Abstract
Although large quantities of glutamate are found in the carotid body, to date this excitatory neurotransmitter has not been assigned a role in chemoreception. To examine the possibility that glutamate and its N-methyl-d-aspartate (NMDA) receptors play a role in acclimatization after exposure to cyclic intermittent hypoxia (CIH), we exposed male Sprague-Dawley rats to cyclic hypoxia or to room air sham (Sham) for 8 h/day for 3 wk. Using RT-PCR, Western blot analysis, and immunohistochemistry, we found that ionotropic NMDA receptors, including NMDAR1, NMDAR2A, NMDAR2A/2B, are strongly expressed in the carotid body and colocalize with tyrosine hydroxylase in glomus cells. CIH exposure enhanced the expression of NMDAR1 and NMDAR2A/2B but did not substantially change the level of NMDAR2A. We assessed in vivo carotid sinus nerve activity (CSNA) at baseline, in response to acute hypoxia, in response to infused NMDA, and in response to infused endothelin-1 (ET-1) with and without MK-801, an NMDA receptor blocker. Infusion of NMDA augmented CSNA in CIH rats (124.61 ± 2.64% of baseline) but not in sham-exposed rats. Administration of MK-801 did not alter baseline activity or response to acute hypoxia, in either CIH or sham animals but did reduce the effect of ET-1 infusion on CSNA (CSNA after ET-1 = 160.96 ± 8.05% of baseline; ET-1 after MK-801 = 118.56 ± 9.12%). We conclude that 3-wk CIH exposure increases expression of NMDA functional receptors in rats, suggesting glutamate and its receptors may play a role in hypoxic acclimatization to CIH.
Keywords: glomus cell, glutamate, acclimatization
the carotid bodies are the primary sensory organs for detecting the changes in arterial blood Po2 that result from exposure to hypoxia. Acute decreases in arterial oxygen levels increase afferent traffic through the carotid sinus nerve (CSN) to the central nervous system and result in increases in ventilatory output. Chronic changes in arterial oxygen levels result in further increases in afferent traffic, beyond the levels expected from the absolute level of arterial Po2. This form of plasticity, commonly termed hypoxic acclimatization (1, 3, 27, 28), has recently been recognized to occur either when the hypoxic exposure is continuous or when cyclic and intermittent (CIH) (5, 11, 29).
Acclimatization refers to the increase in resting ventilation and gain of the ventilatory response to progressive hypoxia that follows exposure to hypoxia of hours or days. Characteristically, this enhanced activity and gain significantly outlast the duration of the hypoxic exposure (10). Originally, this gradual increase in chemosensitivity was attributed to acid-base changes occurring in the central nervous system (3, 10). Considerable evidence now suggests, however, that short-term acclimatization is mediated primarily through changes in the carotid chemoreceptor rather than through central mechanisms (2). Bisgard and colleagues (3, 4) performed an elegant series of investigations involving a model of separate perfusion of the carotid body and the remainder of the circulation in conscious goats. These authors showed that acclimatization occurs with carotid body hypoxia (remainder of the body normoxic) but not with systemic hypoxia when the carotid body is selectively perfused with normoxic blood (3, 4). Additional studies have demonstrated increased firing of the CSN in cats, both during normoxia and in response to a repeat hypoxic challenge, after a 14-day exposure to continuous hypoxia (39). The duration of exposure necessary to induce short-term acclimatization is species dependent. Species such as the goat display acclimatization after exposures as short as 6 h, whereas most investigators have suggested that humans require exposures measured in days to display the increase in chemoreflex response (27, 28). Recently, however, Howard and Robbins (16, 17) found that poikilocapnic hypoxia sustained for as few as 4 h in normal volunteers will lead to statistically significant increases in the ventilatory response to hypoxia. Interestingly, after acclimatization has occurred, removal of the hypoxic stimulus does not result in an immediate return of ventilation to baseline, a phenomenon termed deacclimatization.
There is now considerable evidence that the hypoxic exposure inducing acclimatization need not be continuous. For example, normal human volunteers exposed to hypoxia for 4 h daily for 3 wk in a hypobaric chamber depressurized to 15,000 feet demonstrated an increase in resting ventilation and hypoxic ventilatory response (5). The potential for cyclic exposures to hypoxia to induce acclimatization is less well investigated. Peng et al. (25) have, however, reported an increase in carotid sinus nerve activity (CSNA) during both normoxia and rechallenge to hypoxia in rats exposed to 14 days of cyclic hypoxia (20 s every 5 min, 8 h/day).
The molecular mechanisms by which exposure to hypoxia (continuous or intermittent) produces acclimatization remain obscure. A variety of neuromodulators have been explored as mediators of acclimatization. Recently, evidence has accumulated to support a role for carotid body endothelin-1 (ET-1) as a mediator of acclimatization after both CIH (30, 31) and continuous (6, 7) hypoxia, although other neuromodulators such as ANG II also have support (15, 20). The pathways through which endothelin and angiotensin induce acclimatization are yet to be defined, however.
In the central nervous system, both ET-1 and ANG II (8, 9, 19, 34) have been shown to exert some of their actions through the release of glutamate which, in turn, acts at N-methyl-d-aspartate (NMDA) receptors. Large amounts of glutamate were identified in the carotid body over a decade ago, but glutamate was not believed to play a functional role in hypoxic sensing and was concluded to be primarily a metabolic substrate in the peripheral chemoreceptor (37). We hypothesized, however, that glutamate might be important in the plasticity of the carotid body induced by long-term exposure to CIH acting through NMDA receptors. Our results support a possible functional role for glutamate and NMDA receptors in the carotid body in mediating or modulating acclimatization after exposure to CIH.
METHODS
Ethical Approval
All surgical procedures and experimental protocols were approved by the Institutional Animal Care and Use Committee of Beth Israel Deaconess Medical Center and Harvard Medical School.
Animals
All experiments were performed on male Sprague-Dawley rats (Charles River, Boston, MA) weighing 240–250 g at entry into the protocol. Rats were housed in normal rat cages with a 12:12-h light-dark cycle and were given food and water ad libitum.
Cyclic Hypoxic Exposure
During hypoxic exposure, animals were placed daily in commercial hypoxic chambers (A84XOV, Biospherix, Redfield, NY) that were flushed with 100% nitrogen to inspired O2 fraction (FiO2) nadir of 8.2–10% for 1 min. The FiO2 gradually returned to 21% over the remainder of each cycle. The exposure cycle was repeated every 4 min for 8 h/day, 7 days/wk for 3 wk during the animal's sleeping hours. Sham control animals underwent identical handling and exposure, but chambers were flushed with room air rather than N2. After the exposure cycle was completed, animals were randomly assigned to either physiological investigation or molecular studies.
Arterial blood gases were obtained in a separate cohort of conscious, unrestrained rats exposed to either CIH or sham conditions for 3 wk to assess for evidence of acclimatization (Table 1). Arterial Pco2 was significantly less in CIH-exposed than in sham-exposed animals, consistent with acclimatization.
Table 1.
Arterial blood gas values in unrestrained conscious rats after 3-wk exposure to either room air sham or CIH
| Sham | C | P Level | |
|---|---|---|---|
| pH (n = 6) | 7.46±0.02 | 7.47±0.03 | NS |
| PaCO2, Torr (n = 6) | 40.80±0.73 | 37.92±1.58 | 0.002 |
| HCO3−, mmol/l (n = 6) | 28.37±1.43 | 27.03±2.23 | NS |
| PaO2, Torr (n = 3) | 89.17±2.81 | 92.83±1.98 | NS |
Values are means ± SE. CIH, cyclic intermittent hypoxia; NS, not significant; PaCO2 and PaO2 arterial Pco2 and Po2, respectively.
Molecular Studies
RNA extraction and semi-quantitative RT-PCR.
After intraperitoneal anesthesia with 7% chloral hydrate (4 ml/kg body wt), the carotid bifurcations were removed and placed in ice-cold PBS. The carotid bodies were dissected from the bifurcation and soaked in RNA later buffer (Qiagen, Valencia, CA) at −80°C until extracted. Total RNA was extracted from 10 carotid bodies pooled either from five CIH or five sham-exposed rats using Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. RT was achieved with the use of 40 ng of RNA, 1 μl of Sensiscript reverse transcriptase (Qiagen), 10 U/μl RNase inhibitor (NEB, Ipswich, MA), and 10 μM oligo(dT)20 primers (Invitrogen) in 20 μl of the total volume at 37°C for 1 h. Semi-quantitive PCR was performed in a reaction volume of 20 μl with 2 μl of RT-generated cDNA and 2.5 U of HotStarTaq DNA polymerase (Qiagen). The PCR program included an initial activation of 15 min at 95°C to activate HotStarTaq DNA polymerase, followed by cycles consisting of successive incubation at 94°C for 1 min, proper annealing temperature for 50 s according to different primers annealing temperature, and 72°C for 50 s. The last cycle was followed by an extension step at 72°C for 50 s. Amplification products were analyzed by electrophoresis on 1.2% (wt/vol) agarose gels, stained with ethidium bromide, and visualized under ultraviolet light. Primers used for RT-PCR analysis with their respective annealing temperature and the numbers of cycles chosen for the PCR reactions are shown in Table 2. To confirm the integrity of RNA and equal loading of samples and to gain estimated mRNA levels of NMDA receptor subunits, we also performed RT-PCR of the β-actin gene.
Table 2.
Primer and sequence for NMDA receptors and PSD-95
| Gene Product | GenBank Accession No. | Primer Sequence (5′-3′) | PCR Cycle No. | Annealing Temperature, °C |
|---|---|---|---|---|
| NMDAR1 | NM_017010 | F: 269-AGCACCATGCACCTGCTGACA-289 | 33 | 56 |
| R: 511-GAGGTCCTCACACACTGACAGG-490 | ||||
| NMDAR2A | M91561 | F: 3707-ACTCCACACTGCCCATGAAC-3726 | 34 | 53 |
| R: 4231-AGAGTTTGCTTGAGGGGACA-4212 | ||||
| NMDAR2B | NM_012573 | F: 3574-ACATGAAGTATCCAGTATGG-3593 | 33 | 56 |
| R: 4098-GTTCTGGTTGTAGCTGACAG-4079 | ||||
| NMDAR2C | U08259 | F: 1703-CGATGGCGTCTGGAATGG-1722 | 40 | 52 |
| R: 2316-CTGGCAAGAAAGATGACCGC-2297 | ||||
| NMDAR2D | NM_022797 | F: 2281-CGATGGCGTCTGGAATGG-2298 | 40 | 53 |
| R: 2745-CTGGCAAGAAAGATGACCGC-2726 | ||||
| PSD-95 | M96853 | F: 211-TTGCAGGTGAATGGAACAGAG-231 | 36 | 52 |
| R: 825-AGCATAGCTGTCACTCAGGTA-805 |
NMDA, N-methyl-d-aspartate; F, forward primer; R, reverse primer.
Immunohistochemistry and hematoxylin and eosin staining.
Either CIH-exposed or sham-exposed animals were anesthetized (7% chloral hydrate, 4 ml/kg ip) and killed by transcardial perfusion with saline (pH 7.4) to rinse out the blood, followed by a fixative solution (10% neural buffered formalin) (Fisher Scientific). Carotid bifurcations were removed, cleaned of surrounding connective tissue, immersed in the same fixative solution for 2 h, and stored in 20% sucrose-PBS at 4°C for cryoprotection. Before cryostat sectioning, the carotid body was embedded in 10% gelatin, and the gelatin-embedded block was fixed for 2 h to render the gelatin water insoluble. Cryostat sections at 10 μm thickness were cut with a Leica CM1800 cryostat (Leica Microsystems) and mounted onto Superfrost Plus glass slides (Fisher Scientific). Sections were first immersed to 3% H2O2 to block endogenous peroxidase activity. After sections were blocked with 5% goat serum, they were incubated overnight with antiserum to each of the following proteins (Chemicon International): rabbit anti-NR1 (1:800) and rabbit anti-NMDAR2A/2B (1:500). After sections were washed three times in PBS containing 0.2% Triton X-100, sections were incubated in rabbit anti-biotinylated secondary antibody (Vector Laboratories, Burlingame, CA) diluted 1:400 at room temperature for 3 h and then in avidin-biotin-peroxidase complex (Vector) for another 3 h. Finally, sections were washed, and the antibody-antigen complex was visualized by incubating the sections with 0.01% H2O2 and 0.05% diaminobenzidine (33) (Sigma, St. Louis, MO) to yield a reddish-brown crystalline product. The diaminobenzidine reaction was terminated by extensive rinsing with cold PBS. Finally, slides were cleared in xylenes and coverslipped with p-xylene-bis-pyridinium bromide (Sigma). Negative staining control was prepared by omitting the primary antibody. For hematoxylin and eosin staining, carotid body sections were washed three times with PBS and stained with hematoxylin and then eosin (Richard Aleen Scientific) for 1 min individually. Sections were rinsed three times with tap water and dehydrated with 70, 90, and 100% ethanol and coverslipped. The staining was examined with the Nikon Eclipse E800 microscope and photographed with the Nikon digital camera DXM 1200 (Nikon, Tokyo, Japan).
Double immunofluorescence.
Carotid bodies were removed as above and frozen. After three washes with PBS, cryosections of carotid body were permeabilized in PBS containing 0.2% Triton X-100 for 30 min and incubated with 4 drops of Image-iT FX signal enhancer (Invitrogen) at room temperature for 30 min. Slides were then exposed to a mixture of two primary antibodies obtained from different species described as follows: rabbit anti-NR1 antibody (1:200), rabbit anti-NMDAR2A/2B antibody (1:50; Chemicon International) with mouse anti-tyrosine hydroxylase antibody (1: 500; Sigma), and rabbit anti-NR1 antibody (1:200) with mouse anti-PSD-95 antibody (1:400; Chemicon International). The incubation was performed overnight at room temperature. Slides were washed and then exposed for 3 h at room temperature to a mixture of Alexa Fluor 488 goat anti-mouse IgG (1:400) and Alexa Fluor 594 goat anti-rabbit IgG (1:400; Invitrogen). After they were washed, slides were mounted with Vectashield mounting medium (Vector). Negative staining control was prepared by omitting the primary antibody. The fluorescent sections were observed under a Nikon ECLIPSE E800 microscope and photographed with the Nikon digital camera DXM 1200.
Western blotting.
Carotid bodies from eight sham and eight CIH rats were harvested and homogenized in RIPA buffer. Thirty micrograms of the homogenates were separated by SDS-PAGE and transferred to Immobilon transfer membranes (Millipore). The membrane was blocked with 5% (wt/vol) nonfat milk at room temperature for 2 h and incubated with rabbit anti-NR1 antibody (1:500), rabbit anti-NMDAR2A antibody (1:800), and NMDRA2A/2B (1:500; Chemicon International), as well as mouse monoclonal antibody against β-actin (1:5,000; Sigma) at room temperature for 1 h. After the membrane was washed with PBS containing 0.1% Tween 20, the membrane was incubated with horseradish peroxidase-conjugated anti-rabbit antibody (1: 10,000; Amersham Biosciences) and anti-mouse antibody (1:4,000; Jackson Laboratory) at 4°C overnight. After bands were washed in PBS containing 0.1% Tween 20, the bands were visualized with SuperSignal West Pico chemiluminescent substrate detection system (Pierce) in accordance with the manufacturer's instructions.
Physiological Studies
Physiological preparation.
Rats were anaesthetized initially with isoflurane in a closed chamber and then maintained in an anesthetized state via a nose cone (2.5–3.0% isoflurane, FiO2 = 0.5, balance N2). The trachea was cannulated, and the rats were ventilated mechanically (Harvard Apparatus, Holliston, MA) while maintaining the inspired isoflurane concentration. A right femoral venous catheter was inserted for administration of anesthesia and fluid. A catheter was also placed into the right femoral artery to allow blood pressure measurement and blood sample withdrawal for measurement of arterial blood gases and pH analysis. Blood pressure was recorded with a PowerLab data-acquisition system (AD Instruments, Colorado Springs, CO). Rats were slowly converted from isoflurane to urethane anesthesia (1.6 g/kg iv in distilled water, supplemented as needed), and the adequacy of anesthesia was assessed periodically by testing corneal reflexes and blood pressure responses to toe pinch. A slow infusion of sodium bicarbonate (5%) and Ringer solution with sodium lactate (50:50, ∼1.7 ml·kg−1·h−1) was initiated ∼1 h after induction of anesthesia to maintain fluid and acid-base balance. Rectal temperature was monitored and maintained near 37.5°C using a rectal thermometer and heating pad. End-tidal Pco2 (PetCO2) was monitored in the expired line of the ventilator circuit using a flow-through capnograph (Novametrix, Wallingford, CT) with sufficient response time (<75 ms) to measure PetCO2 in rats. PetCO2 values obtained with this method approximate the Pco2 in arterial blood in most rats (usually within 1–2 Torr). Animals inspired 21% O2 (balance N2) during baseline anesthetic conditions. At the end of the experiments, rats were euthanized by sedative overdose.
In vivo carotid body sensory activity.
Sensory activity of the carotid body in anesthetized rats was recorded with the following technique. First, the nerve to the baroreceptor was cut. The left CSN was dissected where it joins the glossopharyngeal nerve and was desheathed and prepared for recording by placing it on a bipolar platinum electrode connected to a bioelectrical amplifier (CP 511, Grass Astro). The isolated sinus nerve and surrounding structures were immersed in mineral oil mixed with Vaseline. The bioelectrical signal was fed to a polygraph (AD Instruments PowerLab 8SP) and integrated using a low-pass filter with a time constant of 5 s. The integral of sinus nerve activity was obtained and measured. Electrical activity was amplified by an AC amplifier (P511, Grass Instruments), with a bandwidth of 30–1,000 Hz. Carotid body afferent activity was identified by prompt augmentation of sensory discharge in response to 10% O2 and prompt decrease in response to 100% O2. Reducing the pressure in the carotid sinus by occluding the common carotid artery for 10 s caused no change or an increase in sinus nerve activity but never a decrease, indicating that the sensory activity is of carotid body rather than baroreceptor origin.
Experimental protocol.
After completion of the surgical procedures, the preparation was allowed to stabilize for at least 1 h while the inspired gas was maintained at 21% O2-balance N2 before formal data collection. Baseline room air CSN activity was then recorded for ∼30 min, after which the response to hypoxia (10% O2) was determined.
The effect of NMDA receptor stimulation on CSN activity was determined in CIH-exposed (n = 6) and sham-exposed (n = 6) animals. NMDA (10 mM/kg in 0.5 ml of 0.9% saline) was infused into the left common carotid artery. After infusion, CSN activity was allowed to return to baseline, after which MK-801 (6 mg/kg dissolved with DMSO and then in 0.5 ml of 0.9% saline) was infused through the left common carotid artery. Ten minutes after MK-801 infusion, NMDA (10 mM/kg in 0.5 ml) was again infused into the left common carotid artery.
In a second group of rats (CIH = 5, Sham = 5), once baseline data were collected as above, the rats inhaled 10% O2 for 1 min through the ventilator. Once the response to hypoxia was recorded and variables had returned to baseline resting levels, NMDA (10 mM/kg in 0.5 ml) was infused into carotid body through the left common carotid artery. Ten minutes after completion of the infusion, the rats again inhaled 10% O2 for 1 min through the ventilator. After physiological parameters again returned to baseline, MK-801 was infused (6 mg/kg dissolved with DMSO and then in 0.5 ml of 0.9% saline) after which the animals were again exposed to acute hypoxia.
In an additional group of CIH-exposed rats (n = 6), the CSN response to ET-1 (1.0 nmol/kg in 0.5 ml 0.9% saline), which was infused through the left common carotid artery over 10 min, was measured. After CSNA returned to baseline, MK-801 (6 mg/kg dissolved with DMSO and then in 0.5 ml of 0.9% saline) was administered, after which the ET-1 infusion was repeated.
Doses of all agents were determined after preliminary studies in which dose-response testing was performed to determine doses with maximum response with minimum systemic effect.
Statistical Analysis
All CSNA values were normalized as percentage of the baseline nerve activity values. The data were statistically analyzed by two-way ANOVA between sham and CIH groups followed by comparison for individual differences using the Student-Newman-Keuls test. The results in the same group of rats before and after administration of MK-801 were subjected to paired t-test (see Fig. 6). P < 0.05 was considered to indicate statistical significance. Values are means ± SE.
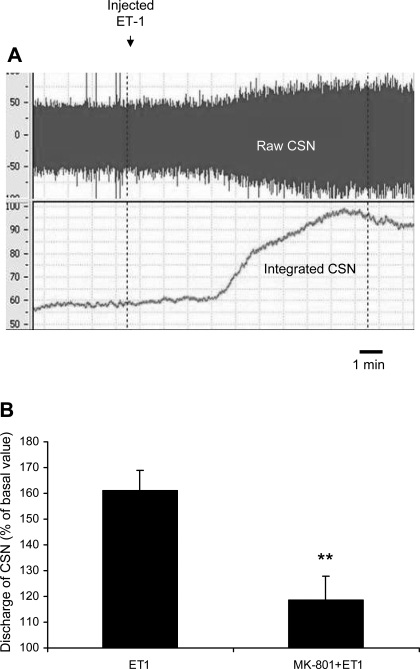
Fig. 6.
A: representative raw tracing of raw and integrated CSN recordings before and after administration of endothelin-1 (ET-1). ET-1 infusion begins at first vertical dashed line and ends 10 min later at second dashed line. B: administration of the NMDA receptor blocker MK-801 substantially reduces the CSN response to ET-1 administration in CIH-exposed rats. ET-1 represents peak response to endothelin administration without MK-801, and MK-801 + ET-1 represents peak response to ET-1 after prior administration of the NMDA receptor blocker. **P < 0.01 vs. ET-1.
Values of densitometric analysis are means ± SD. Statistical analysis of the data was performed with Student's paired t-test. P < 0.05 was considered to indicate statistical significance.
RESULTS
Effect of CIH Exposure on NMDA Receptor Expression
Selective expression of NMDA receptor subunits by RT-PCR in the carotid body.
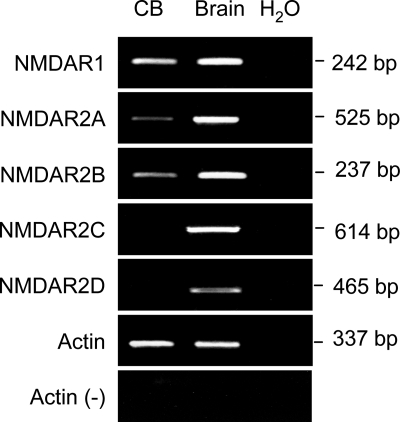
To first determine whether glutamate NMDA receptor subunits are expressed in carotid body, we examined mRNA expression of different NMDA receptor subunits except NMDAR3 by RT-PCR using specific primers that recognize unique sequences of each NMDA receptor subtype. Figure 1 shows ethidium bromide-stained agarose gels of representative experiments. PCR products corresponding to the predicted sizes of NMDA receptor subunits were detected with all primers in rat brain sample taken from rat cerebral cortex, as a positive control. NMDAR1, as well as NMDAR2A and NMDAR2B, was detected in rat carotid body. No applications were obtained in carotid body with NMDAR2C or NMDAR2D even after running 40 cycles of amplification. mRNA for NMDAR1 was generally more abundant than NMDAR2A and NMDAR2B under 33 cycles of amplification.
Fig. 1.
RT-PCR results from representative experiments showing expression of mRNA for N-methyl-d-aspartate (NMDA) receptors NMDAR1, NMDAR2A, and NMDAR2B in carotid body (CB, left lane). RNA isolated from rat brain cerebral cortex was used as positive control (Brain, middle lane). Sterile water instead of cDNA (H2O, right lane) was used as PCR negative control. PCR product was not detected if reverse transcriptase was omitted from the reaction in tubes [Actin (−)].
Protein expression and localization of glutamate NMDA receptors in the carotid body.
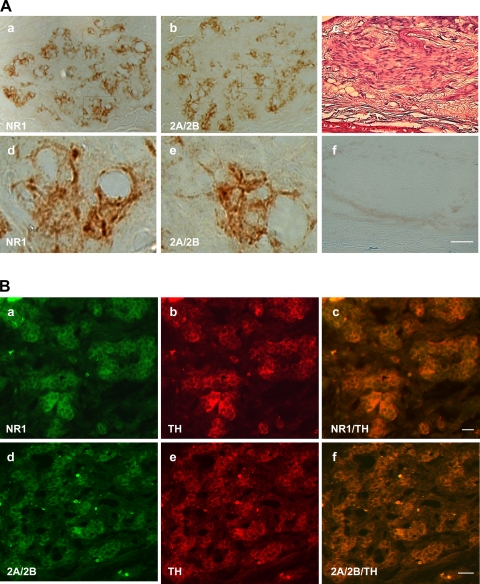
Sucher et al. (35) reported that NMDAR1 mRNA but not NMDAR1 protein is expressed in a rat pheochromocytoma cell line, PC-12, indicating that NMDAR1 mRNA is not always transcribed into NMDAR1 protein. The expression of NMDAR1 protein may be controlled by posttranscriptional mechanisms in some tissues and cells. To examine the protein presence and localization of NMDA receptor subunits in the carotid body, immunohistochemistry was performed on sections of the rat carotid body. Immunohistochemical analysis was focused on the subunits of NMDAR1 and NMDAR2A/2B, which mRNAs were markedly detected by RT-PCR. As shown in Fig. 2A, top, and in Fig. 2Ba and 2Bd, left lane, intense immunoreactive NMDAR1 and NMDAR2A/2B were strongly detected and ubiquitously found clustering in glomeruli in the carotid body sections. Negative control with a primary antibody omitted did not yield specific staining in a consecutive section of the carotid body (Fig. 2Af), which was stained by hematoxylin and eosin to show carotid body (Fig. 2Ac) Tyrosine hydroxylase was localized mainly on the cell clusters of glomus cells (Fig. 2B, a and d). The immunoreactive signals of NMDAR1 and NMDAR2A/2B were colocalized with tyrosine hydroxylase staining in glomus cells (Fig. 2B, c and f).
Fig. 2.
A: immunohistochemical reactive staining for NMDAR1 (a) and NMDAR2A/2B (b) revealed products of these receptors in carotid body; d and e are higher magnifications of framed areas in a and b, respectively. c: Hematoxylin and eosin staining to show carotid body. f: Negative staining control obtained by omitting the primary antibody in a consecutive section adjoining c. Scale bar (applies to all images) = 20 μm. B: additional immunofluorescent staining further revealed NMDAR1 (a–c) and NMDAR2A/2B (d–f) in clusters of glomus cells in rat carotid body. Stain for tyrosine hydroxylase (b and e) confirmed the identification of glomus cells. Double staining for tyrosine hydroxylase and NMDAR1 (c) or NMDAR2A/2B (f) confirmed colocalization of the NMDA receptors and tyrosine hydroxylase in glomus cells. Scale bars = 20 μm.
Expression of PSD-95 in carotid body.
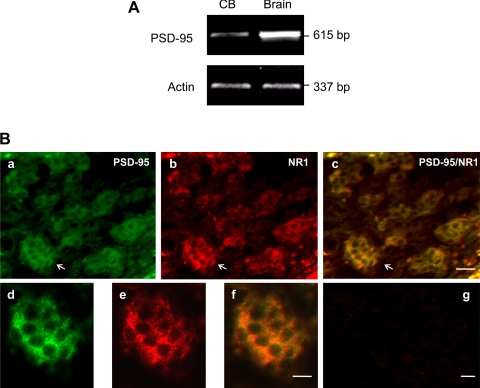
In the central nervous system, glutamate receptors with other structures form a postsynaptic microdomain that is essential for their functions. A key protein involved in this clustering process is PSD-95, a PDZ domain-containing protein that has been shown to bind the COOH-terminal tails of both the NMDAR1 and NMDAR2 subunits. To test the possibility that rat carotid body expresses PSD-95, we first examined mRNA expression of PSD-95 by RT-PCR. Figure 3A shows that carotid body expressed PSD-95 and, to a lesser extent, that this protein is expressed in brain. In addition, we confirmed, by double immunofluorescence staining in Fig. 3B, that PSD-95 protein exists in rat carotid body and colocalizes with NMDAR1 in glomus cells. The presence of PSD-95 in carotid body suggests that the NMDA receptors existing in glomus cells are functional receptors.
Fig. 3.
A: RT-PCR demonstrating message for PSD-95 in carotid body (CB, left lane) as well as in brain cortex (Brain, right lane). B: immunofluorescent staining showing colocalization of PSD-95 and NMDAR1 in glomus cells of the carotid body. a: Intense immunoreactive products for PSD-95 (green). b: Immunoreactive products for NMDAR1 (NR1, red). c: Merged images of a and b. d, e, and f: Higher magnification of the framed areas in a, b, and c, respectively. g: Negative control obtained by omitting the primary antibody in a consecutive section of c in Fig. 1B. The magnifications of a–c and d–f are same. Scale bars = 20 μm.
Effect of CIH on the protein expression of NMDA receptors.
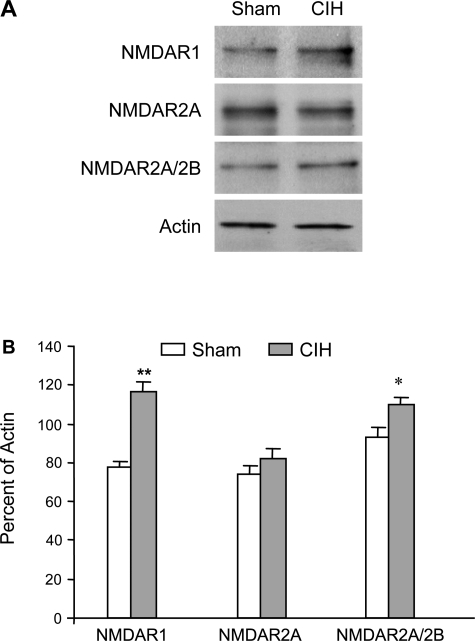
To further assess whether hypoxic exposure regulates NMDA receptor subunits existing in the carotid body, we assessed NMDA receptor protein levels by Western blot on carotid bodies from Sham and CIH-exposed rats. As shown in Fig. 4, NMDAR1, a major NMDA receptor subunit expressed in the rat carotid body, was augmented 1.47-fold (P < 0.001, n = 3) after CIH exposure compared with that shown in the sham-exposed control rats. However, CIH exposure did not substantially change the level of NMDAR2A. With the use of the anti-NMDAR2A/2B antibody that recognizes both NMDAR2A and NMDAR2B in immunoblotting, the level of NMDAR2A/2B was increased 1.12 times (P < 0.05, n = 3) in CIH vs. that shown in sham rats. This indicates that CIH also increases the level of NMDAR2B, although to a lesser extent than it increases the level of NMDAR1.
Fig. 4.
Western blot showing enhanced expression of protein products of NMDAR1 in carotid bodies of cyclic intermittent hypoxia (CIH)-exposed rats relative to sham-exposed animals. Homogenized tissues of carotid bodies pooled from 8 rats in each group were used. A: representative Western blots. B: percentages of NMDAR1, NMDAR2A, and NMDAR2A/2B to actin. Data (means ± SD of densitometric analysis) are from 3 individual Western blots run in triplicate. *P < 0.005, **P < 0.01 vs. sham rats.
Responses to Stimulating NMDA Receptors in the Carotid Body
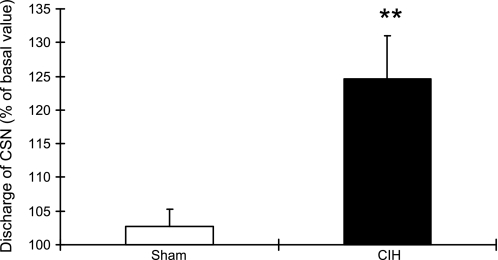
Infusion of NMDA had no effect on basal CSNA in sham animals but produced a significant increase in afferent activity in CIH rats (Fig. 5). The CSNA in CIH rats reached 124.61 ± 2.64% of the control value 10 min after the infusion ended (P < 0.01). This increase was blocked by pretreatment with the NMDA receptor blocker MK-801. Again, MK-801 had no effect in Sham animals. Interestingly, NMDA infusion did not alter the CSN response to acute hypoxia (FiO2 of 0.1) in either sham or CIH rats.
Fig. 5.
Carotid sinus nerve (CSN) activity as a percent of baseline is increased after NMDA administration in CIH-exposed (solid bar) but not in sham-exposed (open bar) rats. **P < 0.01 vs. sham group.
Responses to Blockade of NMDA Receptors in the Carotid Body
Infusion of MK-801 had no effect on basal CSNA in sham and CIH animals. Similarly, there was no difference in the acute response to hypoxia after infusion of MK-801 in either sham or CIH rats (data not shown).
Effect of NMDA Receptor Blockade on the Carotid Body Response to ET-1
As previously demonstrated (31), infusion of ET-1 resulted in a substantial increase in CSNA in CIH animals (Fig. 6A), as demonstrated in Fig. 6B and to a lesser extent in sham animals (data not shown). We tested the response of CIH animals to ET-1 after prior administration of MK-801 and the ET-1 response was significantly reduced by prior administration of MK-801 (peak CSNA after ET-1 = 160.96 ± 8.05%, peak CSNA after ET-1 preceded by MK-801 = 118.56 ± 9.12%; P < 0.01).
Thus these findings show that NMDA receptor proteins are functional in the carotid bodies of CIH-exposed rats and that these receptors may, in part, mediate the actions of ET-1 on CSNA.
DISCUSSION
There are two major findings of this study. First, exposure of rats to CIH increases expression of both message and protein of specific glutamate NMDA receptors in the carotid body. Second, our data indicate that these are functional receptors, as infusion of NMDA increases carotid afferent activity in CIH-exposed but not in sham-exposed rats and MK-801 administration substantially reduced the CSN response to administration of ET-1. These findings suggest that glutamate is more than simply a metabolic substrate in the carotid body and, in fact, is involved in modulating chemoreceptor sensitivity (21).
The carotid body is the primary sensor of arterial Po2 in mammals. An acute decrease in arterial Po2 results in a rapid increase in afferent chemoreceptor activity, resulting in an increase in ventilatory output. The molecular oxygen sensor in the carotid body is still uncertain, however. Recently, attention has focused on reactive oxygen species generated by mitochondria (14), NAD(P)H oxidase (12), or AMP kinase(13) or on hypoxic modulation of ion channels in type 1 or glomus cell (12, 21), the cell believed to contain the oxygen-sensing machinery. With chronic hypoxia lasting hours to days, the output of the chemoreceptor increases. This form of plasticity is termed hypoxic acclimatization. Exposure to CIH appears to induce this form of plasticity as well as the phenomenon of “sensory long-term facilitation” recently described by Peng et al. (24). Sensory long-term facilitation appears to involve 5-hydroxy tryptamine and PKC (26), but the molecular basis for acclimatization remains uncertain.
As with acute hypoxic sensing, the molecular machinery of acclimatization is uncertain; however, several candidates have recently gained support. ET-1 is a 21-amino acid peptide that is found in endothelium and in type 1 cells (glomus cells) in the carotid bodies (22) and is one apparent modulator of chemoreflex activity. Endothelin acts at two receptors, the ETA receptor and the ETB receptor. Functional studies with ETA receptor antagonists suggest that ET-1 causes chemoexcitation at the ETA receptor (6, 41). Chen et al. (6) have presented evidence, based on RT-PCR, that continuous hypoxia (14 days of hypobaric hypoxia, FiO2 of ∼0.1) increases expression of the ETA receptor and of preproendothelin, the precursor of endothelin, in the carotid body. Furthermore, these investigators showed that analysis of chemoreceptor activity using CSN recordings and a selective ETA antagonist suggested that the increases in chemoreceptor activity paralleled the increases in ET-1 and ETA expression. These same investigators showed that the increase in ET-1 and ETA receptor that occurs with chronic hypoxia was associated with enhanced Ca2+ influx. Recent evidence also suggests a role for ET-1 in enhanced chemosensitivity after CIH. Rey et al. (31) examined ET-1 immunoreactivity in cats exposed to CIH for 8 h/day for 4 days. They observed an increase in isolated carotid body response to exogenous ET-1 after CIH and a reduction in activity after treatment with bosentan, a mixed ETA and ETB blocker.
In the central nervous system, a number of studies have suggested that endothelin, acting through specific receptors, influences neural activity in part by acting through release of glutamate. For example, Shihara et al. (34) found that endothelin application to slices of medulla augments the response of single neurons to glutamate, an effect mediated through ETA receptors. Rossi and Chen (32) found that the pressor effect of ET-1 administered into the subfornical organ was blocked by blockade of glutamate receptors, although this effect appeared to be mediated through aminoproprionic acid receptors rather than through NMDA receptors.
Glutamate release in the brain stem has long been recognized as essential in the ventilatory response to hypoxia. In 1993, Kubo et al. (18) reported that NMDA receptor stimulation mediated the pressor effect induced by carotid chemoreceptor stimulation, and Vardhan et al. (38) reported that blockade of NMDA and non-NMDA receptors in the commissural nucleus of the nucleus tractus solitarii abolished the responses to stimulation of the carotid bodies. Mizusawa et al. (23) then demonstrated using microdialysis that carotid body stimulation in consciously behaving rats produced glutamate release in the rostral ventral lateral medulla.
Despite the evidence of a role for glutamate in the brain stem response to peripheral chemoreceptor stimulation, minimal attention has been paid to the neurotransmitter role of glutamate within the carotid body itself. Torrealba (36) first demonstrated significant amounts of glutamate in the carotid bodies of cats using immunohistochemistry. This same investigator later dismissed a role for glutamate in the acute response of the peripheral chemoreceptor to hypoxia, however, when superfusion of the carotid body with a high concentration of potassium failed to elicit glutamate release (37). We are unaware of any subsequent studies investigating a potential role for glutamate in hypoxic acclimatization.
Our data indicate that CIH increases expression of functional glutamate receptors in the carotid body. Although we believe these data suggest a role for glutamate in hypoxic acclimatization after CIH exposure, several aspects of our findings should be noted. First, we performed in vivo recordings of CSNA. Consequently, we cannot exclude the possibility that NMDA administration had a nonspecific systemic effect, mediated through barorecptors that were not eliminated by our procedures or by some other reflex response. Arguing against this possibility, however, are the small dose of NMDA used in our protocol, the finding of specific NMDA-type receptors in the carotid body, and the increase in NMDAR1 and NMDAR2A/2B expression after exposure to CIH. Furthermore, NMDA infusion had no effect on CSNA in sham-exposed animals. A second point that should be made is that our data do not suggest a role for glutamate NMDA receptors in acute oxygen sensing and the precise physiological role of glutamate in the carotid body is yet to be defined. There was no effect of NMDA receptor blockade on the CSN response to acute hypoxia in either CIH- or sham-exposed animals. This finding suggests that glutamate and its receptors are not necessary for the translation of oxygen sensing into afferent nerve activity. It is important to note that we observed no effect of NMDA receptor blockade with MK-801 on baseline CSNA in either CIH-exposed or sham-exposed animals, although MK-801 substantially decreased the response of the chemoreceptor to ET-1. If glutamate and NMDA receptors are important in hypoxic acclimatization, one might expect blockade of the receptors to reduce baseline activity, particularly in CIH-exposed rats. One possible way to reconcile this finding with a role for glutamate and NMDA receptors in hypoxic acclimatization is to hypothesize that activation of these NMDA receptors produces further molecular changes in the carotid body leading to a persistent alteration in CSNA. Downstream structural changes in membrane ion channels, for example, might not reverse immediately with NMDA receptor blockade. Interestingly, our own studies indicate that acute blockade of carotid body endothelin receptors is also without effect on baseline CSNA in hypoxia-exposed animals.
In summary, we provide evidence that exposure of rats to CIH for 3 wk results in increased expression of glutamate NMDA receptor NMDAR1 and NMDAR2A/2B in the carotid body. Furthermore, these receptors are functional since stimulation with infused NMDA results in augmented CSNA in CIH-exposed but not in sham-exposed animals. These findings are consistent with a possible role for glutamate in hypoxic acclimatization to CIH and warrant further investigation.
GRANTS
These studies were performed with support from National Heart, Lung, and Blood Institute Grant HL-075184.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Aaron EA, Powell FL. Effect of chronic hypoxia on hypoxic ventilatory response in awake rats. J Appl Physiol 74: 1635–1640, 1993. [DOI] [PubMed] [Google Scholar]
- 2.Bisgard GE Carotid body mechanisms in acclimatization to hypoxia. Resp Physiol 121: 237–246, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Bisgard GE, Busch MA, Forster HV. Ventilatory acclimatization to hypoxia is not dependent on cerebral hypocapnic alkalosis. J Appl Physiol 60: 1011–1015, 1986. [DOI] [PubMed] [Google Scholar]
- 4.Busch MA, Bisgard GE, Forster HV. Ventilatory acclimatization to hypoxia is not dependent on arterial hypoxemia. J Appl Physiol 58: 1874–1880, 1985. [DOI] [PubMed] [Google Scholar]
- 5.Casas M, Casas H, Pages T, Rama R, Ricart A, Ventura JL, Ibanez J, Rodriguez FA, Viscor G. Intermittent hypobaric hypoxia induces altitude acclimation and improves the lactate threshold. Aviation Space Env Med 71: 125–130, 2000. [PubMed] [Google Scholar]
- 6.Chen J, He L, Dinger B, Stensaas LJ, Fidone S. Role of endothelin and endothelin A-type receptor in adaptation of the carotid body to chronic hypoxia. Am J Physiol Lung Cell Mol Physiol 282: L1314–L1323, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, Tipoe GL, Liong E, Leung PS, Lam SY, Iwase R, Tjong YW, Fung ML. Chronic hypoxia enhances endothelin-1-induced intracellular calcium elevation in rat carotid body chemoreceptors and up-regulates ETa receptor expression. Pflugers Arch 443: 565–573, 2002. [DOI] [PubMed] [Google Scholar]
- 8.D'Amico M, Berrino L, Maione S, Filippelli A, Pizzirusso A, Vitagliano S, Rossi F. Endothelin-1 in rat periaqueductal gray area induces hypertension via glutamatergic receptors. Hypertension 25: 507–510, 1995. [DOI] [PubMed] [Google Scholar]
- 9.Dampney RAL, Horiuchi J, Tagawa T, Fontes MAP, Potts PD, Polson JW. Medullary and supramedullary mechanisms regulating sympathetic vasomotor tone. Acta Physiol Scan 177: 209–218, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Dempsey JA, Forster HV, Bisgard GE, Chosy LW, Hanson PG, Kiorpes AL, Pellegrino DA. Role of cerebrospinal fluid [H+] in ventilatory deacclimatization from chronic hypoxia. J Clin Invest 64: 199–205, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Giulio C, Huang WX, Mokashi A, Roy A, Cacchio M, Macri MA, Lahiri S. Sustained hypoxia promotes hyperactive response of carotid body in the cat. Resp Physiol Neurobiol 134: 69–74, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Dinger B, He L, Chen J, Liu X, Gonzalez C, Obeso A, Sanders K, Hoidal J, Stensaas L, Fidone S. The role of NADPH oxidase in carotid body arterial chemoreceptors. Resp Physiol Neurobiol 157: 45–54, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans AM AMP-activated protein kinase underpins hypoxic pulmonary vasoconstriction and carotid body excitation in mammals. Exp Physiol 91: 821–827, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez C, Agapito MT, Rocher A, Gonzalez-Martin MC, Vega-Agapito V, Gomez-Nino A, Rigual R, Castaneda J, Obeso A. Chemoreception in the context of the general biology of ROS. Resp Physiol Neurobiol 157: 30–44, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Heitman SJ, Jennings DB. Angiotensin II modulates respiratory and acid-base responses to prolonged hypoxia in conscious dogs. Am J Physiol Regul Integr Comp Physiol 275: R390–R399, 1998. [DOI] [PubMed] [Google Scholar]
- 16.Howard LS, Robbins PA. Alterations in respiratory control during 8 h of isocapnic and poikilocapnic hypoxia in humans. J Appl Physiol 78: 1098–1107, 1995. [DOI] [PubMed] [Google Scholar]
- 17.Howard LS, Robbins PA. Ventilatory response to 8 h of isocapnic and poikilocapnic hypoxia in humans. J Appl Physiol 78: 1092–1097, 1995. [DOI] [PubMed] [Google Scholar]
- 18.Kubo T, Amano M, Asari T. N-methyl-d-aspartate receptors but not non-N-methyl-d-aspartate receptors mediate hypertension induced by carotid body chemoreceptor stimulation in the rostral ventrolateral medulla of the rat. Neurosci Lett 164: 113–116, 1993. [DOI] [PubMed] [Google Scholar]
- 19.Latchford KJ, Ferguson AV. ANG II-induced excitation of paraventricular nucleus magnocellular neurons: a role for glutamate interneurons. Am J Physiol Regul Integr Comp Physiol 286: R894–R902, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Leung PS Novel roles of a local angiotensin-generating system in the carotid body. J Physiol 575: 4, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li YL, Sun SY, Overholt JL, Prabhakar NR, Rozanski GJ, Zucker IH, Schultz HD. Attenuated outward potassium currents in carotid body glomus cells of heart failure rabbit: involvement of nitric oxide. J Physiol 555: 219–229, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McQueen DS, Dashwood MR, Cobb VJ, Bond SM, Marr CG, Spyer KM. Endothelins and the rat carotid body: autoradiographic and functional pharmacological studies. J Autonomic Nervous System 53: 115–125, 1995. [DOI] [PubMed] [Google Scholar]
- 23.Mizusawa A, Ogawa H, Kikuchi Y, Hida W, Kurosawa H, Okabe S, Takishima T, Shirato K. In vivo release of glutamate in nucleus tractus solitarii of the rat during hypoxia. J Physiol 478: 55–66, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peng YJ, Overholt JL, Kline D, Kumar GK, Prabhakar NR. Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: implications for recurrent apneas. Proc Natl Acad Sci USA 100: 10073–10078, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peng YJ, Rennison J, Prabhakar NR. Intermittent hypoxia augments carotid body and ventilatory response to hypoxia in neonatal rat pups. J Appl Physiol 97: 2020–2025, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Peng YJ, Yuan G, Jacono FJ, Kumar GK, Prabhakar NR. 5-HT evokes sensory long-term facilitation of rodent carotid body via activation of NADPH oxidase. J Physiol 576: 289–295, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Powell FL, Dwinell MR, Aaron EA. Measuring ventilatory acclimatization to hypoxia: comparative aspects. Resp Physiol 122: 271–284, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Powell FL, Milsom WK, Mitchell GS. Time domains of the hypoxic ventilatory response. Resp Physiol 112: 123–134, 1998. [DOI] [PubMed] [Google Scholar]
- 29.Reeves SR, Gozal E, Guo SZ, Sachleben LR Jr, Brittian KR, Lipton AJ, Gozal D. Effect of long-term intermittent and sustained hypoxia on hypoxic ventilatory and metabolic responses in the adult rat. J Appl Physiol 95: 1767–1774, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Rey S, Corthorn J, Chacon C, Iturriaga R. Expression and immunolocalization of endothelin peptides and its receptors, ETA AND ETB, in the carotid body exposed to chronic intermittent hypoxia. J Histochem Cytochem 55: 167–174, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Rey S, Del Rio R, Iturriaga R. Contribution of endothelin-1 to the enhanced carotid body chemosensory responses induced by chronic intermittent hypoxia. Brain Res 1086: 152–159, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Rossi NF, Chen H. Aminopropionic acid receptors in paraventricular nucleus mediate pressor and vasopressin responses to endothelin-1 in subfornical organ. Exp Biol Med 231: 1075–1080, 2006. [PubMed] [Google Scholar]
- 33.Seyedabadi M, Goodchild AK, Pilowsky PM. Differential role of kinases in brain stem of hypertensive and normotensive rats. Hypertension 38: 1087–1092, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Shihara M, Hirooka Y, Hori N, Matsuo I, Tagawa M, Suzuki S, Akaike N, Takeshita A. Endothelin-1 increases the neuronal activity and augments the responses to glutamate in the NTS. Am J Physiol Regul Integr Comp Physiol 275: R658–R665, 1998. [DOI] [PubMed] [Google Scholar]
- 35.Sucher NJ, Brose N, Deitcher DL, Awobuluyi M, Gasic GP, Bading H, Cepko CL, Greenberg ME, Jahn R, Heinemann SF. Expression of endogenous NMDAR1 transcripts without receptor protein suggests posttranscriptional control in PC12 cells. J Biol Chem 268: 22299–22304, 1993. [PubMed] [Google Scholar]
- 36.Torrealba F Immunocytochemistry of neuroactive substances in visceral receptors: glutamate and CGRP. Arch Biol Med Exp (Santiago) 23: R222, 1990. [Google Scholar]
- 37.Torrealba F, Bustos G, Montero VM. Glutamate in the glomus cells of the cat carotid body: immunocytochemistry and in vitro release. Neurochem Int 28: 625–631, 1996. [DOI] [PubMed] [Google Scholar]
- 38.Vardhan A, Kachroo A, Sapru HN. Excitatory amino acid recptors in the commisural nucleus of the NTS mediate carotid chemoreceptor responses. Am J Physiol Regul Integr Comp Physiol 264: R41–R50, 1993. [DOI] [PubMed] [Google Scholar]
- 39.Vizek M, Pickett CK, Weil JV. Increased carotid body hypoxic sensitivity during acclimatization to hypobaric hypoxia. J Appl Physiol 63: 2403–2410, 1987. [DOI] [PubMed] [Google Scholar]
- 40.Wei Y, Whaley-Connell AT, Chen K, Habibi J, Uptergrove GME, Clark SE, Stump CS, Ferrario CM, Sowers JR. NADPH oxidase contributes to vascular inflammation, insulin resistance, and remodeling in the transgenic (mRen2) rat. Hypertension 50: 384–391, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Yueping C, George LT, Emily L, Po L, Siu-Yin L, Ryu I, Yung-Wui T, Man-Lung F. Chronic hypoxia enhances endothelin-1-induced intracellular calcium elevation in rat carotid body chemoreceptors and up-regulates ETA receptor expression. Pfluegers Arch V443: 565–573, 2002. [DOI] [PubMed] [Google Scholar]