Abstract
We investigated the cellular and molecular bases for the promotion of muscle development and growth by temperature manipulations (TMs) during late-term chick embryogenesis. We show that incubation at 39.5°C (increase of 1.7°C from normal conditions) from embryonic days 16 to 18 (E16 to E18) for 3 or 6 h daily increased diameter of myofibers as of day 13 of age and enhanced absolute muscle growth relative to controls, until day 35 of age. TMs had immediate (E17) and later (up to 2 wk posthatch) effects in elevating muscle cell proliferation relative to controls. This was indicated by higher DNA incorporation of thymidine and a higher number of cells expressing PCNA in intact muscle, accompanied by higher Pax7 levels, all reflecting a higher number of myogenic cells, and suggesting that the increased hypertrophy can be attributed to a higher reservoir of myogenic progeny cells produced in response to the TM. IGF-I levels were higher in the TM groups than in controls, implying a mechanism by which heat manipulations in chicks affect muscle development, with locally secreted IGF-I playing a major role. Whereas hypertrophy was similar in both TM groups, cell proliferation and Pax7 levels were more robust in the 6-h muscle, mainly posthatch, suggesting a differential effect of various TM periods on cell reservoir vs. hypertrophy and a high sensitivity of myoblasts to relatively small changes in heat duration with respect to these processes, which is manifested in the short and long term.
Keywords: myoblasts, thermoregulation, chick embryo
development and histogenesis of skeletal muscle proceed from early embryogenesis through adulthood. In the embryo, muscle progenitors undergo myogenic determination, giving rise to myoblasts that first proliferate and then differentiate and fuse into multinucleate fibers (reviewed in Ref. 21). Previous studies have indicated that, in the chick, embryonic myoblasts are most abundant on embryonic day 5 (E5), whereas fetal myoblasts are most abundant between E8 and E12 (31). From E15 onwards, satellite cells (or adult myoblasts) can be distinguished by their morphology and location under the basal membrane of the myofibers (14). These cells, first identified by Mauro (18), are the primary source of myogenic precursors in the postnatal muscle (29); however, their numbers decrease to <5% of total myofiber nuclei toward the end of the growth phase, and they become largely quiescent (3, 10, 11). Nevertheless, satellite cells are capable of reentering the cell cycle in response to various muscular stresses and undergo proliferation, followed by withdrawal from the cell cycle and fusion into existing or newly formed fibers (reviewed in Refs. 15, 29).
Myogenesis is tightly governed by the basic helix-loop-helix MyoD family members (MyoD, myogenic factor-5, myogenin, and myogenic regulatory factor-4), all of which are transcription factors that act in concert with the myocyte enhancer factor-2 proteins (reviewed in Ref. 20). MyoD and myogenin are sequentially expressed only in activated satellite cells (6, 35). The paired-box transcription factor Pax7 is selectively expressed in quiescent and proliferating satellite cells and is considered to play a role in their self-renewal (22, 30, 38). Recent reports have suggested Pax7 as an early marker of myogenesis during posthatch muscle growth, the expression of which is maintained by satellite cells in adult chicken muscle (3, 11).
Several growth factors and hormones have been reported to affect satellite cell proliferation and differentiation: one is insulin-like growth factor I (IGF-I). The muscle-secreted IGF-I isoform has been shown to stimulate proliferation as well as differentiation of satellite cells and increase myofiber hypertrophy (1, 2, 23). Recently, Halevy and colleagues (10) have demonstrated elevated levels of IGF-I in the muscle in response to mild heat stress for 24 h at 3 days posthatch, resulting in enhanced muscle cell proliferation and differentiation and subsequent muscle growth in broilers. Thermal manipulation (TM) of chick embryos at critical phases of functional system development has been conducted as a way of improving long-term functions, such as thermoregulation (24, 25, 36, 37) or neuronal hypothalamic sensitivity (33). These TMs caused a significant decline in plasma thyroid hormone concentration (25, 37). TM on various embryonic days or at various temperatures revealed that late-term embryonic treatments have the greatest effect on muscle growth. TM on E16 to E18, the period of satellite cell population expansion (14), for 3 h at 38.5°C (13) or 39.5°C (5), increased absolute pectoralis muscle weight in broilers at 42 days of age relative to controls, whereas TM between E8 and E10 had no effect.
The present study was aimed at understanding in detail the mechanisms underlying the enhanced muscle growth following TM of chick embryos. The study shows that the enhanced muscle growth following late-term embryonic TM is due to immediate, as well as long-lasting, effects on myogenic cell proliferation and differentiation and subsequent hypertrophy. Yet the differential effect of the treatments on cell proliferation vs. hypertrophy improvement suggests a high sensitivity of myoblasts to relatively small changes in heat duration with respect to these processes, which are manifested in both the short and long term.
MATERIALS AND METHODS
Experimental procedures.
Fertile Cobb strain broiler eggs (n = 540) were purchased from a local hatchery (Braun, Israel). The eggs were arranged in homologous locations in two incubators. The incubators (Masalles, Spain, Type 65Hs) were identical and automatic. Incubation conditions from day 0 until hatch were 37.8°C and 56% relative humidity (RH) for the control group (4). Eggs in the incubators were turned through 270° every hour. On E10, infertile eggs and those with dead embryos were removed after candling (<5%). Thermal treatments of the eggs from E16 to E18 involved an increase in temperature to 39.5°C and in RH to 65% for 3 h (0900–1200; termed 3H) or 6 h (900–1500; termed 6H) per day. Ten eggs from each treatment were taken daily from each group for analyses. On E19, the eggs were transferred to hatching trays located in each incubator. The hatching chicks were monitored for time of hatch (hatchability was >95% in all groups). Each chick was weighed, sexed, and tagged. Male chicks were placed in cages (3 chicks per cage, per treatment) that measured 40 × 28 × 45 cm (length, width, and height, respectively), with a 2-cm wire mesh floor. The cages were situated in three computer-controlled environmental rooms that maintained a constant temperature with an accuracy of ±1.0°C, RH at ±2.5%, air velocity at ±0.25 m/s, and under continuous fluorescent illumination (10, 11, 25). Chicks from each group were distributed equally in the cages in the rooms (n = 120). Chicks were raised under standard conditions up to day 21, after which chicks were placed individually in cages (25). Water and feed in mesh form were supplied ad libitum. All of the experiments and procedures were carried under the approval of the Animal Welfare Committee of the Faculty of Agriculture, Food and Environmental Quality Sciences at the Hebrew University of Jerusalem, and the Israeli Ethic Committee.
Cell cultures.
Skeletal muscle cells were cultured from the pectoralis muscle (major and minor) of experimental embryos during the TM or from male chicks, hatched in the same time window, at various ages, as previously described (9, 12). Cells were counted using a hemocytometer, and plated at 5 × 104 cells/cm2 on gelatin-coated dishes. The media used was MEM with 10% (vol/vol) horse serum and 3% (vol/vol) chicken embryo extract (CEE, Gibco, Paisley, UK) for cells derived from embryos, or 10% horse serum-containing DMEM for cells derived from posthatch chicks. Cells were maintained at 37°C in a humidified atmosphere containing 95% air and 5% CO2. On all days, cell cultures were prepared from either 1.5 or 6 g of muscle (for embryos and posthatch, respectively) sampled from a pool of chopped muscle from 10 embryos or chicks (12). An enriched population of myogenic cells was recovered, with <5% of these cells being nonmyogenic. The coefficient of variation of cell preparations was ∼5% (9, 12).
Thymidine incorporation.
Cells were cultured in 24-well plates for 17 h, and [3H]thymidine (Amersham, Uppsala, Sweden) was added (2 μCi/well) for an additional 2 h (9, 10). The cells were then detached with 0.25% (wt/vol) trypsin-EDTA and precipitated with 10% (wt/vol) trichloroacetic acid. Radioactivity in the dissolved precipitates was counted in Ultima Gold scintillation fluid (Packard, Downers Grove, IL) using a Tri-Carb 1600CA scintillation counter (Packard).
Muscle sampling and myofiber diameter analysis.
Muscle samples, collected from chicks hatched in the same time window, were excised from the superficial regions of the proximal one-half of the left pectoralis major of each chick (∼0.5 × 0.5 × 1.2 cm in size). The long axis of each sample was parallel to the direction of the muscle fibers.
Myofiber diameter was determined by analyzing the lesser myofiber diameter values (7), as described (11). Briefly, muscle samples were fixed in 4% paraformaldehyde and embedded in paraffin, and muscle sections (5 μm) were cut. Sections were stained with hematoxylin-eosin, and at least 10 arbitrary fields in two to three serial sections of each muscle sample were photographed. In each muscle sample, the lesser fiber diameter was measured for individual myofibers with CELL B software (Olympus, Hamburg, Germany). Since there were no statistically significant differences among chicks, all data for the same treatment were pooled for further analysis.
PCNA analysis.
Muscle sections were immunostained with an antibody against PCNA (a marker for dividing cells), using a commercial kit from Zymed (San Francisco, CA), followed by counterstaining with hematoxylin. Five chicks were analyzed per group; five sections were studied per chick, by monitoring five random fields per section. Analysis of positive cells was based on digitized images (12). Only nuclei within the myofiber perimeter were included in this analysis; regions rich with connective tissue were not included.
RNA preparation and RT-PCR.
Total RNA was prepared using TRIzol Reagent (Invitrogen, Carlsbad, CA). Total RNA (1 μg) was reverse transcribed into cDNA using random primers and SuperScript reverse transcriptase (Invitrogen). PCR was then performed using Taq DNA polymerase (Fermentas, Glen Burnie, MD) for the following primers: GAPDH (F) 5′-AGTCATCCCTGAGCTGAATG-3′, GAPDH (R) 5′-AGGATCAAGTCCACAACACG-3′ (330 bp), IGF-I (F)-5′-GTATGTGGAGACAGA GGCTTC-3′, IGF-I (R)-5′-TTTGGCATATCAGTGTGGCGC-3′ (200 bp). Thirty-five cycles (IGF-I) or 20 cycles (GAPDH) of amplification were performed, each consisting of 1 min at 94°C, 1 min at 55°C, and 1 min at 72°C, followed by a final 10-min extension at 72°C. GAPDH was used as an internal control to normalize the sample mRNA amounts. PCR products were separated by electrophoresis, and bands were visualized by a digital camera (UVItec, Cambridge UK).
Western blot analysis.
Muscle samples were collected from the right half of the pectoralis muscle used in the immunohistochemical analysis and immediately frozen in liquid nitrogen. Samples were homogenized with a Kinematica homogenizer (Lucerne, Switzerland) for 30 s on ice in lysis buffer. All extracts were sonicated and normalized for protein content (BCA kit, Pierce, Rockford, IL). Equal amounts of protein from muscle extracts were separated by SDS-PAGE and transferred to nitrocellulose filters (Bio-Rad Laboratories, Hercules, CA). Membranes were incubated overnight at 4°C with the appropriate antibodies and then washed and incubated for 1 h with horseradish peroxidase-conjugated goat anti-mouse or goat anti-rabbit IgG (Zymed). The primary antibodies were as follows: myogenin (rabbit polyclonal, a kind gift from B. Paterson, National Institutes of Health, Bethesda, MD), IGF-I (mouse monoclonal; Upstate Biotechnology, Lake Placid, NY), and Pax7 (mouse monoclonal) from Hybridoma Bank (University of Iowa, Iowa City, IA). Densitometric analysis was performed on bands using National Institutes of Health software. Band intensity in each lane was normalized to the level of α-tubulin as an internal standard (12).
Statistical analysis.
The data were subjected to one-way ANOVA and to the all-pairs Tukey-Kramer-honestly significant difference test by means of the JMP software (28).
RESULTS
Embryonic TM increases myofiber diameter in posthatch chicks.
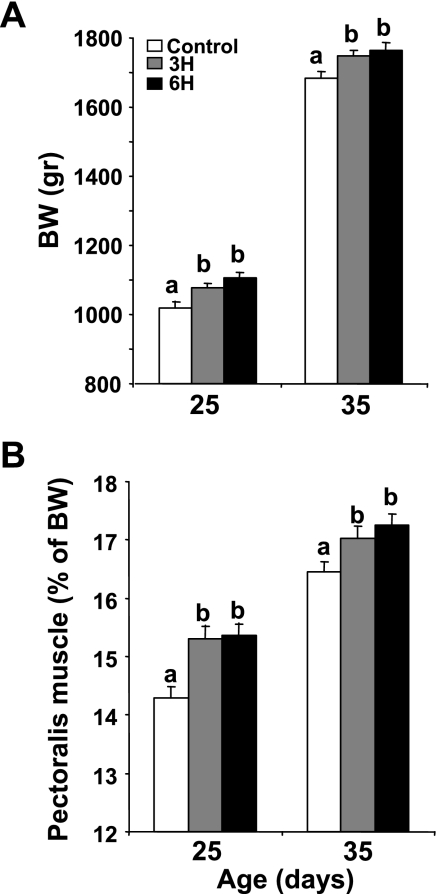
TM was conducted on E16 to E18 at 39.5°C for 3 or 6 h daily (groups 3H and 6H, respectively); controls were kept continuously at 37.8°C. Body weight (BW) was higher in the 3H and 6H groups than in the controls from day 9 onward (data not shown). At 25 and 35 days of age, BW (Fig. 1A) and absolute pectoralis muscle growth, presented as percentage of BW (Fig. 1B), were higher in both the 3H and 6H groups compared with controls (P < 0.05). No significant differences were observed between the treatment groups.
Fig. 1.
Body weight (BW) (A) and pectoralis muscle as percentage of BW (B) of thermally manipulated (TM) [3 h daily (3H), 6 h daily (6H)] and control chickens on days 25 and 35 of age. Results are means ± SE; n = 30. a,bData with different letters differ significantly within the same age group (P < 0.05).
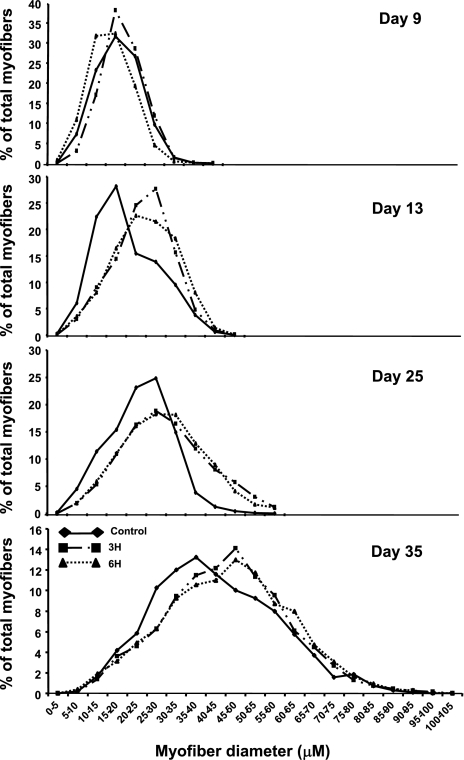
The higher muscle weight raised the possibility that TM might be affecting muscle hypertrophy. To test this, lesser diameter analyses were performed on samples derived from pectoralis muscles of the experimental chicks at various days of age (Fig. 2). An analysis of myofiber distribution demonstrated that the total range of myofiber diameter values was similar within the experimental groups (between 0 and 45 μm, 0 and 50 μm, and 0 and 60 μm on days 9, 13, and 25, respectively, and between 0 and 100 μm on day 35 of age), displaying typical Gaussian curves, which were sharper on days 9 and 13 and became flatter as time went on. A shift in the curve peak toward the higher diameter bins was observed in the 3H and 6H groups from day 13 onward; the percentage of myofibers with higher diameter values was higher in these groups than in controls (75 and 71% vs. 43% within the diameter range of 25–45 μm on day 13, 46 and 47% vs. 20% within the diameter range of 35–60 μm on day 25, and 6.3 and 5.5% vs. 4.4% within the diameter range of 70–100 μm on day 35 in the 3H, 6H, and control groups, respectively). The shift in the curves was reflected in the mean myofiber diameter, which was significantly higher in the treated groups than in controls on days 13, 25, and 35 of age (Table 1; P < 0.05). No significant differences were observed within the TM groups.
Fig. 2.
Myofiber diameter distribution in pectoralis muscle from control and TM (3H, 6H) groups at various days of age. Myofibers are clustered in bin intervals of 5 μm, and myofiber diameter values are ranked in ascending order within each treatment group. Results are presented as percentage of total fibers.
Table 1.
The effect of thermal manipulation for 3 h and 6 h on average myofiber diameter in chicks at various days of age
| Age, days | Myofiber Diameter, μM |
Total Myofiber Number | ||
|---|---|---|---|---|
| Control | 3H | 6H | ||
| 9 | 17.88±0.62* | 19.55±0.62* | 16.28±0.01* | 2,000 |
| 13 | 20.07±2.20* | 24.35±0.31† | 24.35±0.31† | 4,000 |
| 25 | 24.29±0.83* | 31.44±2.77† | 30.62±2.28† | 4,000 |
| 35 | 41.45±1.29* | 46.12±2.16† | 46.54±2.01† | 2,000 |
Values are means ± SE of myofibers derived from 5–7 chickens per day. 3H and 6H, groups receiving thermal manipulation for 3 and 6 h daily, respectively.
Values with different symbols differ significantly within the same age (P < 0.05).
TM at 39.5°C promotes proliferative activity and number of muscle cells in embryonic and posthatch chicks.
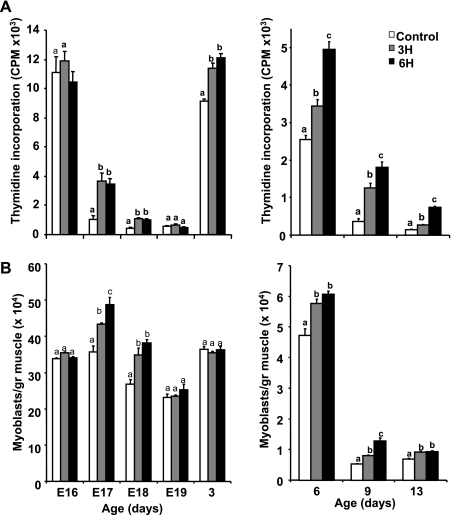
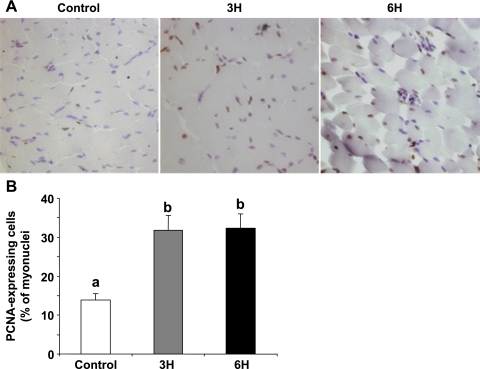
The higher muscle hypertrophy noted in the TM groups until day 35 of age suggested an enhancement in muscle cell proliferation and an increase in the myogenic cell pool in the early growth phase. To evaluate myoblast proliferation, we used the thymidine incorporation assay, which has previously been reported to reflect in vivo proliferative activity in these cells (9, 10, 12). The effect of the TM was immediate: thymidine incorporation was already significantly higher in the 3H and 6H groups relative to the control on E17, 1 day into the TM, and on E18 (Fig. 3A, left; P < 0.05). This was reflected in the number of myoblasts (Fig. 3B, left). It was only on E19 that the differences between the groups became insignificant, with respect to both thymidine incorporation and cell numbers. However, the promotive effect of the TM on thymidine incorporation was evident again in the 3H and 6H groups on the 3rd day posthatch (Fig. 3A, left). As expected, thymidine incorporation and cell numbers declined considerably from day 6 onward, as cells underwent terminal differentiation (10, 11, 13). Nevertheless, these parameters remained higher, even at 2 wk of age, in the TM groups compared with controls, and, for thymidine incorporation, the levels in the 6H group were significantly higher than those in the 3H group (Fig. 3, A and B, right; P < 0.05). The promotive effect of the embryonic TM on myoblast proliferation was seen in vivo by PCNA analysis. The number of cells expressing PCNA, a marker for cycling cells, was more than twofold higher in the 3H and 6H groups (31.9 ± 3.9 and 32.2 ± 3.8%, respectively) than in the control cells, where only 14 ± 1.8% of the total myonuclei expressed PCNA (Fig. 4; P < 0.05).
Fig. 3.
Thymidine incorporation by DNA (A) and number of muscle cells per gram of pectoralis muscle (B) in control and TM (3H, 6H) groups. Muscle was removed from embryos or posthatch chicks on various days and pooled within each group. Left: embryonic days (E) 16–19 and posthatch day 3; right: posthatch days 6–13. Myoblasts were isolated in parallel from each group and counted using a hemocytometer. Cells were incubated for 1 day, after which labeled thymidine was added for 2 h. Results are means ± SE (n = 6) of a representative of three independent experiments. a,b,cData with different letters differ significantly within the same age group (P < 0.05).
Fig. 4.
A: PCNA staining of pectoralis muscle cross sections prepared from the experimental chicks (control and TM: 3H, 6H) at 9 days of age. Magnification ×200. B: quantitation analysis of the PCNA-expressing cells. Results are means ± SE of PCNA-expressing cells within the myofibers and are presented as percentage of total myonuclei. Five sections were studied per five chicks (n = 5), monitoring five random fields per section (total of 1,000 nuclei per chick) (P < 0.05). Only nuclei within the myofiber perimeter were included in this analysis, and regions rich with connective tissue were not included. a,bData with different letters differ significantly (P < 0.05).
Myogenic factors and IGF-I are affected by embryonic TM.
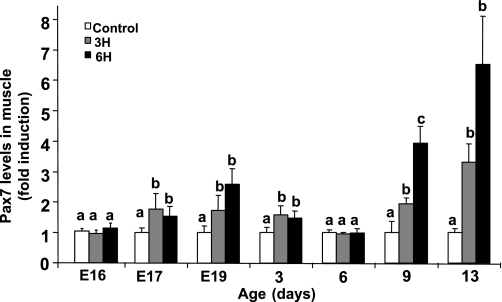
The increase in the myogenic pool due to TM suggested alteration in the expression levels of Pax7, which has been shown to mark proliferating chicken satellite cells (3, 11, 12). Protein expression levels in muscle cell cultures or in muscle samples derived from the experimental groups on late embryonic days and posthatch were evaluated by Western blot analysis followed by densitometry. In view of the large number of samples per time point and the multiple in vivo repeats, samples harvested at the same time were analyzed in parallel on the same blot for each day, and results are expressed as fold induction relative to the control group. In the muscle tissue, Pax7 protein levels were higher in the 3H and 6H groups than in the control group from as early as E17 onward (Fig. 5), reaching a threefold and nearly sevenfold difference on day 13 in the 3H and 6H groups, respectively (P < 0.05). A similar trend in Pax7 expression was observed in muscle cells derived from these groups (data not shown), confirming that the kinetics of these cells reflect that of the satellite cells in vivo.
Fig. 5.
Pax7 levels in pectoralis muscle-derived myoblasts and muscle tissue are upregulated in response to TM (3H, 6H). Pectoralis muscle samples were removed in parallel from individual embryos or chicks from all groups (n = 4) at various days of age. On each day, samples derived from the control and TM groups were electrophoresed side by side on an SDS-polyacrylamide gel, and protein expression levels were evaluated by Western blot analysis. Bands were quantified by densitometry relative to α-tubulin, and results are means ± SE presented as fold induction relative to control. a,b,cData with different letters differ significantly within the same age group (P < 0.05).
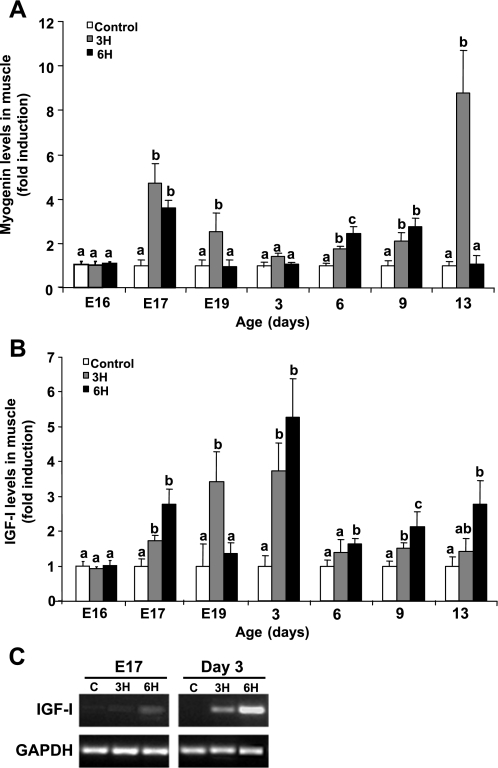
The differentiation state of the myoblasts was evaluated by quantifying the protein levels of myogenin, a marker for differentiating muscle cells, in muscle samples. The TM for 3 h significantly increased myogenin levels in the 3H group relative to controls on E17 and E19 and from day 6 posthatch; the highest increase was observed on day 13 (Fig. 6A; P < 0.05). The myogenin level profile in the 6H group varied somewhat from that in the 3H group; it was higher than the control on E17 and highest on days 6 and 9 posthatch.
Fig. 6.
Densitometric analysis of myogenin (A) and IGF-I (B) expression relative to α-tubulin protein levels in pectoralis muscle samples derived from control and TM (3H, 6H) embryos and chicks at various days of age. Sample collection and Western blot analysis were performed as described in Fig. 5 legend. Results are means ± SE and presented as fold induction of control (n = 4). a,bData with different letters differ significantly within the same age (P < 0.05). C: RT-PCR analysis for IGF-I mRNA expression levels in myoblasts derived from pectoralis muscle of the experimental chicks on E17 and day 3 posthatch. Equal samples were analyzed and electrophoresed as demonstrated by GAPDH mRNA levels.
Locally expressed IGF-I has been shown by us and others to be a prime candidate for inducing myoblast activity following muscle stress (1, 10, 13). Here, compared with controls, IGF-I protein levels were higher in the 3H group mainly at earlier time points, increasing until day 3 posthatch (Fig. 6B; P < 0.05). IGF-I levels tended to be even higher in the 6H group, significantly so (relative to all other groups) from day 3 onward. Figure 6C demonstrates that IGF-I mRNA is expressed in myoblasts derived from pectoralis muscles of controls and TM groups on pre- and posthatch days.
DISCUSSION
The present study examined in detail the cellular and molecular events associated with the effect of embryonic TM on muscle growth in chicken. The results of this study suggest that short periods of heat exposure at 39.5°C, during late-term chick embryogenesis, have immediate and posthatch stimulatory effects on the proliferation and differentiation of myogenic cells, as manifested by increased myofiber hypertrophy and muscle growth in chickens at later ages. Moreover, together with previous studies (13), the results presented here demonstrate the dependency of these processes on incubation temperature and duration of the manipulation.
TM from E16 to E18 induced muscle cell proliferation and cell numbers, as evidenced by thymidine incorporation into the DNA of muscle cells derived from pectoralis muscles. Moreover, the TM's effect on muscle cell proliferation was notable up to nearly 2 wk posthatch, a time at which this activity is normally markedly reduced in broilers as cells undergo terminal differentiation (10, 11). Indeed, a higher population of PCNA-expressing cells, within the perimeter of the myofibers, was detected in cross sections of muscles derived from the 3H and 6H vs. control groups on day 9 posthatch. Moreover, Pax7 levels were higher in muscle cells and tissue derived from the TM groups compared with the control group. Pax7 is known to be expressed in all satellite cells in adults (30, 38) and has been reported as a marker for the proliferative capacity of satellite cells in turkey poults and broilers (12, 13, 19). Together, these results suggest that TM between E16 and E18 promotes a higher reservoir of myogenic progeny cells, which can mainly be attributed to the satellite cell population (14).
TM for 3 and 6 h also enhanced cell differentiation (e.g., myogenin expression levels in the muscle). Our laboratory has previously shown that, during muscle development in pre- and posthatch chickens, there are situations in which at least two different cell populations exist simultaneously: one that is still proliferating, and one that is undergoing differentiation (12). A similar phenomenon was observed in the present study. First, on almost all days examined, a similar pattern of TM-induced cell proliferation and myogenin expression was observed (Figs. 3 and 6). Second, the temporal protein expression was similar within the experimental groups (data not shown), suggesting that TM under these conditions probably does not accelerate differentiation, but more likely enhances the number of cells available for proliferation and subsequently for differentiation and hypertrophy. This is emphasized by the finding that, on day 13, while some myoblasts were still proliferating in the TM groups, muscle hypertrophy was greater than in controls, as reflected by the higher average diameter and increased number of myofibers in the higher diameter bins. Taken together, we conclude that TM of late-term chick embryos promotes a higher reservoir of myogenic progeny that are produced for a longer time posthatch (i.e., up to 2 wk of age). The effect of TM in extending the period for reservoir formation is potentially long lasting, as it contributes additional nuclei to the myofibers, which will result in increased hypertrophy later on (29); indeed, increased hypertrophy and higher muscle weight were observed until day 35 in the TM groups.
Interestingly, the enhanced hypertrophy in the heat-treated chicks was similar, regardless of the length of the TM. Nevertheless, the more robust cell proliferation and Pax7 levels in the muscles of the 6H group, mainly posthatch, imply a greater reservoir of myogenic cells in this group. Together, these findings suggest a differential effect of various periods of TM on cell reservoir enhancement vs. hypertrophy improvement, which implies a high sensitivity of myoblasts to relatively small changes in heat duration manifested in both the short and long term. This phenomenon is also true for other experiments with temperature changes, such as elevating the TM by only 0.7°C (i.e., 38.5°C; Ref. 13), but not raising it to 41°C (unpublished data), on the same embryonic days, suggesting that the inductive effect of TM on muscle cell proliferation is limited to a narrow range of temperatures. We postulate that the longer lasting stimulatory effect on myoblast proliferation (up to 2 wk of age) is specific to TMs in the chick embryo (Figs. 3 and 4), because other in ovo environmental manipulations, such as illumination with green light, while having a promotive effect on muscle growth, have no effect on cell proliferation dynamics (12, 27).
It is conceivable that the increase in temperature has a direct effect on myoblast proliferation. Indeed, incubation of primary myoblasts derived from E17 embryos at 39.5°C for 3 or 6 h increased cell proliferation, whereas this effect was completely abolished at 40.5°C (data not shown). However, it is even more likely that TM at 39.5°C has a prominent effect on systemically or locally induced factors associated with muscle cell proliferation and muscle hypertrophy, one of which is IGF-I (1, 23, 26). The observed immediate stimulation in IGF-I expression in the muscle in response to TM, which persisted until at least day 13, alongside the finding that the TM stimulates IGF-I gene expression in muscle cells derived from the TM groups, support this notion. Moreover, our laboratory has previously reported that the stimulatory effect of subjecting chicks to mild heat exposure on day 3 posthatch (10) or to TM at 38.5°C on E16 to E18 (13) on muscle IGF-I expression (but not on that of other mitogens, such as hepatocyte growth factor or basic fibroblast growth factor) is correlated with myoblast proliferation and muscle growth. IGF-I stimulation has been reported in cases of muscle stress, such as stretch and overload (8). Therefore, it may well be that the heat-mediated increase in IGF-I is a stress response, which is regulated by the conditions of the heat manipulation (e.g., duration or temperature). Interestingly, the increase in IGF-I by TM was still evident 2 wk posthatch. This raises the possibility that TM during critical phases of satellite cell myogenic activity [i.e., in late-term embryos (14) and very early days posthatch (11, 13)] induces genomic modifications. Studies on long-term thermal adaptation achieved by TM in chick embryos have shown changes in brain-derived neurotrophic factor (16) and R-Ras3 gene regulation (17), suggesting that this long-term adaptation occurs via epigenetic adaptation processes (32).
It is worth noting that the immediate differences in cell proliferation and myogenic factor expression observed in the TM vs. control chicks disappeared toward hatch and reappeared from day 3 onward. We do not yet have a definitive explanation for this phenomenon. One possibility may lie in the fact that, overall, the relatively low thymidine incorporation and cell numbers on E19 are associated with the depletion of energetic sources, such as muscle glycogen, toward hatch (34). Another explanation may lie in the TM regime itself, which included repeated heat treatments for 3 days. It may well be that, in parallel to the increase in cell reservoir on E17 and E18, some genomic changes requiring DNA repair occurred in response to the multiple TM, as reflected in the immediate cell cycle arrest observed on E19 and the difference in thymidine incorporation, but not in cell number, that was still observable on day 3 posthatch. Yet the higher Pax7 levels in the TM groups noted on day 3 imply true cell proliferation (11, 38) followed by higher cell numbers on day 6. We believe that the resumption in cell proliferation and differentiation is due to muscle IGF-I protein levels that remain higher in the TM groups throughout the early days posthatch and continue to be synthesized at both the transcriptional and posttranscriptional levels (Fig. 6, B and C).
In view of our findings, we propose that heat manipulations at critical phases of muscle development in the late-term chick embryo have a stimulatory effect on cell proliferation during both late embryogenesis and the posthatch period, creating a higher reservoir of myogenic progeny with a potential for enhanced muscle growth. These findings emphasize the importance of various incubation temperatures and manipulation durations in the fine-tuning of muscle development and growth processes during late-term embryogenesis and in posthatch chickens.
GRANTS
This study was supported by a grant from the United States-Israel Binational Agricultural Research and Development Fund (Agreement No. IS-3836-06R, S. Yahav, O. Halevy, and J. Brake), and in part by the Israeli Poultry Marketing Board. O. Halevy is a holder of the Charles Charkowsky Chair in Poultry Science and Animal Hygiene.
Acknowledgments
We are grateful to Bruce Paterson (National Institutes of Health, Bethesda, MD) for the myogenin antibody.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Adams GR, McCue SA. Localized infusion of IGF-I results in skeletal muscle hypertrophy in rats. J Appl Physiol 84: 1716–1722, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Adams GR, McCue SA, Bodell BW, Zeng M, Baldwin KM. Effects of spaceflight and thyroid deficiency on hindlimb development. I. Muscle mass and IGF-I expression. J Appl Physiol 88: 894–903, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Allouh MZ, Yablonka-Reuveni Z, Rosser BWC. Pax7 reveals a greater frequency and concentration of satellite cells at the ends of growing skeletal muscle fibers. J Histochem Cytochem 56: 77–87, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruzual JJ, Peak SD, Brake J, Peebles ED. Effects of relative humidity during incubation on incubation on hatchability and body weight of broiler chicks from young breeder flocks. Poult Sci 79: 827–830, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Collin A, Berry C, Tesseraud S, Redon FE, Skiba-Cassy S, Crochet S, Duclos MJ, Rideau N, Tona K, Buyse J, Bruggeman V, Decuyper E, Picard M, Yahav S. Effects of thermal manipulation during early and late embryogenesis on thermotolerance and breast muscle characteristics in broiler chickens. Poult Sci 86: 795–800, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Cornelison DD, Wold BJ. Single-cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle satellite cells. Dev Biol 191: 270–283, 1997. [DOI] [PubMed] [Google Scholar]
- 7.Dubowitz V Muscle Biopsy: A Practical Approach. Philadelphia, PA: Balliere-Tindal, 1985.
- 8.Goldspink G Changes in muscle mass and phenotype and the expression of autocrine and systemic growth factors by muscle in response to stretch and overload. J Anat 194: 323–334, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halevy O, Geyra A, Barak M, Uni Z, Sklan D. Early posthatch starvation decreases satellite cell proliferation and skeletal muscle growth in chicks. J Nutr 130: 858–864, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Halevy O, Krispin A, Leshem Y, McMurtry JP, Yahav S. Early-age heat exposure affects skeletal muscle satellite cell proliferation and differentiation in chicks. Am J Physiol Regul Integr Comp Physiol 281: R302–R309, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Halevy O, Piestun Y, Allouh M, Rosser B, Rinkevitch Y, Reshef R, Rozenboim I, Wleklinski-Lee M, Yablonka-Reuveni Z. The pattern of Pax7 expression during myogenesis in the posthatch chicken establishes a model for satellite cell differentiation and renewal. Dev Dyn 231: 489–502, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Halevy O, Piestun Y, Rozenboim I, Yablonka-Reuveni Z. In-ovo exposure to monochromatic green light promotes skeletal muscle cell proliferation and affects myofiber growth in posthatch chicks. Am J Physiol Regul Integr Comp Physiol 290: R1062–R1070, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Halevy O, Rozenboim I, Yahav S. Enhancement of meat production by environmental manipulation in embryo and young broilers. Review. Worlds Poult Sci J 62: 485–497, 2006. [Google Scholar]
- 14.Hartley RS, Bandman E, Yablonka-Reuveni Z. Skeletal muscle satellite cells appear during late chicken embryogenesis. Dev Biol 153: 206–216, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hawke TJ, Garry DJ. Myogenic satellite cells: physiology to molecular biology. J Appl Physiol 91: 534–551, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Katz A, Meiri N. Brain-derived neurotrophic factor is critically involved in thermal-experience-dependent developmental plasticity. J Neurosci 26: 3899–3907, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Labunskay G, Meiri N. R-Ras3/(M-Ras) is involved in thermal adaptation in the critical period of thermal control establishment. J Neurobiol 66: 56–70, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Mauro A Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol 9: 493–495, 1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore DT, Ferket PR, Mozdiak PE. The effect of early nutrition on satellite cell dynamics in the young turkey. Poult Sci 84: 748–756, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Naya FS, Olson E. MEF2: a transcriptional target for signaling pathways controlling skeletal muscle growth and differentiation. Curr Opin Cell Biol 11: 683–688, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Ordahl CP, Williams BA, Denetclaw W. Determination and morphogenesis in myogenic progenitor cells: an experimental embryological approach. Curr Top Dev Biol 48: 319–367, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Oustanina S, Hause G, Braun T. Pax7 directs postnatal renewal and propagation of myogenic satellite cells but not their specification. EMBO J 23: 3430–3439, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paul AC, Rosenthal N. Different modes of hypertrophy in skeletal muscle fibers. J Cell Biol 156: 751–760, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piestun Y, Shinder D, Ruzal M, Halevy O, Yahav S. The effect of thermal manipulations during the development of the thyroid and adrenal axes on in-hatch and post-hatch thermoregulation. J Therm Biol 7: 413–418, 2008. [Google Scholar]
- 25.Piestun Y, Shinder D, Ruzal M, Halevy O, Brake J, Yahav S. Thermal manipulations during broiler embryogenesis: effect of acquisition of thermotolerance. Poult Sci 87: 1–10, 2008. [DOI] [PubMed] [Google Scholar]
- 26.Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, Yancopoulos GD, Glass DJ. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol 11: 1009–1013, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Rozenboim I, Piestun Y, Mobarkey N, Barak M, Hoyzman A, Halevy O. Monochromatic light stimuli during embryogenesis enhance embryo development and posthatch growth. Poult Sci 83: 1413–1419, 2004. [DOI] [PubMed] [Google Scholar]
- 28.SAS JMP Statistics and Graphic Guide. Version 4. Cary, NC: SAS Institute Incorporation, 2002.
- 29.Schultz E, McCormick KM. Skeletal muscle satellite cells. Rev Physiol Biochem Pharmacol 123: 213–257, 1994. [DOI] [PubMed] [Google Scholar]
- 30.Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA. Pax7 is required for the specification of myogenic satellite cells. Cell 102: 777–786, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Stockdale FE Myogenic cell lineages. Dev Biol 154: 284–298, 1992. [DOI] [PubMed] [Google Scholar]
- 32.Tzschentke B, Basta D. Early development of neuronal hypothalamic sensitivity in birds: influence of epigenetic temperature adaptation. Comp Biochem Physiol A 131: 825–832, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Tzschentke B, Plagemann A. Imprinting and critical periods in early development. Worlds Poult Sci J 62: 626–638, 2006. [Google Scholar]
- 34.Uni Z, Ferket PR, Tako E, Kedar O. In ovo feeding improves energy status of late-term chicken embryos. Poult Sci 84: 764–770, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Yablonka-Reuveni Z, Paterson BM. MyoD and myogenin expression patterns in cultures of fetal and adult chicken myoblasts. J Histochem Cytochem 49: 455–462, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Yahav S, Collin A, Shinder D, Picard M. Thermal manipulations during broiler chick embryogenesis: effects of timing and temperature. Poult Sci 83: 1959–1963, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Yahav S, Sasson Rath R, Shinder D. The effect of thermal manipulations during embryogenesis of broiler chicks (Gallus domesticus) on hatchability, body weight and thermoregulation after hatch. J Therm Biol 29: 245–250, 2004. [Google Scholar]
- 38.Zammit PS, Golding JP, Nagata Y, Hudon V, Partridge TA, Beauchamp JR. Muscle satellite cells adopt divergent fates: a mechanism for self-renewal? J Cell Biol 166: 347–357, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]