Abstract
Iron movement between organ pools involves a dynamic equilibrium of iron efflux and uptake, and homeostatic mechanisms are likely involved in providing iron to cells and organs when required. Daily iron levels in the plasma pool fluctuate with the diurnal cycle, but clear explanations regarding the objectives and regulation of the flux are lacking. The association between diurnal cycle and iron flux is relevant in the disease of restless legs syndrome (RLS), where individuals display diurnal deficits in motor control, have impaired brain iron metabolism, and perhaps altered iron uptake from the plasma pool. The goal of the present study was to examine diurnal variations in peripheral and regional brain iron to evaluate iron flux between organs in iron-sufficient and iron-deficient mice. In mice fed control diet, liver iron was elevated 30–40%, and plasma iron was reduced 20–30% in the active dark period compared with the inactive light phase. Dietary iron deficiency eliminated this variation in liver iron in male and female mice and in plasma iron in male mice. Reductions in ventral midbrain and nucleus accumbens iron and ferritin were apparent in iron-deficient mice during both diurnal phases, but only during the light phase was an ∼25% reduction in whole brain iron observed, suggesting different brain iron requirements between phases. These data demonstrate that iron flux between organs is sensitive to diurnal regulatory biology. Importantly, variations in brain iron may have temporal implications regarding neural functioning and may contribute to the diurnal cycle-dependent symptoms of RLS.
Keywords: diurnal cycles, iron deficiency, restless legs syndrome, ventral midbrain, liver iron
proper iron regulation is important for many peripheral and central biological processes, including electron transfer, cell growth, and oxygen transport, and thus iron movement is tightly regulated (4). Daily iron levels in the blood are not static and are known to fluctuate with the diurnal cycle. The low point in serum iron occurs during the typically inactive evening hours, while the high point in serum iron occurs at midday (55, 61). Total iron binding capacity (TIBC) has also been shown to vary with the light cycle (10). It has been hypothesized that the variations in serum iron and TIBC occur as a result of fluctuating iron release from reticuloendothelial cells. The newly described regulator of iron absorption and release from macrophages, hepcidin, may also be implicated (45). This hormone becomes associated with the cellular iron exporter, ferroportin, and determines the extent of iron flux out of cells (3). The rationale for variations in plasma iron relative to fluctuations in tissue needs for iron is interesting but not elucidated by current theories of iron requirements.
The association between diurnal cycle and iron flux is relevant in the disease of restless legs syndrome (RLS), which has been diagnosed in 3–24% of American and European populations (7, 46, 47). Several studies have shown relationships between RLS and peripheral and brain iron metabolism. Individuals diagnosed with RLS display diurnal-based deficiencies in motor control, where the severity of symptoms increases in the evening hours at a time when serum iron is at its lowest (20, 55). It is tempting to speculate that low serum iron is associated with decreased iron uptake by the brain, but blood-brain barrier uptake of iron is not strictly dependent on plasma iron content or transferrin saturation (5, 39). Nighttime levels of ferritin are also lower in cerebrospinal fluid (CSF) of RLS patients with early-onset symptoms, but they are not necessarily related to lower serum ferritin concentrations, thus adding a further suggestion that diurnal variations in brain iron and systemic iron are not completely related events (2). In the brain, MRI and ultrasound imaging and autopsy studies have all indicated that RLS patients have lower substantia nigra iron, but changes in brain iron with respect to diurnal cycle have not been demonstrated (1, 14, 24, 30, 56). Serum ferritin concentrations (48, 59) and substantia nigra H-ferritin levels (14) are lower as well in RLS, furthering the association between iron deficits and this disease.
Several studies from this laboratory have shown that regional brain iron levels are reduced by dietary iron deficiency in rats (9, 25, 26, 43, 51). Gene and protein expression of ferritin, transferrin, and transferrin receptor (TfR) are related to these changes in brain iron, but all of these proteins were measured during the early part of the light/rest cycle (31, 32). Decreased ventral midbrain (VMB) and striatal iron levels are also correlated with altered dopamine neurotransmission and behavioral deficits. Diurnal cycles influence many outcomes, including brain neurotransmitter synthesis and metabolism (13, 57, 58) and behavior (i.e., feeding, locomotion, startle response), but, to date, there are no data indicating that brain iron varies over the diurnal cycle or that variations in brain iron are related to these behaviors. Thus an understanding of diurnal fluctuations in brain iron may provide some clues as to the diurnal-based behaviors in RLS patients.
Mice from the BXD recombinant inbred (RI) strains have been used for the study of many complex behavioral and physiological phenotypes. Previously, a subset of 15 BXD strains was shown to differ in regards to regional brain iron and liver iron content (36). Liver iron, as well as VMB (substantia nigra and ventral tegmental area), caudate, nucleus accumbens, and prefrontal cortex iron, were continuously distributed among these strains. Liver iron did not correlate with VMB iron, suggesting that iron regulation peripherally is independent of VMB iron content. Peripheral iron measurements, including hemoglobin, hematocrit, plasma iron, and spleen iron also vary among the BXD strains (34, 35). All of these measurements have been collected at specific time points and not considered within the context of the diurnal cycle. In addition, these studies in mice did not consider the impact of dietary iron deficiency on the expression of iron proteins. Given the link between RLS, diurnal cycles, and brain iron deficiency, we believe it is important to understand the dynamic movement of iron between organs in the light and dark phases of the light cycle. In this study, strain 40 of the BXD RI strains was used to explore diurnal variations in peripheral and regional brain iron to examine the influence of diurnal cycle on iron movement in the iron-sufficient and iron-deficient (ID) states.
METHODS
Ethical approval.
All experimental protocols were conducted in accordance with the National Institutes of Health Animal Care guidelines and were approved by the Penn State Institutional Animal Care and Use Committee.
Animals, treatment, and housing.
Breeding pairs of strain 40 of the BXD RI panel were purchased from Jackson Laboratories (Bar Harbor, MA) and housed in an isolated environment under the supervision of Dr. Byron Jones. This strain of mouse was chosen based on a previous analysis where 30 strains of the BXD/Ty RI panel, including the C57Bl/6 and DBA parental strains, were analyzed for VMB iron content (unpublished observations). Strain 40 mice showed midrange VMB iron levels among the strains studied. At 21 days after birth, mice were weaned and separated by sex (2–3/cage). Cages were randomly divided into two dietary groups: control (50 μg/g iron) and ID (3 μg/g iron). All attempts were made to have an equal number of control and ID mice from each litter. Control and ID diets were prepared in our laboratory following the recipe of the American Institute of Nutrition-93G diet, with cornstarch as the sole source of carbohydrate (50, 53). The ID diet contained all components of the control diet, with the exception of ferric citrate, and iron levels were verified using atomic absorption spectrophotometry after wet digestion with nitric acid. All rats received food and deionized distilled water ad libitum in a temperature (23 ± 2°C) and humidity (40%) controlled room maintained on a 12:12-h light-dark cycle (lights on 6:00 AM to 6:00 PM). All experimental protocols were conducted in accordance with the NIH Animal Care guidelines and were approved by the Penn State Institutional Animal Care and Use Committee.
Hematology and liver and spleen iron.
At 120 ± 5 days of age, mice were euthanized by CO2 suffocation, either 3–4 h into the lights-on phase (9:00–10:00 AM) or 2–3 h into the lights-off phase (8:00–9:00 PM) (n = 12–15 mice per diet per sex per light cycle). These periods correspond to peak periods of inactivity and activity, respectively (33). Whole blood was collected by cardiac puncture, centrifuged (16,110 g, 4°C, 15 min), and the sera frozen at −80°C. Serum iron and TIBC were determined as previously described (25, 50). Transferrin saturation was calculated as serum iron/TIBC × 100. Hemoglobin values were determined photometrically using cyanmethemoglobin standard solution (Sigma Aldrich, St. Louis, MO), and hematocrit was calculated after 5 min of centrifugation of blood samples in heparinized microcapillary tubes at 13,700 g. Liver and spleen nonheme iron were measured using the technique described by Cook et al. (17).
Brain dissection and homogenate preparation.
The brain was removed from the skull and quickly dissected on ice for frontal cortex, striatum, nucleus accumbens, VMB, pons, and cerebellum via protocols previously utilized in this laboratory (36, 62). The regions were placed immediately in storage tubes, weighed, and frozen at −80°C. All brain tissue remaining after dissection was collected into a storage tube, weighed, and also frozen at −80°C. For iron and ELISA analysis, brain regions were thawed on ice and homogenized 1:10 in phosphate buffered saline (9.1 mM Na2HPO4, 1.7 mM NaH2PO4, 150 mM NaCl, pH 7.4) containing protease inhibitors (Roche, Indianapolis, IN).
Brain iron analysis.
Brain region aliquots (n = 7–10/group) were wet digested by published and standard procedures and analyzed for iron concentration by atomic absorption spectrophotometry (Perkin Elmer AAnalyst 600, Perkin Elmer, Norwalk, CT) (51). Standards were prepared by diluting a Perkin Elmer iron standard (PE no. N9300126) in 0.2% ultrapure nitric acid, and blanks were prepared with digesting and diluting reagents to control for possible contamination. All standard curves exceeded r > 0.99.
ELISA analysis.
Brain ferritin and TfR and liver ferritin protein levels were determined from tissue homogenates using an ELISA (n = 7–10/group), based on a method developed by Dr. Nan Li (32, 51). Briefly, the analysis was performed on 1 mg of protein homogenate from each region in triplicate. The polyclonal ferritin and TfR primary antibodies were used at a dilution of 1:2,000 (produced at the Pennsylvania State University). For both assays, an anti-rabbit IgG (Sigma-Aldrich) secondary antibody was used at 1:4,000. Optical density was measured on a microplate reader (model EL340, BioTek Instruments, Winooski, VT) at 405 and 570 nm.
Statistics.
Group values are expressed as means ± SE. All data were analyzed by three-way ANOVA with treatment, strain, and sex as between-subjects variables using SYSTAT 10.2 (SYSTAT Software, Richmond, CA). Post hoc analyses were performed using the Tukey pairwise multiple-comparison test. Differences were considered statistically significant at P < 0.05.
RESULTS
Body weight, hematology, and liver and spleen iron.
Overall, a low-iron diet significantly altered body weight (F = 15.4, P < 0.001), with a different effect in males vs. females. Body weight was reduced in ID male (P < 0.05), but not female mice, regardless of time of day when the mice were euthanized (Table 1). Hematocrit and hemoglobin levels were significantly lower in mice fed an ID diet (F = 309.3, P < 0.001; F = 555.6, P < 0.001, respectively), but these decrements in red cell indexes were greater in male mice than female mice (F = 6.1, P < 0.05; F = 5.3, P < 0.05). Compared with control mice, ID males showed 49–57% and 60–70% reductions in hematocrit and hemoglobin levels, respectively (P < 0.05 for both), while hematocrit was reduced by ∼40% and hemoglobin was reduced by 52–57% in their ID female littermates (P < 0.05 for both, Table 1). Body weight and hematocrit and hemoglobin levels did not vary with respect to the time of day that the samples were collected (F < 1 for all).
Table 1.
Hematology and spleen iron levels of control and iron-deficient mice in the light and dark phases
| Body Weight, g | Hb, g/l | Hct, % | TIBC | TfSat, % | Spleen Iron, μmol/l | |
|---|---|---|---|---|---|---|
| CNM-light | 27.3±1.6 | 117±4 | 37.4±1.1 | 488.6±43.4 | 48.9±4.3 | 304.1±28.5 |
| CNM-dark | 28.5±1.1 | 114±3 | 37.2±0.7 | 345.9±32.0† | 49.7±3.6 | 412.9±36.8 |
| IDM-light | 20.7±0.9* | 36±4* | 16.9±1.4* | 612.9±25.4* | 10.6±1.1* | 51.4±5.8* |
| IDM-dark | 23.5±1.4*† | 42±4* | 18.9±1.7* | 532.4±41.7*† | 15.8±2.2*† | 68.7±6.3* |
| CNF-light | 24.1±1.6 | 119±3 | 36.4±1.7 | 341.5±30.3 | 60.4±5.1 | 323.9±26.8 |
| CNF-dark | 23.7±1.0 | 112±5 | 36.6±0.9 | 357.9±24.2 | 47.0±3.2† | 324.7±21.3 |
| IDF-light | 24.1±1.2 | 57±4* | 22.5±3.0* | 507.7±24.1* | 18.9±1.3* | 72.7±6.8* |
| IDF-dark | 22.0±0.7 | 51±5* | 21.5±1.7* | 551.2±30.3* | 11.4±1.0*† | 73.0±6.8* |
Values are means ± SE; n = 12–15 mice/group. CNM-light and CNM-dark, control males during the light and dark phase, respectively; IDM-light and IDM-dark, iron-deficient males during the light and dark phase, respectively; CNF-light and CNF-dark, control females during the light and dark phase, respectively; IDF-light and IDF-dark, iron-deficient females during the light and dark phase, respectively; TIBC, total iron-binding capacity; TfSat, transferrin saturation.
P < 0.05 relative to respective control diet-fed group.
P < 0.05 vs. respective light-phase group fed the same diet.
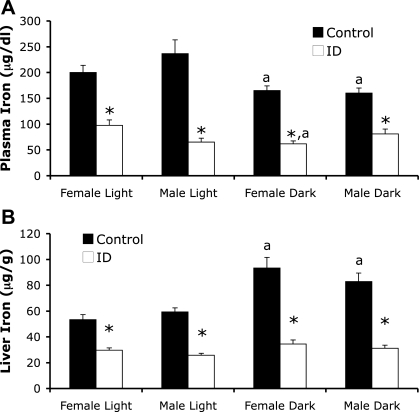
ID male and female mice also showed lower plasma iron levels than their respective control mice (F = 131.0, P < 0.001, Fig. 1A). Light phase influenced plasma iron levels (F = 9.1, P < 0.01). Mice fed a control diet had plasma iron levels that were lower during the dark (active) phase compared with the light (inactive) phase (P < 0.05). ID female mice, but not male mice, also showed the same diurnal response in plasma iron (P < 0.05) as we observed in control mice. TIBC was increased by iron deficiency (F = 52.0, P < 0.001) in both sexes compared with respective controls (Table 1). Light phase also influenced TIBC levels in mice (F = 7.1, P < 0.01), but only control and ID male mice showed lower TIBC levels in the dark compared with the light phase (P < 0.05, Table 1). Transferrin saturation, the ratio of plasma iron to TIBC, also differed significantly by dietary treatment (F = 245.3, P < 0.001; Table 1). Iron deficiency resulted in 30–50% decreases in transferrin saturation in all ID groups compared with controls (P < 0.05; Table 1). The effect of light phase on transferrin saturation was different between male and female mice (F = 7.44, P < 0.01). Control and ID female mice showed lower levels of transferrin saturation, while male ID mice had an increase in transferrin saturation in the dark phase compared with the light phase (P < 0.05).
Fig. 1.
Peripheral iron measurements in strain 40 control and iron-deficient (ID) mice. Plasma iron (A) and liver iron (B) were measured in male and female control and ID mice during the light (Female Light, Male Light) and dark phases (Female Dark, Male Dark) of the diurnal cycle. Values are means ± SE. Significance is denoted as *P < 0.05 vs. respective control diet fed group and aP < 0.05 vs. respective light-phase group fed the same diet.
Dietary iron deficiency significantly lowered both liver and spleen iron in all groups of BXD strain 40 mice (F = 167.5, P < 0.001; F = 289.8, P < 0.001, respectively). Liver iron was also influenced by light phase (F = 32.4, P < 0.001), as liver iron was 43 and 28% higher in control diet-fed male and female mice, respectively, in the dark phase compared with the light phase (P < 0.05; Fig. 1B). ID mice did not show a diurnal variation in liver iron, as they had similar levels of liver iron across the light phases (Fig. 1B). Spleen iron concentration did not vary with phases of the diurnal cycle (F = 3.88, P = 0.062; Table 1). Splenomegaly (enlargement of the spleen) was observed in 61% of the male ID mice and 37% of the female ID mice and did not differ by phase of the diurnal cycle.1
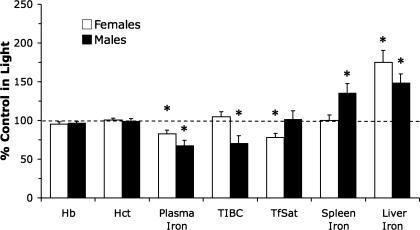
The overall diurnal changes in peripheral iron measures in control diet-fed mice are shown in Fig. 2. These data indicate that the storage compartments for iron are sensitive to the diurnal cycle and that iron movement through the plasma pool differs between sexes. Dietary iron deficiency completely eliminated the diurnal variation in systemic iron status measurements, perhaps suggesting an elimination of the exchangeable pool, or flux, of iron once tissue stores are at low levels.
Fig. 2.
Diurnal changes in peripheral iron levels in male and female strain 40 control mice. Values are presented as percentage of light-phase control diet-fed groups (mean percent ± SE). *Significance is denoted as P < 0.05 vs. respective light phase control group. Hb, hemoglobin; Hct, hematocrit; TIBC, total iron-binding capacity; TfSat, transferrin saturation.
Brain iron.
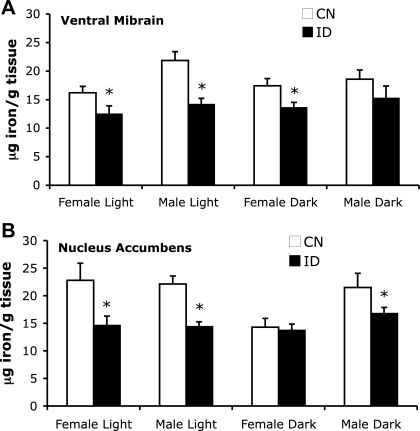
Regional brain iron content was measured in VMB (substantia nigra and ventral tegmental area), nucleus accumbens, striatum, prefrontal cortex, cerebellum, and pons. Iron deficiency resulted in decrements in brain iron in VMB (F = 23.2, P < 0.001) and nucleus accumbens (F = 6.6, P < 0.01), but not in other brain regions (Fig. 3). VMB iron was reduced ∼35% in males during the light phase, ∼25% in females during the light phase, and ∼20% in females during the dark phase compared with respective control groups (P < 0.05; Fig. 3A). A similar trend in VMB iron was found in males during the dark phase, but this effect was not statistically significant. VMB iron content was greater in males than females (F = 6.32, P < 0.05), but did not change significantly between light cycles (F < 1, P = 0.75). In nucleus accumbens, strain 40 male mice showed 25–35% reductions in iron during both phases of the diurnal cycle with dietary iron deficiency (P < 0.05; Fig. 3B). A 35% decrease in nucleus accumbens iron also was detected in female mice during the light phase. Importantly, however, female ID mice did not differ from control female mice in the dark phase, despite having severe anemia and depletion of systemic iron storage pools. In nucleus accumbens, iron levels were not different between sexes (F = 3.22, P = 0.08) or time of day (F < 1, P = 0.65). Brain iron content in striatum (F < 1, P = 0.55), prefrontal cortex (F < 1, P = 0.47), cerebellum (F = 1.4, P = 0.24), and pons (F < 1, P = 0.56) was not affected by dietary iron deficiency (Table 2). Furthermore, regional brain iron did not differ with respect to time of day in any of the brain regions analyzed [VMB (F < 1, P = 0.75); nucleus accumbens (F < 1, P = 0.65); striatum (F < 1, P = 0.57); prefrontal cortex (F = 2.4, P = 0.13); cerebellum (F < 1, P = 0.54); pons (F = 2.3, P = 0.13)].
Fig. 3.
Regional brain iron measurements in strain 40 control (CN) and ID mice. VMB iron (A) and nucleus accumbens iron (B) were measured in male and female control and ID mice during the light (Female Light, Male Light) and dark phases (Female Dark, Male Dark) of the diurnal cycle. Values are presented as means ± SE. *Significance is denoted as P < 0.05 vs. respective control diet-fed group.
Table 2.
Brain iron levels in prefrontal cortex, striatum, pons, and cerebellum of control and iron-deficient mice in the light and dark phases
| Prefrontal Cortex | Striatum | Pons | Cerebellum | |
|---|---|---|---|---|
| CNM-light | 19.4±1.6 | 22.3±1.9 | 25.1±2.6 | 20.3±1.2 |
| CNM-dark | 19.5±1.3 | 21.5±2.4 | 20.5±1.6 | 19.2±1.0 |
| IDM-light | 15.3±1.9 | 21.3±2.5 | 23.7±1.7 | 18.5±1.5 |
| IDM-dark | 17.7±1.3 | 18.2±1.4 | 20.0±1.5 | 19.2±2.4 |
| CNF-light | 15.8±2.3 | 18.6±2.3 | 16.1±2.0 | 23.3±3.4 |
| CNF-dark | 17.7±1.6 | 15.7±1.2 | 18.6±1.4 | 23.5±2.0 |
| IDF-light | 17.7±2.4 | 14.4±1.9 | 18.4±0.7 | 19.9±2.0 |
| IDF-dark | 15.3±1.9 | 15.8±1.9 | 18.8±1.9 | 24.5±2.8 |
Values are means ± SE in μg/g tissue; n = 7–10 mice/group.
After the brains were dissected for VMB, nucleus accumbens, striatum, prefrontal cortex, pons, and cerebellum, the remaining brain tissue was collected, and iron levels determined (Fig. 4). Whole brain iron levels in each animal were attained by summing iron content in each brain region (prefrontal cortex, striatum, nucleus accumbens, VMB, pons, cerebellum) and iron content in the leftover brain tissues. Iron deficiency reduced whole brain iron (F = 10.5, P < 0.01), but this effect differed with respect to time of day (F = 5.2, P < 0.05). Only during the light phase of the diurnal cycle did strain 40 male and female mice show significant decrements in total brain iron (P < 0.05, Fig. 4). During the active part of the diurnal cycle, total brain iron levels did not differ between ID and control treatment groups.
Fig. 4.
Total brain iron measurements in strain 40 control and ID mice. Total brain iron was attained by summing the iron content within each region and the leftover brain tissue (see methods). Values are means ± SE. Significance is denoted as *P < 0.05 vs. respective control diet-fed group, and aP < 0.05 vs. respective light-phase group fed the same diet.
Ferritin protein levels in brain and liver.
ELISA was used to measure relative levels of the iron storage protein, ferritin, in liver, VMB, and striatum. Liver ferritin was lower in ID compared with that in control diet groups (F = 12.1, P < 0.001; Table 3). There were no differences in liver ferritin between male and female mice (F = 2.0, P = 0.17) or between light phases (F < 1, P = 0.61). The mean ferritin levels in strain 40 mice fed the control diet were 15.4 ng/mg protein compared with 12.3 ng/mg protein in ID mice (Table 3). Although male and female control mice displayed >25% increases in liver iron during the active phase relative to the inactive phase of the light cycle, a corresponding increase in ferritin protein was not observed.
Table 3.
Liver, VMB, and striatum ferritin and VMB TfR levels in control and iron-deficient mice in the light and dark phases
| Liver Ferritin, ng/mg protein | VMB Ferritin, OD | Striatum Ferritin, OD | VMB TfR, OD | |
|---|---|---|---|---|
| CNM-light | 15.6±1.0 | 1.021±0.127 | 0.716±0.132 | 0.098±0.008 |
| CNM-dark | 15.4±0.4 | 0.777±0.138 | 0.781±0.204 | 0.061±0.004† |
| IDM-light | 13.1±1.0* | 0.786±0.121* | 0.835±0.124 | 0.086±0.011 |
| IDM-dark | 13.5±0.3* | 0.571±0.136 | 0.557±0.232 | 0.079±0.010 |
| CNF-light | 15.2±1.5 | 1.383±0.132 | 0.934±0.265 | 0.109±0.002 |
| CNF-dark | 15.3±1.9 | 1.298±0.285 | 1.030±0.232 | 0.076±0.011† |
| IDF-light | 10.1±0.3* | 0.598±0.120* | 0.790±0.118 | 0.067±0.007 |
| IDF-dark | 11.8±0.9* | 0.652±0.147* | 0.982±0.229 | 0.087±0.006† |
Values are means ± SE; n = 6–8 mice/group. VMB, ventral midbrain; OD, optical density; TfR, transferrin receptor.
P < 0.05 relative to respective control group.
P < 0.05 vs. respective light-phase group fed the same diet.
Dietary treatment resulted in statistically significant differences in VMB ferritin (F = 19.3, P < 0.001). VMB ferritin levels were lower in ID female mice relative to controls during the light and dark phases and in ID male mice during the light phase (P < 0.05 for all; Table 3). In contrast, when iron levels were not reduced by iron deficiency (males during the active phase), there was not a significant decrease in ferritin. Female ID mice showed a greater reduction in ferritin than male ID mice (F = 4.0, P < 0.05), but VMB ferritin levels did not change with respect to time of day (F = 1.8, P = 0.20). In striatum (Table 3), where iron levels were not affected, dietary iron deficiency had no impact on ferritin protein concentrations (F < 1, P = 0.90). Striatal ferritin levels were not different between sexes (F = 2.1, P = 0.15) and did not change with light cycle (F < 1, P = 0.90).
TfR protein levels in VMB were not different with respect to diet (F = 1.2, P = 0.28) or sex (F < 1, P = 0.54), although there were differences between the light and dark periods (F = 6.2, P < 0.05; Table 3). The effect of light period on TfR levels varied by dietary treatment (F = 13.5, P < 0.001). In mice fed control diet, TfR levels were lower during the dark phase than the light phase (P < 0.05), but in ID female mice, TfR levels were higher during the dark phase (Table 3).
DISCUSSION
This investigation was designed to examine the influence of diurnal cycle on iron distribution in the periphery and the brain in the iron-sufficient and ID states. Daily iron levels in the blood are not static and are known to fluctuate with the diurnal cycle, which is likely important for diurnal changes in oxygen transport, cell growth, and electron transfer (10, 29). Our study extends these previous findings by examining diurnal fluctuations in liver, spleen, and regional brain iron in an iron-sufficient and an ID RI strain of mouse. The association between diurnal cycle and iron flux is relevant in the disease of RLS. This sensorimotor disorder is characterized by an urge to move the legs at the end of the normal active phase and can be successfully treated with iron intravenous injection (1, 2, 14, 20). Females are more susceptible than males to develop early-onset RLS (48); thus we also evaluated diurnal iron distributions in mice with regard to sex.
In the present study, liver iron in male and female mice fed the control diet was elevated 30–40%, and plasma iron was reduced 20–30% in the active dark phase compared with the inactive light phase (Fig. 2). Diurnal fluctuations in hematological measurements, including plasma iron and TIBC, also have been reported in rabbits and humans and attributed to the release of iron from the reticuloendothelial system (10, 28, 29, 61), although more recent evidence demonstrates that hepcidin, an iron-regulatory hormone released from hepatocytes, also regulates iron flux into the blood. During times of inflammation or elevated body iron, hepcidin complexes with the iron exporter ferroportin causing ferroportin to be internalized and degraded, thus preventing release of more iron into the body from both gastrointestinal absorptive cells and macrophages (44, 49, 63). Importantly, both serum and urine hepcidin concentrations follow a diurnal cycle that is inversely related to serum iron levels (37). Studies show that interleukin-6, which is secreted in a biphasic manner through regulation by clock genes in the suprachiasmatic nucleus, induces the synthesis of hepcidin via a JAK/STAT pathway (54). It is possible then that these changes in liver and plasma iron between lighting conditions in strain 40 mice may be promoted by changes in hepcidin/ferroportin interactions. The half-life of hepcidin in the plasma pool is certainly consistent with this explanation (54).
Despite increases in liver iron in control mice during the active phase, significant differences in ferritin protein levels between the dark and the light phases of the diurnal cycle were not detected. There are several possible explanations for these observations. H- and L-ferritin subunits were not independently measured, so isoform-specific increases in ferritin may exist. The other option is that the accumulated iron in the liver did not enter into a cytoplasmic pool that is necessary for the stimulation of ferritin production (19). The ferritin antibody we utilized should have easily recognized an elevation in L-ferritin with increased cytoplasmic iron. Determination of ferritin levels with an antibody does not specify the extent of iron loading into the ferritin core; thus it is likely that liver ferritin iron content was elevated during this accumulation of liver iron (19).
Movement of iron between organs is altered by iron deficiency in strain 40 BXD mice. Dietary iron deficiency completely eliminated the diurnal variation in liver iron status, suggesting that the exchangeable pool of iron is diminished once tissue stores are at a low level. The decrease in liver ferritin levels in the ID mice is reflective of the loss of iron in the storage pool (4). Plasma iron levels in ID female mice were maintained on the diurnal rhythm observed in control mice, although this change in plasma iron was less than that observed in control mice. These observations are consistent with an increase in uptake of iron from the plasma to other organs during the active dark phase and a possible decrease in release of iron from iron-donating cells and organs.
In ID mice, the brain appears to be a site of uptake, since whole brain iron was reduced by ∼25% in ID mice during the inactive phase, but was similar to controls during the active phase of the diurnal cycle. This change in brain iron, however, was not universal, since VMB and nucleus accumbens iron levels were lower in most groups of ID mice during both time periods. A heterogeneity in brain iron requirements is implied from differential responses to both dietary iron depletion and repletion (26, 31, 32, 50). The candidate proteins that may modulate this regional heterogeneity of response include ferritin, TfR, and transferrin (4), but data are generally lacking regarding gene expression of these proteins across the diurnal cycle. The present study does note that brain ferritin but not TfR protein levels were correlated with changes in brain iron. The linkage between iron and ferritin in brain, but not in liver, is suggestive of different regulatory approaches and is consistent with a much greater prevalence of H ferritin in brain than in liver (16). The significant effect of time of day on TfR levels in brain is also consistent with a dynamic model of iron metabolism in the brain.
Iron movement from brain and organ pools across the diurnal cycle has not been documented before; however, it has been suggested that a dynamic equilibrium of iron between organs may exist (15). This concept is derived from the observations that radio-labeled iron appears in the choroid plexus within 1 h of intravenous injection and is distributed throughout the brain within 24 h, indicating that iron uptake is a continuous process (8, 18). There are several proposed mechanisms of brain iron uptake and efflux at the blood-brain barrier (27, 42, 60) and through the ventricular system (6, 40, 41). Recent evidence supports a role for hepcidin and ferroportin in iron movement between the brain and the periphery. It has been suggested that ferroportin-mediated iron export from brain cells at the choroid plexus may be regulated by hepcidin levels in the CSF (12). The source of the signal(s) for controlling iron uptake is unclear, although the observation that hepcidin levels are reduced in substantia nigra in RLS brains makes it an appealing candidate (12). Furthermore, stimulation of the immune system by intravenous injection of lipopolysaccharide is associated with increases in hepcidin protein and gene expression in brain, suggesting that hepcidin-ferroportin regulation of iron flux may also exist at the levels of the blood-brain barrier (62a). Further studies are required to elucidate the potential role of hepcidin in the regulation of brain iron homeostasis.
Deficits in regional brain iron and changes in uptake and efflux of brain iron are currently being investigated as underlying causes of RLS symptoms. MRI, ultrasound imaging, and autopsy studies have all indicated that RLS patients exhibit low brain iron, particularly in the substantia nigra (1, 14, 24, 56). Furthermore, infusion of iron dextran improves RLS symptoms and elevates substantia nigra and prefrontal cortex iron levels (22). Later studies in which a different iron complex was infused did not result in an improvement in nigral iron concentration and did not improve RLS symptoms (23). Patients with early onset, but not late onset, symptoms also have reduced levels of ferritin in CSF and H-ferritin levels in substantia nigra (11, 20, 21), supporting the argument that iron storage is altered in the central nervous system. A more recent study indicates that hepcidin concentrations are elevated in neuromelanin cells of the substantia nigra and in substantia nigra homogenates and reduced in CSF from early-onset RLS patients compared with control subjects (12). The authors hypothesized that a reduction in hepcidin in CSF may be exacerbating RLS by promoting abnormal iron release at the choroid plexus through hepcidin-ferroportin signaling (12).
In conclusion, the present report presents the novel observations that iron movement between storage pools varies across the diurnal cycle in inbred mice. Associated reports also note that dopamine system functioning and responses to pharmacological manipulations of the dopamine system differ across the diurnal cycle in this strain of mouse (unpublished observations). Strain 40 BXD mice were not as susceptible as Sprague-Dawley rats to dietary iron deficiency (25, 26), but it is clear from previous genetic analyses of the BXD panel of mice that brain iron homeostasis is strongly affected by the genotype of the animal (35, 36). Current unpublished data from five more strains from the BXD panel, as well as from Sprague-Dawley rats, show that the diurnal variations in iron are not unique to strain 40 mice or just mice. Ultimately, these studies will attempt to determine whether variations in brain iron across the diurnal cycle offer some explanation for the highly temporal aspects of RLS symptoms.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant NS 35088 with Dr. Christopher Earley as the leading principle investigator.
Acknowledgments
The authors thank Dr. Byron Jones and Cecelia Irvin for providing the mice from the Animal Core for these studies and Sarah Rundle, Denise Konrad, and Lindsay Ghramm for excellent technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Splenomegaly was defined as ID mice having a relative spleen weight >6.9 mg/g body wt, which was the highest observed in control mice (38).
REFERENCES
- 1.Allen RP, Barker PB, Wehrl F, Song HK, Earley CJ. MRI measurement of brain iron in patients with restless legs syndrome. Neurology 56: 263–265, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Allen RP, Dean T, Earley CJ. Effects of rest-duration, time-of-day and their interaction on periodic leg movements while awake in restless legs syndrome. Sleep Med 6: 429–434, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Anderson GJ, Darshan D, Wilkins SJ, Frazer DM. Regulation of systemic iron homeostasis: how the body responds to changes in iron demand. Biometals 20: 665–674, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Beard JL, Connor JR. Iron status and neural functioning. Annu Rev Nutr 23: 41–58, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Beard JL, Wiesinger JA, Li N, Connor JR. Brain iron uptake in hypotransferrinemic mice: influence of systemic iron status. J Neurosci Res 79: 254–261, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Benkovic SA, Connor JR. Ferritin, transferrin, and iron in selected regions of the adult and aged rat brain. J Comp Neurol 338: 97–113, 1993. [DOI] [PubMed] [Google Scholar]
- 7.Bjorvatn B, Leissner L, Ulfberg J, Gyring J, Karlsborg M, Regeur L, Skeidsvoll H, Nordhus IH, Pallesen S. Prevalence, severity and risk factors of restless legs syndrome in the general adult population in two Scandinavian countries. Sleep Med 6: 307–312, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Bradbury MW Transport of iron in the blood-brain-cerebrospinal fluid system. J Neurochem 69: 443–454, 1997. [DOI] [PubMed] [Google Scholar]
- 9.Burhans MS, Dailey C, Beard Z, Wiesinger J, Murray-Kolb L, Jones BC, Beard JL. Iron deficiency: differential effects on monoamine transporters. Nutr Neurosci 8: 31–38, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Casale G, Migliavacca A, Bonora C, Zurita IE, de Nicola P. Circadian rhythm of plasma iron, total iron binding capacity and serum ferritin in arteriosclerotic aged patients. Age Ageing 10: 115–118, 1981. [DOI] [PubMed] [Google Scholar]
- 11.Clardy SL, Earley CJ, Allen RP, Beard JL, Connor JR. Ferritin subunits in CSF are decreased in restless legs syndrome. J Lab Clin Med 147: 67–73, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Clardy SL, Wang X, Boyer PJ, Earley CJ, Allen RP, Connor JR. Is ferroportin-hepcidin signaling altered in restless legs syndrome? J Neurol Sci 247: 173–179, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Collu R, Jequier JC, Letarte J, Leboeuf G, Ducharme JR. Effect of stress and hypothalamic deafferentation on the secretion of growth hormone in the rat. Neuroendocrinology 11: 183–190, 1973. [DOI] [PubMed] [Google Scholar]
- 14.Connor JR, Boyer PJ, Menzies SL, Dellinger B, Allen RP, Ondo WG, Earley CJ. Neuropathological examination suggests impaired brain iron acquisition in restless legs syndrome. Neurology 61: 304–309, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Connor JR, Menzies SL, Burdo JR, Boyer PJ. Iron and iron management proteins in neurobiology. Pediatr Neurol 25: 118–129, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Connor JR, Snyder BS, Arosio P, Loeffler DA, LeWitt P. A quantitative analysis of isoferritins in select regions of aged, Parkinsonian, and Alzheimer's diseased brains. J Neurochem 65: 717–724, 1995. [DOI] [PubMed] [Google Scholar]
- 17.Cook GA, King MT, Veech RL. Changes in liver inorganic pyrophosphate content during ethanol metabolism. Adv Exp Med Biol 132: 433–440, 1980. [DOI] [PubMed] [Google Scholar]
- 18.Crowe A, Morgan EH. Iron and transferrin uptake by brain and cerebrospinal fluid in the rat. Brain Res 592: 8–16, 1992. [DOI] [PubMed] [Google Scholar]
- 19.De Domenico I, McVey Ward D, Kaplan J. Regulation of iron acquisition and storage: consequences for iron-linked disorders. Nat Rev Mol Cell Biol 9: 72–81, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Earley CJ, Allen RP, Beard JL, Connor JR. Insight into the pathophysiology of restless legs syndrome. J Neurosci Res 62: 623–628, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Earley CJ, Connor JR, Beard JL, Clardy SL, Allen RP. Ferritin levels in the cerebrospinal fluid and restless legs syndrome: effects of different clinical phenotypes. Sleep 28: 1069–1075, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Earley CJ, Heckler D, Allen RP. The treatment of restless legs syndrome with intravenous iron dextran. Sleep Med 5: 231–235, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Earley CJ, Horska A, Mohamed MA, Barker PB, Beard JL, Allen RP. A randomized, double-blind, placebo-controlled trial of intravenous iron sucrose in restless legs syndrome. Sleep Med. In press. [DOI] [PMC free article] [PubMed]
- 24.Earley CJ, Barker PB, Horska A, Allen RP. MRI-determined regional brain iron concentrations in early- and late-onset restless legs syndrome. Sleep Med 7: 458–461, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Erikson KM, Jones BC, Beard JL. Iron deficiency alters dopamine transporter functioning in rat striatum. J Nutr 130: 2831–2837, 2000. [DOI] [PubMed] [Google Scholar]
- 26.Erikson KM, Jones BC, Hess EJ, Zhang Q, Beard JL. Iron deficiency decreases dopamine D1 and D2 receptors in rat brain. Pharmacol Biochem Behav 69: 409–418, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Fishman JB, Rubin JB, Handrahan JV, Connor JR, Fine RE. Receptor-mediated transcytosis of transferrin across the blood-brain barrier. J Neurosci Res 18: 299–304, 1987. [DOI] [PubMed] [Google Scholar]
- 28.Fox RR, Laird CW. Biochemical parameters of clinical significance in rabbits. II. Diurnal variations. J Hered 61: 265–268, 1970. [DOI] [PubMed] [Google Scholar]
- 29.Fox RR, Laird CW, Kirshenbaum J, Meier H. Effect of strain, sex, and circadian rhythm on rabbit serum bilirubin and iron levels. Proc Soc Exp Biol Med 145: 421–427, 1974. [DOI] [PubMed] [Google Scholar]
- 30.Godau J, Schweitzer KJ, Liepelt I, Gerloff C, Berg D. Substantia nigra hypoechogenicity: definition and findings in restless legs syndrome. Mov Disord 22: 187–192, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Han J, Day JR, Connor JR, Beard JL. Gene expression of transferrin and transferrin receptor in brains of control vs. iron-deficient rats. Nutr Neurosci 6: 1–10, 2003. [PubMed] [Google Scholar]
- 32.Han J, Day JR, Thomson K, Connor JR, Beard JL. Iron deficiency alters H- and L-ferritin expression in rat brain. Cell Mol Biol (Noisy-le-grand) 46: 517–528, 2000. [PubMed] [Google Scholar]
- 33.Hutchins DA, Rogers KJ. Some observations on the circadian rhythm of locomotor activity of mice after depletion of cerebral monoamines. Psychopharmacologia 31: 343–348, 1973. [DOI] [PubMed] [Google Scholar]
- 34.Johannes F, Blizard DA, Lionikas A, Lang DH, Vandenbergh DJ, Stout JT, Strauss JA, McClearn GE, Vogler GP. QTL influencing baseline hematocrit in the C57BL/6J and DBA/2J lineage: age-related effects. Mamm Genome 17: 689–699, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Jones BC, Beard JL, Gibson JN, Unger EL, Allen RP, McCarthy KA, Earley CJ. Systems genetic analysis of peripheral iron parameters in the mouse. Am J Physiol Regul Integr Comp Physiol 293: R116–R124, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Jones BC, Reed CL, Hitzemann R, Wiesinger JA, McCarthy KA, Buwen JP, Beard JL. Quantitative genetic analysis of ventral midbrain and liver iron in BXD recombinant inbred mice. Nutr Neurosci 6: 369–377, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Kemna EH, Tjalsma H, Podust VN, Swinkels DW. Mass spectrometry-based hepcidin measurements in serum and urine: analytical aspects and clinical implications. Clin Chem 53: 620–628, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Kuvibidila SR, Porretta C. Differential effects of iron deficiency on the expression of CD80 and CD86 co-stimulatory receptors in mitogen-treated and untreated murine spleen cells. J Cell Biochem 86: 571–582, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Malecki EA, Devenyi AG, Beard JL, Connor JR. Existing and emerging mechanisms for transport of iron and manganese to the brain. J Neurosci Res 56: 113–122, 1999. [DOI] [PubMed] [Google Scholar]
- 40.Moos T Immunohistochemical localization of intraneuronal transferrin receptor immunoreactivity in the adult mouse central nervous system. J Comp Neurol 375: 675–692, 1996. [DOI] [PubMed] [Google Scholar]
- 41.Moos T, Morgan EH. The significance of the mutated divalent metal transporter (DMT1) on iron transport into the Belgrade rat brain. J Neurochem 88: 233–245, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Moos T, Morgan EH. Transferrin and transferrin receptor function in brain barrier systems. Cell Mol Neurobiol 20: 77–95, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nelson C, Erikson K, Pinero DJ, Beard JL. In vivo dopamine metabolism is altered in iron-deficient anemic rats. J Nutr 127: 2282–2288, 1997. [DOI] [PubMed] [Google Scholar]
- 44.Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest 113: 1271–1276, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306: 2090–2093, 2004. [DOI] [PubMed] [Google Scholar]
- 46.Nichols DA, Allen RP, Grauke JH, Brown JB, Rice ML, Hyde PR, Dement WC, Kushida CA. Restless legs syndrome symptoms in primary care: a prevalence study. Arch Intern Med 163: 2323–2329, 2003. [DOI] [PubMed] [Google Scholar]
- 47.O'Keeffe ST, Egan D, Myers A, Redmond S. The frequency and impact of restless legs syndrome in primary care. Ir Med J 100: 539–542, 2007. [PubMed] [Google Scholar]
- 48.Patrick LR Restless legs syndrome: pathophysiology and the role of iron and folate. Altern Med Rev 12: 101–112, 2007. [PubMed] [Google Scholar]
- 49.Pietrangelo A, Trautwein C. Mechanisms of disease: the role of hepcidin in iron homeostasis–implications for hemochromatosis and other disorders. Nat Clin Pract Gastroenterol Hepatol 1: 39–45, 2004. [DOI] [PubMed] [Google Scholar]
- 50.Pinero D, Jones B, Beard J. Variations in dietary iron alter behavior in developing rats. J Nutr 131: 311–318, 2001. [DOI] [PubMed] [Google Scholar]
- 51.Pinero DJ, Li NQ, Connor JR, Beard JL. Variations in dietary iron alter brain iron metabolism in developing rats. J Nutr 130: 254–263, 2000. [DOI] [PubMed] [Google Scholar]
- 53.Reeves PG, Nielsen FH, Fahey GC Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 123: 1939–1951, 1993. [DOI] [PubMed] [Google Scholar]
- 54.Rivera S, Nemeth E, Gabayan V, Lopez MA, Farshidi D, Ganz T. Synthetic hepcidin causes rapid dose-dependent hypoferremia and is concentrated in ferroportin-containing organs. Blood 106: 2196–2199, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scales WE, Vander AJ, Brown MB, Kluger MJ. Human circadian rhythms in temperature, trace metals, and blood variables. J Appl Physiol 65: 1840–1846, 1988. [DOI] [PubMed] [Google Scholar]
- 56.Schmidauer C, Sojer M, Seppi K, Stockner H, Hogl B, Biedermann B, Brandauer E, Peralta CM, Wenning GK, Poewe W. Transcranial ultrasound shows nigral hypoechogenicity in restless legs syndrome. Ann Neurol 58: 630–634, 2005. [DOI] [PubMed] [Google Scholar]
- 57.Simon ML, George R. Diurnal variations in plasma corticosterone and growth hormone as correlated with regional variations in norepinephrine, dopamine and serotonin content of rat brain. Neuroendocrinology 17: 125–138, 1975. [DOI] [PubMed] [Google Scholar]
- 58.Sleipness EP, Sorg BA, Jansen HT. Diurnal differences in dopamine transporter and tyrosine hydroxylase levels in rat brain: dependence on the suprachiasmatic nucleus. Brain Res 1129: 34–42, 2007. [DOI] [PubMed] [Google Scholar]
- 59.Sun ER, Chen CA, Ho G, Earley CJ, Allen RP. Iron and the restless legs syndrome. Sleep 21: 371–377, 1998. [PubMed] [Google Scholar]
- 60.Taylor EM, Morgan EH. Role of transferrin in iron uptake by the brain: a comparative study. J Comp Physiol [B] 161: 521–524, 1991. [DOI] [PubMed] [Google Scholar]
- 61.Uchida T, Akitsuki T, Kimura H, Tanaka T, Matsuda S, Kariyone S. Relationship among plasma iron, plasma iron turnover, and reticuloendothelial iron release. Blood 61: 799–802, 1983. [PubMed] [Google Scholar]
- 62.Unger EL, Beard JL, Jones BC. Iron regulation in C57BLI6 and DBA/2 mice subjected to iron overload. Nutr Neurosci 10: 89–95, 2007. [DOI] [PubMed] [Google Scholar]
- 62a.Wang Q, DUF, Qian ZM, Ge XH, Zhu L, Yung WH, Yang L, Ke Y. Lipopolysaccharide induces a significant increase in expression of iron regulatory hormone hepcidin in the cortex and substantia nigra in rat brain. Endocrinology. 149: 3920–3925, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zoller H, Theurl I, Koch R, Kaser A, Weiss G. Mechanisms of iron mediated regulation of the duodenal iron transporters divalent metal transporter 1 and ferroportin 1. Blood Cells Mol Dis 29: 488–497, 2002. [DOI] [PubMed] [Google Scholar]