid entry into the mitochondria (23). Unlike muscle, where energy derived from FAO fuels muscle contraction, an increase in hepatic PGC-1α-mediated FAO provides a key source of ATP for ketogenesis and gluconeogenesis during fasting conditions (48). A limitation of the present study is that we did not measure oxidation of substrates, malate or pyruvate, that are commonly used to assess mitochondrial function.
Exercise causes an acute and transient increase in skeletal muscle PGC-1α gene expression (2, 37). Conversely, we show here that the cessation of daily physical activity reduces skeletal muscle PGC-1α expression in 173 h, supporting earlier work in which more extreme forms of physical inactivity reduced skeletal muscle PGC-1α in humans (45). We also witnessed that CPT-1 activity, a downstream target of PGC-1α, decreased in red gastrocnemius skeletal muscle after the cessation of daily running. Reduced PGC-1α expression in skeletal muscle has been linked to insulin resistance in SED subjects (33, 36), leading us to speculate that a physical inactivity-induced drop in PGC-1α is linked to skeletal muscle insulin resistance.
To our knowledge, this is the first report to measure changes in hepatic PGC-1α expression after chronic voluntary exercise or after a transition to inactivity. We speculate that daily exercise increases hepatic PGC-1α in a similar manner to fasting, as both stimuli rely on increased hepatic glucose output to restore or sustain plasma glucose homeostasis. Interestingly, in contrast to what was found in muscle, the reduction in hepatic PGC-1α expression found after wheel lock was associated with increased complete hepatic FAO and no change in CPT-1 enzyme activity. Further research is needed to fully understand hepatic PGC-1α gene expression, its regulation by exercise, and its role in controlling hepatic FAO.
Intracellular accumulation of malonyl-CoA reduces fat and increases glucose utilization by the mitochondria (3, 32). The present findings show a greater reduction in CPT-1 activity in the presence of 10 μM malonyl-CoA in the WL5 group, suggesting that skeletal muscle mitochondrial fatty acid entry may be more tightly regulated in wheel-running rats than SED rats. Potentially, this would allow for skeletal muscle to be more responsive in switching substrate utilization to match present energy demands. In addition, the CPT-1 responses to 10 μM malonyl CoA were similar between SED and WL173 skeletal muscle, suggesting that the enhanced exercise-induced regulation of CPT-1 by malonyl-CoA is quickly lost if daily physical activity is not maintained. A lack of malonyl-CoA inhibition is also noted with Type 2 diabetes, in which isolated skeletal muscle mitochondria have greater FAO than healthy controls, despite having elevated malonyl-CoA levels (3). Collectively, these observations suggest that physical activity modulates plasticity for malonyl-CoA's effects on skeletal muscle CPT-1 activity.
We speculate that the observed increase in complete FAO in liver during 7-day periods of decreased physical activity, in addition to the expansion of adipose tissue, may help prevent excess lipid storage in lean animals on a low-fat diet. The findings that complete hepatic FAO is increased after the cessation of wheel running is opposite to our laboratory's previous findings in hyperphagic, obese Otsuka Long-Evans Tokushima Fatty rats in which the cessation of wheel running for 7 days reduced hepatic palmitate oxidation to SED levels (38). These differences may be due to the larger body fat stores and/or older age in the Otsuka Long-Evans Tokushima Fatty rat. It is important to emphasize that the witnessed changes were for complete FAO ([14C]palmitate to 14CO2) or the complete breakdown of fatty acids through the TCA cycle, rather than the incomplete oxidation of fatty acids ([14C]palmitate to 14C-labeled ASM) in which fatty acids are only processed through β-oxidation. The difference between complete and incomplete FAO is significant for two reasons: 1) only complete FAO is coupled to significant ATP production, and 2) as suggested by Koves et al. (25), complete FAO is less likely to produce intermediate metabolites that may negatively impact metabolic processes. In addition, our FAO measurements represent the maximal capacity or potential to oxidize fatty acids, and, because they are not in vivo measures, they should be interpreted cautiously.
Unlike skeletal muscle and liver, adipocyte FAO remained elevated over the SED group with 7 days of physical inactivity. Mitochondrial DNA number from human subcutaneous fat is positively associated with increased lipogenic enzymes (19), and our laboratory has previously demonstrated that cessation of voluntary wheel running increased protein and enzyme activity for mitochondrial glycerol-3-phosphate acyltransferase, a rate-limiting lipogenic enzyme, in adipose tissue in rats (27). In addition, although our unpublished preliminary work has found no significant change in red gastrocnemius or liver triglyceride content, there is a significant increase in adipocyte triglyceride deposition after the cessation of wheel running in wild-type rats on a low-fat diet (26, 30). The exercise-induced increase in adipose FAO in adipose tissue with ceased running may partially supply ATP for FFA reesterfication, an energy costly process, or the increased ATP may be used to synthesize adipokines like adiponectin, a process shown to require functional mitochondria (22).
To our knowledge, this is the first evidence that the cessation of voluntary running acutely (for at least 173 h) attenuates lean body mass growth. We have also recently reported that lean body mass significantly decreases in young healthy men who decrease their daily walking from 10,501 to 1,344 steps for 2 wk (35). The apparent change in lean mass in both studies leads us to question if the attenuated growth or loss of muscle mass in both studies is linked to a repartitioning of glucose and amino acids away from skeletal muscle (loss of insulin sensitivity) and/or decreased FAO. Because of the importance of lean body mass in metabolic health, future research is needed to study these interactions.
In conclusion, we show that FAO in skeletal muscle, liver, and adipocytes display strikingly different responses to a 1-wk transition from high to low daily physical activity, a period in which fat pad mass increases rapidly. In addition, we found that two factors associated with metabolic disease, skeletal muscle FAO and PGC-1α, were both reduced after the cessation of daily activity. These results support our overall hypothesis that the cessation of daily physical activity quickly alters metabolic function in a manner that likely precedes the later development of chronic disease, should physical inactivity continue.
GRANTS
M. J. Laye was supported by a Life Science Fellowship (University of Missouri); S. J. Borengasser was fully supported and S. P. Naples was partially supported by National Institutes of Health Grant T32-AR48523; J. P. Thyfault was supported by a Veterans Affairs Career Development Grant. Partial research support was provided by the University of Missouri College of Veterinary Medicine. In addition, the research presented here is the result of work supported with resources and the use of facilities at the Harry S. Truman Memorial Veterans Hospital in Columbia, MO.
Acknowledgments
The authors thank Drs. Timothy Koves, Robert Noland, and Michael Carper for providing protocols and technical advice used in the collection of the data.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Astrup A Healthy lifestyles in Europe: prevention of obesity and type II diabetes by diet and physical activity. Public Health Nutr 4: 499–515, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M, Kelly DP, Holloszy JO. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J 16: 1879–1886, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Bandyopadhyay GK, Yu JG, Ofrecio J, Olefsky JM. Increased malonyl-CoA levels in muscle from obese and type 2 diabetic subjects lead to decreased fatty acid oxidation and increased lipogenesis; thiazolidinedione treatment reverses these defects. Diabetes 55: 2277–2285, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Bass A, Brdiczka D, Eyer P, Hofer S, Pette D. Metabolic differentiation of distinct muscle types at the level of enzymatic organization. Eur J Biochem 10: 198–206, 1969. [DOI] [PubMed] [Google Scholar]
- 5.Blaak EE, van Aggel-Leijssen DP, Wagenmakers AJ, Saris WH, van Baak MA. Impaired oxidation of plasma-derived fatty acids in type 2 diabetic subjects during moderate-intensity exercise. Diabetes 49: 2102–2107, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Chakravarthy MV, Booth FW. Eating, exercise, and “thrifty” genotypes: connecting the dots toward an evolutionary understanding of modern chronic diseases. J Appl Physiol 96: 3–10, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Choo HJ, Kim JH, Kwon OB, Lee CS, Mun JY, Han SS, Yoon YS, Yoon G, Choi KM, Ko YG. Mitochondria are impaired in the adipocytes of type 2 diabetic mice. Diabetologia 49: 784–791, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Dahlman I, Forsgren M, Sjogren A, Nordstrom EA, Kaaman M, Naslund E, Attersand A, Arner P. Downregulation of electron transport chain genes in visceral adipose tissue in type 2 diabetes independent of obesity and possibly involving tumor necrosis factor-alpha. Diabetes 55: 1792–1799, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Dohm GL, Barakat HA, Tapscott EB, Beecher GR. Changes in body fat and lipogenic enzyme activities in rats after termination of exercise training. Proc Soc Exp Biol Med 155: 157–159, 1977. [DOI] [PubMed] [Google Scholar]
- 10.Dohm GL, Huston RL, Askew EW, Weiser PC. Effects of exercise on activity of heart and muscle mitochondria. Am J Physiol 223: 783–787, 1972. [DOI] [PubMed] [Google Scholar]
- 11.Fitts RH, Booth FW, Winder WW, Holloszy JO. Skeletal muscle respiratory capacity, endurance, and glycogen utilization. Am J Physiol 228: 1029–1033, 1975. [DOI] [PubMed] [Google Scholar]
- 12.Hancock CR, Han DH, Chen M, Terada S, Yasuda T, Wright DC, Holloszy JO. High-fat diets cause insulin resistance despite an increase in muscle mitochondria. Proc Natl Acad Sci USA 105: 7815–7820, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hickner RC, Privette J, McIver K, Barakat H. Fatty acid oxidation in African-American and Caucasian women during physical activity. J Appl Physiol 90: 2319–2324, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Houmard JA Intramuscular lipid oxidation and obesity. Am J Physiol Regul Integr Comp Physiol 294: R1111–R1116, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu G, Lakka TA, Kilpelainen TO, Tuomilehto J. Epidemiological studies of exercise in diabetes prevention. Appl Physiol Nutr Metab 32: 583–595, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Hulver MW, Berggren JR, Cortright RN, Dudek RW, Thompson RP, Pories WJ, MacDonald KG, Cline GW, Shulman GI, Dohm GL, Houmard JA. Skeletal muscle lipid metabolism with obesity. Am J Physiol Endocrinol Metab 284: E741–E747, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Ibdah JA, Perlegas P, Zhao Y, Angdisen J, Borgerink H, Shadoan MK, Wagner JD, Matern D, Rinaldo P, Cline JM. Mice heterozygous for a defect in mitochondrial trifunctional protein develop hepatic steatosis and insulin resistance. Gastroenterology 128: 1381–1390, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Ji H, Friedman MI. Reduced hepatocyte fatty acid oxidation in outbred rats prescreened for susceptibility to diet-induced obesity. Int J Obes (Lond) 27: 1331–1334, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Kaaman M, Sparks LM, van H, V, Smith SR, Sjolin E, Dahlman I, Arner P. Strong association between mitochondrial DNA copy number and lipogenesis in human white adipose tissue. Diabetologia 12: 2526–2533, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Kelley DE, Simoneau JA. Impaired free fatty acid utilization by skeletal muscle in non-insulin-dependent diabetes mellitus. J Clin Invest 94: 2349–2356, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim JY, Hickner RC, Cortright RL, Dohm GL, Houmard JA. Lipid oxidation is reduced in obese human skeletal muscle. Am J Physiol Endocrinol Metab 279: E1039–E1044, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Koh EH, Park JY, Park HS, Jeon MJ, Ryu JW, Kim M, Kim SY, Kim MS, Kim SW, Park IS, Youn JH, Lee KU. Essential role of mitochondrial function in adiponectin synthesis in adipocytes. Diabetes 56: 2973–2981, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Koves TR, Li P, An J, Akimoto T, Slentz D, Ilkayeva O, Dohm GL, Yan Z, Newgard CB, Muoio DM. Peroxisome proliferator-activated receptor-gamma co-activator 1 alpha-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. J Biol Chem 280: 33588–33598, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Koves TR, Noland RC, Bates AL, Henes ST, Muoio DM, Cortright RN. Subsarcolemmal and intermyofibrillar mitochondria play distinct roles in regulating skeletal muscle fatty acid metabolism. Am J Physiol Cell Physiol 288: C1074–C1082, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, Lopaschuk GD, Muoio DM. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 7: 45–56, 2008. [DOI] [PubMed] [Google Scholar]
- 26.Kump DS, Booth FW. Sustained rise in triacylglycerol synthesis and increased epididymal fat mass when rats cease voluntary wheel running. J Physiol 565: 911–925, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kump DS, Laye MJ, Booth FW. Increased mitochondrial glycerol-3-phosphate acyltransferase protein and enzyme activity in rat epididymal fat upon cessation of wheel running. Am J Physiol Endocrinol Metab 290: E480–E489, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Kump DS, Booth FW. Alterations in insulin receptor signalling in the rat epitrochlearis muscle upon cessation of voluntary exercise. J Physiol 562: 829–838, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Labarca C, Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem 102: 344–352, 1980. [DOI] [PubMed] [Google Scholar]
- 30.Laye MJ, Thyfault JP, Stump CS, Booth FW. Inactivity induces increases in abdominal fat. J Appl Physiol 102: 1341–1347, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Louet JF, Hayhurst G, Gonzalez FJ, Girard J, Decaux JF. The coactivator PGC-1 is involved in the regulation of the liver carnitine palmitoyltransferase I gene expression by cAMP in combination with HNF4 alpha and cAMP-response element-binding Protein (CREB). J Biol Chem 277: 37991–38000, 2002. [DOI] [PubMed] [Google Scholar]
- 32.McGarry JD, Brown NF. The mitochondrial carnitine palmitoyltransferase system. From concept to molecular analysis. Eur J Biochem 244: 1–14, 1997. [DOI] [PubMed] [Google Scholar]
- 33.Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstrale M, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, Groop LC. PGC-1 alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 34: 267–273, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Morris RT, Laye MJ, Lees SJ, Rector RS, Thyfault JP, Booth FW. Exercise-induced attenuation of obesity, hyperinsulinemia, and skeletal muscle lipid peroxidation in the OLETF rat. J Appl Physiol 104: 708–715, 2008. [DOI] [PubMed] [Google Scholar]
- 35.Olsen RH, Krogh-Madsen R, Thomsen C, Booth FW, Pedersen BK. Metabolic responses to reduced daily steps in healthy nonexercising men. JAMA 299: 1261–1263, 2008. [DOI] [PubMed] [Google Scholar]
- 36.Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, Landaker EJ, Goldfine AB, Mun E, DeFronzo R, Finlayson J, Kahn CR, Mandarino LJ. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes. Potential role of PGC1 and NRF1. Proc Natl Acad Sci USA 100: 8466–8471, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pilegaard H, Saltin B, Neufer PD. Exercise induces transient transcriptional activation of the PGC-1 alpha gene in human skeletal muscle. J Physiol 546: 851–858, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rector RS, Thyfault JP, Laye MJ, Morris RT, Borengasser SJ, Uptergrove GM, Chakravarthy MV, Booth FW, Ibdah JA. Cessation of daily exercise dramatically alters precursors of hepatic steatosis in Otsuka Long-Evans Tokushima Fatty (OLETF) rats. J Physiol 586: 4241–4249, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rector RS, Thyfault JP, Morris RT, Laye MJ, Borengasser SJ, Booth FW, Ibdah JA. Daily exercise increases hepatic fatty acid oxidation and prevents steatosis in Otsuka Long-Evans Tokushima Fatty rats. Am J Physiol Gastrointest Liver Physiol 294: G619–G626, 2008. [DOI] [PubMed] [Google Scholar]
- 40.Rodbell M Metabolism of isolated fat cells. I. Effects of hormones on glucose metabolism and lipolysis. J Biol Chem 239: 375–380, 1964. [PubMed] [Google Scholar]
- 41.Ryan DH Diet and exercise in the prevention of diabetes. Int J Clin Pract Suppl 134: 28–35, 2003. [PubMed] [Google Scholar]
- 42.Simoneau JA, Veerkamp JH, Turcotte LP, Kelley DE. Markers of capacity to utilize fatty acids in human skeletal muscle: relation to insulin resistance and obesity and effects of weight loss. FASEB J 13: 2051–2060, 1999. [DOI] [PubMed] [Google Scholar]
- 43.Srere PA Citrate synthase. Methods Enzymol 13: 3–5, 1969. [Google Scholar]
- 44.Thyfault JP, Kraus RM, Hickner RC, Howell AW, Wolfe RR, Dohm GL. Impaired plasma fatty acid oxidation in extremely obese women. Am J Physiol Endocrinol Metab 287: E1076–E1081, 2004. [DOI] [PubMed] [Google Scholar]
- 45.Timmons JA, Norrbom J, Scheele C, Thonberg H, Wahlestedt C, Tesch P. Expression profiling following local muscle inactivity in humans provides new perspective on diabetes-related genes. Genomics 87: 165–172, 2006. [DOI] [PubMed] [Google Scholar]
- 46.Valerio A, Cardile A, Cozzi V, Bracale R, Tedesco L, Pisconti A, Palomba L, Cantoni O, Clementi E, Moncada S, Carruba MO, Nisoli E. TNF-alpha downregulates eNOS expression and mitochondrial biogenesis in fat and muscle of obese rodents. J Clin Invest 116: 2791–2798, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wende AR, Huss JM, Schaeffer PJ, Giguere V, Kelly DP. PGC-1α coactivates PDK4 gene expression via the orphan nuclear receptor ERRα: a mechanism for transcriptional control of muscle glucose metabolism. Mol Cell Biol 25: 10684–10694, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoon JC, Puigserver P, Chen G, Donovan J, Wu Z, Rhee J, Adelmant G, Stafford J, Kahn CR, Granner DK, Newgard CB, Spiegelman BM. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature 413: 131–138, 2001. [DOI] [PubMed] [Google Scholar]