Abstract
The primary goal of this study was to determine the acute glycemic and endocrine responses to the reduction of fat content from a meal. On three separate occasions, nine overweight subjects (body mass index = 30 ± 1 kg/m2; 5 men, 4 women) consumed 1) a control meal (∼800 kcal; 100 g of carbohydrate, 31 g of fat, and 30 g of protein), 2) a low-fat meal (∼530 kcal; 100 g of carbohydrate, 1 g of fat, and 30 g of protein), or 3) a low-fat meal plus lipid infusion [same meal as low-fat meal, but the total energy provided was the same as control (800 kcal), with the “missing” fat (∼30 g) provided via an intravenous lipid infusion]. All three meals contained [13C]glucose (3 mg/kg body wt) to assess the bioavailability of ingested glucose. During the 5-h period after each meal, we measured the recovery of [13C]glucose in plasma, plasma glucose, and insulin concentrations. We also measured plasma concentration of the gastrointestinal peptides: glucose-dependent insulinotropic polypeptide (GIP), glucagon-like peptide-1 (GLP-1), and peptide YY3-36 (PYY3-36). The recovery of the ingested [13C]glucose in the hour after ingestion was greater (P < 0.05) after the low-fat than after the control meal [area under the curve (AUC): 1,206 ± 252 and 687 ± 161 μM·h, respectively]. However, removing dietary fat from the meal did not affect the plasma concentration of glucose or insulin. Importantly, [13C]glucose recovery was not different during the low-fat and lipid infusion trials (AUC: 1,206 ± 252 and 1,134 ± 247 μM·h, respectively), indicating that the accelerated delivery of exogenous glucose found after removing fat from the meal is due exclusively to the reduction of fat in the gastrointestinal tract. In parallel with these findings, the reduction in fat calories from the meal reduced plasma concentration of GIP, GLP-1, and PYY3-36. In summary, these data suggest that removing fat from the diet expedited exogenous glucose delivery into the systemic circulation and reduced the concentration of key gastrointestinal peptides, yet maintained plasma glucose concentration at control levels.
Keywords: glucose metabolism, incretins, gastrointestinal peptides, low-fat diet, obesity
metabolic alterations after a single meal (e.g., hyperglycemia, hyperlipidemia) have been found to be important independent risk factors for development of obesity-related diseases (19, 31, 37). However, the impact of the acute response to meal ingestion often gets little attention. For example, despite the fact that low-fat diets have been advocated for weight loss for decades, surprisingly little is understood about the acute metabolic effects of reducing fat calories from the meals. Findings from studies evaluating the metabolic and endocrine responses to the removal of fat from a meal are equivocal. Often the metabolic responses to a low-fat meal are assessed when carbohydrate and/or protein content of the diet are increased to compensate for the reduction in fat calories (9, 40). Although this approach controls for energy content of the diets, it does not distinguish the metabolic effects of removing fat calories from the effects of adding the other macronutrients. This approach is particularly confounding when attempting to assess the effect of removing fat from the diet on plasma glycemia, because the greater plasma glucose response is most likely an artifact of the greater amount of ingested carbohydrate (9). In addition, understanding the effects of reducing dietary fat from the meal without compensating for energy content is most relevant in the context of diets prescribed for weight loss. Reducing dietary fat from a mixed meal without adding additional nutrients (i.e., lower total caloric content) has been found to augment the plasma glucose response (6–8, 18, 29), although this finding is not universal (9, 30).
It has become increasingly evident that the gastrointestinal tract plays a key role in the interplay between the contents of the meal and the subsequent metabolic and hormonal responses. Over the past several years, the gastrointestinal tract has been found to release important peptide hormones into the systemic circulation in response to the contents of the meal (1, 14, 21). These gastrointestinal peptides, such as glucose-dependent insulinotropic polypeptide (GIP), glucagon-like peptide-1 (GLP-1), and peptide YY3-36 (PYY3-36) have several important metabolic actions, including mediating pancreatic insulin secretion and effecting the sensation of hunger and satiety (3, 27, 36). However, many of the mechanisms governing gastrointestinal peptide secretion remain unclear. Fat content in the diet has been found to be a trigger for the secretion of these gastrointestinal peptides (14, 32, 36), but the impact of removing fat calories from the diet on the magnitude of change in the secretion of these peptides is not known.
The primary goal of this study was to determine the acute glycemic and endocrine responses to the reduction of fat content from a meal. To better address the influence of ingested fat on postprandial glucose response, subjects ingested tracer-labeled carbohydrate ([13C]glucose) to measure the delivery of the exogenous glucose to the systemic circulation.
METHODS
Subjects
Nine young adults (5 men and 4 women) participated in this study (average age: 25 ± 3 yr). Subjects were overweight to mildly obese (average body mass index: 30.5 ± 1.2 kg/m2; average body mass: 89.4 ± 5.1 kg; average body fat: 38.8 ± 3.5%) and otherwise considered to be in good health after completion of a medical history and physical examination, 12-lead electrocardiogram, and measurement of fasting plasma glucose, insulin, and triglyceride concentrations. Subjects engaged in no more than 2 h/wk of physical exercise, did not have any evidence of metabolic or cardiovascular disorders, and were not taking prescription medications. Subjects were informed of the procedures and the possible risks and signed an informed consent document that was approved by the University of Michigan Institutional Review Board.
Preliminary Procedures
Before each experiment, subjects were instructed to consume a standardized diet for 2 days before being admitted to the hospital for the study. This diet consisted of 30 kcal/kg body wt per day and had a macronutrient content of ∼50% carbohydrate, 35% fat, and 15% protein. A description of meals and snacks in accordance with this diet was provided as a guide for subjects to follow. Subjects were also asked to keep a detailed record of their food intake for the 2 days before each experiment. All subjects reported close adherence to the standard diet during the 2 days before each experiment. All female subjects were studied during the self-reported follicular phase of their menstrual cycle.
During the subjects' first screening visit, resting oxygen consumption (V̇o2) and carbon dioxide production (V̇co2) were measured (DeltaTrac II; Sensormedics) for 20–30 min. Resting V̇o2 and V̇co2 measurements from this test were used to calculate the subjects' resting metabolic rate (RMR) using the Weir equation (41).
Experimental Protocol
Subjects underwent three separate, randomized trials (separated by at least 5 days). For each trial, they were admitted to the General Clinical Research Center at the University of Michigan Hospital at 1800 the evening before each experimental trial and were given a standardized meal (12 kcal/kg body wt; 0.5 g fat/kg, 1.5 g carbohydrate/kg, and 0.5 g protein/kg) at 1830. After the evening meal, subjects were allowed only water, and they were required to spend the remainder of the evening restricted to their beds.
The next morning, a Teflon catheter was inserted into an antecubital vein of one arm for blood sampling, and a basal blood sample was obtained at 0600. After the baseline blood draw, body composition was assessed using dual-energy X-ray absorptiometry. At 0900 (after a 14-h fast), subjects consumed one of three test meals/treatments: 1) control, a meal containing ∼800 kcal (50% carbohydrate, 35% fat, and 15% protein); 2) low fat, a meal containing exactly the same absolute amount (i.e., grams) of carbohydrate and protein as the control meal but with nearly all of the dietary fat removed (∼530 kcal; 75% carbohydrate, 2% fat, 23% protein); or 3) lipid infusion, where the subjects ingested exactly the same low-fat meal as described above, but the “missing” fat (i.e., missing compared with the control meal, ∼30 g of fat) was provided via an intravenous lipid infusion of a 20% lipid emulsion. The lipid solution (Liposyn II 20%; Abbott Laboratories, Chicago, IL) was infused through an intravenous catheter placed in the arm opposite to the arm used for blood sampling. The lipid infusion started at the beginning of the meal, and the amount of lipid infused was calculated to exactly match the amount of fat ingested during the control trial. The lipid was infused at a variable rate in attempt to mimic the expected increase in plasma triglyceride concentration when the fat was ingested during the control trial. In addition, to control for the type of fat ingested/infused, during the control trial, subjects ingested Liposyn as their primary source of dietary fat. Therefore, subjects received the same exact amount and type of fat during the control and lipid infusion trials; only the mode of delivery was different (i.e., enteral vs. intravenous, respectively). Caloric content for the control meal was determined as one-third of total daily energy requirements estimated as 1.4 × RMR. This estimate for total daily energy expenditure has been found to approximate total daily energy expenditure when subjects are inactive (38). Caloric content of the low-fat and lipid infusion meals was determined as 65% of the total caloric content of the control meal (i.e., one-third of 65% × 1.4 × RMR). To match the volume of all meals, subjects drank ∼140 ml of water with their low-fat and lipid infusion meals to match the volume of Liposyn ingested during the control trial. Exactly 3 mg/kg body wt of [U-13C]glucose (Cambridge Isotopes, Andover, MA) were mixed in with the meal to allow us to trace the entry of the ingested glucose into the circulation. Specifically, the tracer was dissolved in water and was consumed by the subjects while they ate the test meal. Subjects remained resting in bed, and blood samples were taken 15, 30, 45, 60, 90, 120, 150, 180, 210, 240, 270, and 300 min after the meal.
Blood Sample Preparation
Blood samples were collected into chilled glass test tubes containing EDTA (0.03 mM) and dipeptidyl peptidase IV inhibitor (10 μl/ml blood; Linco Research, St. Charles, MO) for analysis of glucose, insulin, triglyceride, fatty acid, GIP, GLP-1, and PYY3-36 concentrations. All samples were kept on ice and then centrifuged (1,600 g for 15 min at 4°C) within 30 min of collection. After centrifugation, the plasma from each sample was transferred into 12 × 75-mm glass culture tubes, immediately frozen, and stored at −80°C for later analysis.
Hunger Assessment
Before and after the meal, subjects were asked to complete a survey to identify their perception of hunger and satiety [i.e., visual analog scale (VAS)] (17). More specifically, the VAS survey consisted of four questions: 1) How hungry do you feel? 2) How much do you think you can eat? 3) How satisfied do you feel? and 4) How full do you feel? Below each question on the survey was a horizontal 100-mm line with qualifying statements on the extreme left and right side of this line. In response to each question, subjects were asked to draw a vertical mark on the horizontal line to represent the magnitude of their response to the question. A value for each response was quantified by measuring the distance of their mark (in mm) relative to the left end of the line. Therefore, the values (or “scores”) for each question ranged from 0 to 100. VAS scores for each question were measured before the meal and 0.5, 2.5, and 5 h after the meal. The area under the curve (AUC) for VAS vs. time (after normalizing for each subject's baseline VAS score for each question) was calculated to represent the response to each treatment. To ensure consistency, all VAS ratings were measured by the same member of the research team.
Analytical Procedures
Plasma substrate, hormone, and gastric peptide concentrations.
Commercially available colorimetric assays were used to measure plasma concentrations of glucose (ThermoTrace, Melbourne, Australia), triglyceride (Sigma, St. Louis, MO), and fatty acid (Wako Chemicals USA, Richmond, VA). Plasma insulin concentration was measured by radioimmunoassay (Linco Research). Plasma gastrointestinal peptide concentrations (GIP, GLP-1, and PYY3-36) were measured by multisphere assay (LINCOplex kit; Linco Research) using Luminex technology (Luminex100; Luminex, Austin, TX).
Isotope tracer enrichment.
The tracer-to-tracee ratio (TTR) of plasma glucose (13C:12C ratio) was measured by electron impact ionization gas chromatography-mass spectrometry (Agilent 5973Networks, Mass Selective Detector; Agilent Technologies, Palo Alto, CA) after conversion to the aldonitrile derivative of glucose in deproteinized plasma (39).
Calculations
[13C]glucose concentration (μM) was determined by multiplying the TTR of plasma glucose by the plasma glucose concentration (mM) at each corresponding time point. AUC was calculated using the trapezoidal method. Average concentrations were also calculated over the periods 0–1 and 1–5 h after the meal to allow for comparison of the early phase and later phase responses between trials.
Statistical Analysis
A two-way analysis of variance (ANOVA) (treatment × time) with repeated measures and Tukey's post hoc analysis were used to assess statistical differences in [13C]glucose recovery in plasma, plasma substrate, hormone, and gut peptide concentrations before and during the hours after the first meal on day 1. A one-way ANOVA was used to assess statistical differences in AUC measurements among the different meal treatments. For the VAS measurements, a one-way ANOVA on ranks was performed to assess significant differences in the relative sensations of hunger and satiety among the different treatments. Statistical analyses were performed using SigmaStat for Windows (version 3.0.1a; Systat Software, Point Richmond, CA). Statistical significance was defined as P < 0.05. All results are means ± SE.
RESULTS
Recovery of Exogenous [13C]Glucose and Plasma Concentrations of Substrates and Insulin
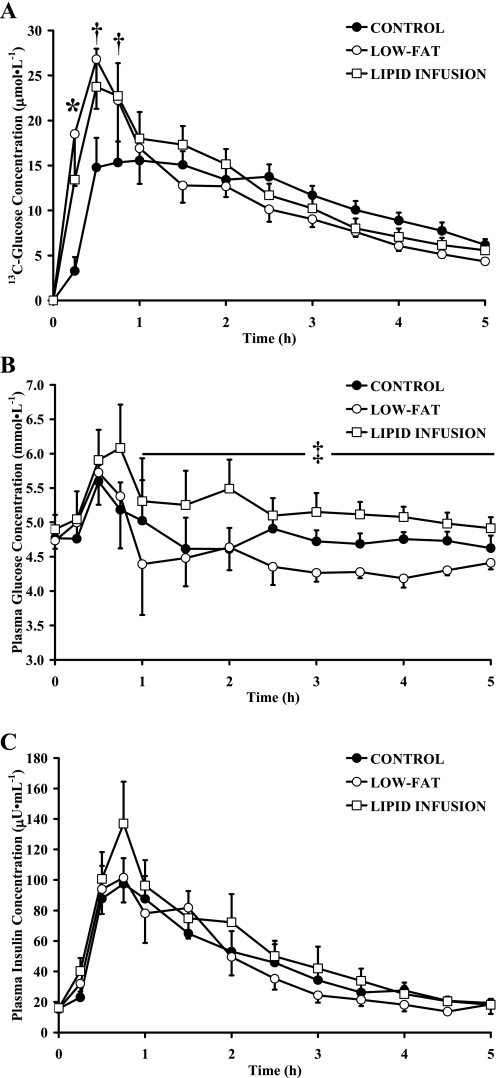
Removing nearly all of the dietary fat from the meal significantly increased the recovery of the ingested [13C]glucose during the hour after ingestion (Fig. 1A). However, total plasma glucose concentration was not different during the hour after ingestion of the low-fat meal compared with the control meal (Fig. 1B). Although there was a trend for a greater plasma glucose concentration toward the end of the first hour of the lipid infusion compared with low fat (Fig. 1B), this was largely due to the response of one subject (statistical power was calculated to be 0.14 for this comparison, which is far below the acceptable power level of 0.8). Importantly, during the period 1–5 h after the meal, the average [13C]glucose recovery in plasma tended to be greater during control compared with low-fat trials (11.3 ± 1.0 and 9.3 ± 0.9 M, respectively; P = 0.07). As a result, average total [13C]glucose recovery over the 5-h period after the meal was not different between control and low-fat trials (10.4 ± 0.8 and 11.7 ± 1.4 μM, respectively; P = 0.24). Interestingly, average plasma glucose concentration was slightly yet significantly lower during the period 1–5 h after low-fat compared with control meals (4.4 ± 0.2 and 4.7 ± 0.2 mM, respectively; P < 0.05). Despite this difference in plasma glucose concentration between trials during the 1- to 5-h period, plasma insulin concentration was the same throughout low-fat compared with control trials (Fig. 1C).
Fig. 1.
Plasma [13C]glucose (A), glucose (B), and insulin concentrations (C) before and during 5 h after ingestion of control, low-fat, or lipid infusion meals. Values are means ± SE. *P < 0.05, significant difference among all trials. †P < 0.05, low-fat and lipid infusion significantly different from control. ‡P < 0.05, at 1–5 h area under the curve (AUC), significant difference among all trials.
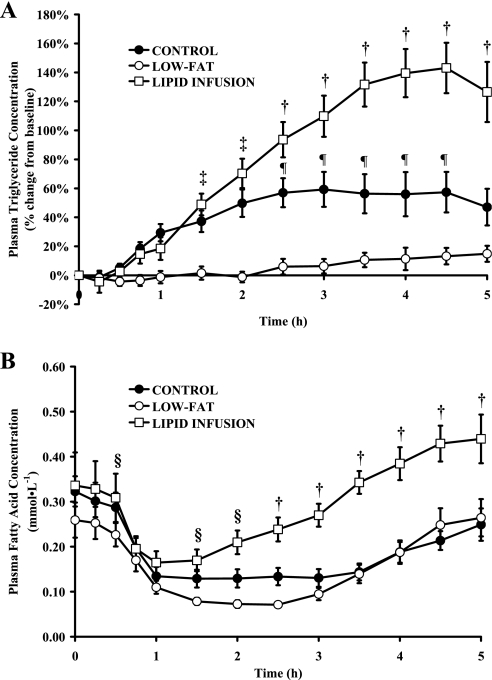
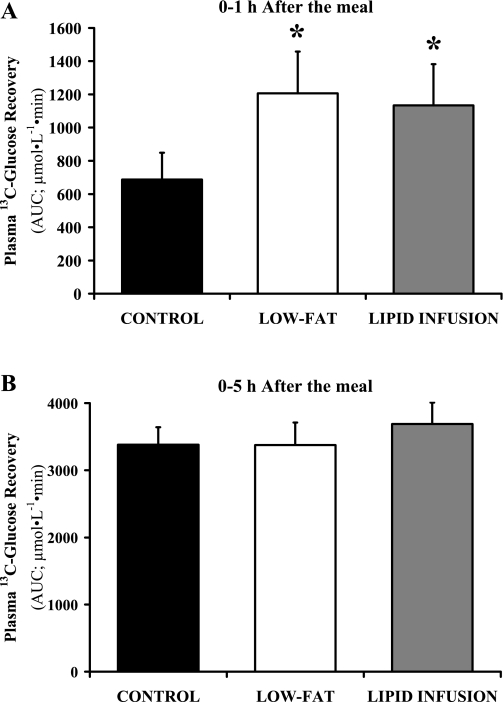
Although we infused exactly the same amount and type of lipid during the lipid infusion trial as was ingested during the control trial, and we infused the lipid solution at a variable rate in attempt to mimic the postprandial rise in plasma triglyceride concentration, the increase in plasma triglyceride concentration was markedly greater during the lipid infusion than the control trial (P < 0.05) (Fig. 2A). Similarly, plasma fatty acid concentration was higher during lipid infusion than both the control and low-fat trials (Fig. 2B). Therefore, our lipid infusion intervention did not match the postprandial lipemic response of the control trial as originally intended. However, despite the augmented plasma triglyceride and fatty acid concentration during the lipid infusion trial, information from this trial still provides important insight into the influence of fat availability on postprandial glucose metabolism. Indeed, [13C]glucose recovery during the first hour after the meal was nearly identical during low-fat and lipid infusion trials (Fig. 3A), indicating that the lipid infusion did not affect exogenous glucose delivery into the systemic circulation. Importantly, these data indicate the expedited delivery of exogenous glucose found after removing fat from the meal is due exclusively to the absence of fat in the gastrointestinal tract. Although exogenous glucose recovery was blunted in the control trial during the first hour after the meal (Fig. 3A), total [13C]glucose recovery over the entire 5-h period after the meal was the same in all three trials (Fig. 3B).
Fig. 2.
Percent change from baseline in plasma triglyceride (A) and plasma fatty acid concentrations (B) before and during 5 h after control, low-fat, or lipid infusion meals. Values are means ± SE. †P < 0.05, lipid infusion significantly different from control and low fat. ‡P < 0.05, lipid infusion and control significantly different from low fat. ¶P < 0.05, control significantly different from low fat and lipid infusion. §P < 0.05, lipid infusion significantly different from low fat.
Fig. 3.
Recovery of ingested [13C]glucose in plasma 0–1 h after the meal (A) and 0–5 h after the meal (B). Values are means ± SE. Recovery is reported as AUC for plasma [13C]glucose concentration vs. time. *P < 0.05, low fat and lipid infusion significantly greater than control.
Effect of Reducing Dietary Fat on Plasma Concentration of Gastrointestinal Peptides
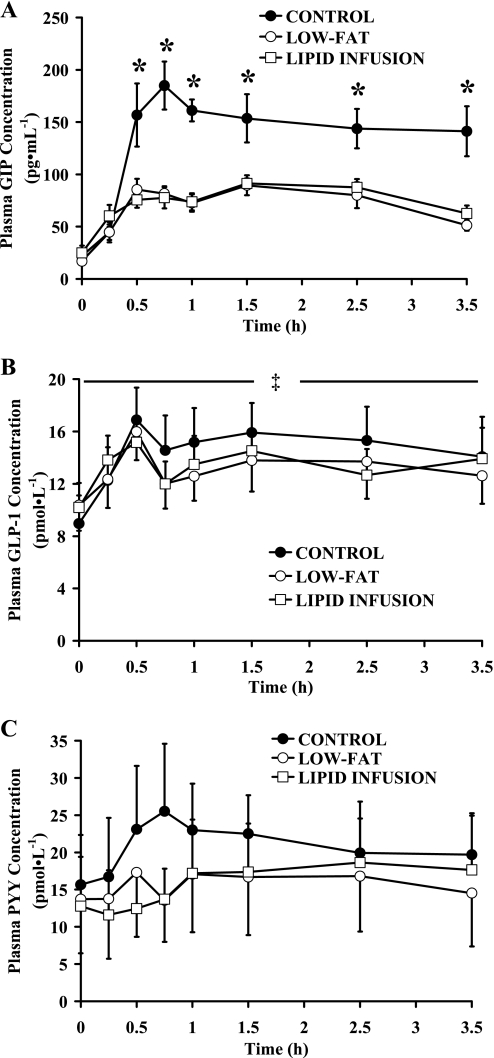
In general, removing fat calories from the meals attenuated the increase in the plasma concentration of gastrointestinal peptides in response to the meal (Fig. 4). We found a robust increase in plasma GIP concentration after the control meal, but this response was significantly lower when fat was removed from the meals during low-fat and lipid infusion trials (P < 0.05) (Fig. 4A). Similarly, the increase in plasma GLP-1 (0–5 h AUC, P < 0.05) and PYY3-36 were attenuated by the removal of fat from the meal (Fig. 4, B and C), although the difference in plasma PYY3-36 concentration during control vs. low-fat and lipid infusion trials did not reach statistical significance. Importantly, plasma concentrations of all measured gastrointestinal peptides were nearly identical in the hours after the meal during low-fat and lipid infusion trials (Fig. 4). Therefore, the similar peptide responses between low-fat and lipid infusion and the observation that the responses during both of these trials were often different from those of the control trial support the premise that delivery of fat calories through the gastrointestinal tract is a key regulator for the release of gastrointestinal peptides into systemic circulation.
Fig. 4.
Plasma glucose-dependent insulinotropic polypeptide (GIP; A), glucagon-like peptide-1 (GLP-1; B), and peptide YY3-36 (PYY3-36; C) concentrations before and during 5 h after control, low-fat, and lipid infusion meals. Values are means ± SE. *P < 0.05, control significantly different from low fat and lipid infusion. ‡P < 0.05, at 0–5 h AUC, control significantly different from low fat and lipid infusion.
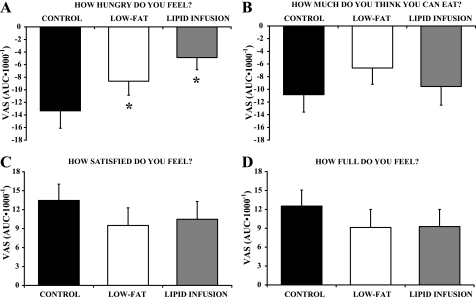
Hunger and Satiety Ratings
Not surprisingly, reducing fat calories (and total calories) from the meal increased hunger and decreased satiety, although some of these differences did not reach statistical significance. In Fig. 5, we present the hunger and satiety data as the AUC for the responses to the four different VAS questions during the 5-h period after the meals. The VAS scores after the meal were normalized to each subject's baseline VAS score, so a negative value indicates that the meal lowered the response below premeal levels. For all questions, VAS scores were not different between low-fat and lipid infusion trials. Removing fat calories from the meal during both low-fat and lipid infusion trials attenuated the reduction in the VAS score for “How hungry do you feel?” compared with the control trial (P < 0.05 for low-fat and lipid infusion vs. control). Removing fat from the meal during low-fat and lipid infusion trials tended to blunt the increase in VAS score for “How satisfied do you feel?” and “How full do you feel?” compared with the control trial, but the difference did not reach statistical significance (P = 0.34 and P = 0.23, respectively).
Fig. 5.
Visual analog scale (VAS) scores in response to hunger and satiety questionnaires. VAS scores are presented as AUC for scores recorded during the 5-h period after the meal. Values are means ± SE. VAS scores after the meal are expressed relative to the premeal score, so a negative value indicates the meal reduced the VAS score for that question. *P < 0.05, significantly different from control.
DISCUSSION
Because a reduction in dietary fat is among the most common weight loss prescriptions, a better understanding of the metabolic consequences of reducing fat from a meal provides valuable information for optimizing dietary interventions. One major finding from this study was that reducing the fat content from a meal accelerates the bioavailability of exogenous carbohydrate after the meal without altering plasma glucose or insulin concentrations. We also found generally greater gastrointestinal peptide responses after delivery of fat calories through the gastrointestinal tract compared with fat delivered directly into the systemic circulation, indicating the importance of ingested fat on the release of gastrointestinal peptides.
Our finding that the augmented exogenous glucose availability after removing fat from the meal did not affect plasma glucose concentration necessitates that either glucose uptake was accelerated or hepatic glucose production was suppressed. Although lowering systemic lipid availability is known to enhance insulin-mediated glucose uptake (2), plasma triglyceride and fatty acid concentrations in our study were not significantly different between low-fat and control trials until at least 1 h after the meal. It is also important to note that the effect of lipids on glucose uptake takes at least a few hours (35). Therefore, we believe that the maintenance of euglycemia despite an augmented delivery of exogenous glucose during the low-fat trial is due to a compensatory reduction in hepatic glucose production, rather than enhanced glucose uptake. Recent studies in animals and humans have provided evidence for the existence of a portal glucose sensor that regulates hepatic glucose production (4, 42). Therefore, hepatoportal sensing of the greater delivery of exogenous carbohydrate to the liver during the hour after ingestion of the low-fat meal may have reduced hepatic glucose production proportionally such that plasma glucose concentration was similar in response to the mixed meal. Interestingly, it appears that the accuracy of this proportional exchange between exogenous glucose availability and endogenous glucose production is relatively short-lived. Our observation that plasma glucose concentration was lower in the later hours of the experiment during the low-fat compared with the control trial suggests that if hepatic glucose production was indeed suppressed in the first hour after the low-fat meal, this suppression may have persisted for several hours.
Contrary to our findings, Normand et al. (29) found that reducing the fat content from the meal did not accelerate the rate of glucose entry into the circulation. This discrepancy may be due to the difference in tracer that was used between the two studies. We used readily absorbed [13C]glucose as our tracer (because the majority of the carbohydrate in our test meal was in the form of simple carbohydrates), whereas Normand et al. (29) used a 13C-labeled starch, which is less readily digested and absorbed (25). In addition, in contrast to our findings, some studies have reported that reducing almost all of the dietary fat from the meal increased plasma glucose concentration during the hour after eating (7, 8, 18, 29). Importantly, the fat content of the mixed meals in these studies (i.e., ∼60% of total caloric content) was about twofold greater than we provided in our study (i.e., ∼30% of total caloric content), and it has been found that dietary fat has a more profound effect on suppressing the glycemic response to the meal when it comprises more than ∼40% of the total energy content of the meal (30).
Reducing fat from the diet increases the rate of exogenous glucose delivery into the circulation largely by increasing the rate of gastric emptying and absorption (5, 7, 11). Indeed, plasma glucose levels after a meal have been found to be related to the rate of emptying (22). In turn, the rates of gastric emptying and absorption have been found to be controlled by many factors, including the osmolality of the ingested meal (26), the viscous property of the food mixture within the small intestines (13), and the enzymatic breakdown of the food mixture within the epithelium (10). Reducing dietary fat from meals can affect all of these parameters.
Clearly, the gastrointestinal tract plays a major role in the interaction between the contents of a meal and the subsequent metabolic and hormonal responses. We found that delivery of fat calories through the gastrointestinal tract compared with fat delivered directly into the systemic circulation is important for release of GIP, GLP-1, and PYY3-36. Fat content in the diet has been found to be an important trigger for the secretion of these gastrointestinal peptides (14, 32, 36). However, whereas previous studies have found that dietary fat is a potent stimulus for gastrointestinal peptides secretion, we found a potent reduction in only GIP, with just a modest reduction in GLP-1 and PYY3-36, when most of the fat was removed from the diet. This may be explained in part by the overweight status of our subjects, because secretion of GLP-1 and PYY3-36 in response to fat ingestion has been found to be attenuated in obesity (24, 33). Unlike GLP-1 and PYY3-36, GIP does not appear to be affected by an individual's body weight (33), which may explain why we found a more profound attenuation in GIP concentration when fat was removed from the meal. It is important to acknowledge that the effect of removing fat from the meal on the secretion of these peptides cannot clearly be differentiated from the effect of the reduction in total energy that was delivered through the gastrointestinal tract.
An important action of some of the gastrointestinal peptides, specifically the incretins GIP and GLP-1, is to augment the insulin response to the meal. Several studies have reported that despite augmented plasma glucose response to meals with reduced dietary fat, plasma insulin concentration did not increase accordingly (6–9, 29) or may even be lower (18). The blunted insulin response to the meal may be a consequence of a reduction in the plasma concentration of GIP and GLP-1. It has been reported that the effect of incretins can account for roughly 20–60% of the total insulin secretory response to a meal (28). Interestingly, despite significantly greater incretin response (especially a robust increase in GIP) during the first hour after our control compared with low-fat meals, plasma insulin concentration was not different. Although plasma glucose concentration was similar during control and low-fat trials, insulin secretion has been found to be sensitive to the rate of change in glucose presentation to the pancreas (20, 23) and not just to the static plasma glucose concentration. Therefore, the greater rate of exogenous glucose entry into the systemic circulation during the low-fat trial may have counteracted the lower incretin response, resulting in similar insulin concentrations between these trials.
Our original goal for the lipid infusion trial was to mimic the postprandial rise in plasma triglyceride concentration found during the control trial. Unfortunately, although we infused exactly the same amount and type of lipid that was ingested during the control trial, we were unable to match the plasma triglyceride response found in the control trial. It is not clear why this occurred, but we expect the entrapment of ingested lipids within the intestinal endothelial cells and/or the lymphatic system (15, 16) was far greater than we anticipated. This unexpected result does not allow us to make the direct comparisons between the control and lipid infusion trials that we originally intended. However, data from the lipid infusion trial do demonstrate that the accelerated delivery of exogenous glucose found after removing fat from the meal is due exclusively to the absence of fat in the gastrointestinal tract and is not responsive to systemic lipid and/or energy availability.
It is known that the regulation of food intake and sensations of hunger and satiety are regulated by several factors, including the bioavailability of energy and macronutrients ingested. We found that a reduction in dietary fat calories from the meals resulted in perceptions of greater hunger and less satiety in our overweight participants. By itself, this finding simply suggests that eating fewer calories and less fat induced a greater hunger response. However, an important finding of this study was that replacing the fat calories “missing” from the meals via an intravenous infusion (thereby bypassing the gastrointestinal tract) did not reverse the increased sensation of hunger or decreased sensation of satiety. This supports the assertion that the physical presence of calories, and specifically calories from fat, in the gastrointestinal tract is important for the perception of hunger and satiety sensations. Importantly, we found a temporal association between the attenuated hunger response when fat was removed from the ingested meal, and the blunted change in gastrointestinal peptides (i.e., GIP, GLP-1, and PYY3-36) known to be associated with alterations in the perception of hunger and satiety (12). However, our data do not allow us to assess whether this relationship is causal.
We recognize that there are some limitations to the interpretation of our findings. For example, we cannot clearly differentiate the effect of reducing fat calories from the effect of reducing total energy content from the meals. Infusing lipids during the lipid infusion trial allowed us to maintain systemic energy balance, but this does not account for differences in energy content passing through the gastrointestinal tract. However, it is important to note that simply increasing dietary carbohydrate and/or protein in an effort to compensate for the reduction in total calories brought about by the removal of fat from the meal is unacceptable, because the addition of these macronutrients will alter the glycemic and endocrine responses to the meal. In addition, we recognize that our relatively small sample size could certainly have contributed to our inability to detect any sex-related differences in our study, but a comparison of data from the men and women in our study did not reveal any notable trends for differences among our major outcome measurements. We acknowledge that a previous study has reported a difference in the rate of exogenous glucose appearance in women and men in response to a carbohydrate meal (34), but this result has not been replicated.
In summary, a major finding from this study is that removing fat from a meal accelerated the bioavailability of exogenous carbohydrate during the first hour after the meal without altering plasma glucose concentration. We interpret this to suggest that the liver sensed the increased entry of exogenous carbohydrate and compensated by suppressing endogenous glucose production. In addition, we found that it is the delivery of fat directly into the gastrointestinal tract that is key to this process. Also, we found that fat passing through the gastrointestinal tract, compared with fat delivered directly into the systemic circulation, is essential for the secretion of important peptides that are involved in the regulation of metabolic processes and linked with sensations of hunger. Because a reduction of fat from the diet is often prescribed for weight loss, the findings in this study improve our understanding about the metabolic and endocrine responses to low-fat meals.
GRANTS
This study was supported by National Institutes of Health (NIH) Grant R0–1 DK071955 and by University of Michigan General Clinical Research Center NIH Grant M01-RR00042.
Acknowledgments
We are thankful to Dan Faden and Kristin Thomas for technical assistance, Sacha Hamady for dietetic consultation and study coordination, and the nursing and dietary staff of the General Clinical Research Center at The University of Michigan Hospital for the excellent assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Adrian TE, Ferri GL, Bacarese-Hamilton AJ, Fuessl HS, Polak JM, Bloom SR. Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology 89: 1070–1077, 1985. [DOI] [PubMed] [Google Scholar]
- 2.Bajaj M, Suraamornkul S, Romanelli A, Cline GW, Mandarino LJ, Shulman GI, DeFronzo RA. Effect of a sustained reduction in plasma free fatty acid concentration on intramuscular long-chain fatty acyl-CoAs and insulin action in type 2 diabetic patients. Diabetes 54: 3148–3153, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, Cone RD, Bloom SR. Gut hormone PYY(3–36) physiologically inhibits food intake. Nature 418: 650–654, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Burcelin R, Dolci W, Thorens B. Glucose sensing by the hepatoportal sensor is GLUT2-dependent: in vivo analysis in GLUT2-null mice. Diabetes 49: 1643–1648, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Cherbut C, Bruley des Varannes S, Schnee M, Rival M, Galmiche JP, Delort-Laval J. Involvement of small intestinal motility in blood glucose response to dietary fibre in man. Br J Nutr 71: 675–685, 1994. [DOI] [PubMed] [Google Scholar]
- 6.Collier G, Greenberg G, Wolever T, Jenkins D. The acute effect of fat on insulin secretion. J Clin Endocrinol Metab 66: 323–326, 1988. [DOI] [PubMed] [Google Scholar]
- 7.Collier G, McLean A, O'Dea K. Effect of co-ingestion of fat on the metabolic responses to slowly and rapidly absorbed carbohydrates. Diabetologia 26: 50–54, 1984. [DOI] [PubMed] [Google Scholar]
- 8.Collier G, O'Dea K. The effect of coingestion of fat on the glucose, insulin, and gastric inhibitory polypeptide responses to carbohydrate and protein. Am J Clin Nutr 37: 941–944, 1983. [DOI] [PubMed] [Google Scholar]
- 9.Collier G, Wolever T, Jenkins D. Concurrent ingestion of fat and reduction in starch content impairs carbohydrate tolerance to subsequent meals. Am J Clin Nutr 45: 963–969, 1987. [DOI] [PubMed] [Google Scholar]
- 10.Crowe TC, Seligman SA, Copeland L. Inhibition of enzymic digestion of amylose by free fatty acids in vitro contributes to resistant starch formation. J Nutr 130: 2006–2008, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham KM, Read NW. The effect of incorporating fat into different components of a meal on gastric emptying and postprandial blood glucose and insulin responses. Br J Nutr 61: 285–290, 1989. [DOI] [PubMed] [Google Scholar]
- 12.Druce M, Bloom SR. The regulation of appetite. Arch Dis Child 91: 183–187, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edwards CA, Johnson IT, Read NW. Do viscous polysaccharides slow absorption by inhibiting diffusion or convection? Eur J Clin Nutr 42: 307–312, 1988. [PubMed] [Google Scholar]
- 14.Elliott RM, Morgan LM, Tredger JA, Deacon S, Wright J, Marks V. Glucagon-like peptide-1 (7–36)amide and glucose-dependent insulinotropic polypeptide secretion in response to nutrient ingestion in man: acute post-prandial and 24-h secretion patterns. J Endocrinol 138: 159–166, 1993. [DOI] [PubMed] [Google Scholar]
- 15.Evans K, Kuusela PJ, Cruz ML, Wilhelmova I, Fielding BA, Frayn KN. Rapid chylomicron appearance following sequential meals: effects of second meal composition. Br J Nutr 79: 425–429, 1998. [DOI] [PubMed] [Google Scholar]
- 16.Fielding B, Callow J, Owen R, Samra J, Matthews D, Frayn K. Postprandial lipemia: the origin of an early peak studied by specific dietary fatty acid intake during sequential meals. Am J Clin Nutr 63: 36–41, 1996. [DOI] [PubMed] [Google Scholar]
- 17.Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord 24: 38–48, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Gannon MC, Nuttall FQ, Westphal SA, Seaquist ER. The effect of fat and carbohydrate on plasma glucose, insulin, C-peptide, and triglycerides in normal male subjects. J Am Coll Nutr 12: 36–41, 1993. [DOI] [PubMed] [Google Scholar]
- 19.Hanefeld M, Fischer S, Julius U, Schulze J, Schwanebeck U, Schmechel H, Ziegelasch HJ, Lindner J. Risk factors for myocardial infarction and death in newly detected NIDDM: the Diabetes Intervention Study, 11-year follow-up. Diabetologia 39: 1577–1583, 1996. [DOI] [PubMed] [Google Scholar]
- 20.Heart E, Corkey RF, Wikstrom JD, Shirihai OS, Corkey BE. Glucose-dependent increase in mitochondrial membrane potential, but not cytoplasmic calcium, correlates with insulin secretion in single islet cells. Am J Physiol Endocrinol Metab 290: E143–E148, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Herrmann C, Goke R, Richter G, Fehmann HC, Arnold R, Goke B. Glucagon-like peptide-1 and glucose-dependent insulin-releasing polypeptide plasma levels in response to nutrients. Digestion 56: 117–126, 1995. [DOI] [PubMed] [Google Scholar]
- 22.Horowitz M, Edelbroek MA, Wishart JM, Straathof JW. Relationship between oral glucose tolerance and gastric emptying in normal healthy subjects. Diabetologia 36: 857–862, 1993. [DOI] [PubMed] [Google Scholar]
- 23.Jonkers FC, Henquin JC. Measurements of cytoplasmic Ca2+ in islet cell clusters show that glucose rapidly recruits β-cells and gradually increases the individual cell response. Diabetes 50: 540–550, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Le Roux CW, Batterham RL, Aylwin SJB, Patterson M, Borg CM, Wynne KJ, Kent A, Vincent RP, Gardiner J, Ghatei MA, Bloom SR. Attenuated peptide YY release in obese subjects is associated with reduced satiety. Endocrinology 147: 3–8, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Mathers JC, Daly ME. Food polysaccharides, glucose, absorption and insulin sensitivity. In: Advanced Dietary Fibre Technology, edited by McCleary B and Prosky L. Oxford, UK: Blackwell Science, 2001, p. 186–197.
- 26.Meeroff JC, Go VL, Phillips SF. Control of gastric emptying by osmolality of duodenal contents in man. Gastroenterology 68: 1144–1151, 1975. [PubMed] [Google Scholar]
- 27.Naslund E, King N, Mansten S, Adner N, Holst JJ, Gutniak M, Hellstrom PM. Prandial subcutaneous injections of glucagon-like peptide-1 cause weight loss in obese human subjects. Br J Nutr 91: 439–446, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Nauck MA, Baller B, Meier JJ. Gastric inhibitory polypeptide and glucagon-like peptide-1 in the pathogenesis of type 2 diabetes. Diabetes 53: S190–S196, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Normand S, Khalfallah Y, Louche-Pelissier C, Pachiaudi C, Antoine J, S, Desage M, Riou JP, Laville M. Influence of dietary fat on postprandial glucose metabolism (exogenous and endogenous) using intrinsically 13C-enriched durum wheat. Br J Nutr 86: 3–11, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Owen B, Wolever TM. Effect of fat on glycaemic responses in normal subjects: a dose-response study. Nutr Res 23: 1341–1347, 2003. [Google Scholar]
- 31.Patsch JR, Miesenbock G, Hopferwieser T, Muhlberger V, Knapp E, Dunn JK, Gotto AMJ, Patsch W. Relation of triglyceride metabolism and coronary artery disease. Studies in the postprandial state. Arterioscler Thromb Vasc Biol 12: 1336–1345, 1992. [DOI] [PubMed] [Google Scholar]
- 32.Pedersen-Bjergaard U, Host U, Kelbaek H, Schifter S, Rehfeld JF, Faber J, Christensen NJ. Influence of meal composition on postprandial peripheral plasma concentrations of vasoactive peptides in man. Scand J Clin Lab Invest 56: 497–503, 1996. [DOI] [PubMed] [Google Scholar]
- 33.Ranganath LR, Beety JM, Morgan LM, Wright JW, Howland R, Marks V. Attenuated GLP-1 secretion in obesity: cause or consequence? Gut 38: 916–919, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robertson MD, Livesey G, Mathers JC. Quantitative kinetics of glucose appearance and disposal following a 13C-labelled starch-rich meal: comparison of male and female subjects. Br J Nutr 87: 569–577, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Roden M, Price TB, Perseghin G, Petersen KF, Rothman DL, Cline GW, Shulman GI. Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest 97: 2859–2865, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schirra J, Katschinski M, Weidmann C, Schafer T, Wank U, Arnold R, Goke B. Gastric emptying and release of incretin hormones after glucose ingestion in humans. J Clin Invest 97: 92–103, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharrett AR, Chambless LE, Heiss G, Paton CC, Patsch W. Association of postprandial triglyceride and retinyl palmitate responses with asymptomatic carotid artery atherosclerosis in middle-aged men and women: The Atherosclerosis Risk in Communities (ARIC) study. Arterioscler Thromb Vasc Biol 15: 2122–2129, 1995. [DOI] [PubMed] [Google Scholar]
- 38.Stubbs RJ, Hughes DA, Johnstone AM, Horgan GW, King N, Blundell JE. A decrease in physical activity affects appetite, energy, and nutrient balance in lean men feeding ad libitum. Am J Clin Nutr 79: 62–69, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Tserng KY, Kalhan SC. Estimation of glucose carbon recycling and glucose turnover with [U-13C] glucose. Am J Physiol Endocrinol Metab 245: E476–E482, 1983. [DOI] [PubMed] [Google Scholar]
- 40.Van Amelsvoort JMM, Van Stratum P, Kraal JH, Lussenburg RN, Houtsmuller UMT. Effects of varying the carbohydrate: fat ratio in a hot lunch on postprandial variables in male volunteers. Br J Nutr 61: 267–283, 1989. [DOI] [PubMed] [Google Scholar]
- 41.Weir JB New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 109: 1–9, 1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zangeneh F, Basu R, Shah P, Arora P, Camilleri M, Rizza RA. Enteral infusion of glucose at rates approximating EGP enhances glucose disposal but does not cause hypoglycemia. Am J Physiol Endocrinol Metab 285: E280–E286, 2003. [DOI] [PubMed] [Google Scholar]