Abstract
Overweight and physical inactivity are associated with elevated reactive oxygen species and chronic low-grade inflammation. Exercise training studies have measured changes in systemic inflammatory and oxidative/antioxidative biomarkers but predominantly at moderate-high intensities. Few low-intensity, lifestyle-based physical activity (PA) studies have been conducted. The purpose of this study was to determine whether improvements in lifestyle-oriented PA resulting from a 16-wk Internet-delivered PA program [Active Living Every Day-Internet (ALED-I)] elicit cardioprotective improvements in measures of inflammation, oxidation, or antioxidant enzyme capacity. Forty-one men and women (age 23–62 yr) were randomized to either the ALED-I intervention [n = 19; age = 40.4 ± 1.9 yr; body mass index (BMI) = 31.4 ± 1.1 kg/m2] or a delayed intent-to-treat control condition (n = 22; age = 46.6 ± 1.3 yr; BMI = 31.0 ± 0.7 kg/m2). TNF-α, C-reactive protein, myeloperoxidase, superoxide dismutase, catalase, total antioxidative capacity, change in PA, and other cardiometabolic disease risk factors were measured at baseline and postintervention. The ALED-I group increased PA and decreased central adiposity without changes in the control group. There was no change in the control group for any inflammation, oxidation, or antioxidant biomarkers. TNF-α decreased (P = 0.01) in the intervention group but was not statistically different from the control group. In conclusion, modest improvements in daily low-intensity ambulatory PA as a result of an Internet-delivered lifestyle PA intervention may be cardioprotective in sedentary and overweight adults through reductions in central adiposity and inflammation. However, the absence of favorable changes in other inflammation, oxidation, and antioxidant biomarkers highlights the need for further attention to the dose response of lifestyle-structured PA promotion strategies for health maintenance/improvement.
Keywords: overweight, oxidation, tumor necrosis factor-α
more than 50% of the U.S. population is not meeting current physical activity (PA) recommendations (22), and ∼25% engage in no leisure-time PA (63a) despite the known health benefits of leading a habitually physically active lifestyle (33, 65). Such health benefits have recently been highlighted in the American Heart Association and American College of Sports Medicine's recommendations and their jointly sponsored Exercise Is Medicine initiative (22, 40, 67). Specifically, greater PA is known to prevent and reduce the risk of many cardiometabolic diseases (5, 10, 33). However, the underlying cardioprotective mechanisms of habitual exercise continue to be elucidated. Recent efforts have supported a PA-induced alleviation of oxidative stress and chronic low-grade inflammation as a possible mechanism for disease risk reduction (1, 20).
Imbalance between oxidative molecules and antioxidant capacity and proinflammatory and anti-inflammatory states are both associated with overweight and physical inactivity (18, 28, 46, 74). Proinflammatory cytokines and reactive oxygen species (ROS) from both visceral and accumulated subcutaneous fat compartments are implicated in increased cardiometabolic disease risk (17, 18). Conversely, PA is associated with elevations in anti-inflammatory cytokines (26, 43) and antioxidant enzyme capacity (16, 50), as well as diminished systemic inflammation (46) and oxidative stress (44), all of which support the cardioprotective actions of PA. However, the effects of chronic PA on basal levels of inflammation, oxidation, and antioxidant capacity biomarkers are still controversial (16, 19, 50), largely due to PA intensity discrepancies. Moreover, the majority of previous applied human research has focused primarily on the effects of moderate- to high-intensity exercise training and/or the effects of acute bouts of exercise. To our knowledge, only one study has included low (25% maximal oxygen consumption)-intensity PA (20), and no studies have investigated lifestyle-oriented PA intervention/programs, which are often of low intensity, focused on reducing physical inactivity, and gaining clinical- and wellness-based popularity in part due to the obesity epidemic (14).
Recently, significant research efforts have focused on the use of theory-based and Internet-delivered PA behavior change programs due to their 1) demonstrated efficacy; 2) potential delivery and reach to community/population levels; and 3) adoption feasibility and safety for diverse populations (overweight, elderly) necessitating low-intensity volume-based approaches to increasing PA (21, 39, 69). The Active Living Every Day (ALED; Human Kinetics) PA behavior change program is a theory-based [transtheoretical model (51) and social cognitive theory (2)] and commercially available program that has been shown to favorably increase self-reported PA (73) and aerobic fitness (15) when delivered in small-group face-to-face formats. An Internet-delivered version of ALED (ALED-I) is also commercially available, and through a randomized control trial, we recently demonstrated its efficacy in increasing objectively measured PA levels (pedometer) and decreasing central adiposity, triglycerides, and coronary risk ratio (CRR) over 16 wk in a group of sedentary and overweight adults (9).
The Task Force on Community Preventive Services strongly recommends theory-based and individually adapted and targeted health behavior-change programs for increasing PA in communities (63a). The ALED-I behavior change program adheres to these recommendations and has demonstrated efficacy in rural, geographically isolated populations devoid of PA resources more common to urban/metropolitan areas (9). However, the efficacy of this program and most other theoretically based programs, regardless of delivery method, has not been extended to physiological mechanisms (e.g., inflammation) etiologically pinned to cardiometabolic diseases. The present study is a preliminary investigation aimed at determining whether the 16-wk ALED-I PA behavior change program elicited further cardiometabolic disease protection through favorable modulation of biomarkers assessing inflammation, oxidation, and antioxidant capacity.
METHODS
The reader is referred to Carr et al. (9) for a full description of the initial randomized control trial recruitment/enrollment process, power determination, and detailed descriptive data collection procedures from which this preliminary investigation stemmed. The initial randomized control trial achieved a power of 0.82 based on the primary outcome measure of PA (steps per day). Due to this a priori design, the present study does not achieve similar power with respect to the selected biomarker outcome measures but rather provides pilot evidence regarding the influence of a lifestyle-oriented and Internet-delivered PA behavior change program on selected biomarkers of inflammation, oxidation, and antioxidant capacity.
Subjects.
Briefly, forty-one sedentary, overweight [body mass index (BMI) ≥ 25.0 kg/m2] adults (8 men, 33 women) were recruited and randomized to either the 16-wk Internet-delivered ALED-I PA intervention (n = 19; age = 40.4 ± 1.9 yr; BMI = 31.4 ± 1.1 kg/m2) or the delayed intent-to-treat control condition (n = 22; age = 46.6 ± 1.3 yr; BMI = 31.0 ± 0.7 kg/m2). Descriptive, anthropometric, metabolic, and primary biomarker measures were assessed at baseline and following the 16-wk intervention period and were conducted 24–36 h following each participant's most recent bout of PA. Participants randomized to the control condition maintained their present lifestyle throughout the duration of the 16-wk experimental period. Participants were nonsmokers, devoid of ambulatory/exercise limitations, and free of overt, complicated, or acute cardiovascular, metabolic, respiratory, or neurological diseases as assessed by medical history. Female participants were predominantly premenopausal (2 perimenopausal and 1 postmenopausal, surgical). None of the latter used any form of hormone replacement therapy, and three premenopausal women used oral contraception. All experimental protocols were approved by the Institutional Review Board for projects involving human subjects at the University of Wyoming, Laramie, WY, and voluntary written informed consent was obtained from each participant.
Physical activity intervention and measurement.
Participants randomized to the PA intervention group were provided access to the ALED website and given a copy of the complimentary workbook (3) following their baseline testing session. The ALED website content and functionality are based on the Transtheoretical Model and Social Cognitive Theory and are designed to increase daily PA by guiding participants through a 16-wk, self-paced program that utilizes interactive activities and behavior modification strategies. The ALED website/workbook (3) content were developed based on Project ACTIVE (31), and the material is similar “…including issues of barriers, self-monitoring, stimulus control, social support, self-efficacy, relapse prevention, consequences, rewards, and time management, among others” (31). A description of the website is available at: (http://www.activeliving.info/AboutUs.cfm) under “Take a Course.” For all participants, PA was objectively measured using a previously validated pedometer (Yamax Digiwalker SW-200, Lee's Summit, MO) (56). Intensity of PA was determined by self-report at baseline and postintervention according to previously validated procedures and instrumentation by the Seven-Day Physical Activity Recall Questionnaire (4). From the Seven-day Physical Activity Recall Questionnaire, time (h/wk) spent in sleep, low, moderate, hard, and very hard activities is reported. Aerobic fitness was estimated at baseline and postintervention according to sex-specific prediction equations of Kline et al. (30) following a timed, supervised 1-mile walk test on a premeasured track.
Dietary intake measurement.
Three-day food diaries (2 working days and 1 nonworking day) were completed 1 wk before baseline testing and during week 16 of the study in 28 participants (13 food diaries not complete or not returned) to characterize potential group differences and/or time course changes in micro- and macronutrient intake. Food diaries, which were analyzed using Nutritionist Pro software, are demonstrated to be reliable and valid (48) and are a practical and feasible measure in free-living adults (34).
Metabolic and biomarker measurements.
In an attempt to remain consistent with previous best-practice profiling recommendations for characterization of the complex and interrelated effects of PA and overweight on oxidative and inflammatory homeostasis (12), six biomarkers, measurable in venous serum/plasma samples, were selected. These biomarkers included TNF-α, high-sensitivity C-reactive protein (CRP), myeloperoxidase (MPO), catalase (CAT), superoxide dismutase (SOD), and total antioxidant capacity (TAC), and all were measured at baseline and postintervention (16 wk). The biomarkers were selected based on existing evidence linking/implicating them with cardiometabolic diseases, overweight/obesity, and physical activity as follows. TNF-α is a proinflammatory cytokine produced by adipocytes (35), is inversely associated with PA (26), and is a predictor of cardiovascular disease outcomes (32). TNF-α was measured in plasma by sandwich ELISA (Alpco Diagnostics, Salem, NH). CRP is an acute-phase protein produced by the liver (47), is associated with cardiovascular disease (53), glycemic control (29), and overweight/obesity (70). CRP was measured by an outside clinical laboratory (LabCorp, Denver, CO) as previously reported (9). MPO is a peroxidase enzyme and biomarker of lipid peroxidation that is active in atherosclerotic lesions and predicts early risk of myocardial infarction (7). It is mechanistically linked to both inflammation and oxidation, catalyzing nitration of tyrosine residues during inflammation and contributing to depletion of endothelium-derived NO, respectively (24, 68), and was determined in plasma by ELISA (Alpco Diagnostics). SOD is a cytosolic, mitochondrial, and extracellular antioxidant enzyme that promotes the dismutation of superoxide anion to hydrogen peroxide and oxygen. In obesity models of aerobic exercise, elevated levels of SOD activity have been demonstrated (58). SOD enzymatic activity was measured in serum (Cayman Chemical, Ann Arbor, MI). CAT is a ubiquitous antioxidant enzyme found in most aerobic cells that catalyzes the decomposition of hydrogen peroxide to water and oxygen (50). Differences in CAT expression by extent of adiposity have been documented (59) but activity level responsiveness to exercise training is mixed (19). CAT activity was measured in serum (Cayman Chemical). TAC is a global measure of antioxidant capacity (enzymatic and nonenzymatic) measuring hydrogen peroxide sequestering by available serum/plasma antioxidants (27). Greater abdominal adiposity is associated with lower TAC (11); however, the influence of exercise (physical activity) is mixed (8, 45, 71). TAC was determined in serum by colorimetric assay (Alpco Diagnostics). All biomarkers were determined in duplicate, and the intra- and interassay coefficients of variation were 3.6% and 3.5% for TNF-α, 3.6% and 3.2% for MPO, 9.6% and 8.8% for SOD, 10.7% and 10.2% for CAT, and 3.7% and 2.1% for TAC, respectively. Lipid/lipoprotein, glucose, and insulin concentrations were determined by an outside clinical laboratory (LabCorp) as previously reported (9). Insulin sensitivity and resistance were estimated using the inversely correlated homeostasis model assessment (HOMA-IR) and the quantitative insulin sensitivity check index (QUICK-I) (41, 55).
Blood samples were collected at the research laboratory in Laramie, WY, and a medical facility in Riverton, WY, according to standard phlebotomy techniques with minimal venostasis following an overnight fast (∼10 h) with the exception of water consumption. An infection/inflammation questionnaire was administered to rule out underlying acute illness or infection before collection of 20 ml of blood between 6:00 A.M. and 9:00 A.M. into two vacutainer tubes containing either 0.081 ml 15% (K3) EDTA solution or clot activator. Samples were centrifuged at 4,000 rpm for 15 min, aliquoted, and frozen at −70° until biochemical analyses.
Statistical analyses.
All data, including physiological/dietary descriptors, PA, and biomarker outcome measures, were analyzed by two-way repeated-measures ANOVA (mixed model: group × time). ANOVA models for the specific measure of interest included the groups (control and intervention) and time (baseline and post-16 wk). Before these analyses and due to group differences in baseline age (attrition following randomization) (9), adjustment was made accordingly. Additionally, baseline TNF-α levels were different between groups, so the TNF-α model was adjusted for this baseline difference. Of the biomarker outcome measures, only TAC met the assumption of normality; thus TNF-α, high-sensitivity CRP, CAT, MPO, and SOD were log transformed before analyses and reported as such. When indicated by a significant F value (group × time interaction), post hoc procedures were performed (Tukey). Linear regression analysis was performed with the observed changes in PA (steps/day) and waist circumference (Δ = postintervention − baseline) as independent response variables and the observed changes in inflammatory and oxidation/antioxidative biomarkers (Δ = postintervention − baseline) as potential predictors. Statistical significance was set a priori at P < 0.05. All data are presented as means ± SE. (SigmaStat 3.11; 2004).
RESULTS
Descriptive and metabolic measures at baseline and postintervention for the control and interventions groups are provided in Table 1. At baseline, PA levels and most descriptive and metabolic measures were similar between groups (Table 1). Significant between-group differences were present for coronary risk ratio (CRR), triglycerides, waist circumference, and age although both groups were within the same decade of life (Table 1). Following completion of the 16-wk ALED-I PA behavior change intervention, the ALED-I group increased PA (+1,404 steps/day; P = 0.01), and there was no change in the control group (Table 1). Controlling for age and baseline differences, waist circumference decreased (−4.0 cm; P = 0.01) in the intervention group, whereas no change occurred in the control group (Table 1). A small but significant gain in body fat percentage (+1.1%; P = 0.03) and a reduction in percent lean mass (−1.1%; P = 0.03) occurred in the control group, but both remained stable in the ALED-I group (Table 1). Aerobic fitness increased in both groups (Table 1), which is attributable to the previously documented (64) acquired familiarity with the submaximal aerobic fitness test at postintervention. At baseline and 16 wk, both overweight and obese groups were similar and tended toward apparently healthy for cholesterol levels, glucose and insulin concentrations, insulin resistance/sensitivity, and blood pressure (Table 1). Group-specific attrition rates during the 16-wk study were 30% for the ALED-I group and 10% for the control group.
Table 1.
Descriptive and physiological characteristics of participants (n =41)
| Control Group (n = 22) |
ALED-I Group (n = 19) | |||||
|---|---|---|---|---|---|---|
| Baseline | Post 16-wk intervention | Pa | Baseline | Post 16-wk intervention | Pa | |
| Age, yr | 46.6±1.3 | 40.4±1.9* | ||||
| Predicted V̇o2peak, ml·kg−1·min−1 | 29.6±1.3 | 33.5±1.4† | 0.01 | 34.9±1.9* | 37.8±2.2† | 0.01 |
| Physical activity, steps/day | 6,826±509 | 7,165±540 | 0.50 | 7,223±469 | 8,627±554† | 0.01 |
| Systolic BP, mmHg | 125±3 | 123±2 | 0.31 | 122±2 | 124±3 | 0.50 |
| Diastolic BP, mmHg | 77±2 | 78±1 | 0.59 | 79±2 | 78±2 | 0.94 |
| Waist circumference, cmb | 99.2±2.2 | 99.8±2.1 | 0.66 | 100.6±2.4* | 96.6±2.7† | <0.01 |
| BMI, kg/m2 | 31.0±0.7 | 31.0±0.8 | 0.89 | 31.4±1.1 | 31.2±1.1 | 0.44 |
| Weight, kg | 85.6±2.8 | 85.5±3.0 | 0.85 | 89.6±3.8 | 89.3±3.9 | 0.75 |
| Body fat, % | 43.6±1.4 | 44.7±1.6† | 0.03 | 44.3±1.8 | 44.2±2.0 | 0.67 |
| %Lean mass | 56.4±1.4 | 55.3±1.6† | 0.03 | 55.6±1.8 | 55.8±2.0 | 0.67 |
| Total cholesterol, mg/dl | 188±8 | 182±7 | 0.15 | 207±11 | 197±11 | 0.27 |
| LDL, mg/dl | 110±7 | 107±6 | 0.36 | 124±10 | 120±9 | 0.63 |
| HDL, mg/dl | 51±3 | 51±3 | 0.63 | 45±2 | 46±3 | 0.48 |
| Triglycerides, mg/dl | 123±12 | 103±11 | 0.06 | 168±13* | 148±18‡ | 0.10 |
| Fasting glucose, mg/dl | 94±3 | 94±2 | 0.69 | 93±2 | 94±2 | 0.78 |
| Fasting insulin, μIU/ml | 6.9±1.1 | 9.4±1.1 | 0.61 | 10.5±1.4 | 9.3±1.2 | 0.51 |
| HOMA | 1.6±0.2 | 1.8±0.2 | 0.69 | 2.5±0.4 | 2.2±0.3 | 0.55 |
| QUICK-I | 3.4±0.1 | 3.4±0.1 | 0.76 | 3.2±0.2 | 3.4±0.1 | 0.23 |
| Coronary risk ratio | 3.9±0.2 | 3.8±0.2 | 0.18 | 4.8±0.3* | 4.5±0.3‡ | 0.14 |
| Reynold's risk score (model B)c | 1.3±0.2 | 1.2±0.1 | 0.33 | 2.2±0.6 | 1.9±0.5 | 0.19 |
Values are means ± SE. ALED-I, Active Living Every Day-Internet; V̇o2peak, peak oxygen consumption; BP, blood pressure; BMI, body mass index; LDL, low-density lipoprotein; HDL, high-density lipoprotein; HOMA, homeostatic model assessment; QUICK-I, quantitative insulin-sensitivity check index. aStatistical level within group (baseline to postintervention). bn = 32 (18 control; 14 ALED-I). cFemale participants only.
P < 0.05 compared with baseline in control group.
P < 0.05 compared with baseline within same group.
P < 0.05, between-group comparison vs. postintervention in the control group.
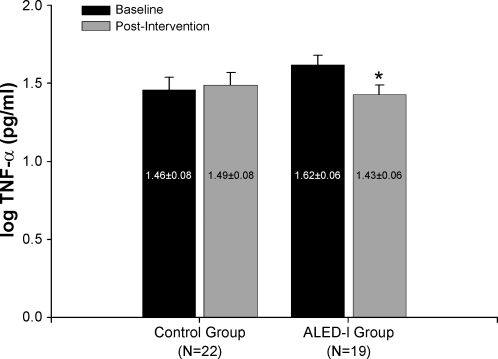
For the control and ALED-I groups no significant main effect (group × time interaction) was found for CRP (P = 0.9), CAT (P = 0.5), MPO (P = 0.6), SOD (P = 0.8), or TAC (P = 0.5), and thus they were similar at baseline and 16 wk (Table 2). Within the intervention group, TNF-α declined significantly after adjustment for baseline group differences (F: 8.74; P < 0.01; Fig. 1); however, this reduction was not significantly different from the control group (P = 0.29). No change in TNF-α was evident in the control group after 16 wk (P = 0.41). For CAT, sufficient sample volume was only available on 32 of the 41 participants (14 intervention and 19 control participants); thus statistical analysis was performed on this subgroup of participants whose descriptive characteristics did not differ from that of the total sample (n = 41). Neither change in PA nor waist circumference was significantly associated with change in any of the measured inflammation, oxidation, or antioxidative biomarkers.
Table 2.
Mean biomarker levels and dietary factors at baseline and 16 wk
| Control Group (n =22) |
ALED-I Group (n =19) | |||||
|---|---|---|---|---|---|---|
| Baseline | Post-16-wk intervention | P‡ | Baseline | Post-16-wk intervention | P‡ | |
| Catalase, nmol·min−1·ml−1* | 1.61±0.04 | 1.75±0.04 | 0.07 | 1.34±0.14 | 1.56±0.11 | 0.07 |
| CRP, mg/l* | 0.29±0.08 | 0.30±0.09 | 0.91 | 0.27±0.11 | 0.29±0.10 | 0.63 |
| Myeloperoxidase, ng/ml* | 1.55±0.06 | 1.60±0.07 | 0.46 | 1.59±0.08 | 1.48±0.07 | 0.84 |
| Superoxide dismutase, U/ml* | −1.21±0.05 | −1.24±0.04 | 0.51 | −1.26±0.04 | −1.20±0.03 | 0.09 |
| Total antioxidant capacity, μmol/l | 307.9±8.2 | 311.6±8.2 | 0.78 | 295.1±9.0 | 315.4±8.9 | 0.18 |
| Carbohydrate, % of dietary intake† | 45.9±1.6 | 44.2±1.8 | 0.39 | 50.0±1.5 | 48.1±1.5 | 0.27 |
| Fat, % of dietary intake† | 37.0±1.7 | 37.5±1.8 | 0.81 | 33.1±1.4 | 33.6±1.5 | 0.70 |
| Protein, % of dietary intake† | 17.1±1.0 | 17.9±1.3 | 0.51 | 16.0±1.0 | 17.6±1.4 | 0.07 |
| Vitamin A, RE† | 817.4±131.3 | 725.7±111.7 | 0.49 | 750.0±117.5 | 688.0±128.3 | 0.71 |
| Beta-carotene, μg† | 491.8±420.9 | 428.0±398.9 | 0.89 | 481.6±376.5 | 473.6±306.4 | 0.72 |
| Vitamin C, mg† | 58.1±10.0 | 73.4±9.0 | 0.48 | 81.0±9.1 | 57.4±12.3 | 0.51 |
| Vitamin E, mg† | 4.9±1.6 | 4.5±1.5 | 0.73 | 7.1±1.4 | 7.9±1.7 | 0.82 |
| Alpha-tocopherol, mg† | 0.3±0.3 | 0.6±0.3 | 0.07 | 0.9±0.2 | 0.5±0.2 | 0.06 |
Values are means ± SE. CRP, C-reactive protein; RE, retinol equivalents (equivalent to micrograms).
Log10 values.
n = 28 participants (ALED-I group, n =15; control group, n =13).
Statistical level within group (baseline to postintervention).
Fig. 1.
TNF-α levels for control and Active Living Every Day-Internet (ALED-I) groups at baseline and postintervention (n = 41). *P < 0.01 compared with baseline in same group.
Following completion of the 16-wk experimental period, significant between-group differences were present for reported time (h/wk) spent in moderate-intensity activities (P = 0.01; Table 3). In both groups, greater than 54% of time was reportedly spent in light activities. Contrasting changes were observed in the two groups following the 16-wk experimental period. Time spent in moderate activities increased in the control group but decreased in the intervention group, and time spent in light-intensity activities increased in the intervention group (P = 0.055) and decreased in the control group (Table 3). There were no significant associations between change in intensity of activity, light or moderate, and changes in TNF-α. Analyses of 3-day food diaries in 28 of the 41 participants revealed no baseline or 16-wk between-group differences or within-group differences in any of the measured micro- or macronutrients. Table 2 shows that macronutrient intake was similar between groups ranging from 46–50% carbohydrate, 33–37% fat, and 16–18% protein. Importantly, potential sources of exogenous dietary antioxidants remained constant throughout the 16-wk study in both groups (Table 2).
Table 3.
Change (postintervention − baseline) in hours per week spent in sleep and in light, moderate, hard, and very hard activities during the 16-wk experimental period (Seven-Day Physical Activity Recall Questionnaire)
| Control Group (n =22) | ALED-I Group (n =19) | P† | |
|---|---|---|---|
| Sleep | −1.9±1.3 | −0.3±1.3 | 0.41 |
| Light activities | −4.7±4.3* | 6.8±4.1 | 0.06 |
| Moderate activities | 7.2±3.2* | −4.8±2.6* | 0.01 |
| Hard activities | 1.6±1.0 | −2.4±2.1 | 0.09 |
| Very hard activities | 0.2±0.2 | 0.6±0.3 | 0.24 |
Values are means ± SE.
P < 0.05, within-group change, baseline to 16 wk.
Significance between groups.
DISCUSSION
The aim of this preliminary study was to determine whether previously demonstrated improvements in PA resulting from an Internet-delivered lifestyle-oriented PA behavior change program also elicited favorable improvements in inflammation, oxidation, and antioxidant capacity in sedentary overweight adults. Consistent with profiling recommendations (12), several biomarkers associated with PA, obesity, and cardiometabolic diseases were measured, including TNF-α, CRP, CAT, SOD, MPO, and TAC. The PA intervention resulted in favorable modification of only one inflammatory biomarker, TNF-α, and this improvement was not significantly different from the control condition. With the exception of TNF-α, these largely null findings are not entirely surprising due to the lifestyle-oriented volume-based approach of the ALED-I PA behavior change program that is devoid of individualized PA intensity prescription. The popularity and use of lifestyle-oriented Internet-delivered PA programs is increasing (39, 69); to our knowledge, this is the first study to expand the efficacy testing of such a program to disease-related physiological mechanisms such as inflammation and antioxidant capacity. The observed benefit of increased PA volume on TNF-α extends previous evidence that has predominantly focused on the influence of moderate- to vigorous-intensity exercise training (20, 75). Additionally, the reduction in TNF-α through a non-intensity-based PA intervention was specific to overweight/obese adults who may possess greater chronic low-grade inflammation due to their excess adiposity (49).
The observed improvement in TNF-α is complementary to findings from previous cross-sectional (1) and moderate- to higher-intensity PA intervention studies (26, 61). Adipose tissue has been identified as a major source for circulating TNF-α (25), and a positive relation between inflammation and excess adiposity has been reported (49). Combined with increased adiposity as a consequence of leading a sedentary lifestyle (36), the 4% reduction in central adiposity and the 19% increase in PA likely contribute, at least in part, to explaining the reduced TNF-α concentrations in the ALED-I group. It is important to highlight that the associations between change in adiposity or PA and TNF-α were weak and insignificant, which has also been reported previously (52). The absence of change in TNF-α in the nonactive control group is supportive of the well-documented deleterious changes in body composition due to a sedentary lifestyle (6) and the corresponding deleterious changes in body composition (central adiposity, increased percent body fat, and reduced lean tissue mass) that occurred in the control participants over a relatively short period of time (9). Whether the small but statistically significant increase in percent body fat observed in the control group represents a clinically significant concern is arguable. Nonetheless, the observed increase in percent body fat occurred over a short time frame (16 wk) and if extrapolated over a longer duration, would likely achieve clinical significance should such sedentary lifestyles be maintained.
Collectively, the TNF-α findings for both groups may be important as TNF-α is a moderately strong predictor of future coronary events (54) and appears to be a primary instigator of insulin resistance and dyslipidemia (46), but several important considerations exist. The Internet-delivered PA intervention reduced triglyceride concentrations, but improvements in insulin resistance, sensitivity, and fasting insulin and glucose concentrations were absent. The lack of intervention-induced changes in these metabolic parameters may be due to 1) the relatively normal metabolic profile of these overweight adults, 2) the relatively short observation duration, and 3) the low-intensity nature of the volume-focused intervention. Moreover, the TNF-α findings are potentially confounded by largely unexplainable baseline differences between the groups. At baseline, both groups were similar in most physical and physiological descriptors with exception of age, CRR, triglycerides, and waist circumference. Although not statistically significant, the intervention group also had fasting insulin concentrations that were ∼35% greater than the control group and were more insulin resistant (HOMA). A positive association between TNF-α and triglyceride concentrations and insulin resistance has been documented (60), which may help explain the higher baseline TNF-α levels in the ALED-I group. Likewise, the postintervention reduction in triglyceride levels may have contributed, similarly, to the significant reduction in TNF-α; however, the association between change in TNF-α and change in triglycerides was insignificant (P = 0.79). While our findings are encouraging, in context of an efficacious public health PA strategy that may also elicit improvements in TNF-α and subsequently cardiometabolic disease risk, the aforementioned limitations, small sample size, and attrition rate inherent within this preliminary investigation necessitate cautious interpretation.
The lifestyle-oriented PA behavior change program and its favorable PA and central adiposity effects did not significantly influence any other inflammation, oxidation, or antioxidant biomarkers. In addition to small sample size, other explanations for these largely null findings exist, namely intensity of PA. First, TAC is stratified from low to high capacity (1a) where TAC less than 280 μmol/l is considered low antioxidative capacity, 280 to 320 μmol/l is moderate antioxidative capacity, and greater than 320 μmol/l is high antioxidative capacity. In this relatively healthy sample of overweight and sedentary adults, baseline TAC levels for both groups were in the moderate antioxidative capacity range. Thus a greater dose (volume and intensity) of PA may be necessary to elicit favorable change in the already moderate antioxidant capacity levels as has been documented by others using greater exercise training doses (8). Second, with respect to CAT activity, no significant change was observed (+14%; P = 0.07) following the PA intervention, which is contrary to previous reports of exercise-induced increases in skeletal muscle (37), erythrocyte (42) and mRNA (19) CAT activity. Diminished antioxidant defense has been documented in overweight populations compared with normal weight counterparts such that overweight adults possess lower tissue levels of available antioxidant vitamins (β-carotene and vitamin C), antioxidant enzymes (SOD and glutathione peroxidase), and thiol-containing molecules (63). It is speculated that the antioxidant enzyme defense system in overweight individuals may be impaired or simply unable to overcome elevated levels of oxidation, further augmenting inflammation and leading to increased cardiometabolic disease risk (28). Based on the trend toward elevated CAT activity in this group of overweight adults, it may be worthwhile to consider modification of the Internet-delivered PA behavior change program to include more individualized attention to increasing intensity in conjunction with the increase in PA volume. This modification, which is not unrealistic, may serve to augment the beneficial effect of the Internet-delivered program on other disease-related biomarkers in addition to CAT activity.
The absence of individualized PA intensity prescription in the commercially available Internet-delivered ALED PA behavior change program is a limitation rooted in necessary safety considerations related to initiating nonmedically supervised PA programs in sedentary populations and plausibly contributed to our null findings for CRP, CAT, MPO, SOD, and TAC. We employed a self-report measure to quantify PA intensity in an effort to identify relations between PA intensity and improvements in the selected biomarkers. The increase in volume of PA for the ALED-I group includes an increase of ∼1 h/day spent in low-intensity activities (4% increase from baseline to 16 wk) and a reduction of ∼41 min/day spent in moderate-intensity activities. While these changes may seem counterintuitive in the ALED-I group, they are aligned with self-reported changes in PA intensity perception that accompany improved aerobic fitness, body composition, and energy costs of PA. Specifically, activities once perceived as moderate intensity were perceived as lower intensity after 16 wk of becoming more physically active. As with the change in volume of PA, neither time spent in light activities nor moderate activities were associated with change in TNF-α. However, from a public health perspective, the observed effects of a modest increase in volume of low-intensity PA may have important implications as segments of the population, such as the overweight, elderly, or diseased, may be unable to achieve the current PA duration and intensity recommendations (23, 66).
Interpretation of our findings should be considered in the context of this preliminary investigation's strengths and limitations. As addressed previously, the small attrition-related sample size is a limitation that likely influenced several of our findings such as CAT activity; however, this attrition is consistent with other Internet-delivered PA intervention studies (69). Acknowledging total attrition (postrandomization) at 51% (9), a strength of the study is maintenance of relatively similar metabolic and physical characteristics between the groups, protecting against other confounding variables such as hypertension or insulin resistance. None of the selected biomarkers assessed protein expression, and a direct measure of oxidative stress (e.g., lipid peroxidation) would have been informative. Assessment of 8-hydroxydeoxyguanosine (8-OHdG) was attempted (27) but unsuccessful due to technical issues with the ELISA kit and lack of available serum/plasma sample for repeat analysis. Participants were fasted, but alcohol consumption was not withheld more than 10 h before blood collection. Of the 28 participants submitting food diaries, only 3 reported alcohol consumption in the week before blood collection (2 at baseline and 1 at 16 wk). Three premenopausal women using hormone-based oral contraception were included in the final analyses as their exclusion did not alter any conclusions for the biomarker outcome measures, but the influence of hormone-based therapy is acknowledged (62, 72). The sample was predominantly female (81%) and ethnically homogeneous (Caucasian). All of these limit the overall generalizability of our preliminary findings.
The evidence demonstrating increased PA levels, reduced central adiposity, and reduced TNF-α do substantiate the potential efficacy of this Internet-delivered PA behavior change program. Due to the significant reach of Internet-delivered programs combined with high Internet use for health information, which continues to grow (38), the target populations for this type of PA intervention may only be limited by Internet access. The individualized nature of the ALED-I program, its emphasis on walking modes of activity and activities of daily living and adoptability by overweight adults make it well suited for populations without access to PA promotion resources and venues more common in urban/metropolitan areas, specifically rural populations that may be geographically isolated.
Recommendations and conclusions.
With escalating use and attention to Internet-delivered and theory-based PA programs, such as ALED-I to increase population PA levels, larger more robust trials are necessary to clearly elucidate the dose response of PA on inflammation, oxidative stress, and antioxidant capacity. Specifically, inclusion of more direct and objective measures of PA intensity (accelerometry) to clarify the minimum volume-intensity dose of PA necessary to achieve health benefits may be necessary. The consequences of a sedentary lifestyle are well-documented and include increased risk for cardiometabolic diseases (6) which may be mediated by an imbalance between oxidative and antioxidative as well as pro-inflammatory and anti-inflammatory mechanisms. Our findings indicate that modest improvements in daily low-intensity PA as a result of a 16-wk Internet-delivered PA intervention may restore some of this balance through reduced TNF-α in overweight adults.
GRANTS
This project was supported by National Institutes of Health Grant P20-RR-016474.
Acknowledgments
We thank Christy Lohof, Mandolyn Vendela, Jill Korenke, Kevin Bretting, Dr. Jamie Broomfield, Dr. Todd Bartee, and Debbie McClure for contributions to these projects. We also thank our participants.
Data were collected at the Human Integrative Physiology Laboratory at the University of Wyoming in Laramie, WY, and a medical clinic in Riverton, WY.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abramson JL, Vaccarino V. Relationship between physical activity and inflammation among apparently healthy middle-aged and older US adults. Arch Intern Med 162: 1286–1292, 2002. [DOI] [PubMed] [Google Scholar]
- 1a.Alpco Diagnostics. Antioxidative Capacity EIA: Colorimetric Test System for the Determination of Antioxidative Capacity in Serum and EDTA-Plasma. Catalog No. 30–5200. Salem, NH: Alpco, 2007. (www.alpco.com).
- 2.Bandura A Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood Cliffs, NJ: Prentice-Hall, 1986.
- 3.Blair S, Dunn AL, Marcus BH, Carpenter RA, Jaret P. Active Living Every Day: Get Active with a 20-Step Program. Champaign, IL: Human Kinetics, 2001.
- 4.Blair SN, Haskell WL, Ho P, Paffenbarger RS Jr, Vranizan KM, Farquhar JW, Wood PD. Assessment of habitual physical activity by a seven-day recall in a community survey and controlled experiments. Am J Epidemiol 122: 794–804, 1985. [DOI] [PubMed] [Google Scholar]
- 5.Blair SN, Kampert JB, Kohl HW 3rd, Barlow CE, Macera CA, Paffenbarger RS Jr, Gibbons LW. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA 276: 205–210, 1996. [PubMed] [Google Scholar]
- 6.Bouchard C (Editor). Physical Activity and Obesity. Champaign, IL: Human Kinetics, 2000, p. 312.
- 7.Brennan ML, Penn MS, Van Lente F, Nambi V, Shishehbor MH, Aviles RJ, Goormastic M, Pepoy ML, McErlean ES, Topol EJ, Nissen SE, Hazen SL. Prognostic value of myeloperoxidase in patients with chest pain. N Engl J Med 349: 1595–1604, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Burneiko RC, Diniz YS, Galhardi CM, Rodrigues HG, Ebaid GM, Faine LA, Padovani CR, Cicogna AC, Novelli EL. Interaction of hypercaloric diet and physical exercise on lipid profile, oxidative stress and antioxidant defenses. Food Chem Toxicol 44: 1167–1172, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Carr LJ, Bartee RT, Dorozynski C, Broomfield JF, Smith ML, Smith DT. Internet-delivered behavior change program increases physical activity and improves cardiometabolic disease risk factors in sedentary adults: results of a randomized controlled trial. Prev Med 46: 431–438, 2008. [DOI] [PubMed] [Google Scholar]
- 10.CDC. Surgeon General's Report on Physical Activity and Health from the Centers for Disease Control and Prevention. JAMA 276: 522, 1996. [PubMed] [Google Scholar]
- 11.Chrysohoou C, Panagiotakos DB, Pitsavos C, Skoumas I, Papademetriou L, Economou M, Stefanadis C. The implication of obesity on total antioxidant capacity in apparently healthy men and women: the ATTICA study. Nutr Metab Cardiovasc Dis 17: 590–597, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Cutler RG, Plummer J, Chowdhury K, Heward C. Oxidative stress profiling. II. Theory, technology, and practice. Ann NY Acad Sci 1055: 136–158, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Dunn AL, Andersen RE, Jakicic JM. Lifestyle physical activity interventions. History, short- and long-term effects, and recommendations. Am J Prev Med 15: 398–412, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Dunn AL, Marcus BH, Kampert JB, Garcia ME, Kohl HW 3rd, Blair SN. Comparison of lifestyle and structured interventions to increase physical activity and cardiorespiratory fitness: a randomized trial. JAMA 281: 327–334, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Elosua R, Molina L, Fito M, Arquer A, Sanchez-Quesada JL, Covas MI, Ordonez-Llanos J, Marrugat J. Response of oxidative stress biomarkers to a 16-week aerobic physical activity program, and to acute physical activity, in healthy young men and women. Atherosclerosis 167: 327–334, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Fain JN Release of interleukins and other inflammatory cytokines by human adipose tissue is enhanced in obesity and primarily due to the nonfat cells. Vitam Horm 74: 443–477, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Fujita K, Nishizawa H, Funahashi T, Shimomura I, Shimabukuro M. Systemic oxidative stress is associated with visceral fat accumulation and the metabolic syndrome. Circ J 70: 1437–1442, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Lopez D, Hakkinen K, Cuevas MJ, Lima E, Kauhanen A, Mattila M, Sillanpaa E, Ahtiainen JP, Karavirta L, Almar M, Gonzalez-Gallego J. Effects of strength and endurance training on antioxidant enzyme gene expression and activity in middle-aged men. Scand J Med Sci Sports 17: 595–604, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Goto C, Higashi Y, Kimura M, Noma K, Hara K, Nakagawa K, Kawamura M, Chayama K, Yoshizumi M, Nara I. Effect of different intensities of exercise on endothelium-dependent vasodilation in humans: role of endothelium-dependent nitric oxide and oxidative stress. Circulation 108: 530–535, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Haskell WLJB Wolffe Memorial Lecture: Health consequences of physical activity: understanding and challenges regarding dose-response. Med Sci Sports Exerc 26: 649–660, 1994. [DOI] [PubMed] [Google Scholar]
- 22.Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, Macera CA, Heath GW, Thompson PD, Bauman A. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc 39: 1423–1434, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, Macera CA, Heath GW, Thompson PD, Bauman A. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation 116: 1081–1093, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Hazen SL, Zhang R, Shen Z, Wu W, Podrez EA, MacPherson JC, Schmitt D, Mitra SN, Mukhopadhyay C, Chen Y, Cohen PA, Hoff HF, Abu-Soud HM. Formation of nitric oxide-derived oxidants by myeloperoxidase in monocytes: pathways for monocyte-mediated protein nitration and lipid peroxidation in vivo. Circ Res 85: 950–958, 1999. [DOI] [PubMed] [Google Scholar]
- 25.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 259: 87–91, 1993. [DOI] [PubMed] [Google Scholar]
- 26.Kadoglou NP, Iliadis F, Angelopoulou N, Perrea D, Ampatzidis G, Liapis CD, Alevizos M. The anti-inflammatory effects of exercise training in patients with type 2 diabetes mellitus. Eur J Cardiovasc Prev Rehabil 14: 837–843, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Karlsson J Antioxidants and Exercise. Champaign, IL: Human Kinetics, 1997.
- 28.Keaney JF, Larson MG, Vasan RS, Wilson PW, Lipinska I, Corey D, Massaro JM, Sutherland P, Vita JA, Benjamin EJ. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol 23: 434–439, 2003. [DOI] [PubMed] [Google Scholar]
- 29.King DE, Mainous AG 3rd, Buchanan TA, Pearson WS. C-reactive protein and glycemic control in adults with diabetes. Diabetes Care 26: 1535–1539, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Kline GM, Porcari JP, Hintermeister R, Freedson PS, Ward A, McCarron RF, Ross J, Rippe JM. Estimation of V̇o2max from a one-mile track walk, gender, age, and body weight. Med Sci Sports Exerc 19: 253–259, 1987. [PubMed] [Google Scholar]
- 31.Kohl HW, 3rd Dunn AL, Marcus BH, Blair SN. A randomized trial of physical activity interventions: design and baseline data from project active. Med Sci Sports Exerc 30: 275–283, 1998. [DOI] [PubMed] [Google Scholar]
- 32.Kritchevsky SB, Cesari M, Pahor M. Inflammatory markers and cardiovascular health in older adults. Cardiovasc Res 66: 265–275, 2005. [DOI] [PubMed] [Google Scholar]
- 33.LaMonte MJ, Blair SN. Physical activity, cardiorespiratory fitness, and adiposity: contributions to disease risk. Curr Opin Clin Nutr Metab Care 9: 540–546, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Lansky D, Brownell KD. Estimates of food quantity and calories: errors in self-report among obese patients. Am J Clin Nutr 35: 727–732, 1982. [DOI] [PubMed] [Google Scholar]
- 35.Lau DC, Dhillon B, Yan H, Szmitko PE, Verma S. Adipokines: molecular links between obesity and atheroslcerosis. Am J Physiol Heart Circ Physiol 288: H2031–H2041, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Laye MJ, Thyfault JP, Stump CS, Booth FW. Inactivity induces increases in abdominal fat. J Appl Physiol 102: 1341–1347, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Linke A, Adams V, Schulze PC, Erbs S, Gielen S, Fiehn E, Mobius-Winkler S, Schubert A, Schuler G, Hambrecht R. Antioxidative effects of exercise training in patients with chronic heart failure: increase in radical scavenger enzyme activity in skeletal muscle. Circulation 111: 1763–1770, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Madden M Internet Evolution: Internet Penetration and Impact. PEW Internet and American Life Project Report. http://www.pewinternet.org/PPF/r/182/report_display.asp [September 2, 2008].
- 39.Marcus BH, Nigg CR, Riebe D, Forsyth LH. Interactive communication strategies: implications for population-based physical-activity promotion. Am J Prev Med 19: 121–126, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Marcus BH, Williams DM, Dubbert PM, Sallis JF, King AC, Yancey AK, Franklin BA, Buchner D, Daniels SR, Claytor RP. Physical activity intervention studies: what we know and what we need to know: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity); Council on Cardiovascular Disease in the Young; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research. Circulation 114: 2739–2752, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419, 1985. [DOI] [PubMed] [Google Scholar]
- 42.Miyazaki H, Oh-ishi S, Ookawara T, Kizaki T, Toshinai K, Ha S, Haga S, Ji LL, Ohno H. Strenuous endurance training in humans reduces oxidative stress following exhausting exercise. Eur J Appl Physiol 84: 1–6, 2001. [DOI] [PubMed] [Google Scholar]
- 43.Mora S, Cook N, Buring JE, Ridker PM, Lee IM. Physical activity and reduced risk of cardiovascular events: potential mediating mechanisms. Circulation 116: 2110–2118, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nojima H, Watanabe H, Yamane K, Kitahara Y, Sekikawa K, Yamamoto H, Yokoyama A, Inamizu T, Asahara T, Kohno N. Effect of aerobic exercise training on oxidative stress in patients with type 2 diabetes mellitus. Metabolism 57: 170–176, 2008. [DOI] [PubMed] [Google Scholar]
- 45.Parise G, Phillips SM, Kaczor JJ, Tarnopolsky MA. Antioxidant enzyme activity is up-regulated after unilateral resistance exercise training in older adults. Free Radic Biol Med 39: 289–295, 2005. [DOI] [PubMed] [Google Scholar]
- 46.Pedersen BK The anti-inflammatory effect of exercise: its role in diabetes and cardiovascular disease control. Essays Biochem 42: 105–117, 2006. [DOI] [PubMed] [Google Scholar]
- 47.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest 111: 1805–1812, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pietinen P, Hartman AM, Haapa E, Rasanen L, Haapakoski J, Palmgren J, Albanes D, Virtamo J, Huttunen JK. Reproducibility and validity of dietary assessment instruments. I. A self-administered food use questionnaire with a portion size picture booklet. Am J Epidemiol 128: 655–666, 1988. [DOI] [PubMed] [Google Scholar]
- 49.Pou KM, Massaro JM, Hoffmann U, Vasan RS, Maurovich-Horvat P, Larson MG, Keaney JF Jr, Meigs JB, Lipinska I, Kathiresan S, Murabito JM, O'Donnell CJ, Benjamin EJ, Fox CS. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation 116: 1234–1241, 2007. [DOI] [PubMed] [Google Scholar]
- 50.Powers SK, Ji LL, Leeuwenburgh C. Exercise training-induced alterations in skeletal muscle antioxidant capacity: a brief review. Med Sci Sports Exerc 31: 987–997, 1999. [DOI] [PubMed] [Google Scholar]
- 51.Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol 51: 390–395, 1983. [DOI] [PubMed] [Google Scholar]
- 52.Reynolds THt Brown MD, Supiano MA, Dengel DR. Aerobic exercise training improves insulin sensitivity independent of plasma tumor necrosis factor-alpha levels in older female hypertensives. Metabolism 51: 1402–1406, 2002. [DOI] [PubMed] [Google Scholar]
- 53.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med 336: 973–979, 1997. [DOI] [PubMed] [Google Scholar]
- 54.Ridker PM, Rifai N, Pfeffer M, Sacks F, Lepage S, Braunwald E. Elevation of tumor necrosis factor-alpha and increased risk of recurrent coronary events after myocardial infarction. Circulation 101: 2149–2153, 2000. [DOI] [PubMed] [Google Scholar]
- 55.Sarafidis PA, Lasaridis AN, Nilsson PM, Pikilidou MI, Stafilas PC, Kanaki A, Kazakos K, Yovos J, Bakris GL. Validity and reproducibility of HOMA-IR, 1/HOMA-IR, QUICKI and McAuley's indices in patients with hypertension and type II diabetes. J Hum Hypertens 21: 709–716, 2007. [DOI] [PubMed] [Google Scholar]
- 56.Schneider PL, Crouter SE, Lukajic O, Bassett DR Jr. Accuracy and reliability of 10 pedometers for measuring steps over a 400-m walk. Med Sci Sports Exerc 35: 1779–1784, 2003. [DOI] [PubMed] [Google Scholar]
- 58.Shih LY, Liou TH, Chao JC, Kau HN, Wu YJ, Shieh MJ, Yeh CY, Han BC. Leptin, superoxide dismutase, and weight loss: initial leptin predicts weight loss. Obesity 14: 2184–2192, 2006. [DOI] [PubMed] [Google Scholar]
- 59.Silver AE, Beske SD, Christou DD, Donato AJ, Moreau KL, Eskurza I, Gates PE, Seals DR. Overweight and obese humans demonstrate increased vascular endothelial NAD(P)H oxidase-p47(phox) expression and evidence of endothelial oxidative stress. Circulation 115: 627–637, 2007. [DOI] [PubMed] [Google Scholar]
- 60.Skoog T, Dichtl W, Boquist S, Skoglund-Andersson C, Karpe F, Tang R, Bond MG, de Faire U, Nilsson J, Eriksson P, Hamsten A. Plasma tumour necrosis factor-alpha and early carotid atherosclerosis in healthy middle-aged men. Eur Heart J 23: 376–383, 2002. [DOI] [PubMed] [Google Scholar]
- 61.Smith JK, Dykes R, Douglas JE, Krishnaswamy G, Berk S. Long-term exercise and atherogenic activity of blood mononuclear cells in persons at risk of developing ischemic heart disease. JAMA 281: 1722–1727, 1999. [DOI] [PubMed] [Google Scholar]
- 62.Stauffer BL, Hoetzer GL, Smith DT, DeSouza CA. Plasma C-reactive protein is not elevated in physically active postmenopausal women taking hormone replacement therapy. J Appl Physiol 96: 143–148, 2004. [DOI] [PubMed] [Google Scholar]
- 63.Strauss RS Comparison of serum concentrations of alpha-tocopherol and beta-carotene in a cross-sectional sample of obese and nonobese children (NHANES III). National Health and Nutrition Examination Survey. J Pediatr 134: 160–165, 1999. [DOI] [PubMed] [Google Scholar]
- 63a.Task Force on Community Preventive Services. Recommendations to increase physical activity in communities. Am J Prev Med 22: 67–72, 2002.11985935 [Google Scholar]
- 64.Taylor HL, Wang Y, Rowell L, Blomqvist G. The standardization and interpretation of submaximal and maximal tests of working capacity. Pediatrics 32, Suppl: 703–722, 1963. [PubMed] [Google Scholar]
- 65.Thompson PD, Buchner D, Pina IL, Balady GJ, Williams MA, Marcus BH, Berra K, Blair SN, Costa F, Franklin B, Fletcher GF, Gordon NF, Pate RR, Rodriguez BL, Yancey AK, Wenger NK. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity). Circulation 107: 3109–3116, 2003. [DOI] [PubMed] [Google Scholar]
- 66.Tudor-Locke CE, Myers AM, Rodger NW. Development of a theory-based daily activity intervention for individuals with type 2 diabetes. Diabetes Educator 27: 85–93, 2001. [DOI] [PubMed] [Google Scholar]
- 67.United States Department of Health and Human Services. National Center for Health Statistics: Deaths/Mortality. U.S. Department of Health and Human Services. http://www.cdc.gov/nchs/faststats/death.htm [November 12, 2006, 2006].
- 68.van der Vliet A, Eiserich JP, Halliwell B, Cross CE. Formation of reactive nitrogen species during peroxidase-catalyzed oxidation of nitrite. A potential additional mechanism of nitric oxide-dependent toxicity. J Biol Chem 272: 7617–7625, 1997. [DOI] [PubMed] [Google Scholar]
- 69.Vandelanotte C, Spathonis KM, Eakin EG, Owen N. Website-delivered physical activity interventions a review of the literature. Am J Prev Med 33: 54–64, 2007. [DOI] [PubMed] [Google Scholar]
- 70.Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA 282: 2131–2135, 1999. [DOI] [PubMed] [Google Scholar]
- 71.Watson TA, MacDonald-Wicks LK, Garg ML. Oxidative stress and antioxidants in athletes undertaking regular exercise training. Int J Sport Nutr Exerc Metab 15: 131–146, 2005. [DOI] [PubMed] [Google Scholar]
- 72.White T, Ozel B, Jain JK, Stanczyk FZ. Effects of transdermal and oral contraceptives on estrogen-sensitive hepatic proteins. Contraception 74: 293–296, 2006. [DOI] [PubMed] [Google Scholar]
- 73.Wilcox S, Dowda M, Rheaume CE, Ory MG, Leviton L, Dunn A, King AC, Estabrooks P, Castro CM, Dowdy D, Bazzarre T, Campbell-Voytal K, Buchner DM, Bartlett-Prescott J. Results of the third year of Active for Life. Med Sci Sports Exerc 39, Suppl: S80, 2007. [Google Scholar]
- 74.Yudkin JS Adipose tissue, insulin action and vascular disease: inflammatory signals. Int J Obes Relat Metab Disord 27, Suppl 3: S25–S28, 2003. [DOI] [PubMed] [Google Scholar]
- 75.Zoppini G, Targher G, Zamboni C, Venturi C, Cacciatori V, Moghetti P, Muggeo M. Effects of moderate-intensity exercise training on plasma biomarkers of inflammation and endothelial dysfunction in older patients with type 2 diabetes. Nutr Metab Cardiovasc Dis 16: 543–549, 2006. [DOI] [PubMed] [Google Scholar]