Abstract
Lung morphogenesis requires precise coordination between branching morphogenesis and vascularization to generate distal airways capable of supporting respiration at the cell-cell interface. The specific origins and types of blood vessels that initially form in the lung, however, remain obscure. Herein, we definitively show that during the early phases of lung development [i.e., embryonic day (E) 11.5], functional vessels, replete with blood flow, are restricted to the mesenchyme, distal to the epithelium. However, by day E14.5, and in response to epithelial-derived VEGF signals, functional vessels extend from the mesenchyme to the epithelial interface. Moreover, these vessels reside adjacent to multipotent mesenchymal stromal cells that likely play a regulatory role in this process. As well as and distinct from the systemic vasculature, immunostaining for EphrinB2 and EphB4 revealed that arterial and venous identity is not distinguishable in emergent pulmonary vasculature. Collectively, this study provides evidence that lung vascularization initially originates in the mesenchyme, distal to the epithelium, and that arterial-venous specification does not exist in the early lung. At a mechanistic level, we show that basilar epithelial VEGF prompts endothelial cells to move toward the epithelium where they undergo morphogenesis during the proliferative, canalicular stage. Thus our findings challenge existing notions of vascular origin and identity during development.
Keywords: vascular, vessel, EphrinB2, EphB4, platelet endothelial cell adhesion molecule-1, lectin, blood flow, fate specification
during embryonic development, lung morphogenesis is initiated by the outpouching, elongation, and penetration of the ventrolateral foregut wall into the underlying splanchnopleuric mesoderm at the laryngotracheal groove. This results in the formation of two rudimentary lung buds composed of inner epithelial cells and surrounding mesoderm. Within this microenvironment, cells communicate with one another, the extracellular matrix, and soluble growth factors to give rise to progressive remodeling and development of the immature lung. Recent studies have shown a direct synergism between neovascularization and lung morphogenesis, one that is supported by lung morphological abnormalities following vascular alteration (10, 28, 44, 51). Such studies have significantly contributed to the conclusion that vascular regulatory factors play a crucial role in lung morphogenesis.
Vessel formation within the embryo is governed by interactions between endothelial cells, mural cells, and the extracellular matrix (27). It is believed that angiogenic and vasculogenic forces (6, 42) work in concert to form the lung vasculature. However, the precise way in which vessels form during lung development remains obscure. Serial reconstruction of human embryonic fetal lungs suggests that during the pseudoglandular stage pulmonary arteries are formed from the splanchnopleural mesenchyme via vasculogenesis, whereas later canalicular and alveolar stages use angiogenic mechanisms (18). In contrast, methacrylate vessel casting and electron microscopy studies indicate that the two vascular networks, vasculogenesis and angiogenesis, form simultaneously but independently from each other with rare communication between the vascular networks early in mouse lung development [embryonic days (E) 13–14], with communication between the two networks gradually increasing until a complete vascular circuit is established by day E17 just before term in the mouse embryo (term = day E18.5; Ref. 11). Vascular-specific reporter transgenic mice studies that use a precursor endothelial marker fetal liver kinase-1 [Flk-1; VEGF receptor-2 (VEGFR2)] suggests that neovascularization occurs throughout lung formation and in conjunction with morphological development (43, 48) in regions where mesenchymal cells closely appose epithelial cells (16). Alternatively, studies examining vascular formation in the vascular-specific marker Tie2-LacZ expression in transgenic mice suggest that the vasculature does not reside initially within the mesenchyme but rather forms by angiogenesis from vessels that grow into the lung mesenchyme (41).
Despite these significant contributions to our understanding of pulmonary neovascularization, it is unclear whether the developing pulmonary vasculature has blood flow during early lung morphogenesis, originates from predominately arterial or venous origins, and is contained within the mesenchyme during early lung morphogenesis. Our studies sought to expand our understanding of pulmonary neovascularization by identifying regions within the lung with vascular blood flow and the fate specification of the pulmonary endothelial cells. To this end, the goals of this present study were: 1) to identify the pulmonary vasculature by staining it with the vascular perfusion of fluorescein-labeled Lycopersicon esculentum (tomato) lectin (25, 31); 2) morphometric and FACS analysis of the arterial EphrinB2 receptor and its venous ligand, EphB4; 3) to identify the endothelial cells responding to the epithelial secretion of VEGF; and 4) to determine whether pluripotent perivascular mesenchymal stromal cells contribute to the endothelial cell population by transdifferentiation.
Here, we demonstrate that pulmonary vascular flow is found initially in the mesenchyme during early pulmonary development (days E11.5–E13.5) and extends toward the epithelial/mesenchymal interface in response to basilar epithelial VEGF resulting in endothelial cell activation. On days E14.5–E15.5, PECAM-1/VEGFR2 vessels containing blood flow reach the epithelial/mesenchymal interface. Intravascular perfusion of fluorescein-labeled L. esculentum lectin indicates that PECAM-1-positive vessels are functional and replete with blood flow. EphrinB2 and EphB4 are coexpressed on PECAM-1-positive endothelial cells and cells that are negative for endothelial cell markers. These studies indicate that initial pulmonary vessel formation occurs within the mesenchyme, is predominately angiogenic, and is guided by basilar epithelial VEGF activation of endothelial cells and that the emerging pulmonary vasculature lacks fate specification.
EXPERIMENTAL PROCEDURES
Fetal lungs were isolated from timed pregnant CD1 pathogen-free mice housed and handled according to the Institutional Animal Care and Use Committee, which approved this study. Animals were mated overnight, and those females who were found with a plug were isolated in separate cages, and the day was noted as day E0.5. On noted days, the fetuses were removed and placed in ice-cold PBS, and the lungs were isolated using microdissection techniques.
Pulmonary blood flow was determined using fluorescein-labeled L. esculentum (tomato) lectin (Vector Laboratories, Burlingame, CA). In contrast to some lectins, intravascular perfusion of fluorescein-labeled L. esculentum lectin binds uniformly to the endothelial surface of normal and inflamed vessels (45). At the designated embryological stage, CD1 pregnant mice were sedated with ketamine/xylazine and received an intracardiac vascular injection of 100 μl of lectin (1 mg/ml). Verification of intracardiac location was confirmed before and after injection by aspiration of blood. After 5 min, the CD1 pregnant mice were euthanized, and the fetuses were removed and placed in ice-cold PBS. Using microdissection, the lungs were isolated and fixed with 4% paraformaldehyde followed by incubation in 30% sucrose 4°C and embedded in optimum cutting temperature compound (OCT). Light exposure was minimized at all steps. Studies were performed on two separate occasions, and multiple fetal lungs were examined.
FACS analysis of fetal lung populations were performed on single cells from dissociated lungs. In brief, microdissection techniques were used to isolate the lung and remove the heart and trachea. Lungs were then rinsed in PBS, cut into smaller pieces, incubated in 0.5% collagenase and 20 μg/ml DNase I for 15–20 min at 37°C with occasional agitation, and gently pipetted. The collagenase was inactivated using 2 ml of ice-cold FBS, centrifuged at 2,000 rpm for 5 min, and suspended in 5 ml of red blood cell (RBC) lysis buffer. Following a 15-min incubation, 2 ml of ice-cold FBS was added, and cells were passed through a 100-μm filter and centrifuged. Cells were then suspended in PBS and 2% FBS solution at a density of 1 × 107 cells per milliliter. Cells (1 × 106) were placed into a 5-ml polystyrene tube, exposed to the labeled conjugated primary antibody [PECAM-1 PE-conjugated (ab23635; Abcam, Cambridge, MA), PECAM-1 FITC-conjugated (ab23359; Abcam), CD90/Thy-1 PE-conjugated (ab25777; Abcam), CD90/Thy-1 FITC-conjugated (ab226; Abcam), biotinylated EphrinB2/Fc chimeric (BT496; R&D Systems, Minneapolis, MN), and biotinylated EphB4 (BAF446; R&D Systems)] for 30 min on ice with agitation every 10 min. After incubation, 2 ml of PBS/2% FBS solution was added to the cells, the cells were spun down, the supernatant was aspirated, and the cell pellet was resuspended in 0.1 ml of PBS/2% FBS. Cells incubated with the conjugated biotinylated primary antibody were then incubated with secondary streptavidin phycoerythrin (1:1,000; Invitrogen) for 30 min on ice in the dark and agitated every 10 min. Following incubation, 2 ml of PBS/2% FBS were added, the cells were pelleted, the supernatant was aspirated, and the cell pellet was resuspended in 1-ml PBS/2% FBS. Cells were analyzed on a Becton Dickinson FACS caliber with sort option (dual beam with 4 color). Cells incubated with no antibody served as the control population. Studies were performed on a minimum of three separate occasions for each condition and time point.
Histological analysis of vascular markers was performed using immunofluorescent techniques. Fetal lungs were rinsed in PBS before fixation with 4% paraformaldehyde and then incubated in 30% sucrose overnight at 4°C. Lungs were then mounted in OCT and placed at −80°C. Sections were permeabilized with 0.1% Triton X-100, rinsed with PBS, blocked using CSA (Zymed, San Francisco, CA), and exposed to the primary antibody for 1 h per manufacturer's instructions [PECAM-1, 1:100, BD Biosciences, San Jose, CA; Thy-1, 1:100, Abcam; EphrinB2, 1:100, R&D Systems; EphB4, 1:100, R&D Systems; VEGF monoclonal antibody, 1:100, Calbiochem, San Diego, CA; VEGFR2, 1:100, Cell Signaling Technology, Danvers, MA; phosphorylated (P) VEGFR2, 1:100, Cell Signaling Technology]. Following washing with PBS, tissues were exposed to the appropriate secondary Cy3 or Alexa 488 fluorescent antibody (Chemicon, Temecula, CA and Molecular Probes/Invitrogen, Carlsbad, CA) for 1 h. For dual localization, primary antibodies from different species were incubated together, whereas same species antibodies were performed separately following repeat blocking and a separate incubation period. This was followed by a 6-min incubation with membrane-permeable 4′,6′-diamidino-2-phenylindole (DAPI; 5 mg/ml at 1:1,000 dilution; Sigma, St. Louis, MO), rinsing with PBS, and mounting. The signal was viewed by fluorescent microscopy at the appropriate wavelength for the secondary antibody on an IX81 Olympus microscope, and images were captured with a Hamamatsu ORCA digital camera using SlideBook software. For lectin blood flow studies, images were captured using a Zeiss LSM 510 META confocal microscope system of the W. M. Keck Center for Collaborative Neuroscience. Studies were performed on two different occasions, and a minimum of four lungs were examined for each immunofluorescence staining procedure.
RESULTS
Early lung vessel formation and extension occurs within the lung mesenchyme and is marked by extensive endothelial cell remodeling and proliferation.
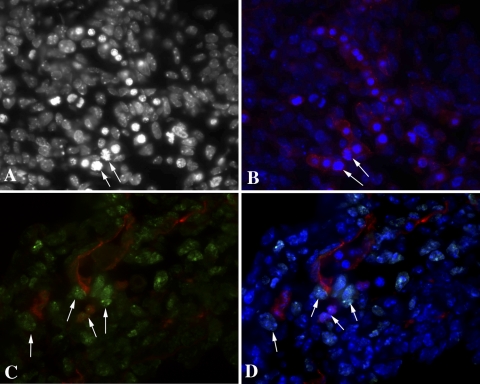
Rudimentary lung tissue outpouches from the ventrolateral foregut wall into the underlying splanchnopleuric mesoderm at the laryngotracheal groove. To determine the initial location of vessel formation, we examined PECAM-1 expression in day E11.5 fetal lung. Vessel formation was limited to the mesenchymal tissue at day E11.5. Indeed, the PECAM-1-positive endothelial cell population was actively dividing and extending within the undifferentiated mesenchyme (Fig. 1B). Interestingly, many of the tip endothelial cells were undergoing mitosis as noted by the DAPI staining (Fig. 1A) and cells adjacent to the last PECAM-1-positive endothelial cell expressing the proliferation marker Ki67 (Fig. 1, C and D).
Fig. 1.
PECAM-1 (Cy3) distribution in embryonic day (E) 11.5 fetal lungs is localized to the mesenchymal regions and proliferating tip endothelial cells (B–D). Mitotic figures are denoted by arrows [A, B, and D: 4′,6′-diamidino-2-phenylindole (DAPI); C and D: Ki67-FITC]. Magnification, ×600.
PECAM-1-positive vessels contain blood flow within the mesenchyme during days E11.5–E13.5 with extension to the epithelial/mesenchymal junction at days E14.5–E15.5.
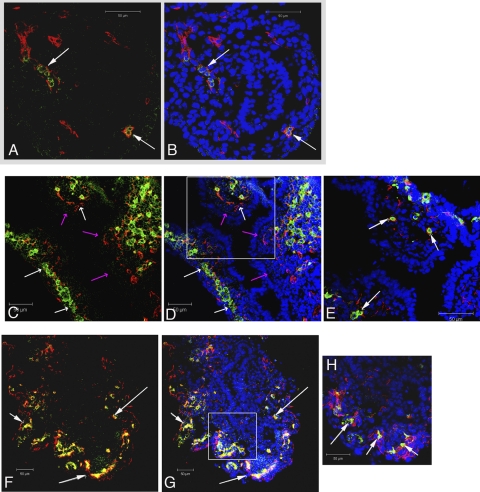
Lung morphogenesis initiates on the ventral aspect of the foregut note by thickening of the foregut epithelium and the subsequent evagination of the laryngotracheal groove. Morphogenetic changes of the endodermal epithelium result in the formation of two small lung buds, composed of inner epithelial pouches surrounded by a thick layer of mesoderm. Vessel casting (11) and documentation of one of the earliest precursor endothelial cell markers, VEGFR2, suggests that vessel in growth occurs in areas where mesenchyme closely apposes epithelial cells (16, 43) without connecting to vasculogenic pools until the later stages of lung development. Because PECAM-1-positive endothelial cell extensions in day E11.5 fetal murine lungs lacked epithelial/mesenchymal cell proximity, we examined blood flow and PECAM-1 staining during the pseudoglandular stage of lung development (days E11.5–E16.5) to define the relationship between epithelial/mesenchymal proximity and vessel formation. This stage of development is defined by epithelial-lined airways (preacinar bronchi) that undergo repeated dichotomous branching (mouse: days E11.5–E16) surrounded by mesenchyme. To overcome the challenges faced in determining fetal lung blood flow patterns, we utilized intravascular injections of fluorescein-labeled L. esculentum lectin in conjunction with PECAM-1 immunohistochemistry for precise endothelial cell localization. Blood flow in days E11.5–E13.5 (Fig. 2, A–H) fetal lungs was limited to the mesenchymal tissue. Furthermore, most of the regions positive for PECAM-1 expression also contained fluorescent lectin. Blood flow and PECAM-1-positive cells were first noted in close proximity to the epithelial/mesenchymal region on day E14.5 (Fig. 3, A and B) and were found in all epithelial/mesenchymal regions by day E15.5 (Fig. 3C).
Fig. 2.
Intravascular-injected fluorescein-labeled Lycopersicon esculentum (tomato) lectin (FITC) identifies blood flow in day E11.5 within the fetal lung mesenchyme surrounded by cells expressing the endothelial cell marker PECAM-1 (Cy3; arrows in A and B). The lectin signal increases in day E12.5 (white arrows in C–E) and day E13.5 (white arrows in F–H) fetal lung mesenchyme but is not found at epithelial/mesenchymal interface (red arrows in C and D; E). Bar = 50 μm.
Fig. 3.
Intravascular-injected fluorescein-labeled L. esculentum (tomato) lectin (FITC) and PECAM-1 (Cy3; white arrows) identify blood flow at the epithelial/mesenchymal interface at day E14.5 (A and B) and day E15.5 (C). Bar = 50 μm.
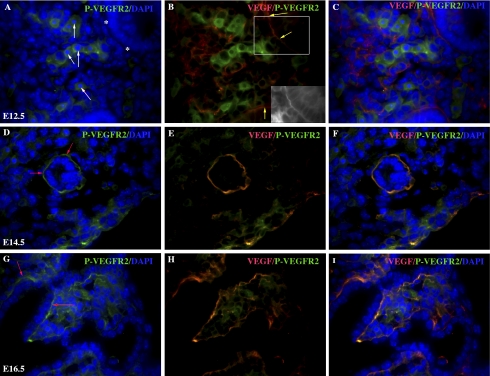
Vessel extension to the epithelial mesenchymal junction is associated with VEGF protein expression at the basilar region of the epithelial cell.
Angiogenesis and vasculogenesis are, in part, a direct response to endothelial mitogen signals such as VEGF. VEGF induces endothelial cell differentiation (49), proliferation, and migration through interaction with its tyrosine kinase receptors and is expressed at the subepithelial matrix during embryonic lung neovascularization (19). To determine whether VEGF expression was one of the factors responsible for attracting endothelial cell extension into the epithelial/mesenchymal region, coimmunofluorescence examined VEGF expression in relationship to phosphorylation of VEGFR2. At day E12.5, VEGF expression localized to the basilar epithelial cell region (Fig. 4B, inset) within the subepithelial matrix as previously reported (available in the data supplement online at the AJP-Lung Cellular and Molecular Physiology web site) and P-VEGFR2 regions with no noted dual localization. In addition, colocalization of VEGF and P-VEGFR2 was noted in some mesenchymal regions consistent with endothelial cell VEGF expression and VEGFR2 phosphorylation (Fig. 4, B and H). In contrast, on days E14.5–E16.5 (Fig. 4, D–F and G–I, respectively), corresponding with blood flow of the epithelial/mesenchymal region, we noted that there was dual localization of VEGF and P-VEGFR2 in this region.
Fig. 4.
Vessel extension in the mesenchyme is in response to basilar epithelial cell VEGF (Cy3). Fetal mouse lungs from timed pregnant mothers indicate VEGF expression (Cy3) at the basilar region of the day E12.5 epithelial cells (yellow arrows and B, inset) as well as the epithelium of day E14.5 (E and F) and day E16.5 (H and I). VEGF-activated endothelial cells, identified by VEGF receptor-2 (VEGFR2) phosphorylation (FITC) surrounding DAPI-positive nuclei (A, white arrows), were found only in the mesenchyme at day E12.5 (A–C) and not at the epithelial/mesenchymal interface (“*” in A). Phosphorylated (P) VEGFR2 colocalized with basilar epithelial VEGF expression at day E14.5 (D, red arrows; D–F) and day E16.5 (G, red arrows; G–I). DAPI denotes nuclear staining. Magnification, ×600.
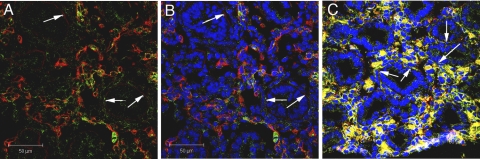
Mesenchymal stromal cells surround and precede extending vessels.
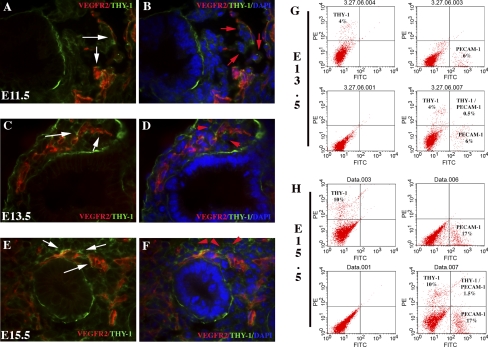
Although the formation of a vascular tube is fundamental to vessel establishment, vessel maturation also relies on the structural stability of smooth muscle cells and pericytes. To determine whether vessel maturation occurred in tandem with vessel formation, we examined the distribution of one mesenchymal stromal marker coexpressed in pericytes, CD90/Thy-1. From days E11.5 to E15.5, Thy-1 distribution was limited to (α-smooth muscle actin-positive; Supplemental Fig. 2) cells surrounding the extending vessel (Fig. 5). Importantly, endothelial markers (VEGFR2 and PECAM-1) did not colocalize with Thy-1 during this period as noted by both dual immunofluorescence and FACS analysis (Fig. 5).
Fig. 5.
Mesenchymal stromal cells (Thy-1-FITC; nuclei noted by red arrows) surround extending vessels at days E11.5–E15.5. VEGFR2 (Cy3; A–F) colocalization indicates that independent cell populations express the 2 different cell surface markers during days E11.5–E15.5. Extending vessels surrounded by stromal cells reach for the epithelial/mesenchymal interface (white arrows in C and E). DAPI denotes nuclear staining. FACS analysis confirms PECAM-1 and Thy-1 are found on separate cell populations at day E13.5 (G) and day E15.5 17% (H). Magnification, ×600.
EphrinB2 and EphB4 are coexpressed on a large number of cells during early lung development with only a small percentage of the cells also expressing the endothelial marker PECAM-1.
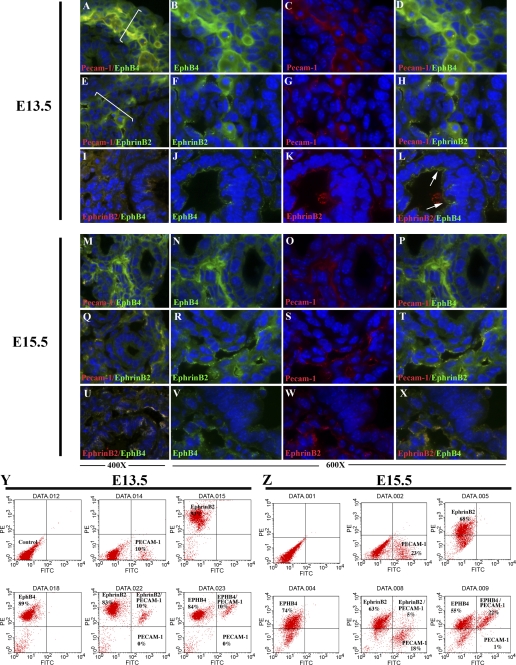
Studies have shown that EphrinB2 and EphB4 reciprocal interactions and bidirectional signaling provide critical guidance cues at cell-cell contact points and are an essential component of vascular morphogenesis (1, 7, 13, 46). EphrinB2 is predominately expressed in arterial endothelial cells, whereas one of its binding partners, the EphB4 receptor, is restricted to venous endothelial cells (1, 46). Recently, EphrinB2 expression was noted on nonendothelial cell populations and found to have properties that regulate smooth muscle cell migration, focal adhesion formation, and spreading that are independent of its cell-cell contact function (14, 15). At days E13.5–E15.5, EphrinB2 was expressed in three predominate regions, endothelial cells, perivascular (Fig. 6, white bars), and in the epithelium (Fig. 6, E–H and Q–T) (but expression is not bound to basement membrane laminin; Supplemental Fig. 3). Furthermore, the cells expressing EphrinB2 and EphB4 are ringed on the basilar edge by the extracellular matrix protein laminin and line a pseudogland, supporting their epithelial cell origin (Supplemental Fig. 3). Similarly, EphB4 expression also localized to these regions (Fig. 6, A–D and M–P). Flow cytometry determined that, at day E13.5, 80–90% of the cells expressed EphrinB2 and EphB4 on their cell surface. Of this population, all PECAM-1-positive cells (9–14%) also coexpressed EphrinB2 and EphB4 (Fig. 6Y). In contrast, by day E15.5, EphrinB2 was represented on 60–65% of the total cell population, whereas EphB4 was expressed on 70–75% of the cells. The number of PECAM-1-positive cells was noted to be 16–23% with a greater ratio of these cells coexpressing the venous marker EphB4 (22%) and a smaller ratio of cells coexpressing EphrinB2 (5%) (Fig. 6Z). The increase in PECAM-1-positive cells suggests that EphrinB2/EphB4-positive cells later develop the expression of this endothelial cell surface marker.
Fig. 6.
EphrinB2 (FITC; E–H and Q–T) and EphB4 (FITC; A–D and M–P) pronounced expression is found in cells encompassing (white line) vascular structures (PECAM-1-Cy3; A–H and M–T) in day E13.5 (A–L) and day E15.5 (M–X) lungs. PECAM-1 (Cy3)-positive cells coexpress EphrinB2 (E–H and Q–T) and EphB4 (A–D and M–P). Epithelial and vascular coexpression of EphrinB2 and EphB4 is found at day E13.5 (I–L) and day E15.5 (U–X). EphrinB2 (Cy3) and EphB4 (FITC) are noted. FACS analysis indicates coexpression of EphrinB2 and EphB4 in PECAM-1-positive cells at day E13.5 (Y). By day E15.5 (Z), PECAM-1-positive cells predominately express the EphB4. Magnification, ×400–600.
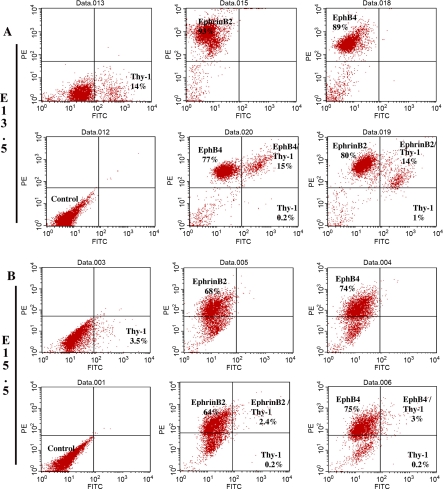
Coimmunofluorescence indicated that EphrinB2 and EphB4 were coexpressed (day E13.5, Fig. 6, I–L; day E15.5, Fig. 6, U–X). Because the perivascular region was noted to also express the stromal marker Thy-1, we performed additional FACS analysis to determine whether either EphrinB2 or EphB4 coexpressed this stromal marker. FACS analysis indicated that a portion of the coexpressing EphrinB2 and EphB4 cell population at day E13.5 (15%) and on day E15.5 (∼3%) also express the cell surface stromal marker Thy-1 (Fig. 7) and was confirmed by dual immunofluorescence of EphrinB2 or EphB4 with Thy-1 (data not shown).
Fig. 7.
FACS analysis of day E13.5 (A) and day E15.5 (B) indicates coexpression of EphrinB2 and EphB4 in a subpopulation of cells that have Thy-1 cell surface expression.
DISCUSSION
The endothelium is the first tissue to differentiate during embryogenesis from a totipotent extraembryonic mesoderm cell population (7.5 days gestational age). During endothelial cell differentiation, there is an ordered progression of identifying cell surface markers beginning with Flk-1/VEGFR2, and this is subsequently followed by the appearance of vascular endothelial cadherin (VE-cadherin), PECAM-1, and CD34+ (39). Importantly, it is the environmental factors, such as hypoxia, the extracellular matrix, growth factors, and other cell populations, that are the main determining factors in endothelial cell differentiation (27). The presence of VEGF promotes pluripotent Flk-1 cells to differentiate into an endothelial cell. In contrast, pluripotent Flk-1 cells differentiate into smooth muscle cells and pericytes following exposure to PDGF-BB (49). Studies have shown that vessel formation is influenced by the cellular microenvironment and epigenetics (2). Although it is known that the lung vasculature is comprised of vessels that arise from intrapulmonary and extrapulmonary sources to form the pulmonary vascular bed (3), it is not clear whether the formation is predominately vasculogenic (18) or a combination of angiogenic and vasculogenic forces (11). Our histological examination of pulmonary blood flow, identification of tip endothelial cell proliferation, and VEGF/VEGFR2 phosphorylation signaling suggests that angiogenesis is the predominate method of vessel formation during early stages of lung development. Although this does not rule out vasculogenic methods of vessel formation during these early stages, the presence of blood flow in the majority of PECAM-1-positive endothelial cells suggests that angiogenesis is the dominating force during early lung vascular development. An alternative explanation is that during early stages of vasculogenic initiated vessel formation, PECAM-1 is not an ideal marker of committed endothelial cells. Although examination of an earlier endothelial cell marker such as VEGFR2 is attractive, it is limited in its usefulness by its distribution to uncommitted endothelial cells and the pluripotent nature of the cells expressing this marker to differentiate into an endothelial cell, smooth muscle cell, or pericyte (49). However, if one examines the colocalization of VEGFR2 phosphorylation and its endothelial differentiating protein, VEGF, one can surmise that this VEGFR2-expressing cell is committing to an endothelial phenotype. Our findings suggest that a cell population expressing P-VEGFR2 in association with VEGF is found in the pulmonary mesenchyme extending toward the epithelial/mesenchymal junction on day E12.5. By days E14.5–E15.5, cells expressing P-VEGFR2 and VEGF are found at the epithelial/mesenchymal interface corresponding with the arrival of PECAM-1-positive blood containing vessels. These findings support a prominent role for angiogenesis in early pulmonary vessel formation.
Vessel remodeling, blood flow, and angiogenesis have been shown to play an important role in embryological development as well as the pathophysiology of ischemic cardiovascular disease and cancer. For example, characteristics such as arterial/venous specification and network patterning are influenced by these forces (29). Although early vessel formation during embryonic development is genetically hardwired through neural guidance genes, Jones et al. (29) recently determined that blood flow is a dynamic process that evokes a high degree of vessel plasticity resulting in vessel identity and network remodeling in the late stages of embryogenesis. Their findings are supported by studies suggesting that dynamic blood flow plays a pivotal role in the formation of the arterial system where flow velocity and vessel branching angle determine the number of arterial branches (33). To better understand whether blood flow has an impact on lung formation, we delivered intravascular fluorescein-labeled lectin to determine blood flow during early lung development. Although there are many types of lectins, studies indicate that L. esculentum lectin tightly binds uniformly to the endothelial surface of normal and inflamed vessels resulting in demarcation of the perfused vessel (45). Three important observations were obtained from the examination of lung blood flow. The first was the location of the vessels that contain blood flow. Blood flow was found in the mesenchymal tissue but not in regions of epithelial/mesenchymal contact during early lung development (days E11.5–E13.5). This is contrary to standard belief the vessel formation occurs simultaneous with lung bud formation at the epithelial/mesenchymal interface. The lack of vessel formation at the epithelial/mesenchymal interface corresponded with the absence of PECAM-1 expression in this region. This suggests that, during the initial phases of lung development, vessel formation has little influence on lung branching. However, blood vessel proximity to the epithelial/mesenchymal junction changes at days E14.5–E15.5. It is during this period that we first note blood flow and PECAM-1-positive cells adjacent to the epithelial cells. There are several possible explanations for the timing of the vessel to reach this interface at this time. One possibility is the need for nutrients and oxygen to this region of the lung. As noted in tumor formation, vessel in growth into the tumor is associated tissue size surpassing 1–2 mm in diameter. Alternatively, it is at this time during lung development that we note the strong basilar epithelial expression of VEGF that may function as a vessel beacon and/or induce differentiation of VEGFR2-positive cells into endothelial cells. Another possibility is that during this phase of lung development, these regions are devoid of perfusion due to pulmonary vascular shunting. The second observation that we noted was that the arrival of blood flow to the epithelial/mesenchymal interface corresponds with the onset of marked epithelial cell proliferation and differentiation. Our third observation was that blood flow is found in lumens surrounded by PECAM-1-positive cells. This provided an endothelial cell marker that could be used to examine arterial vs. venous origin of the blood-containing vessel. Examination of endothelial cells that coexpress PECAM-1 and the venous marker EphB4 or the arterial marker EphrinB2 using immunofluorescence and FACS analysis indicates that, during early lung development, day E13.5 PECAM-1 endothelial cells lack arterial/venous designation as they dual express EphrinB2 and EphB4. This suggests that blood flow has a minimal role in early lung vessel arterial/venous designation. In contrast, by day E15.5, the PECAM-1-positive cells coexpressed distinct arterial or venous markers suggesting a broader role for blood flow in the later stages of lung formation.
VEGF is a key modulator of endothelial cell behavior throughout fetal development, providing guidance for vascular patterning in organ development where variable ratios of the VEGF isoforms are expressed throughout development before arriving ultimately at different levels in adult organs (38). In lung development, the epithelial cell has been identified as being a significant source of VEGF expression (17, 19, 24, 36, 38). Furthermore, recent studies indicate that mesenchymal FGF9 and SHH signaling are essential to capillary plexus formation and VEGFA expression during the early stages of lung development (47). In our studies, we show that VEGF is not only found in the mesenchyme and epithelial cells, but also is localized to the basilar cell surface. In this basilar region, we propose that it serves as an attractant to a PECAM-1-positive cell population that is responding to the VEGF signal as defined by the activated phosphorylated state of VEGFR2. Importantly, the basilar epithelial expression of VEGF and the corresponding P-VEGFR2 signal present on the leading vascular edge suggest that the epithelial basilar VEGF serves as a guidance and endothelial differentiation signal (49). Through this interactive attraction, the vessel migrates toward the epithelial/mesenchymal interface arriving at days E14.5–E15.5. Interruption in this tightly regulated process can result in marked abnormalities in lung morphogenesis, as shown in studies where dysregulation of the amount and cellular phenotype that produces VEGF significantly alters lung morphogenesis (38, 51, 53). In addition, the VEGF basilar epithelial location suggests a morphological role where a reciprocal interaction between the VEGF-expressing basilar epithelial surface and the endothelial cells set in motion the developmental process. Studies have shown that endothelial cell signals are an important component of the morphogenic process. For example, during pancreatic development, key events of pancreatic differentiation occur only in close association with endothelial cells (26, 32). We propose a similar mechanism for lung development. Before day E16.5, cell differentiation is limited at best. This is followed by an intensive period of cell proliferation, vessel extension, and epithelial cell differentiation. VEGF and its spatiotemporal delivery have been shown to pattern and coordinate epithelial/vascular morphogenesis (5, 53), and blockade of lung vascular growth at day E14.5 before the establishment of adequate blood flow to the epithelial/mesenchymal interface results in the blockade of epithelial cell differentiation (44). This supports the concept that the vasculature needs to develop a close physiological relationship with the epithelium at the days E14.5–E15.5 stage to facilitate the mutual signaling between the two cell types and the initiation of lung morphogenic progression.
Working in concert with VEGF in vessel growth are the ephrins, receptor tyrosine kinases that comprise the largest known family of growth factor receptors (50). Key roles for the two EphrinB ligands and the three EphB receptors in demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis have been identified (1). EphrinB2 has been shown to be predominately confined in expression to arterial endothelial cells, whereas its binding partner, the EphB4 receptor, is found in the venous endothelial cells, suggesting defined boundaries between arterial and venous domains. Using these two vessel markers to differentiate between arterial and venous vessel formation, we examined their distribution and frequency in the developing lung. In contrast to prior reports (1, 46), we found that EphrinB2 and EphB4 are coexpressed on the cell surface of 80–90% of the cell population during the early pseudoglandular stage of pulmonary development (day E13.5). To understand the EphrinB2 and EphB4 distribution, we initially determined what percentage of the cell population were endothelial cells. FACS analysis indicated that only 10% of the cell population of the lung expresses the endothelial marker PECAM-1. Interestingly, this population of endothelial cells also coexpressed EphrinB2 and EphB4, suggesting that, at this stage of lung development, endothelial cells have not committed to an arterial or venous designation. However, by day E15.5, the PECAM-1-positive endothelial cells exhibit selective expression of EphrinB2 or EphB4, indicating acquired venous/arterial designation. Interestingly, the vast majority of the endothelial cell population was venous during this stage of lung development. However, the lack of endothelial markers on the other 70% of the cell population suggests that role of EphrinB2/EphB4 is not purely endothelial.
In addition to their specified vascular location, the ephrin-Eph signaling has been implicated in capillary sprouting and having a stimulatory role in the developing vascular system (1). This, in part, is a function of the unique ability of the ephrins and their Eph receptors to signal in a bidirectional fashion resulting in a cell being able to send as well as receive a signal. This type of signaling has been routinely employed in lung morphogenesis where push-pull forces of attractive vs. repulsive functions between semaphorins, netrins, and their receptors express cues that shape the architecture of lung bud morphogenesis (30), thus allowing for the establishment of permissive and restrictive zones during early steps of branching morphogenesis (21). Similarly, in the vasculature, the bidirectional signaling between the ephrins and their Eph receptors has been shown to contribute to vessel formation (40), thus allowing cell motility to be directed by the dual intracellular signaling that occurs in the ligand- or receptor-expressing cells as both can send as well as receive signals (21). However, this does not explain the coexpression of EphrinB2/EphB4 in nonendothelial cells during early development. One possibility is that the bidirectional signaling of the EphrinB2/EphB4 has a role outside vessel formation. This is supported by the recent findings that bidirectional signaling is not limited to vascular structures as EphrinB2 and EphB2 have been shown to play a role in midline cell-cell adhesion and fusion of the endoderm to the underlying mesoderm during urorectal development (13) or axon pathfinding (7). It is therefore plausible that the focal coexpression of EphrinB2/EphB4 in the epithelium during the early stages of lung development is functioning as a bidirectional signaling force that may have an influence on cell-cell adhesion of the epithelium (that arises from the foregut endoderm) to its underlying mesoderm, functions as a pluripotent vessel related-cell, or plays a role in axon guidance within this region.
Following initial vessel formation, it is important that the vessel recruits associated mural cells that will ultimately remodel the vascular bed in order for the vessel to retain stability and functionality (8, 22). Mural cell recruitment and retention is based on a VEGF-stimulated endothelial cell expression of PDGF-B (4, 9, 20, 27, 35). To determine whether the lung neovasculature also was associated with mural cells, we examined the distribution of one such marker, Thy-1 (CD90). Thy-1 is found on the cell surface of mesenchymal stromal cell populations that demonstrate a proliferative response to PDGF (37), express pericyte markers during development, and facilitate progenitor cell differentiation (23). Cells expressing the surface glycoprotein Thy-1 were found in close proximity to endothelial cells. The close proximity of Thy-1-positive cells to the endothelium is consistent with previous reports of mural cell recruitment during retinal vessel formation (12). Interestingly, Thy-1 marker is expressed on certain precursor cell populations that can be selectively transdifferentiated into endothelial cells. Indeed, during adult angiogenesis, differentiating endothelial cells also express Thy-1 surface antigen. However, blood vessels during embryonic angiogenesis were not found to share this characteristic (34). Consistent with these reports, we found that PECAM-1-positive cells did not coexpress the Thy-1 surface marker during lung development. In contrast, Thy-1 cells do coexpress EphrinB2 and EphB4. EphrinB2 and EphB4 have previously been found on stromal cells that can directly influence vessel formation and endothelial cell growth. Stromal cells expressing EphrinB2 promote vascular network formation, endothelial cell proliferation, and induced recruitment and proliferation of α-smooth muscle actin-positive cells, whereas stromal cells expressing EphB4 inhibit these actions (52). This suggests that mural cells may have a regulatory role on vessel formation in this region.
In conclusion, our studies indicate that during early lung development vascular formation is predominately angiogenic in nature, is confined to the mesenchyme, and lacks fate specification. Early pulmonary vessel extension requires extensive vascular remodeling as endothelial cells, surrounded by a stromal cell population, accompanies the vessel toward the epithelial/mesenchymal interface. During the late pseudoglandular stage, in response to basilar epithelial expression of angiogenic factors such as VEGF, vessels extend into the epithelial/mesenchymal interface. Lastly, broad perivascular distribution of EphrinB2 and its receptor, EphB4, suggests a broader role for the ephrins and their Eph receptors in fetal lung development.
GRANTS
Research was supported, in part, by National Heart, Lung, and Blood Institute Grants HL-60061 and HL-75764.
Supplementary Material
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Adams RH, Wilkinson GA, Weiss C, Diella F, Gale NW, Deutsch U, Risau W, Klein R. Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev 13: 295–306, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aird WC Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res 100: 158–173, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Aird WC Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ Res 100: 174–190, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Benjamin LE, Hemo I, Keshet E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development 125: 1591–1598, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Chen RR, Silva EA, Yuen WW, Mooney DJ. Spatio-temporal VEGF and PDGF delivery patterns blood vessel formation and maturation. Pharm Res 24: 258–264, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Coffin JD, Harrison J, Schwartz S, Heimark R. Angioblast differentiation and morphogenesis of the vascular endothelium in the mouse embryo. Dev Biol 148: 51–62, 1991. [DOI] [PubMed] [Google Scholar]
- 7.Cowan CA, Yokoyama N, Saxena A, Chumley MJ, Silvany RE, Baker LA, Srivastava D, Henkemeyer M. Ephrin-B2 reverse signaling is required for axon pathfinding and cardiac valve formation but not early vascular development. Dev Biol 271: 263–271, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Darland DC, D'Amore PA. Cell-cell interactions in vascular development. Curr Top Dev Biol 52: 107–149, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Darland DC, Massingham LJ, Smith SR, Piek E, Saint-Geniez M, D'Amore PA. Pericyte production of cell-associated VEGF is differentiation-dependent and is associated with endothelial survival. Dev Biol 264: 275–288, 2003. [DOI] [PubMed] [Google Scholar]
- 10.DeLisser HM, Helmke BP, Cao G, Egan PM, Taichman D, Fehrenbach M, Zaman A, Cui Z, Mohan GS, Baldwin HS, Davies PF, Savani RC. Loss of PECAM-1 function impairs alveolarization. J Biol Chem 281: 8724–8731, 2006. [DOI] [PubMed] [Google Scholar]
- 11.deMello DE, Sawyer D, Galvin N, Reid LM. Early fetal development of lung vasculature. Am J Respir Cell Mol Biol 16: 568–581, 1997. [DOI] [PubMed] [Google Scholar]
- 12.Dorrell MI, Friedlander M. Mechanisms of endothelial cell guidance and vascular patterning in the developing mouse retina. Prog Retin Eye Res 25: 277–295, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Dravis C, Yokoyama N, Chumley MJ, Cowan CA, Silvany RE, Shay J, Baker LA, Henkemeyer M. Bidirectional signaling mediated by ephrin-B2 and EphB2 controls urorectal development. Dev Biol 271: 272–290, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Foo SS, Turner CJ, Adams S, Compagni A, Aubyn D, Kogata N, Lindblom P, Shani M, Zicha D, Adams RH. Ephrin-B2 controls cell motility and adhesion during blood-vessel-wall assembly. Cell 124: 161–173, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Gale NW, Baluk P, Pan L, Kwan M, Holash J, DeChiara TM, McDonald DM, Yancopoulos GD. Ephrin-B2 selectively marks arterial vessels and neovascularization sites in the adult, with expression in both endothelial and smooth-muscle cells. Dev Biol 230: 151–160, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Gebb SA, Shannon JM. Tissue interactions mediate early events in pulmonary vasculogenesis. Dev Dyn 217: 159–169, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Greenberg JM, Thompson FY, Brooks SK, Shannon JM, McCormick-Shannon K, Cameron JE, Mallory BP, Akeson AL. Mesenchymal expression of vascular endothelial growth factors D and A defines vascular patterning in developing lung. Dev Dyn 224: 144–153, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Hall SM, Hislop AA, Haworth SG. Origin, differentiation, and maturation of human pulmonary veins. Am J Respir Cell Mol Biol 26: 333–340, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Healy AM, Morgenthau L, Zhu X, Farber HW, Cardoso WV. VEGF is deposited in the subepithelial matrix at the leading edge of branching airways and stimulates neovascularization in the murine embryonic lung. Dev Dyn 219: 341–352, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Hellstrom A, Perruzzi C, Ju M, Engstrom E, Hard AL, Liu JL, Albertsson-Wikland K, Carlsson B, Niklasson A, Sjodell L, LeRoith D, Senger DR, Smith LE. Low IGF-I suppresses VEGF-survival signaling in retinal endothelial cells: direct correlation with clinical retinopathy of prematurity. Proc Natl Acad Sci USA 98: 5804–5808, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hinck L The versatile roles of “axon guidance” cues in tissue morphogenesis. Dev Cell 7: 783–793, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Hirschi KK, D'Amore PA. Pericytes in the microvasculature. Cardiovasc Res 32: 687–698, 1996. [PubMed] [Google Scholar]
- 23.Hoppo T, Fujii H, Hirose T, Yasuchika K, Azuma H, Baba S, Naito M, Machimoto T, Ikai I. Thy1-positive mesenchymal cells promote the maturation of CD49f-positive hepatic progenitor cells in the mouse fetal liver. Hepatology 39: 1362–1370, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Hutchings H, Ortega N, Plouet J. Extracellular matrix-bound vascular endothelial growth factor promotes endothelial cell adhesion, migration, and survival through integrin ligation. FASEB J 17: 1520–1522, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Inai T, Mancuso M, Hashizume H, Baffert F, Haskell A, Baluk P, Hu-Lowe DD, Shalinsky DR, Thurston G, Yancopoulos GD, McDonald DM. Inhibition of vascular endothelial growth factor (VEGF) signaling in cancer causes loss of endothelial fenestrations, regression of tumor vessels, and appearance of basement membrane ghosts. Am J Pathol 165: 35–52, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacquemin P, Yoshitomi H, Kashima Y, Rousseau GG, Lemaigre FP, Zaret KS. An endothelial-mesenchymal relay pathway regulates early phases of pancreas development. Dev Biol 290: 189–199, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Jain RK Molecular regulation of vessel maturation. Nat Med 9: 685–693, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Jakkula M, Le Cras TD, Gebb S, Hirth KP, Tuder RM, Voelkel NF, Abman SH. Inhibition of angiogenesis decreases alveolarization in the developing rat lung. Am J Physiol Lung Cell Mol Physiol 279: L600–L607, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Jones EA, le Noble F, Eichmann A. What determines blood vessel structure? Genetic prespecification vs. hemodynamics. Physiology (Bethesda) 21: 388–395, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Kagoshima M, Ito T. Diverse gene expression and function of semaphorins in developing lung: positive and negative regulatory roles of semaphorins in lung branching morphogenesis. Genes Cells 6: 559–571, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Kwan ML, Gomez AD, Baluk P, Hashizume H, McDonald DM. Airway vasculature after mycoplasma infection: chronic leakiness and selective hypersensitivity to substance P. Am J Physiol Lung Cell Mol Physiol 280: L286–L297, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Lammert E, Cleaver O, Melton D. Induction of pancreatic differentiation by signals from blood vessels. Science 294: 564–567, 2001. [DOI] [PubMed] [Google Scholar]
- 33.le Noble F, Fleury V, Pries A, Corvol P, Eichmann A, Reneman RS. Control of arterial branching morphogenesis in embryogenesis: go with the flow. Cardiovasc Res 65: 619–628, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Lee WS, Jain MK, Arkonac BM, Zhang D, Shaw SY, Kashiki S, Maemura K, Lee SL, Hollenberg NK, Lee ME, Haber E. Thy-1, a novel marker for angiogenesis upregulated by inflammatory cytokines. Circ Res 82: 845–851, 1998. [DOI] [PubMed] [Google Scholar]
- 35.Lindblom P, Gerhardt H, Liebner S, Abramsson A, Enge M, Hellstrom M, Backstrom G, Fredriksson S, Landegren U, Nystrom HC, Bergstrom G, Dejana E, Ostman A, Lindahl P, Betsholtz C. Endothelial PDGF-B retention is required for proper investment of pericytes in the microvessel wall. Genes Dev 17: 1835–1840, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miquerol L, Gertsenstein M, Harpal K, Rossant J, Nagy A. Multiple developmental roles of VEGF suggested by a LacZ-tagged allele. Dev Biol 212: 307–322, 1999. [DOI] [PubMed] [Google Scholar]
- 37.Muller I, Kordowich S, Holzwarth C, Spano C, Isensee G, Staiber A, Viebahn S, Gieseke F, Langer H, Gawaz MP, Horwitz EM, Conte P, Handgretinger R, Dominici M. Animal serum-free culture conditions for isolation and expansion of multipotent mesenchymal stromal cells from human BM. Cytotherapy 8: 437–444, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Ng YS, Rohan R, Sunday ME, Demello DE, D'Amore PA. Differential expression of VEGF isoforms in mouse during development and in the adult. Dev Dyn 220: 112–121, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Nishikawa SI, Nishikawa S, Hirashima M, Matsuyoshi N, Kodama H. Progressive lineage analysis by cell sorting and culture identifies FLK1+VE-cadherin+ cells at a diverging point of endothelial and hemopoietic lineages. Development 125: 1747–1757, 1998. [DOI] [PubMed] [Google Scholar]
- 40.Palmer A, Klein R. Multiple roles of ephrins in morphogenesis, neuronal networking, and brain function. Genes Dev 17: 1429–1450, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Parera MC, van Dooren M, van Kempen M, de Krijger R, Grosveld F, Tibboel D, Rottier R. Distal angiogenesis: a new concept for lung vascular morphogenesis. Am J Physiol Lung Cell Mol Physiol 288: L141–L149, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Poole TJ, Coffin JD. Vasculogenesis and angiogenesis: two distinct morphogenetic mechanisms establish embryonic vascular pattern. J Exp Zool 251: 224–231, 1989. [DOI] [PubMed] [Google Scholar]
- 43.Schachtner SK, Wang Y, Scott Baldwin H. Qualitative and quantitative analysis of embryonic pulmonary vessel formation. Am J Respir Cell Mol Biol 22: 157–165, 2000. [DOI] [PubMed] [Google Scholar]
- 44.Schwarz MA, Zhang F, Gebb S, Starnes V, Warburton D. Endothelial monocyte activating polypeptide II inhibits lung neovascularization and airway epithelial morphogenesis. Mech Dev 95: 123–132, 2000. [DOI] [PubMed] [Google Scholar]
- 45.Thurston G, Baluk P, Hirata A, McDonald DM. Permeability-related changes revealed at endothelial cell borders in inflamed venules by lectin binding. Am J Physiol Heart Circ Physiol 271: H2547–H2562, 1996. [DOI] [PubMed] [Google Scholar]
- 46.Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell 93: 741–753, 1998. [DOI] [PubMed] [Google Scholar]
- 47.White AC, Lavine KJ, Ornitz DM. FGF9 and SHH regulate mesenchymal Vegfa expression and development of the pulmonary capillary network. Development 134: 3743–3752, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamamoto Y, Shiraishi I, Dai P, Hamaoka K, Takamatsu T. Regulation of embryonic lung vascular development by vascular endothelial growth factor receptors, Flk-1 and Flt-1. Anat Rec (Hoboken) 290: 958–973, 2007. [DOI] [PubMed] [Google Scholar]
- 49.Yamashita J, Itoh H, Hirashima M, Ogawa M, Nishikawa S, Yurugi T, Naito M, Nakao K. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature 408: 92–96, 2000. [DOI] [PubMed] [Google Scholar]
- 50.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature 407: 242–248, 2000. [DOI] [PubMed] [Google Scholar]
- 51.Zeng X, Wert SE, Federici R, Peters KG, Whitsett JA. VEGF enhances pulmonary vasculogenesis and disrupts lung morphogenesis in vivo. Dev Dyn 211: 215–227, 1998. [DOI] [PubMed] [Google Scholar]
- 52.Zhang XQ, Takakura N, Oike Y, Inada T, Gale NW, Yancopoulos GD, Suda T. Stromal cells expressing ephrin-B2 promote the growth and sprouting of ephrin-B2(+) endothelial cells. Blood 98: 1028–1037, 2001. [DOI] [PubMed] [Google Scholar]
- 53.Zhao L, Wang K, Ferrara N, Vu TH. Vascular endothelial growth factor co-ordinates proper development of lung epithelium and vasculature. Mech Dev 122: 877–886, 2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.