Abstract
Historical accounts of alcohol administration to patients with breathing problems suggest that alcohol may have bronchodilating properties. We hypothesized that acute alcohol exposure will alter airway responsiveness (AR) in mice. To test this hypothesis, C57BL/6 mice were fed either 20% alcohol in drinking water (fed) or received a single intraperitoneal (ip) injection of alcohol (3 g/kg). Control groups received regular drinking water or ip saline. AR was assessed by means of ventilation or barometric plethysmography and reported as either total lung resistance or enhanced pause for each group of mice. To confirm alcohol exposure, elevated blood alcohol levels were documented. Alcohol feeding significantly blocked methacholine-triggered AR compared with water-fed controls. Comparable blunting of AR was also accomplished through a single ip injection of alcohol when compared with saline-injected controls. The alcohol response was slowly reversible in both routes of administration after withdrawal of alcohol: AR attenuation by alcohol persisted 12–20 h (ip) or up to 2 wk (fed) after blood alcohol cleared consistent with a sustained bronchodilator effect. These data demonstrate that brief alcohol exposure blunts AR in this murine model of alcohol exposure suggesting a role for alcohol in the modulation of bronchial motor tone.
Keywords: enhanced pause, bronchodilator effect, Penh
alcohol is one of the most commonly used and abused substances in the United States. With the rapidly growing number of people who consume alcohol on a regular basis (66%), there is a coinciding increase in the number of people seeking medical attention for alcohol-induced problems (13). While heavy alcohol intake can clearly damage brain and liver, the lung is also a target for the toxic effects of alcohol. For example, alcohol greatly increases the risk of developing upper respiratory infections, pneumonia, and acute respiratory distress syndrome (20). The exposure of the airways through the volatility of alcohol likely accounts for many of the biological effects of alcohol on lung airway functions (11).
Airflow in normal lungs is directed to zones of the lung that are well perfused by blood to preserve a normal ventilation (V) to perfusion (Q) ratio (V/Q). As regional blood flow in the lungs change, reflex bronchoconstriction and/or bronchodilation occurs to maintain normal V/Q matching. When inappropriate bronchoconstriction or bronchodilation occurs, such as that seen with asthma or pulmonary emboli, abnormal V/Q matching occurs and causes impaired gas exchange. Airway responsiveness (AR) occurs if the airways narrow too easily and too much (34, 38). AR is a cardinal feature of asthma, but the mechanisms of AR remain poorly understood. Acute narrowing of the airway lumen is caused by contraction of the airway smooth muscle (ASM) (12, 22). The myosin motor drives contraction of the ASM cell, and myosin exerts its mechanical effects within integrated cytoskeletal scaffolding. The ASM cell also plays an important role in the pathophysiology of asthma, including AR, but evidence for a causal link between AR and an altered ASM contractility has been at best equivocal (25, 32). Although a modest number of clinical studies have focused on the role of alcohol in the treatment of asthma (2, 3), it has also been demonstrated to have potential bronchoconstrictive effects (9). With these contradicting findings reported, no one has examined the effects of alcohol on AR.
Our laboratory, and others (10, 11, 36, 42), have demonstrated that brief alcohol feeding of mice stimulates airway cAMP levels in various tissues, including lung, through activation of an alcohol-sensitive adenylyl cyclase, AC-7. Because cAMP is a major second messenger involved in the regulation of airway motor tone, we hypothesized that brief alcohol exposure would modulate AR, resulting in altered airway responsiveness that is reversible and independent of the route of administration. We sought to test this hypothesis in alcohol-exposed mice through the use of the common bronchoprovocant, methacholine.
MATERIALS AND METHODS
Mice
Male C57BL/6 mice 6–8 wk old were obtained from the National Cancer Institute (Bethesda, MD). The mice were kept in community cages with 12-h periods of light and dark cycles and were maintained on standard rodent chow with access to water ad libitum. All animal care and experimentation were approved by and carried out in accordance with the University of Nebraska Medical Center and Columbia University College of Physicians and Surgeons institutional animal care and use committees and in accordance with the principles and guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Chemicals
Ethyl alcohol dehydrated (200 proof) was obtained from PHARMCO-AAPER Alcohol and Chemical, Louisville, KY. Acetyl-β-methylcholine chloride, NG-methyl-l-arginine acetate salt, pancuronium bromide, and pentobarbital sodium were obtained from Sigma Chemical, St. Louis, MO.
Ethanol Administration
For exposure via the oral route, mice were fed alcohol ad libitum directly in their drinking water using a ramping method where the alcohol group drank 10% wt/vol alcohol for 2 days, 15% wt/vol alcohol for 5 days, and 20% wt/vol alcohol for 1–12 wk, whereas the control mice drank water only (10, 11, 33). As previously reported (11), no significant weight loss or nutritional disturbances were observed in mice fed this ad libitum diet. For exposure via the intraperitoneal (ip) route, a single injection of ethanol (3.0 g/kg; 20% wt/vol in 0.9% saline) was administered, and control mice received a single ip injection of saline (16). Following the injection, an accommodation period of 1 h was allowed before experiments were initialized. All doses were sufficient to achieve a blood alcohol concentration (BAC) level of at least 45 mg/dl, which is equivalent to a human consuming one to two alcoholic beverages within 1 h.
Noninvasive Pulmonary Function Measurement
To test the pulmonary function of mice exposed to various agents, barometric plethysmography was utilized (Max II; Buxco Electronics, Troy, NY). This whole body plethysmography system operates without the use of anesthesia or restraint to allow real-time recordings of airway responsiveness via a dimensionless parameter known as enhanced pause (Penh) to estimate the total pulmonary resistance. This method has been demonstrated to accurately reflect airway resistance, expressed as Penh units (14). This noninvasive parameter has been shown to correlate with direct invasive measures of airway obstruction, namely, airway resistance and dynamic compliance (1, 8, 21). AR was assessed following a standard protocol (26). Briefly, unrestrained, non-anesthetized mice were placed in separate chambers and allowed 10 min to accommodate to their surroundings. After the accommodation period, a 5-min recording was performed to obtain a baseline measure of their AR. Following this recording, 0.9% isotonic saline was introduced to the chambers via ultrasonic nebulization for 2 min immediately followed by a 5-min recording period to capture the AR to the inhaled vehicle control. Following the saline challenge, mice were exposed to the common bronchoprovocant, methacholine (MCh), in cumulative doses (1.5–48.0 mg/ml) to establish a dose-response relationship. Readings were taken for 5 min immediately following drug aerosolization. Each consecutive dose was not administered until the mice returned to baseline Penh levels. AR (Penh values) was reported as an average raw value from three separate experiments.
Invasive Pulmonary Function Measurement
The linear first-order compartment model is the standard model of respiratory mechanics. It produces the widely used dynamic resistance and compliance parameters where increased resistance values signal constriction of the lungs. This parameter reflects not only central airway resistance, but is also influenced by the lung periphery (the tissues). In these studies, mice were injected ip with saline or ethanol (3 g/kg) 1 h before being anesthetized with pentobarbital sodium (50 mg/kg), tracheostomized, and mechanically ventilated at a rate of 350 breaths/min, tidal volume of 0.15 ml, and PEEP (3–4 cmH2O) using a computerized small animal ventilator (FlexiVent; SCIREQ, Montreal, Quebec, Canada; Finepoint; Buxco Electronics, Wilmington, NC) as previously described (19, 28, 30). Dose-response curves to aerosolized methacholine (1.5–48.0 mg/ml) were then obtained as previously reported (24, 30) and reported as lung resistance.
Reversibility Experiments
For the alcohol-fed exposure, mice received ethanol in their drinking water for 6 wk and were weaned off ethanol gradually. They drank 15% ethanol in their water for the first 5 days, 10% for the next 2 days, and then regular drinking water until week 12. Following the weaning period, AR was measured. The ip-exposed mice were injected with 3 g/kg ethanol in normal saline and allowed to recover for the designated time periods of 4, 24, and 48 h. After each time point, airway responsiveness to MCh was measured. Separate groups of mice were used in each set of experiments. To avoid possible tachyphylaxis to methacholine, AR experiments were performed on each mouse one time only.
BAC
The alcohol concentration of the blood was monitored closely to verify that the mice had elevated levels of alcohol following each experiment. Upon euthanization, 0.8–1.0 ml of whole blood was collected into plasma separator tubes (BD Scientific, Franklin Lakes, NJ). The tubes were placed on ice for 15 min and then centrifuged at 800 rpm for 10 min. Serum was removed, transferred to microcentrifuge tubes, and frozen at −80°C until assayed. The serum was assayed using an alcohol reagent set and alcohol control (Pointe Scientific, Canton, MI). Briefly, samples and controls were added to reconstituted reagent at 30°C, mixed, and incubated in a shaking water bath for 5 min. Samples and controls were then transferred to a 96-well flat bottom plate, and the absorbance was read at 340 nm. The ethanol concentration in mg/dl was calculated according to the manufacturer's defined procedure.
Statistics
All experimental data are expressed as means ± SE. Data were plotted using GraphPad Prism 4.0a (GraphPad Software, San Diego, CA), and the average airway response between different groups was analyzed by Student's t-test or analysis of variance, where applicable, followed by Bonferroni post hoc analysis for multiple comparisons. These tests were used to determine the level of significance between all treatment groups. P < 0.05 was considered to be statistically significant.
RESULTS
Ethanol Feeding Blocks Methacholine-Induced AR
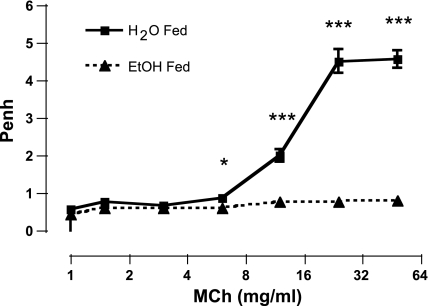
We examined baseline lung function and assessed AR to the inhaled bronchoprovocant MCh. In mice exposed to ethanol in the drinking water, baseline Penh values were the same in control and ethanol-exposed groups of mice (0.57 ± 0.04; 0.46 ± 0.02, respectively). Six weeks of ethanol feeding completely blocked MCh-induced AR (Fig. 1). Administration of MCh caused a dose-dependent increase in AR in the control group of mice, with a highly significant attenuation in the ethanol-fed mice (P < 0.0001) following administration of the 12.0, 24.0, and 48.0 mg/ml doses (Fig. 1). These data demonstrate that short-term ethanol consumption results in a significant attenuation of AR to MCh.
Fig. 1.
Ethanol (EtOH) feeding blocks methacholine (MCh)-induced airway responsiveness (AR). MCh-induced AR was measured in mice drinking water alone (solid line, solid squares) or 20% ethanol (dashed line, solid triangles) for 6 wk. The vertical axis represents enhanced pause (Penh), an indirect measurement of AR. The horizontal axis represents the dose of MCh administered. Ethanol feeding completely blocked AR. ***P < 0.0001, *P < 0.05. Data are represented as means ± SE (n = 8–10 mice/group).
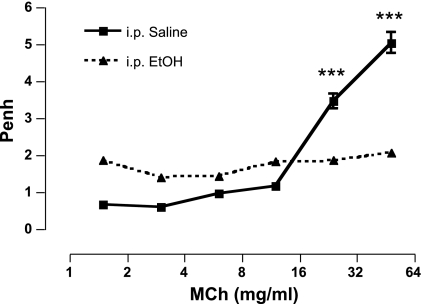
A Single ip Injection of Ethanol Attenuates Methacholine-Induced AR Similar to 6 Wk of Oral Alcohol Feeding
Having established that ingested alcohol blocks AR, we next sought to determine how rapidly this effect occurs. To accomplish this, we exposed groups of mice to ethanol or saline ip. One hour after ip injection, MCh administration resulted in a significant increase in AR of the saline-injected mice, whereas ethanol-injected mice did not respond (5.05 ± 0.81 vs. 2.08 ± 0.23; P < 0.0001). We also noted that 1 h following a single ip injection of ethanol (3 g/kg), baseline values of the ethanol-treated mice were significantly elevated (P < 0.0001) compared with control mice (1.41 ± 0.14 vs. 0.47 ± 0.03, respectively; Fig. 2). BAC analysis revealed that the ethanol-treated mice, in both routes of administration, had elevated blood alcohol levels (>45 mg/dl), whereas water-/saline-exposed control mice had no ethanol present (data not shown). Figures 1 and 2 therefore demonstrate that ethanol-induced attenuation of MCh responsiveness is comparable regardless of the route of administration.
Fig. 2.
Ethanol injection blocks MCh-induced AR. One hour following a single intraperitoneal (ip) dose of ethanol (3 g/kg) or saline, ethanol-injected mice displayed a significant attenuation of AR to aerosolized MCh compared with saline-injected controls. ***P < 0.0001. Data are represented as means ± SE (n = 8–10 mice/group).
Direct Measures of Airway Resistance Corroborate Whole Body Plethysmography AR Following Ethanol Consumption or Single-Dose ip Injection
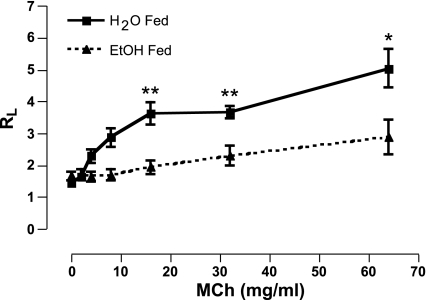
Ethanol feeding model.
Lung resistance values of mice exposed to MCh following 6 wk of 20% EtOH consumption were significantly attenuated compared with mice that drank water for 6 wk (5.08 ± 0.58 vs. 2.88 ± 0.55; P < 0.05; Fig. 3).
Fig. 3.
Direct lung resistance (RL) is decreased in EtOH-fed mice following MCh challenge. Total lung resistance measurements, using a mechanically ventilated mouse system, confirm that 6 wk of EtOH consumption attenuates MCh-induced bronchoconstriction. *P < 0.05, **P < 0.001. Data are expressed as means ± SE (n = 7–9 mice/group).
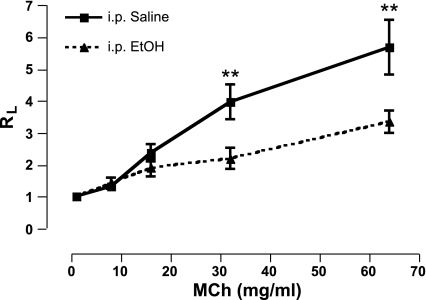
Single-dose ip injection model.
Administration of MCh caused a dose-dependent increase in airway resistance in the saline-injected mice, whereas alcohol-injected mice demonstrated a significant attenuation of lung resistance (5.69 ± 1.7 vs. 3.35 ± 0.69; P < 0.05; Fig. 4). The airway responses to alcohol using an invasive direct measurement of lung resistance method (Figs. 3 and 4) complimented our noninvasive whole body plethysmography findings (Figs. 1 and 2). Because alcohol rapidly affected AR, it was important to determine if this was a transient or persistent effect.
Fig. 4.
Direct lung resistance is decreased in ethanol-injected mice following MCh challenge. Total lung resistance measurements, using a mechanically ventilated mouse system, confirm that ethanol injection attenuates MCh-induced bronchoconstriction. **P < 0.05. Data are expressed as means ± SE (n = 7–9 mice/group).
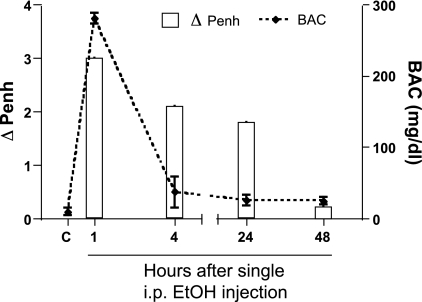
AR Slowly Normalizes To Control Levels Within 48 H of a Single ip Ethanol Injection
AR was assessed at 1, 4, 24, and 48 h after a single ethanol injection. Much to our surprise, and long after alcohol was cleared from the bloodstream, blunted AR persisted for at least 24 h after ip-ethanol injection. The divergence between the two groups decreased with time and was absent at 48 h after injection. Interestingly, the BAC was extremely elevated (∼280 mg/dl) at 1-h postinjection, and by 4 h, this value dropped to near control values (Fig. 5). The behavior of the mice was also changed following the ip injection. At 1-h postinjection, the mice were extremely intoxicated, exhibited an elevated baseline, and attenuated airway response compared with saline-injected control mice. By 4 h after injection, however, the mice were moving around as before they were exposed to the ethanol, even though the attenuated airway response persisted. Twenty-four hours after injection, the mice exhibited normal behavior, and again, the attenuated airway response persisted. Forty-eight hours after injection, the airway responses returned to control values. To determine the relevance of the ip reversal studies, we also examined AR in mice fed alcohol for 6 wk and then weaned back to drinking water.
Fig. 5.
AR blunting persists up to 24 h after alcohol injection. The left vertical axis (bars) represents the change in Penh between saline- and ethanol-injected mice at the 48 mg/ml MCh dose for each time point. The right vertical axis (dashed line) represents the blood alcohol concentrations (BAC) for each time point. The horizontal axis represents the time (hours) after a single injection of saline or alcohol. The BAC levels in the mice peak around 1 h and are rapidly declined to near control levels by 4 h after injection. C, control mice that were not exposed to any ethanol. AR is blunted in the alcohol-injected mice for at least 24 h and long after BAC levels have dropped. Data are represented as means ± SE (n = 8–10 mice/group).
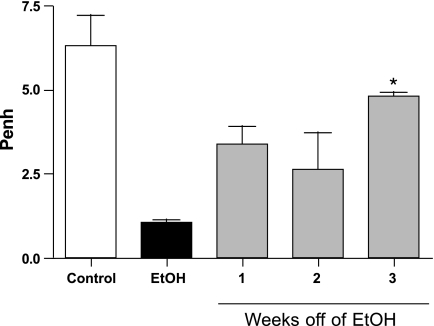
Ethanol-Induced Attenuation of AR Normalizes When Alcohol Is Removed From the Drinking Water
To determine if the attenuated AR observed after 6 wk of alcohol feeding was reversible, ethanol was slowly removed from the drinking water, and AR was assessed at 1, 2, and 3 wk following removal. Mice weaned off of ethanol demonstrated significantly (P < 0.05) elevated AR compared with ethanol-consuming mice (Fig. 6). The attenuated AR gradually normalized and returned to the level of the water-drinking control mice after a 3-wk time period. We also observed that when exposure to ethanol persists for extended periods of time (12 wk), the AR remains attenuated as long as the mice continue to consume ethanol (data not shown). Blood alcohol levels in these weaned mice are indistinguishable from the control mice after alcohol removal.
Fig. 6.
Attenuated AR is slowly reversible. Mice that drank 20% ethanol showed an attenuation of responsiveness to MCh challenge. After a 3-wk time period, the mice weaned off of ethanol showed a recovery in their response to MCh challenge with significant differences observed between the EtOH only and the 3-wk weaned groups (*P < 0.01). The 1- and 2-wk weaned groups demonstrated no significant difference from the EtOH-only group (P > 0.05). Data are represented as means ± SE (n = 8–10 mice/group).
DISCUSSION
The effect of ethanol on the body is well known and includes an increasing number of effects on the lung. Some effects that ethanol exerts on the lungs include inhibition of clearance (4), impaired ciliary motility (4, 10, 40), inflammation (37), suppression of pulmonary neutrophil recruitment (7), and suppression of lung chemokine production (5). While much is known about how alcohol alters lung clearance and the susceptibility to infection, little is known about the effects of alcohol on other airway functions, such as the regulation of bronchial motor tone and AR.
For centuries, alcohol has been used as a treatment for asthma, dating as far back as the Egyptian papyri (23). Several small clinical studies have shown benefits of alcohol as a treatment for asthma. In 1863, Hyde Salter (29) reported improvement in asthma symptoms in three of his patients who self-administered high amounts of oral alcohol. One-hundred years later, Herxheimer and Stresemann (17) saw improvements in the vital capacity of asthmatics after alcohol consumption. In 1947, Brown (6) administered alcohol (intravenous) for the first time to children that were unresponsive to current asthma therapy and noted a bronchodilator effect and a rapid improvement in their symptoms. Because alcohol has been used as an experimental treatment for asthma, we explored the effects of alcohol on bronchial motor tone and AR in a mouse model of alcohol exposure.
Our data demonstrate that feeding ethanol to mice attenuates AR. In mice that drank ethanol, we found a significant attenuation of AR after 1 wk of consumption and a total block following 6 wk of consumption compared with water-drinking control mice. This blocking effect continues as long as the mice continue to consume alcohol. We also examined the effects of short-term alcohol exposure on bronchial reactivity using ip-injected mice. AR attenuation was also observed in the groups of mice that were ip injected with ethanol compared with ip-injected saline control mice. When utilizing the more invasive method of lung resistance, we observed attenuation in the total lung resistance of mice that either consumed ethanol for 6 wk or mice that received a single ip injection of ethanol. We can therefore conclude that the degree of ethanol-mediated attenuation of MCh-induced hyperresponsiveness caused by ethanol is equivalent when these two independent methods are compared in this model. Together, we have shown that alcohol exposure, via two different routes of administration, and two separate methods of analysis of airway response, yields the same outcome and supports historical evidence that the effect of alcohol as a bronchodilator is dependent on the concentration, duration, and route of exposure (31).
The attenuated AR caused by ethanol was reversed over time. To our surprise, however, this alcohol-mediated effect persisted for days (ip) or for weeks (fed) after removal of alcohol. When the ethanol was replaced with water in the alcohol-feeding model, the attenuated AR slowly returned to that of the control mice. As long as ethanol remained in the water, an attenuated/blocked response was observed, suggesting that the alcohol had modified a pathway or changed how the airway cells were reacting to the methacholine challenge. The same finding was also observed in mice that were injected with ethanol. These data demonstrate a sustained alcohol-induced change in the behavior of the airway. Blood alcohol levels were monitored throughout the experiments and showed elevated levels of alcohol in the ethanol groups of mice. At 24 h after alcohol injection, the attenuated AR persisted long after alcohol was absent from the blood and cleared from the tissues, suggesting an unknown mechanism that requires further exploration.
Several possible mechanisms for the ethanol-induced attenuation of airway responses in these mice may include: 1) a change in membrane potential; 2) an altered sensitivity to calcium; 3) altered cAMP-dependent protein kinase (PKA) levels; and 4) a change in nitric oxide (NO)/PKG levels. Ethanol-induced central nervous system suppression of lung responsiveness to methacholine is not the likely mechanism, as we observed similar ethanol-mediated relaxation responses in both whole lung slices and isolated cultures of ASM cells.
One study in canine tracheal smooth muscle demonstrated that alcohol caused hyperpolarization and a suppression of membrane action potentials (27). By suppressing the membrane action potentials, a larger signal would be required to produce contraction.
Various studies also support alcohol-induced modifications in calcium sensitivity as possibly having a role (15, 35). By altering the sensitivity to calcium, the ASM will contract or relax accordingly. In our studies, alcohol exposure may change how the cell utilizes calcium enough to allow the cell to remain in a more relaxed state.
Increased relaxation through the modification of the regulatory kinases including protein kinase A (PKA) and protein kinase G (PKG) (40, 41) may also be a productive mechanism to pursue. By altering PKA or PKG activation in the ASM cells, alcohol may directly affect a key step in multiple known relaxation pathways.
Increased NO production could lead to increased PKG activation resulting in enhanced relaxation. Accordingly, the release of NO represents an additional feasible mechanism for this attenuated response to MCh.
NO has been shown to be a weak bronchodilator in asthmatics, but not in normal subjects (18). Since NO can act as a weak bronchodilator, and increased production of NO with alcohol stimulation in cultured bronchial epithelial cells has been reported (39), the cGMP/NO pathway seems a strong candidate for this response.
In summary, we have demonstrated, for the first time, that ethanol blocks AR regardless of the route of exposure. This effect is slowly reversible over time and surprisingly persists for at least 24 h after alcohol is absent in the blood. Our data have intriguingly demonstrated a novel approach to utilizing alcohol to modify the responsiveness of the airways in mice. Further studies will be necessary to define the specific mechanism(s) of alcohol-induced attenuation of AR.
GRANTS
This work was supported by a Ruth K. Kirschstein National Research Service Award Grant 1F32-AA-017024-01 and National Institute on Alcohol Abuse and Alcoholism Grant 5R37-AA-008769-16.
Acknowledgments
We greatly acknowledge Susmit Das for technical assistance with the mouse ventilator studies and Lisa Chudomelka for help with the preparation of this manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Agrawal DK, Hopfenspirger MT, Chavez J, Talmadge JE. Flt3 ligand: a novel cytokine prevents allergic asthma in a mouse model. Int Immunopharmacol 1: 2081–2089, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Ayres J, Ancic P, Clark TJ. Airways responses to oral ethanol in normal subjects and in patients with asthma. J R Soc Med 75: 699–704, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayres JG, Clark TJ. Intravenous ethanol can provide bronchodilatation in asthma. Clin Sci (Lond) 64: 555–557, 1983. [DOI] [PubMed] [Google Scholar]
- 4.Ballenger JJ Experimental effect of cigarette smoke on human respiratory cilia. N Engl J Med 263: 832–835, 1960. [DOI] [PubMed] [Google Scholar]
- 5.Boe DM, Nelson S, Zhang P, Bagby GJ. Acute ethanol intoxication suppresses lung chemokine production following infection with Streptococcus pneumoniae. J Infect Dis 184: 1134–1142, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Brown EA The use of intravenous ethyl alcohol in the treatment of status asthmaticus. Ann Allergy 5: 193–195, 1947. [PubMed] [Google Scholar]
- 7.Carvalho EM, Brito GA, Pessoa BB, Ribeiro RA, Capaz FR. Long-term ethanol intoxication reduces inflammatory responses in rats. Braz J Med Biol Res 38: 81–89, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Chong BT, Agrawal DK, Romero FA, Townley RG. Measurement of bronchoconstriction using whole-body plethysmograph: comparison of freely moving versus restrained guinea pigs. J Pharmacol Toxicol Methods 39: 163–168, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Cuddy R, Li G. The role of alcohol in asthma: a review of clinical and experimental studies. Am J Emerg Med 19: 501–503, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Elliott MK, Sisson JH, West WW, Wyatt TA. Differential in vivo effects of whole cigarette smoke exposure versus cigarette smoke extract on mouse ciliated tracheal epithelium. Exp Lung Res 32: 99–118, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elliott MK, Sisson JH, Wyatt TA. Effects of cigarette smoke and alcohol on ciliated tracheal epithelium and inflammatory cell recruitment. Am J Respir Cell Mol Biol 36: 452–459, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fredberg JJ Bronchospasm and its biophysical basis in airway smooth muscle. Respir Res 5: 2, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grant BF Prevalence and correlates of alcohol use and DSM-IV alcohol dependence in the United States: results of the National Longitudinal Alcohol Epidemiologic Survey. J Stud Alcohol 58: 464–473, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Hamelmann E, Schwarze J, Takeda K, Oshiba A, Larsen GL, Irvin CG, Gelfand EW. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am J Respir Crit Care Med 156: 766–775, 1997. [DOI] [PubMed] [Google Scholar]
- 15.Hanazaki M, Jones KA, Perkins WJ, Warner DO. The effects of ethanol on Ca2+ sensitivity in airway smooth muscle. Anesth Analg 92: 767–774, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Happel KI, Odden AR, Zhang P, Shellito JE, Bagby GJ, Nelson S. Acute alcohol intoxication suppresses the interleukin 23 response to Klebsiella pneumoniae infection. Alcohol Clin Exp Res 30: 1200–1207, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Herxheimer H, Stresemann E. Ethanol and lung function in bronchial asthma. Arch Int Pharmacodyn Ther 144: 310–314, 1963. [PubMed] [Google Scholar]
- 18.Hogman M, Frostell C, Arnberg H, Hedenstierna G. Inhalation of nitric oxide modulates methacholine-induced bronchoconstriction in the rabbit. Eur Respir J 6: 177–180, 1993. [PubMed] [Google Scholar]
- 19.Humbles AA, Lloyd CM, McMillan SJ, Friend DS, Xanthou G, McKenna EE, Ghiran S, Gerard NP, Yu C, Orkin SH, Gerard C. A critical role for eosinophils in allergic airways remodeling. Science 305: 1776–1779, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Joshi PC, Guidot DM. The alcoholic lung: epidemiology, pathophysiology, and potential therapies. Am J Physiol Lung Cell Mol Physiol 292: L813–L823, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Justice JP, Shibata Y, Sur S, Mustafa J, Fan M, Van Scott MR. IL-10 gene knockout attenuates allergen-induced airway hyperresponsiveness in C57BL/6 mice. Am J Physiol Lung Cell Mol Physiol 280: L363–L368, 2001. [DOI] [PubMed] [Google Scholar]
- 22.King GG, Pare PD, Seow CY. The mechanics of exaggerated airway narrowing in asthma: the role of smooth muscle. Respir Physiol 118: 1–13, 1999. [DOI] [PubMed] [Google Scholar]
- 23.Leake C The Old Egyptian Medical Papyri. Lawrence, KS: University of Kansas Press, 1952.
- 24.Lu FL, Johnston RA, Flynt L, Theman TA, Terry RD, Schwartzman IN, Lee A, Shore SA. Increased pulmonary responses to acute ozone exposure in obese db/db mice. Am J Physiol Lung Cell Mol Physiol 290: L856–L865, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Ma X, Cheng Z, Kong H, Wang Y, Unruh H, Stephens NL, Laviolette M. Changes in biophysical and biochemical properties of single bronchial smooth muscle cells from asthmatic subjects. Am J Physiol Lung Cell Mol Physiol 283: L1181–L1189, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Oldenburg PJ, Mustafa SJ. Involvement of mast cells in adenosine-mediated bronchoconstriction and inflammation in an allergic mouse model. J Pharmacol Exp Ther 313: 319–324, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Richards IS, Kulkarni AP, Brooks SM. Ethanol-induced bronchodilatation in TEA-treated canine tracheal smooth muscle is mediated by a beta-adrenoceptor-dependent mechanism. Eur J Pharmacol 167: 155–160, 1989. [DOI] [PubMed] [Google Scholar]
- 28.Rivera-Sanchez YM, Johnston RA, Schwartzman IN, Valone J, Silverman ES, Fredberg JJ, Shore SA. Differential effects of ozone on airway and tissue mechanics in obese mice. J Appl Physiol 96: 2200–2206, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Salter H On the treatment of asthmatic paroxysm by fulkl doses of alcohol. Lancet ii: 558–559, 1863.
- 30.Shore SA, Rivera-Sanchez YM, Schwartzman IN, Johnston RA. Responses to ozone are increased in obese mice. J Appl Physiol 95: 938–945, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Sisson JH Alcohol and airways function in health and disease. Alcohol 41: 293–307, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Solway J, Fredberg JJ. Perhaps airway smooth muscle dysfunction contributes to asthmatic bronchial hyperresponsiveness after all. Am J Respir Cell Mol Biol 17: 144–146, 1997. [DOI] [PubMed] [Google Scholar]
- 33.Song K, Coleman RA, Zhu X, Alber C, Ballas ZK, Waldschmidt TJ, Cook RT. Chronic ethanol consumption by mice results in activated splenic T cells. J Leukoc Biol 72: 1109–1116, 2002. [PubMed] [Google Scholar]
- 34.Sterk PJ, Bel EH. Bronchial hyperresponsiveness: the need for a distinction between hypersensitivity and excessive airway narrowing. Eur Respir J 2: 267–274, 1989. [PubMed] [Google Scholar]
- 35.Swartz MH, Repke DI, Katz AM, Rubin E. Effects of ethanol on calcium binding and calcium uptake by cardiac microsomes. Biochem Pharmacol 23: 2369–2376, 1974. [DOI] [PubMed] [Google Scholar]
- 36.Tabakoff B, Nelson E, Yoshimura M, Hellevuo K, Hoffman PL. Phosphorylation cascades control the actions of ethanol on cell cAMP signalling. J Biomed Sci 8: 44–51, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Trevisani M, Gazzieri D, Benvenuti F, Campi B, Dinh QT, Groneberg DA, Rigoni M, Emonds-Alt X, Creminon C, Fischer A, Geppetti P, Harrison S. Ethanol causes inflammation in the airways by a neurogenic and TRPV1-dependent mechanism. J Pharmacol Exp Ther 309: 1167–1173, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Woolcock AJ, Peat JK. Epidemiology of bronchial hyperresponsiveness. Clin Rev Allergy 7: 245–256, 1989. [DOI] [PubMed] [Google Scholar]
- 39.Wyatt TA, Forget MA, Sisson JH. Ethanol stimulates ciliary beating by dual cyclic nucleotide kinase activation in bovine bronchial epithelial cells. Am J Pathol 163: 1157–1166, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wyatt TA, Gentry-Nielsen MJ, Pavlik JA, Sisson JH. Desensitization of PKA-stimulated ciliary beat frequency in an ethanol-fed rat model of cigarette smoke exposure. Alcohol Clin Exp Res 28: 998–1004, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wyatt TA, Sisson JH. Chronic ethanol downregulates PKA activation and ciliary beating in bovine bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 281: L575–L581, 2001. [DOI] [PubMed] [Google Scholar]
- 42.Yao L, Asai K, Jiang Z, Ishii A, Fan P, Gordon AS, Diamond I. Dopamine D2 receptor inhibition of adenylyl cyclase is abolished by acute ethanol but restored after chronic ethanol exposure (tolerance). J Pharmacol Exp Ther 298: 833–839, 2001. [PubMed] [Google Scholar]