Abstract
Clinical and experimental studies have shown that trauma combined with hemorrhage shock (T/HS) is associated with myocardial contractile dysfunction. However, the initial events triggering the cardiac dysfunction are not fully elucidated. Thus we tested the hypothesis that factors carried in intestinal (mesenteric) lymph contribute to negative inotropic effects in rats subjected to a laparotomy (T) plus hemorrhagic shock (HS; mean arterial blood pressure of 30–40 Torr for 90 min) using a Langendorff isolated heart preparation. Left ventricular (LV) function was assessed 24 h after trauma plus sham shock (T/SS) or T/HS by recording the LV developed pressure (LVDP) and the maximal rate of LVDP rise and fall ( ± dP/dtmax) in five groups of rats: 1) naive noninstrumented rats, 2) rats subjected to T/SS, 3) rats subjected to T/HS, 4) rats subjected to T/SS with mesenteric lymph duct ligation (T/SS+LDL), or 5) rats subjected to T/HS+LDL. Cardiac function was comparable in hearts from naive, T/SS, and T/SS+LDL rats. Both LVDP and ± dP/dtmax were significantly depressed after T/HS. The T/HS hearts also manifested a blunted responsiveness to increases in coronary flow rates and Ca2+, and this was prevented by LDL preceding T/HS. Although electrocardiograms were normal under physiological conditions, when the T/HS hearts were perfused with low Ca2+ levels (∼0.5 mM), prolonged P-R intervals and second-degree plus Wenckebach-type atrioventricular blocks were observed. No such changes occurred in the control or T/HS+LDL hearts. The effects of T/HS were similar to those of the Ca2+ channel antagonist diltiazem, indicating that an impairment of cellular Ca2+ handling contributes to T/HS-induced cardiac dysfunction. In conclusion, gut-derived factors carried in mesenteric lymph are responsible for acute T/HS-induced cardiac dysfunction.
Keywords: myocardial contractility, Langendorff techniques, electrocardiogram
primary cardiac diseases represent the most common cause of acute heart failure in intensive care units. However, it is now recognized that an acute acquired state of myocardial dysfunction can also occur in septic patients as well as in patients sustaining major trauma, hemorrhage, or burns (5, 21, 22, 35) and contribute to a state of circulatory collapse. This acquired condition of acute cardiac dysfunction has been observed even in patients without intrinsic cardiac disease, indicating that these major stress states can lead to myocardial dysfunction even in patients with healthy hearts.
In the last decades, excellent animal models have been developed to study hemorrhagic shock (24) and significant progress has been made in understanding the pathophysiological mechanisms of myocardial contractile dysfunction associated with trauma combined with hemorrhagic shock (T/HS) (26, 34). Experimental hemodynamic studies indicate that the signaling pathways and effector molecules involved in cardiac depression are multifactorial. However, the source(s) of the initial factors involved in the development of altered myocardial function following various forms of shock have not been well established. Yet, identification of the initial (primary) factors that initiate the processes leading to myocardial contractile dysfunction and the development of therapies to block their effects is likely to be more successful than attempts at blocking secondary cardiodepressant factors.
The concept that the gastrointestinal tract (i.e., gut) plays a pivotal pathogenic role in the pathogenesis of the systemic inflammatory response syndrome and multiple organ dysfunction (MODS) following shock is well established (6, 12, 17). Initially, these studies implicated loss of gut barrier function and the subsequent translocation of bacteria and endotoxin as being involved in gut-induced MODS. However, more recently, there is increasing evidence that it is the egress of nonbacterial gut-derived factors carried in the mesenteric lymph that lead to the development of postshock organ failure (25, 42). In fact, Cox et al. (8) recently reported that an isolated episode of intestinal ischemia will lead to myocardial dysfunction in dogs and that this gut-induced effect also appears to be transduced via the intestinal lymphatics. The mechanisms by which these lymphatic borne factors are produced as well as the organs involved in their production seem to involve pancreatic enzymes interacting with the ischemic gut (1, 14, 28). Furthermore, our recent studies measuring cardiac contractility in isolated hearts and myocytes from rats subjected to burn injury (20, 32) have shown that mesenteric lymph generated following burn injury directly causes changes in cardiac contractility, thereby supporting the role of mesenteric lymph as a key factor involved in regulating myocardial contractile dysfunction.
It is conceivable that the same mechanisms of burn injury can be active in T/HS-induced cardiac dysfunction. However, no attempts have been made to date to fully investigate the effects of mesenteric lymph duct ligation (LDL) on T/HS-induced myocardial contractile dysfunction. Thus, to improve our understanding of signaling factors that trigger cardiac dysfunction following T/HS, it becomes important to examine whether mesenteric LDL, which prevents gut-derived intestinal lymph from reaching the systemic circulation, would be protective. Therefore, the primary goal of this study was to test the hypothesis that the gut is the source of these myocardial depressant-inducing factors and that they exit the gut primarily via the intestinal lymphatic system. To accomplish this, we tested the ability of LDL to limit T/HS-induced cardiac contractile dysfunction, since LDL prevents intestinal lymph from reaching the systemic circulation.
Since cellular Ca2+ homeostasis is an important regulator of cardiac contractility (4, 18), and altered Ca2+ signaling plays an important role in injury conditions such as burns or sepsis (33), the second goal was to determine whether the mechanism of T/HS-induced cardiac dysfunction involves impaired cellular Ca2+ handling. In addition, since cardiac stresses such as ischemia and reperfusion are often associated with abnormalities of cardiac rhythm, we also monitored the electrical rhythm (ECG) of the hearts under baseline conditions and in response to changing Ca2+. Last, since Ca2+ influx through L-type Ca2+ channel is a key determinant of cardiac contractility, we examined the effects of the pharmacological L-type Ca2+ channel blocker diltiazem on left ventricular developed pressure (LVDP) and ECG simultaneously, to investigate a potential mechanism for T/HS-induced myocardial dysfunction.
MATERIALS AND METHODS
Animals.
Male Sprague-Dawley rats (250–350g) were used in this study. The animal maintenance protocols and the experiments were approved by the New Jersey Medical School Animal Care and Use Committee. The care and handling of these animals conformed to all guidelines for animal care as outlined by the National Institutes of Health.
Mesenteric lymph ligation and T/HS model.
All surgical procedures are performed as previously described (13, 25). The model we utilized was a fixed-pressure hemorrhage shock model combined with tissue trauma in the form of a laparotomy. This model was chosen to better reflect the clinical setting of trauma, where patients experience tissue injury plus blood loss. Briefly, rats were anesthetized intraperitoneally with pentobarbital sodium (50 mg/kg), and the femoral artery was isolated using aseptic techniques and cannulated with polyethylene (PE-50) tubing containing heparinized saline (10 U/ml). This catheter was attached in-line to a blood pressure analyzer (BP-2 blood pressure; Columbus Instruments, Columbus, OH) for measurement and monitoring of mean arterial pressure (MAP) and heart rate (HR) during the shock period. The internal jugular vein was used for withdrawal of blood. Before the induction of hemorrhagic shock, the rats underwent a midline laparotomy and were randomly subjected to mesenteric LDL or sham duct ligation as previously described (32). Once the laparotomy was closed, the arterial pressure was reduced to 30–40 Torr and maintained at this level for 90 min by withdrawing or reinfusing shed blood (kept at 37°C). A heat lamp was positioned over the animal to prevent hypothermia. At the end of the shock period, animals were resuscitated by reinfusing all of the shed blood. We chose shed blood resuscitation because we have previously shown that T/HS-induced organ injury after shed blood resuscitation is equivalent to or less than that observed with crystalloid or blood-crystalloid resuscitation regimens (13). No other fluids were infused. After resuscitation, the rats were placed in restraining cages where animals were gently restrained in a natural posture without stress to prevent dislodgment of the catheters. The sham shock (T/SS) rats were anesthetized and underwent femoral artery and jugular vein cannulation; however, no blood was withdrawn.
Rat isolated heart preparation.
Twenty-four hours after the T/HS or T/SS operation, the rats were treated with heparin (200 IU/kg ip) to prevent the formation of thrombi in the excised heart and anesthetized with pentobarbital sodium (50 mg/kg ip). Each heart was rapidly excised, and the ascending aorta was cannulated. The cannula was attached to a Langendorff system (ADInstruments, Colorado Springs, CO). The hearts were perfused in a retrograde fashion with Krebs-Henseleit buffer (KHB; in mM): 118.0 NaCl, 4.7 KCl, 2.0 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, 25.0 NaHCO3, and 11.0 glucose, pH 7.4. During the initial equilibration period, the hearts were allowed to stabilize for 10–20 min at constant LV end-diastolic pressure (LVEDP) adjusted to 5–10 Torr, and the perfusion flow rate was determined for each heart (36). For studies of Ca2+-dependent contractile responses, the perfusate was switched to solutions with different Ca2+ concentrations at the constant flow rate with a peristaltic pump until they appeared to be at steady state (for ∼1–2 min). The perfusion buffer was filtered through a 45-μm filter and continuously gassed with 95% O2 and 5% CO2. The whole system was water jacketed and maintained at 37°C.
Experimental protocols.
Five groups of rats were studied: 1) naive noninstrumented controls as well as rats subjected to 2) T/SS, 3) T/SS+LDL, 4) T/HS, and 5) T/HS+LDL. LV systolic pressure and LVEDP were assessed by measuring the intraventricular pressure with a fluid-filled balloon (polyethylene film) that had been inserted into the left ventricle via the mitral valve from the left atrium. This balloon was connected to a pressure transducer (ADInstruments MLT 844). LVDP was calculated as the difference between the peak systolic pressure and LVEDP. The baseline LVEDP was comparable in hearts isolated from all groups.
The maximum rates of LVDP rise (+dP/dtmax) and fall (−dP/dtmax) were obtained using an electronic differentiator. The ECG was monitored by electrodes attached to the apex and base of the heart (ADInstruments ML 136 Animal Bio Amp). All parameters were stored and analyzed off-line using PowerLab software (ADInstruments). LV myocardial function was measured at 24 h after T/SS or T/HS. This time point was selected because earlier in vivo heart studies have shown that cardiac output, stroke volume, and ±dP/dtmax are significantly depressed in rat hearts 24 h after T/HS (40). Although the Langendorff preparation provides highly reproducible information on the LV systolic and diastolic pressure and their derivatives, one potential limitation is that the assessment of cardiac work parameters that are dependent on intact circulation is not available.
Statistical analysis.
Data are means ± SE. Statistical significance was determined using ANOVA to assess differences among the groups for each of the variables. Between-group/condition analyses were conducted using a Student's t-test. Statistical analysis for ECG parameters reported in Fig. 6B was performed using the Fisher's exact probability test. P < 0.05 were considered statistically significant.
RESULTS
Because the anesthesia and/or the various procedures performed in the T/SS and T/SS+LDL hearts could have affected cardiac pump function, our initial experiments compared LV contractile function in isolated hearts from naive noninstrumented (control), T/SS, and T/SS+LDL rats. In this experiment, we measured baseline HR, LVDP, and ±dP/dtmax under conditions where the hearts were perfused with KHB (2 mM Ca2+) at a constant coronary flow rate of 8 ml/min (36). As summarized in Table 1, we found that none of these LV contractile parameters differed among these groups. Consequently, we pooled the data obtained from naive, T/SS, and T/SS+LDL hearts into one group designated as the control group.
Table 1.
Baseline LV function measured in control rats and rats 24 h after T/SS or T/SS+LDL
| HR, beat/min | LVP, Torr | +dP/dtmax, Torr/s | −dP/dtmax, Torr/s | n | |
|---|---|---|---|---|---|
| Control | 256.1±8.8 | 116.3±3.3 | 3,524±213 | 2,160±86 | 12 |
| T/SS | 273.5±4.6 | 105.5±6.6 | 3,320±220 | 1,841±109 | 4 |
| T/SS+LDL | 261.6±12.3 | 113.6±9.0 | 3,223±256 | 2,131±249 | 4 |
Values are means ± SE; n = no. of rats. The baseline left ventricular (LV) heart rate (HR), pressure (LVP), and the maximum rise (+dP/dtmax) and fall (−dP/dtmax) were measured in naive noninstrumented (control) rats and in rats subjected to trauma and sham shock (T/SS) with or without lymph duct ligation (LDL).
Effects of T/HS on cardiac contractile function.
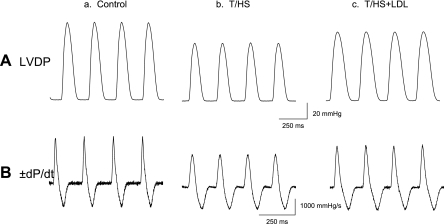
Figure 1 shows representative baseline LVDP (A) and ±dP/dtmax waveforms (B) recorded from Langendorff-perfused hearts isolated from control (a), T/HS (b), and T/HS+LDL (c) rats. The baseline LVDP of the T/HS hearts were significantly depressed (83 ± 4.2 Torr, n = 9, P < 0.05) compared with those of the control (116 ± 3.3 Torr, n = 15) or T/HS+LDL hearts (117 ± 2.5 Torr, n = 9) as was the rate of contraction and relaxation rates (±dP/dtmax) (Fig. 2). These findings in isolated heart preparations were consistent with significant depression of in vivo cardiac function following T/HS (40). In contrast, LDL significantly prevented T/HS-induced changes in LVDP and ±dP/dtmax.
Fig. 1.
Representative left ventricular (LV) tracings showing trauma combined with hemorrhage shock (T/HS)-induced contractile depression and prevention by mesenteric lymph ligation. Studies were performed in Langendorff-perfused rat hearts at 24 h following T/HS. Typical examples are shown of LV developed pressure (LVDP; A) and the rate of LVDP rise and fall (±dP/dt; B) recorded from control (a), T/HS (b), and T/HS with lymph duct ligation (T/HS+LDL) hearts (c) during perfusion with Krebs-Henseleit buffer (KHB) with a constant coronary flow rate of 8 ml/min.
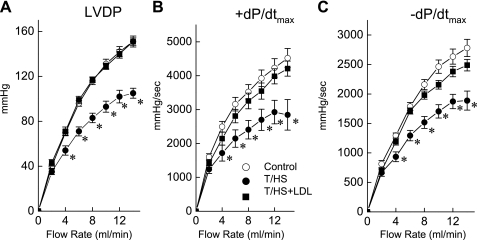
Fig. 2.
LV performance to increasing coronary flow rates is blunted in T/HS hearts. Changes in LVDP (A) and the maximum rates of rise (+dP/dtmax; B) and fall (−dP/dtmax; C) in LVDP are shown in response to changes in coronary flow rate. In control rat hearts, LVDP and ±dP/dtmax were increased with increasing coronary flow rates. However, the responses to increasing flow rates were depressed in T/HS hearts but preserved in the T/HS+LDL hearts. Data are means ± SE (n = 15, 9, and 9 for control, T/HS, and T/HS+LDL groups, respectively). *P < 0.05 vs. control and T/HS+LDL groups.
We next examined changes in LV contractile parameters in response to changes in coronary flow rates. In this experiment, the hearts were exposed to different coronary flow rates and LV function was analyzed at a steady state (Fig. 2). In all groups, LVDP increased as coronary flow rates increased (Fig. 2A); however, the magnitude of increase was significantly less in the T/HS group than in the control or T/HS+LDL groups. Similarly, in all groups, both the rise (+dP/dtmax; Fig. 2B) and fall (−dP/dtmax; Fig. 2C) of LVDP were increased with increasing flow rates. However, the hearts from the T/HS group showed significantly lower amplitudes of LVDP, +dP/dtmax, and −dP/dtmax than the control or T/HS+LDL groups.
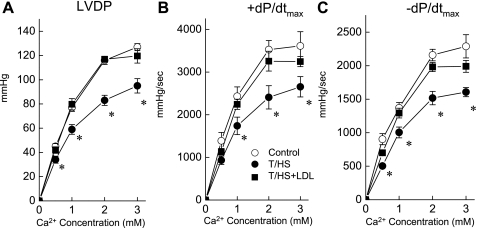
Since there are strong links among Ca2+ regulation, Ca2+ signaling, and heart failure (4, 18), we examined whether T/HS-induced LV dysfunction is associated with altered Ca2+ handling. This was accomplished by examining the contractile response to altered perfusate Ca2+ (Fig. 3). The control and T/HS+LDL hearts showed a normal physiological response where LVDP, +dP/dtmax, and −dP/dtmax increased as extracellular Ca2+ is acutely elevated. Although the T/HS hearts showed increases in these contractile parameters as the external Ca2+ concentration was increased, the magnitudes of the increases were blunted significantly compared with those of the control or T/HS+LDL groups. These results indicate that LV contractile reserve is reduced in T/HS hearts, presumably because responses to Ca2+ are impaired, and that lymph duct ligation prevents this response.
Fig. 3.
LV performance to increasing Ca2+ concentrations in the perfusate was blunted in the T/HS hearts but not in the T/HS+LDL hearts. Changes in LVDP (A), +dP/dtmax (B), and −dP/dtmax (C) are shown in response to Ca2+ in the perfusate. In control rat hearts, LVDP and ±dP/dtmax were increased with an acute increase in Ca2+ concentration. The responses to Ca2+ were depressed in T/HS but not in the T/HS+LDL hearts. Data are means ± SE (n = 15, 9, and 9 for control, T/HS, and T/HS+LDL groups, respectively). *P < 0.05 vs. control and T/HS+LDL groups.
Effects of T/HS on ECG parameters.
To determine whether intrinsic cardiac conduction abnormalities are associated with T/HS induced stress, we continuously recorded ECG signals and studied the effects of changes in perfusate Ca2+ concentrations in the control and T/HS hearts (Figs. 4 and 5). For ECG analysis, averaged measurements of 10 ECG records were made, and R-R interval (to monitor HR), PR intervals [to monitor atrioventricular (AV) conduction], and peak amplitude of the R wave were measured.
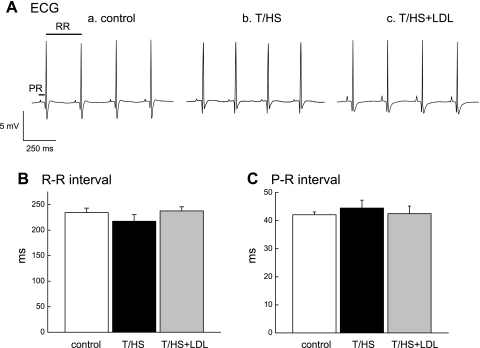
Fig. 4.
ECG recordings or ECG parameters measured in hearts perfused with 2 mM Ca2+ KHB. A typical example of ECG recordings in hearts from control (a), T/HS (b), and T/HS+LDL rats (c) is shown (A). Mean R-R interval (B) and mean PR interval (C) is shown in control, T/HS, and T/HS+LDL hearts. Data are means ± SE (n = 10, 6, and 6 for control, T/HS, and T/HS+LDL groups, respectively).
Fig. 5.
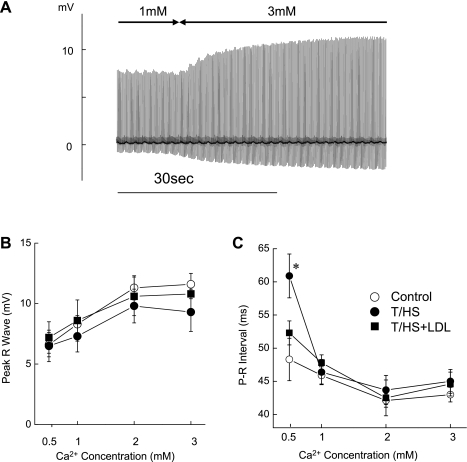
Effects of changes in perfusate Ca2+ concentrations (0.5–3 mM) on ECG parameters showing that PR interval was increased in T/HS hearts at 0.5 mM Ca2+ concentrations. A typical example of continuous ECG recordings during the change of perfusate Ca2+ from 1 to 3 mM in a control heart is shown (A). The mean voltage of peak R wave (B) and mean PR interval (C) are plotted against perfusate Ca2+ concentrations. Data are means ± SE (n = 7, 6, and 5 for control, T/HS, and T/HS+LDL groups, respectively). *P < 0.05 vs. control and T/HS+LDL groups.
Figure 4A shows typical examples of ECG recorded from Langendorff-perfused hearts isolated from the control (a), T/HS (b), or T/HS+LDL hearts (c). When the hearts were perfused with physiological solution (2 mM Ca2+), no significant abnormalities were observed in the ECG of the T/HS hearts. The average times of R-R intervals (HR) and the PR intervals (AV conduction) recorded in the control, T/HS, and T/HS+LDL heart preparations were similar (Fig. 4, B and C).
To further examine the ECG responses to Ca2+ concentration, we perfused the hearts with solutions containing different Ca2+ concentrations (ranging from 0.5 to 3 mM; Fig. 5). As shown in Fig. 5A, the ECG was recorded continuously during equilibration and analyzed when new steady-state levels were reached. In all groups, increasing Ca2+ concentrations increased the peak amplitude of the R wave (Fig. 5B) and shortened the PR interval (Fig. 5C). Interestingly, the T/HS hearts exhibited significantly (P < 0.05) prolonged PR interval at a lower Ca2+ concentration (0.5 mM). The peak R-wave amplitude also appeared lower in T/HS hearts compared with control or T/HS+LDL hearts, indicating an alteration of the transmembrane potential. However, the difference was not significant, presumably due to high heart-to-heart variation.
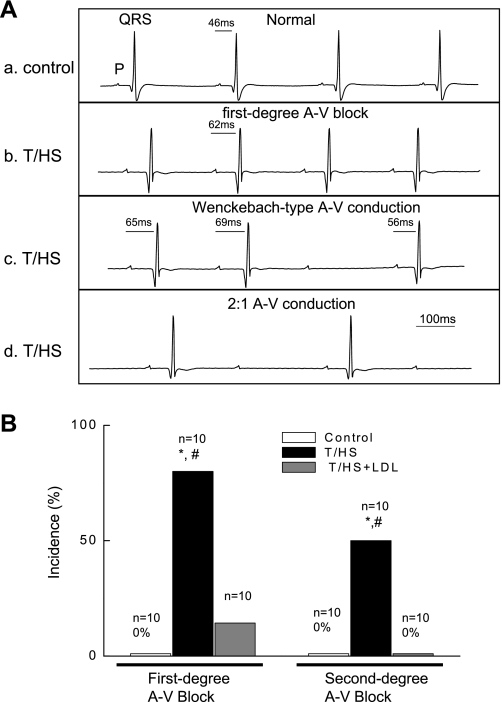
Changes in perfusate Ca2+ concentrations (0.5–3 mM) did not cause abnormalities of cardiac rhythm such as premature beats or ventricular tachycardia in either the control or the T/HS hearts. However, a significant prolongation in electrical conduction (prolonged PR interval) occurred in the T/HS group when hearts were perfused with lower Ca2+ concentration (Fig. 5C). Therefore, we further analyzed ECG patterns using 0.5 mM Ca2+. As shown in Fig. 6A, T/HS hearts demonstrated first-degree AV block (PR interval >60 ms) and Wenckebach-type AV block as well as episodes of 2:1 AV conduction blockade. The results are summarized in Fig. 6B. Although the majority of T/HS hearts manifested significant evidence of abnormal ECG with AV conduction block, this did not occur in any of the 10 control or 10 T/HS+LDL heart preparations examined. These ECG results indicate that an impairment of cardiac myocyte Ca2+ handling develops following T/HS, and this may contribute to the contractile defect and altered ECG dynamics.
Fig. 6.
T/HS hearts exhibit abnormal atrioventricular (AV) conduction at lower external Ca2+ (0.5 mM). A: ECG in control and T/HS hearts: a, normal conduction in control heart; b, first-degree AV block in T/HS heart showing prolonged PR interval; c, second-degree AV block in T/HS heart with a Wenckebach-type AV conduction; and d, 2:1 AV conduction in T/HS heart. B: incidence of first- and second-degree AV block. Numbers (n) correspond to the total number of hearts measured. *P < 0.05 vs. control group. #P < 0.05 vs. T/HS+LDL group.
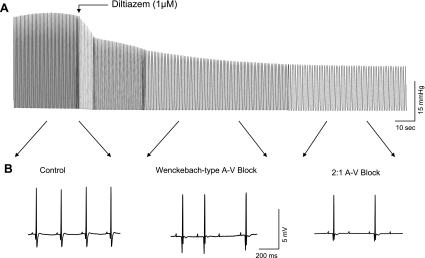
Under physiological conditions, Ca2+ influx through voltage-gated L-type Ca2+ channels is a key determinant of cardiac contractility and a major factor in AV conduction (4, 29). It is therefore possible that depressed cardiac output and ECG changes observed in T/HS hearts are mediated though TH/S-induced downregulation of L-type Ca2+ channel activity. We thus compared the characteristic effects of a Ca2+ channel blocker, diltiazem, known to inhibit the voltage-gated L-type subclass of Ca2+ channel (37), on LVDP and the ECG of normal hearts with those abnormalities observed in T/HS hearts. A typical tracing of the simultaneous measurement of LVDP and the ECG shows that diltiazem decreased LVDP and induced AV conduction defects in normal hearts (Fig. 7). The magnitude of the decrease in LVDP in normal hearts exposed to a 1 μM concentration of diltiazem (40 ± 5%, n = 8) was very similar to that observed in T/HS hearts (Fig. 2). Furthermore, the diltiazem-induced decrease in LVDP was also associated with a prolongation of the PR interval similar to what was observed in the T/HS hearts. In addition, diltiazem induced a Wenckebach-type second-degree AV block in three of the six hearts and 2:1 AV conduction blockade in two of the six hearts tested. These diltiazem effects are in good agreement with the hypothesis that impaired cellular Ca2+ handling is associated with downregulation of L-type Ca2+ channel and is at least partially responsible for the depressed myocardial contractility and altered ECG dynamics observed in T/HS hearts.
Fig. 7.
Effects of diltiazem (1 μM) on LVDP and ECG in Langendorff-perfused normal rat hearts. A representative trace of LVDP (A) and ECG (B) were recorded simultaneously during the application of diltiazem. ECG recordings taken at the indicated times are shown below continuous recordings on the expanded time scale.
We next examined whether pharmacological reduction of L-type Ca2+ channels in control hearts can produce a myocardial contractile phenotype similar to that observed in T/HS hearts. As presented in Figs. 1 and 2, LVDP and ±dP/dtmax were significantly reduced in T/HS hearts. Because cardiac contraction is activated by Ca2+ release from the sarcoplasmic reticulum (SR) Ca2+ channels, and this process is reversed by the active reuptake of Ca2+ into the SR by a Ca2+-ATPase and outward Ca2+ transport via the sarcolemmal pump and the Na+/Ca2+ exchanger (4), slower velocity of LVDP could be related to depressed SR function. In fact, recent evidence indicates that contractile dysfunction in cardiac hypertrophy and failure is accompanied by prolonged contraction and relaxation velocities due to a reduction of SR function (4, 18). On the other hand, the slower rate of relaxation observed in T/HS hearts could have been secondary to reduced amplitude of LVDP. As shown in Table 2, we found that diltiazem, which reduced ∼30–40% of LVDP, resulted in a quantitatively similar inhibition of ±dP/dtmax. These results suggest that the cellular mechanisms for altered contractility in T/HS may be different from the common pattern of changes (e.g., loss of SR function) observed in cardiac hypertrophy and heart failure.
Table 2.
LV function measured in isolated rat hearts from control, T/HS, or diltiazem-treatd rats
| LVP, Torr | +dP/dtmax, Torr/s | −dP/dtmax, Torr/s | n | |
|---|---|---|---|---|
| Control | 121.0±1.7 | 3,690±167 | 2,226±66 | 15 |
| T/HS | 83.0±4.2* | 2,408±273* | 1,517±10* | 9 |
| Diltiazem | 79.3±4.9* | 2,196±108* | 1,414±53* | 9 |
Values are means ± SE; n = no. of rats. LV function was measured in control rats, rats subjected to trauma and sham shock (T/SS), and after application of diltiazem (1 μM).
P < 0.05 vs. control group.
DISCUSSION
Myocardial depression following T/HS has been well established in the literature (21, 30, 41). Although the presence of cardiac dysfunction is well described, the mechanism(s) responsible for acute T/HS-induced myocardial contractile dysfunction remain to be fully elucidated. Two major theories have been proposed to explain the pathogenesis of acute myocardial dysfunction in these circumstances. However, because of a lack of supporting experimental evidence, the first theory, which was that burn, trauma, shock, or sepsis-induced myocardial dysfunction was due to decreased myocardial perfusion leading to ischemic injury, has been replaced by a second theory focusing on the inflammatory response (5, 22). The inflammatory theory of myocardial depression postulates that functional rather than structural changes are responsible for depressed contractility and that myocardial depression is mediated by endogenously produced proinflammatory factors (5, 7, 22, 27, 35, 38). This notion of an inflammatory cardiomyopathy is consistent with this fact that these conditions provoke a profound systemic inflammatory response characterized by increased levels of circulating proinflammatory mediators, leukocyte activation, microvascular leakage, and organ failure (12). Based on our experimental studies implicating gut-derived factors carried in the mesenteric lymph as contributing factors to lung and other organ injuries as well as the induction of a systemic inflammatory state after T/HS or burn injury (15), we hypothesized that gut-derived factors might also be involved in acute T/HS-induced cardiac dysfunction.
The current studies documenting that T/HS-induced myocardial contractile dysfunction can be totally abrogated by LDL support the hypothesis that factors contained in T/HS lymph are necessary for the induction of acute myocardial dysfunction after T/HS. This notion that gut-derived factors carried in the mesenteric lymph can cause acute myocardial dysfunction is further supported by our studies in a burn model where LDL prevented ex vivo burn-induced myocardial depression and where burn lymph recreated this myocyte depressant state in vitro (20, 32). On the basis of our previous burn studies showing that the contractile dysfunction of postburn hearts is linked with a decrease in ventricular myocyte Ca2+ transients caused by a decrease in the Ca2+ entry through L-type Ca2+ channels (20), we examined Ca2+ responsiveness of the T/HS hearts. The T/HS hearts had a blunted contractile response to increases in extracellular Ca2+ concentration compared with the control or T/HS+LDL hearts, indicating an impairment of Ca2+ handling. Furthermore, under conditions of low Ca2+, the T/HS hearts, but not the control or T/HS+LDL hearts, displayed ECG evidence of first- and second-degree AV block. The decreased contractile responsiveness to Ca2+ observed in the T/HS hearts is consistent with studies of failing hearts in other conditions, such as chronic heart failure, ventricular hypertrophy, and ischemia-reperfusion injuries, where contractile dysfunction is associated with impaired myocardial Ca2+ homeostasis (18).
Likewise, the ECG findings of a prolonged PR interval and AV conduction abnormalities in the presence of reduced Ca2+ concentration further implicate a role of impaired Ca2+ handling in T/HS-induced myocardial contractile dysfunction. One potential explanation for these Ca2+-related observations in the T/HS hearts is that L-type Ca2+ channels are altered. This notion is based on the fact that in the mammalian heart, the L-type Ca2+ channel is critically involved in excitation-contraction (EC) coupling and that depolarizations of the sinoatrial and AV nodes are largely dependent on the movement of Ca2+ through the L-type Ca2+ channel (4, 29). Although we are emphasizing the idea that depressed L-type Ca2+ channel function may be present in T/HS hearts, alterations in other components of EC coupling also could lead to electrophysiological and contractile changes. However, consistent with the notion of impaired L-type Ca2+ channel function in the T/HS hearts, we found that normal hearts exposed to the L-type Ca2+ channel blocker diltiazem manifested a decrease in contractility and ECG conduction abnormalities similar to the T/HS hearts. This observation that diltiazem mimicked the effects of T/HS supports the notion that reduced L-type Ca2+ channel function may play a role in T/HS-induced myocardial dysfunction.
The mechanism of the functional downregulation of the L-type Ca2+ channel is unknown, but it may involve altered L-type Ca2+ channel gene transcription, translation, membrane trafficking, or posttranslational modification such as channel phosphorylation or a combination of factors. It is interesting to note that changes in intracellular Ca2+ trafficking due to cytokine production have been reported (31), and certain proinflammatory cytokines, such as TNF-α and IL-1, as well as nitric oxide production have been shown to suppress L-type Ca2+ currents in rat myocytes and thereby reduce Ca2+ transients, resulting in myocyte contractile depression (31). In this study, we are suggesting the idea that changes in cellular Ca2+ handling, possibly downregulation of L-type Ca2+ channel, may contribute to depressed contractility and abnormal ECG in T/HS hearts. However, there could be alternative explanations for abnormal cardiac function, and further studies that include direct examination of ionic channels involved in the cardiac contraction as well as conduction system are required to prove or refute this point.
The notion that myocardial depression may be caused by circulating factors was proposed as early as 1966 (3), and since then, unidentified myocardial depressant factors have been described in animals subjected to hemorrhagic, cardiogenic, splanchnic, ischemic, and traumatic as well as burn shock, with the gut and/or pancreas being incriminated as their originating source (11, 23). Thus our studies showing that LDL protects against T/HS-induced myocardial dysfunction is consistent with the notion that gut-derived myocardial depressant factors are involved in acute postshock myocardial dysfunction syndromes. These results also support a recent study in dogs showing that ligation of the cisterna chylae protected against the development of myocardial dysfunction after superior mesenteric artery occlusion (8) as well as studies from the laboratory of Schmid-Schönbein implicating the action of pancreatic enzymes on the ischemic gut as the genesis of factors leading to shock and cardiac dysfunction (1).
Although no attempts have been made to date to isolate the factors in burn or T/HS lymph that are responsible for the acquired contractile dysfunction observed in these conditions, our studies and the work of others indicate that T/HS lymph contains both biologically active, tissue injurious and proinflammatory lipid and protein factors (10, 16, 19). Although we do not know what the biologically active factors are in T/HS lymph, we have excluded a number of putative candidates, including cytokines (9), bacteria, and bacterial products (2). Whatever the factors are in lymph, on the basis of our in vitro burn studies, burn lymph appears to exert a direct depressant effect on normal myocyte contractility and Ca2+ transients that are similar to the changes observed in myocytes harvested from burned rats (39). Further studies investigating the effects of T/HS lymph on in vivo cardiac and in vitro myocyte contractility and cellular Ca2+ handling as well as on the inflammatory signaling pathways associated with myocardial contractile dysfunction after shock and trauma are required to better elucidate the mechanisms responsible for T/HS-induced myocardial dysfunction.
In summary, the results of this study indicate that acute T/HS-induced myocardial dysfunction is likely due to gut-derived factors contained in mesenteric lymph and that the impaired contractility of T/HS hearts is associated with abnormal myocardial Ca2+ handling. As such, these studies support work done in a burn model and support the notion that gut-induced acute myocardial contractile dysfunction may be a reproducible response to conditions leading to splanchnic hypoperfusion such as T/HS or major burns. However, future studies to examine the interactions between mesenteric lymph isolated from animals subjected to T/HS and cardiac myocyte function are required to further support this notion. In addition, because the present experiments were performed in isolated rat hearts, it will be important to expand and validate these results as well as the potential clinical importance of these observations utilizing large animal models.
GRANTS
This work was supported by National Institutes of Health Grants HL-77480 (to A. Yatani), T32 GM-069330 (to E. A. Deitch), and P50 GM-069790 (to E. A. Deitch).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Acosta JA, Hoyt DB, Schmid-Schönbein GW, Hugli TE, Anjaria DJ, Frankel DA, Coimbra R. Intraluminal pancreatic serine protease activity, mucosal permeability, and shock: a review. Shock 26: 3–9, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Adams CA, Xu DZ, Lu Q, Deitch EA. Factors larger than 100 kd in post-hemorrhagic shock mesenteric lymph are toxic for endothelial cells. Surgery 129: 351–363, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Baxter CR, Cook WA, Shires GT. Serum myocardial depressant factor of burn shock. Surg Forum 17: 1–2, 1966. [PubMed] [Google Scholar]
- 4.Bers DM Cardiac excitation-contraction coupling. Nature 415: 198–205, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Carlson DL, Horton JW. Cardiac molecular signaling after burn trauma. J Burn Care Res 27: 669–675, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Charalambous BM, Stephens RC, Feavers IM, Montgomery HE. Role of bacterial endotoxin in chronic heart failure: the gut of the matter. Shock 28: 15–23, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Chen D, Assad-Kottner C, Orrego C, Torre-Amione G. Cytokines and acute heart failure. Crit Care Med 36: S9–S16, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Cox CS, Fischer UM, Allen SJ, Laine GA. Lymphatic diversion prevents myocardial edema following mesenteric ischemia/reperfusion. Microcirculation 11: 1–8, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Davidson MT, Deitch EA, Lu Q, Osband A, Feketeova E, Nemeth ZH, Hasko G, Xu DZ. A study of the biologic activity of trauma-hemorrhagic shock mesenteric lymph over time and the relative role of cytokines. Surgery 136: 32–41, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Dayal SD, Hasko G, Lu Q, Xu DZ, Caruso JM, Sambol JT, Deitch EA. Trauma/hemorrhagic shock mesenteric lymph upregulates adhesion molecule expression and IL-6 production in human umbilical vein endothelial cells. Shock 17: 491–495, 2002. [DOI] [PubMed] [Google Scholar]
- 11.De Santis D, Phillips P, Spath MA, Lefer AM. Delayed appearance of a circulating myocardial depressant factor in burn patients. Ann Emerg Med 10: 22–24, 1981. [DOI] [PubMed] [Google Scholar]
- 12.Deitch EA Multiple organ failure. Pathophysiology and potential future therapy. Ann Surg 216: 117–134, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deitch EA, Adams C, Lu Q, Xu DZ. A time course study of the protective effect of mesenteric lymph duct ligation on hemorrhagic shock-induced pulmonary injury and the toxic effects of lymph from shocked rats on endothelial cell monolayer permeability. Surgery 129: 39–47, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Deitch EA, Shi HP, Lu Q, Feketeova E, Xu DZ. Serine proteases are involved in the pathogenesis of trauma-hemorrhagic shock-induced gut and lung injury. Shock 19: 452–456, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Deitch EA, Xu D, Kaiser VL. Role of the gut in the development of injury- and shock induced SIRS and MODS: the gut-lymph hypothesis, a review. Front Biosci 11: 520–528, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez RJ, Moore EE, Biffl WL, Ciesla DJ, Silliman CC. The lipid fraction of post-hemorrhagic shock mesenteric lymph (PHSML) inhibits neutrophil apoptosis and enhances cytotoxic potential. Shock 14: 404–408, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Hassoun HT, Kone BC, Mercer DW, Moody FG, Weisbrodt NW, Moore FA. Post-injury multiple organ failure: the role of the gut. Shock 15: 1–10, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Houser SR, Piacentino V 3rd, Weisser J. Abnormalities of calcium cycling in the hypertrophied and failing heart. J Mol Cell Cardiol 32: 1595–1607, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Kaiser VL, Sifri ZC, Dikdan GS, Berezina T, Zaets S, Lu Q, Xu DZ, Deitch EA. Trauma-hemorrhagic shock mesenteric lymph from rat contains a modified form of albumin that is implicated in endothelial cell toxicity. Shock 23: 417–425, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Kawai K, Kawai T, Sambol JT, Xu DZ, Yuan Z, Caputo FJ, Badami CD, Deitch EA, Yatani A. Cellular mechanisms of burn-related changes in contractility and its prevention by mesenteric lymph ligation. Am J Physiol Heart Circ Physiol 292: H2475–H2484, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Kline JA, Thornton LR, Lopaschuk GD, Barbee RW, Watts JA. Heart function after severe hemorrhagic shock. Shock 12: 454–461, 1999. [DOI] [PubMed] [Google Scholar]
- 22.Krishnagopalan S, Kumar A, Parrillo JE, Kumar A. Myocardial dysfunction in the patient with sepsis. Curr Opin Crit Care 8: 376–388, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Lefer AM Properties of cardioinhibitory factors produced in shock. Fed Proc 37: 2734–2740, 1978. [PubMed] [Google Scholar]
- 24.Lomas-Niera JL, Perl M, Chung CS, Ayala A. Shock and hemorrhage: an overview of animal models. Shock 24, Suppl 1: 33–39, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Magnotti LJ, Upperman JS, Xu DZ, Lu Q, Deitch EA. Gut-derived mesenteric lymph but not portal blood increases endothelial cell permeability and promotes lung injury after hemorrhagic shock. Ann Surg 228: 518–527, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDonough KH, Giaimo M, Quinn M, Miller H. Intrinsic myocardial function in hemorrhagic shock. Shock 11: 205–210, 1999. [DOI] [PubMed] [Google Scholar]
- 27.Meng X, Ao L, Song Y, Raeburn CD, Fullerton DA, Harken AH. Signaling for myocardial depression in hemorrhagic shock: roles of Toll-like receptor 4 and p55 TNF-α receptor. Am J Physiol Regul Integr Comp Physiol 288: R600–R606, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Mitsuoka H, Kistler EB, Schmid-Schönbein GW. Generation of in vivo activating factors in the ischemic intestine by pancreatic enzymes. Proc Natl Acad Sci USA 97: 1772–1777, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noma A, Morad M, Irisawa H. Does the “pacemaker current” generate the diastolic depolarization in the rabbit SA node cells? Pflügers Arch 397: 190–194, 1983. [DOI] [PubMed] [Google Scholar]
- 30.Robinson DA, Wang P, Chaudry IH. Pentoxifylline restores the depressed cardiac performance after trauma-hemorrhage and resuscitation. J Surg Res 66: 51–56, 1996. [DOI] [PubMed] [Google Scholar]
- 31.Rudiger A, Singer M. Mechanisms of sepsis-induced cardiac dysfunction. Crit Care Med 35: 1599–1608, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Sambol JT, White J, Horton JW, Deitch EA. Burn-induced impairment of cardiac contractile function is due to gut-derived factors transported in mesenteric lymph. Shock 18: 272–276, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Sayeed MM Signaling mechanisms of altered cellular responses in trauma, burn, and sepsis: role of Ca2+. Arch Surg 135: 1432–1442, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Shahani R, Marshall JG, Rubin BB, Li RK, Walker PM, Lindsay TF. Role of TNF-α in myocardial dysfunction after hemorrhagic shock and lower-torso ischemia. Am J Physiol Heart Circ Physiol 278: H942–H950, 2000. [DOI] [PubMed] [Google Scholar]
- 35.Sharma AC Sepsis-induced myocardial dysfunction. Shock 28: 265–269, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Sutherland FJ, Hearse DJ. The isolated blood and perfusion fluid perfused heart. Pharmacol Res 41: 613–627, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Triggle DJ L-type calcium channels. Curr Pharm Des 12: 443–457, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Yang S, Hu S, Hsieh YC, Choudhry MA, Rue LW, 3rd Bland KI, Chaudry IH. Mechanism of IL-6-mediated cardiac dysfunction following trauma-hemorrhage. J Mol Cell Cardiol 40: 570–579, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Yatani A, Xu DZ, Irie K, Sano K, Jidarian A, Vatner SF, Deitch EA. Dual effects of mesenteric lymph isolated from rats with burn injury on contractile function in rat ventricular myocytes. Am J Physiol Heart Circ Physiol 290: H778–H785, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Yu HP, Shimizu T, Choudhry MA, Hsieh YC, Suzuki T, Bland KI, Chaudry IH. Mechanism of cardioprotection following trauma-hemorrhagic shock by a selective estrogen receptor-beta agonist: up-regulation of cardiac heat shock factor-1 and heat shock proteins. J Mol Cell Cardiol 40: 185–194, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Yu HP, Yang S, Choudhry MA, Hsieh YC, Bland KI, Chaudry IH. Mechanism responsible for the salutary effects of flutamide on cardiac performance after trauma-hemorrhagic shock: upregulation of cardiomyocyte estrogen receptors. Surgery 138: 85–92, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Zallen G, Moore EE, Johnson JL, Tamura DY, Ciesla DJ, Silliman CC. Posthemorrhagic shock mesenteric lymph primes circulating neutrophils and provokes lung injury. J Surg Res 83: 83–88, 1999. [DOI] [PubMed] [Google Scholar]