Abstract
Shortened telomeres are a normal consequence of cell division. However, telomere shortening past a critical point results in cellular senescence and death. To determine the effect of telomere shortening on lung, four generations of B6.Cg-Terctm1Rdp mice, null for the terc component of telomerase, the holoenzyme that maintains telomeres, were bred and analyzed. Generational inbreeding of terc−/− mice caused sequential shortening of telomeres. Lung histology from the generation with the shortest telomeres (terc−/− F4) showed alveolar wall thinning and increased alveolar size. Morphometric analysis confirmed a significant increase in mean linear intercept (MLI). terc−/− F4 lung showed normal elastin deposition but had significantly decreased collagen content. Both airway and alveolar epithelial type 1 cells (AEC1) appeared normal by immunohistochemistry, and the percentage of alveolar epithelial type 2 cells (AEC2) per total cell number was similar to wild type. However, because of a decrease in distal lung cellularity, the absolute number of AEC2 in terc−/− F4 lung was significantly reduced. In contrast to wild type, terc−/− F4 distal lung epithelium from normoxia-maintained mice exhibited DNA damage by terminal deoxynucleotidyltransferase (TdT)-mediated dUTP nick end labeling (TUNEL) and 8-oxoguanine immunohistochemistry. Western blotting of freshly isolated AEC2 lysates for stress signaling kinases confirmed that the stress-activated protein kinase (SAPK)/c-Jun NH2-terminal kinase (JNK) stress response pathway is stimulated in telomerase-null AEC2 even under normoxic conditions. Expression of downstream apoptotic/stress markers, including caspase-3, caspase-6, Bax, and HSP-25, was also observed in telomerase-null, but not wild-type, AEC2. TUNEL analysis of freshly isolated normoxic AEC2 showed that DNA strand breaks, essentially absent in wild-type cells, increased with each successive terc−/− generation and correlated strongly with telomere length (R2 = 0.9631). Thus lung alveolar integrity, particularly in the distal epithelial compartment, depends on proper telomere maintenance.
Keywords: terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling, DNA damage, alveolar epithelial type 2 cell
the telomeric sequences that cap the ends of vertebrate chromosomes are maintained by the holoenzyme telomerase, a highly conserved DNA repair enzyme that contains multiple components, including a catalytic subunit (TERT), an RNA priming molecule (Terc), and a number of regulatory subunits (16). In addition to the shortening that occurs as a normal consequence of cell division, telomeres are known targets for chronic oxidative stress, which in turn can compromise chromosomal integrity (22, 36). Multiple studies, both in naturally aging models and in telomerase-null models with genetically manipulated knockdown of the tert or terc genes, have shown that acutely shortened or impaired telomeres can enhance cellular vulnerability to damage (28, 38, 45, 55, 56). In humans, mutations in terc, tert, and telomerase holoenzyme regulatory proteins, such as Nop10 and NHP2, as well as members of the telomere assembly regulatory shelterin complex, including POT1, have been linked to several debilitating syndromes that are characterized by, among other pathologies, excessive telomere shortening. These include autosomal dominant dyskeratosis congenita (DC) and aplastic anemia (4, 5, 25, 43, 63–65).
It is hypothesized that these syndromes and other conditions in which tissue shows chronic damage and inability to repair, including normal aging, manifest because of telomere insufficiency in stem/progenitor cell populations (23, 24, 48, 61). In telomerase-null mouse models, including the B6.Cg-Terctm1Rdp strain created by knockout of the terc gene that codes for the RNA component of the holenzyme, early homozygous null generations are grossly normal (9). However, succeeding generations, carrying shortened telomeres, exhibit anomalies in multiple organs, and the homozygous line eventually becomes infertile (26, 29, 57). These defects can be directly traced to loss of both telomeres and proper telomere maintenance by telomerase, because crossbreeding with wild-type animals restores both telomere length and normal phenotype (59). These studies underlie the hypothesis that a correlation exists between telomere length and the ability of a cell to resist and/or repair injury.
Aging in both mice and humans correlates with natural shortening of chromosomal telomeres (6). It has been speculated that this puts the organism at risk for damage due to chronic oxidative stress, even under normal oxygen saturation (37, 55, 58). Although not directly linked to telomere shortening, systemic susceptibility to the chronic effects of oxidative stress, even as experienced under normoxic conditions, has been observed in multiple mouse aging models, including naturally aged mice and in the Klotho and senescence-accelerated prone mouse (SAM-P) strains, both models for accelerated aging (11, 30). As has been observed by others, we have found (39) that wild-type C57BL/6J mice, whose chromosomes naturally possess extremely long telomeres, are more resistant to the effects of acute hyperoxia than rats, which possess somewhat shorter telomeres. Oxidative stress due to hyperoxia exposure targets many cellular and noncellular components of the lung, including alveolar epithelial cell types 1 (AEC1) and 2 (AEC2) (6, 10, 27, 50, 54, 66, 68). Recovery from oxidative stress, regardless of the nature of the initial injury, is thought to occur via remodeling, requiring participation of multiple cell types. In the case of hyperoxia exposure, we have observed (10, 39) that participation of the surviving AEC2 that are still capable of proliferation is a critical requirement for maintaining lung tissue integrity. A number of genes that contribute to proliferation and differentiation are upregulated during this phase (14). We showed previously (21, 39, 52) that telomerase expression and activity both increase significantly in whole lung and in isolated AEC2 in the repair phase that follows in vivo hyperoxia exposure, although both are negligible in normoxia and during the acute phase of exposure.
To determine the impact that telomere shortening due to telomerase knockdown might have on pulmonary integrity, we obtained first-generation (F1) B6.Cg-Terctm1Rdp mice, which are homozygous null for terc, carry wild-type-length telomeres, and are phenotypically normal. terc−/− F1 mice were inbred for three successive generations to produce F2, F3, and F4 animals. Interestingly, examination of late-generation lung tissue revealed alveolar simplification, decreased collagen content, and, most strikingly, chronic epithelial cell DNA damage and stress. The level of DNA damage in AEC2 freshly isolated from terc−/− mice of each generation correlated strongly with telomere length. Thus we hypothesize that susceptibility to oxidative stress even under normoxia, at both the whole lung and alveolar epithelial cell levels, is directly proportional to chromosomal telomere length.
METHODS
Animals and AEC2 isolation.
Breeding pairs of C57BL/6J wild-type mice and homozygous terc-null B6.Cg-Terctm1Rdp (terc−/−) mice, purchased at age 6–8 wk from Jackson Laboratories (Bar Harbor, ME), were the founders for either wild-type (WT) or terc−/− F2, F3, and F4 animals. For all experiments, mice were used at age 8 ± 1 wk. Animals were housed in pathogen-free conditions and bred and maintained according to a Childrens Hospital Los Angeles Institutional Animal Care Committee-approved protocol. Isolation of murine AEC2 followed our published protocol (39).
Fluorescence-activated cell sorting analysis for telomere length.
Freshly isolated AEC2 from both WT and terc−/− mice were hybridized with FITC-tagged, telomere-specific peptide nucleic acid (PNA) probes for quantitative fluorescence in situ hybridization (Q-FISH) with reagents and protocol provided in the Telomere PNA Kit/FITC for Flow Cytometry (DakoCytomation). A set number of sample cells were mixed with an equivalent number of control cells provided as an internal control, and aliquots of this mixture were hybridized to the telomere-specific probe. Hybridized samples were washed and counterstained for total DNA, and then analyzed by fluorescence-activated cell sorting (FACS) with a logarithmic scale FL1-Height for probe fluorescence and a linear scale FL3-Height for DNA content. Forward scatter, side scatter, FL1-H, and FL3-H values were saved for analysis. The Becton Dickinson FACScan used for acquisition was calibrated for each experiment with FITC-labeled Quantum MESF beads (Bangs Laboratories, Fishers, IN) of standardized fluorescence to create a standard curve, designed to correlate fluorescence intensity to telomere length. All calculations for telomere length were performed on the Bangs Laboratories web site, with software provided by the manufacturer.
Histology and morphometric analysis.
Lungs were inflated and fixed in situ with 10% neutral buffered formalin delivered via the cannulated trachea under fixed water pressure (25 cm) and then ligated, excised, immersed en bloc in formalin, and fixed overnight at 4°C. Standard paraffin embedding was used to prepare lungs for histology and morphometric analysis. Morphometric analyses utilized four 5-μm paraffin-embedded, hematoxylin and eosin (H & E)-stained sections of the lower left lobe from each of three animals. Sections containing large bronchioles and vessels were not included. Each section was subjected to mean linear intercept (MLI) analysis according to published methods (27); 18.0-cm × 12.5-cm digital images at ×40 were overlaid with a grid (lines at 1-cm intervals). Blinded samples were counted for the number of septa that intersected grid lines. MLI for each sample was calculated as the sum of the length of all counting lines divided by the total number of counted intercepts.
Collagen assay.
Analyses of whole lung collagen content, both recently synthesized and insoluble covalently cross-linked fractions, were performed with the Sircol Soluble Collagen Assay Kit from Biocolor. Briefly, the lung was removed from the chest cavity en bloc, weighed, diced, and subjected to acid hydrolysis with overnight incubation in 0.5 M acetic acid at 4°C. After digestion, the acetic acid-soluble fraction was removed and stored at 4°C, while the remaining insoluble fraction was subjected to further digestion by overnight incubation in 6 M hydrochloric acid at 85°C for denaturation to gelatin. Solubilized collagen and gelatin fractions were then analyzed with Sircol kit reagents. Sirius red dye binding of collagen standards was used to create a standard curve that correlated spectrophotometric values (optical density 540 nm) to collagen concentration. Sample soluble and insoluble collagen concentrations were then converted to micrograms of soluble or insoluble collagen per gram of lung wet weight for comparison of whole lung collagen content between WT and terc−/− animals.
TUNEL in situ immunohistochemical analyses and detection of elastin deposition.
Sections fixed and prepared as described were analyzed for DNA damage with terminal deoxynucleotidyltransferase (TdT)-mediated dUTP nick end labeling (TUNEL) in situ with reagents and protocols provided by the In Situ Cell Death Detection (fluorescein) kit (Roche Diagnostics, Indianapolis, IN) according to manufacturer's instructions. Expression of 8-oxoguanine (8-hydroxy-2-deoxyguanosine, 8-OHdG) was detected with the OxyDNA Assay kit from Calbiochem (EMD Bioscience, La Jolla, CA), also according to manufacturer's instructions. Surfactant protein-C (SP-C) and Clara cell secretory protein (CCSP) expression in situ were determined with specific rabbit antibodies from Seven Hills Bioreagents (Cincinnati, OH). Syrian hamster monoclonal antibody to T1a (hybridoma no. 8.1.1) was from the Developmental Studies Hybridoma Bank, University of Iowa. Mouse monoclonal antibody to smooth muscle actin was from Sigma (St. Louis, MO). Cy3-labeled secondary antibodies for rabbit and mouse primary staining were from Molecular Probes/Invitrogen (Carlsbad, CA). Cy3-labeled secondary antibody for hamster primary staining was from Sigma, as were purified rabbit or mouse IgG used as nonspecific primary antibody controls. Elastin fibers were stained with Hart's resorcin-fuchsin. Briefly, deparaffinized and hydrated lung tissue sections were stained with Hart's resorcin-fuchsin working solution overnight at 25°C (42). After washing, sections were counterstained with 0.5% tartrazine-0.25% acetic acid and mounted with Permount. Microscopy and image acquisition were performed with a Leica DM IV microscope and either OpenLab (fluorescence) or SPOT Image (light) software.
FACS analysis of TUNEL-positive AEC2.
Fresh, uncultured AEC2 were fixed with 1% paraformaldehyde in PBS for 15 min on ice. After washing, cells were incubated in 70% ethanol at −20°C for at least 24 h before TUNEL analysis was performed according to the Apo-Direct kit manufacturer's instructions (Pharmingen, San Diego, CA). Briefly, fixed cells were washed and then incubated with TdT enzyme and substrate (FITC-dUTP) for 1 h at 37°C. After washing, cells were counterstained with a propidium iodide-RNase solution. Samples were analyzed with a Becton Dickinson FACScan and CellQuest software. Parameters for TUNEL were set by using the positive and negative control cells supplied with the Apo-Direct kit. For these experiments, 10,000 events were acquired and the nonclumped cells were gated for analysis.
Western blotting.
Fresh, uncultured AEC2 were isolated from age-matched WT F4 and terc−/− F2, F3, and F3 mice as described previously (39). Cells were lysed by incubation in radioimmunoprecipitation assay (RIPA) buffer for 30 min on ice. Insoluble material was pelleted by centrifugation at 13,000 g for 10 min. Forty micrograms of total soluble cellular protein was then analyzed by Western blotting. Mouse monoclonal antibody to β-actin was from ICN Biomedicals (Costa Mesa, CA). Rabbit polyclonal antibodies to murine stress-activated protein kinase (SAPK)/c-Jun NH2-terminal kinase (JNK), phospho-SAPK/JNK (Thr183/Tyr185), phospho-c-Jun (Ser63), p38K, phospho-p38K (Thr180/Tyr182), caspase-3, caspase-6, and Bax were from Cell Signaling Technology (Danvers, MA). Rabbit polyclonal antibody to heat shock protein (HSP)-25 was from StressGen Biotechnologies (Ann Arbor, MI). All antibodies were used at a concentration of 1–2 μg/ml. Horseradish peroxidase-labeled goat anti mouse IgG and goat anti-rabbit IgG from Sigma were used as secondary antibodies at 1:10,000. Specific antibody binding was visualized by using ECL reagents from Amersham to detect chemiluminescence. To analyze differences in protein expression, specific bands from three separate blotting experiments were subjected to densitometric scanning and normalization to actin. Analysis was performed on a Macintosh computer with the public domain NIH Image program (developed at the National Institutes of Health and available at http://rsb.info.nih.gov/nih-image/).
Statistics.
One-way analyses of data where more than two samples were compared were performed with the nonparametric Kruskal-Wallis test or Spearman nonparametric rank correlation. Within these groups, pairwise tests were done with nonparametric rank tests. Where only two sets of data were compared, Student's t-test was used. Data were deemed significant at P < 0.05.
RESULTS
Generational inbreeding of terc−/− mice shortens telomeres.
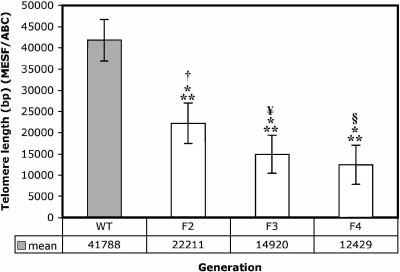
To generate mice that possessed acutely shortened telomeres, terc-null homozygous founders (F1) were bred to produce terc−/− F2 siblings, which in turn were brother-sister mated to produce terc−/− F3 mice. F3 sibling mating produced terc−/− F4 animals. To ensure that inbreeding alone did not cause significant telomere loss, wild-type C57BL/6J mice, the founder strain for the knockout, were bred concurrently to produce WT F2, F3, and F4 generations. A FACS-based telomere length assay that generates data comparable to Southern blotting (31) was used to determine the impact of generational inbreeding on both WT and telomerase-null cohorts. AEC2 freshly isolated from WT F2, F3, and F4 and terc−/− F2, F3, and F4 lungs were hybridized to FITC-labeled, telomere-specific PNA probes. Hybridized AEC2 were then FACS analyzed for level of fluorescence. Mean fluorescence for each cell type was converted to telomere length in base pairs (bp) with a standard curve generated by fluorescein-labeled Quantum MESF beads. Curves were generated each time analyses were performed to limit the influence of acquisition error between data sets. Using this method, we found that telomeres of terc−/− F2, F3, and F4 mice were significantly shorter than those of WT mice of every generation. These data are presented in Fig. 1. (Note that for clarity, data for WT F2 and WT F3, which showed no significant difference compared with WT F4, are not included.) Mean telomere length for WT F4 mice was 41,788 bp (SE ±4,897), while the length of terc−/− F2 telomeres was reduced to a mean of 22,211 bp (SE ±4,769). Further inbreeding produced F3 mice with a mean telomere length of 14,920 bp (SE ±4,499) and F4 animals with a mean telomere length of 12,429 bp (SE ±4,599). This difference from WT was significant for all three terc−/− generations analyzed (P = 0.001). In addition, pairwise analyses showed a significant difference in telomere length between WT F4 and terc−/− F2 (P = 0.0455), terc−/− F3 (P = 0.014), and terc−/− F4 (P = 0.007), and rank correlation showed that the trend toward telomere shortening in the terc−/− animals when analyzed as a group was also significant (P = 0.0142). While the mean telomere length of terc−/− F4 mice was still slightly longer than the average for human chromosomes, reported to range from 6 to 20 kb (mean ∼10 kb) (8), they were considerably shortened from the 40- to 50-kb length reported for inbred C57BL/6J mice and observed in AEC2 isolated from our WT F2, F3, and F4 animals.
Fig. 1.
Determination of telomere length on chromosomes isolated from wild-type (WT) F4 and terc−/− F2, F3, and F4 alveolar epithelial type 2 cells (AEC2). Mean terminal restriction size of telomeres on chromosomes from WT F4 (n = 7) and terc−/− F2 (n = 6), F3 (n = 4), and F4 (n = 5) AEC2 hybridized with a fluorescein-tagged PNA telomere probe is shown. Values are derived from histogram data plotted onto a standard curve generated by FITC-labeled Quantum MESF beads. By Spearman nonparametric rank correlation, **P = 0.001 for differences between WT and terc−/− samples, *P = 0.0142 for differences among terc−/− samples. Nonparametric rank testing was used to compare WT F4 to terc−/−samples: †P = 0.0455, WT F4 vs. terc−/−F2; #P = 0.014, WT F4 vs. terc−/−F3; §P = 0.007, WT F4 vs. terc−/− F4.
Morphometric analysis reveals loss of tissue integrity in terc−/− lung even under normoxic conditions.
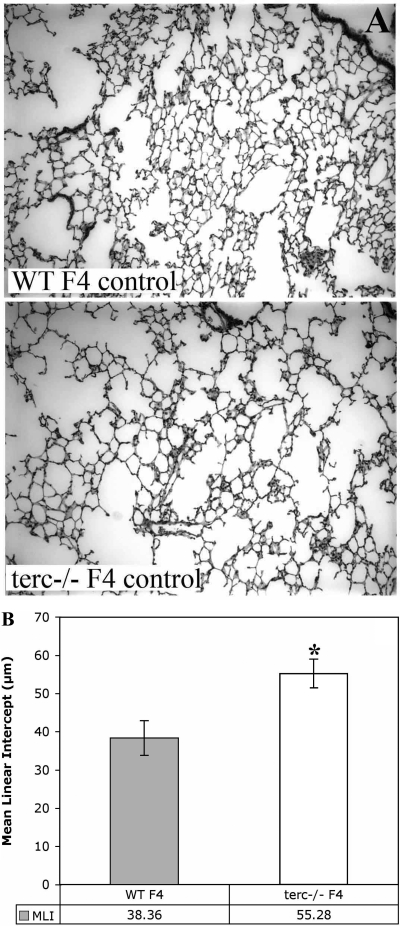
To determine whether decreased telomere length had an impact on the baseline condition of terc−/− lung, we examined distal lung tissue for compromise of structural and/or cellular components. First, histological examination of lung sections from all generations of terc−/− mice revealed some changes in lung parenchyma, trending from mild to more severe in parallel with telomere shortening. To clarify the difference between WT and terc−/− lung, we compared those animals with normal telomeres (WT F4) to those with the most severely shortened telomeres (terc−/− F4). Tissue from normoxia-maintained animals in both groups were analyzed with a number of parameters. First, by histology, the parenchyma of terc−/− F4 mice exhibited increased air space in the form of reduced numbers of alveoli of increased size. Alveolar wall thinning was also observed. Figure 2A shows representative examples from multiple H & E-stained sections obtained from these normoxia-maintained (labeled “control”) mice. Quantitation of changes in alveolar space was performed with MLI analysis of multiple sections for each condition. These data are presented in Fig. 2B. A pairwise nonparametric rank test (where n = 8–12 sections; 3–4 separate regions/section from 2–3 sections of whole left lobe from 2 animals) confirmed a significant increase in MLI, corresponding to increased air space, in terc−/− F4 mice compared with WT F4 mice (P = 0.001). These data indicated a significant loss of tissue integrity and a chronic level of alveolar simplification in terc−/− F4 mice, even under normoxic conditions.
Fig. 2.
Morphometric analysis of distal lung tissue shows an increase in mean linear intercept (MLI) in terc−/− lung under normoxic conditions. A: lung tissue from terc−/− F4 and WT F4 mice maintained in normoxia. Lungs from terc−/− F4 and WT F4 mice were sectioned and stained with hematoxylin and eosin. Sections were prepared from lungs harvested from animals maintained in normoxia (control) and observed at ×20. Representative sections from the 8–12 observed for each sample are presented. B: MLI analysis of lung tissue samples from WT F4 and terc−/− F4 normoxia-maintained mice. Hematoxylin and eosin-stained lung sections from terc−/− F4 and WT F4 animals maintained in normoxia were analyzed for MLI. For each sample, n = 8–12. *P = 0.001.
terc−/− F4 lung produces similar levels of elastin and smooth muscle actin, but lower levels of collagen, than wild type.
To determine whether the changes observed in terc−/− F4 lung were due to an underlying compromise of structural integrity, lung tissue from normoxia-maintained mice were examined for any changes in elastin deposition, smooth muscle actin expression in myofibroblasts, or collagen content. First, sections were fixed and stained with Hart's resorcin-fuchsin solution and then counterstained with tartrazine to detect elastin fibers, according to the method described by Luna (42). As can be seen in Fig. 3, although septal deposition was occasionally decreased in terc−/− F4 alveoli, overall, by densitometry scanning of sections, differences in elastin deposition were not significant (not shown). Likewise, an examination of smooth muscle actin expression in myofibroblasts that surround and support large airways and blood vessels did not show any definitive difference when tissue from WT F4 and terc−/−F4 was examined by immunohistochemistry (not shown). However, biochemical analysis of whole lung collagen content did show a difference between WT and terc−/− strains. Results of whole lung acid hydrolysis of freshly isolated tissue are presented in Fig. 4. Lungs were first assessed for wet weight, which showed no significant difference between WT and terc−/− samples that could be attributed to edema. Both the pool of recently synthesized collagen (designated “soluble collagen”) and the covalently cross-linked fraction (designated “insoluble collagen”) were then measured in tissue harvested from six individual WT F4 or terc−/− F4 animals. These data show a mean insoluble collagen content for WT F4 lung of 207.32 (SE ±21.69) μg/g wet weight, in contrast to 166.6 μg/g wet weight (SE ±28.43) in terc−/− F4 lung. By Student's t-test, this difference was not significant (P = 0.289). However, when soluble collagen content, which represents non-cross-linked, newly synthesized collagen, was analyzed, terc−/− F4 lung contained significantly less than did WT F4 lung [mean 67.53 μg/g (SE ±7.50) vs. mean 123.9 μg/g (SE ±6.34); by Student's t-test, P = 0.002].
Fig. 3.
Elastin deposition in lung tissue from normoxia-maintained WT F4 and terc−/− F4 mice. Lung sections from WT F4 and terc−/− F4 lung were stained with Hart's resorcin-fuchsin, counterstained with tartrazine-acetic acid, mounted with Permount, and observed at ×20. Hart's staining produces dark brown elastin fibers. Elastin deposition was observed along alveolar walls and at the tips of alveolar septa (arrows).
Fig. 4.
Collagen content of lung tissue from normoxia-maintained WT F4 and terc−/− F4 mice. Whole lung from normoxia-maintained WT F4 and terc−/− F4 mice was analyzed for collagen content by acid hydrolysis. For each strain, n = 6. Mean insoluble collagen content in WT F4 lung was 207.32 (SE ±21.69) μg/g wet wt, while mean content of terc−/− F4 lung was 166.60 (SE ±28.43) μg/g wet wt. This difference was not significant. Mean soluble collagen content in WT F4 lung was 123.90 (SE ±6.34) μg/g wet wt, while mean content of terc−/−F4 lung was 67.53 (SE ±7.50) μg/g wet wt. By Student's t-test, this difference was highly significant (*P = 0.002).
terc−/− F4 lung shows a decrease in the absolute number of SP-C-positive alveolar epithelial type 2 cells.
To determine whether the loss of tissue integrity observed in terc−/− F4 mice was also due to an underlying compromise of lung epithelium, lung sections were analyzed for the presence of CCSP-positive airway epithelial cells, T1a-positive AEC1 and SP-C-positive AEC2. To control for nonspecific antibody staining, in some sections purified rabbit or hamster IgG was used as a nonspecific primary antibody controls (not shown). Figure 5A, top, shows no significant difference in the pattern of CCSP-positive cells that line large airways when WT F4 and terc−/− F4 tissue are compared. Both the level of CCSP staining and the ordered arrangement of airway cells appeared similar in the two strains. Likewise, Fig. 5A, middle, shows that the pattern and intensity of T1a staining in AEC1 is similar in WT and terc−/− lung, although this analysis does confirm the altered overall morphology of distal lung tissue in the late-generation knockout animal. Figure 5A, bottom, shows that while lung sections from WT F4 mice exhibited the expected scattered pattern of brightly staining SP-C-positive AEC2, interestingly, the number of SP-C-positive cells per section from the terc−/− F4 mice appeared much lower. This observation was confirmed by quantitation of the number of positive cells per field by counting and averaging data from four fields at ×20 in left lobe sections obtained from two animals (Fig. 5B). These data showed that the absolute number of SP-C-positive cells in terc−/− F4 tissue (mean 19.2/field; SE ±2.88) was significantly lower than the number in WT F4 lung (mean 42.6/field; SE ±6.53) (P = 0.0095 by Student's t-test; n = 8). However, because the cellularity of the terc−/− lung is so compromised, as shown by both morphometry and the reduced numbers of DAPI-positive nuclei in all terc−/− F4 sections examined, we also calculated the percentage of SP-C-positive cells per total number of cells in both WT and telomerase-null lung. These data showed no significant difference in AEC2 percentage as part of the total lung cell population (Fig. 5C). In this analysis, the mean percentage of SP-C-positive cells per total number of cells/field in normoxic WT lung was 12.4% (SE ±0.89), while in normoxic terc−/− F4 lung the number was 10.6% (SE ±4.39). The two-tailed P value for comparison of these populations was not significant (P = 0.6773; n = 8). However, lower absolute numbers of AEC2 in the telomerase-null lung, which implies possible chronic damage to this population and a diminished ability for these cells to serve as a cellular pool for damage repair, could potentially contribute to a reduced defense against oxidative stress.
Fig. 5.
Analysis of Clara cell secretory protein (CCSP)-positive airway cells, T1a-positive AEC1, and surfactant protein-C (SP-C)-positive AEC2 in normoxia-maintained WT F4 and terc−/− F4 samples in situ. A: lung tissue from WT F4 and terc−/− F4 mice maintained in normoxia was analyzed for CCSP and SP-C expression by immunohistochemistry. To control for nonspecific antibody staining, purified rabbit or hamster IgG was used at the same concentration as primary anti-CCSP, anti-T1a, and anti-SP-C antibodies (not shown). Sections were fixed and subjected to immunohistochemistry with the primary antibodies indicated and a Cy3-labeled anti-rabbit IgG secondary antibody. Top: CCSP-positive cells are shown lining the large airways in each sample. Middle: long, thin, T1a-positive AEC1 can be observed lining alveolar walls. Bottom: arrows indicate brightly staining SP-C-positive AEC2 scattered through lung parenchyma. In the WT F4 section, arrows point to a portion of all SP-C-positive cells, while in the terc−/− F4 section, arrows point to all SP-C-positive cells identified. All panels were observed at ×20. B: quantitation of SP-C positive AEC2 present in WT F4 and terc−/− F4 lung tissue. SP-C-labeled sections were observed microscopically, and the number of SP-C-positive cells was counted per microscopic field at ×20. For each sample, n = 8. The mean number of SP-C positive cells per field in normoxic WT lung was 42.6 (SE ±2.88), while in normoxic terc−/− F4 lung, the number was 19.2 (SE ±6.53). The 2-tailed P value for comparison of these populations was highly significant (*P = 0.0095) C: quantitation of SP-C-positive AEC2 present in WT F4 and terc−/−F4 lung tissue as % of total cell number. SP-C-labeled sections were observed microscopically, and the number of SP-C-positive cells was counted per total number of cells (identified by positive DAPI staining) per microscopic field at ×20. For each sample, n = 8. Mean % of SP-C positive cells per field in normoxic WT lung was 12.4% (SE ±0.89), while in normoxic terc−/−F4 lung, the mean was 10.6% (SE ±4.39). The 2-tailed P value for comparison of these populations was not significant (P = 0.6773).
Parenchymal cells in telomerase-null lung exhibit markers for DNA damage.
DNA attacked by reactive oxygen species (ROS) can exhibit both TUNEL, which signifies phosphodiester backbone breakage, and elevated levels of 8-OHdG, a marker for base oxidation (8, 54). Both of these lesions can be repaired, but the presence of TUNEL can also signal the onset of apoptosis, while unrepaired base adducts can be mutagenic and/or lethal (2, 50). To determine why the number of AEC2 and, potentially, other cell populations in normoxia-maintained telomerase-null lung parenchyma was reduced, we examined tissue for TUNEL and for the presence of 8-OHdG adducts. Figure 6A shows representative sections of WT F4 and terc−/− F4 lung from normoxia-maintained animals analyzed for TUNEL in situ. In agreement with our previous observations, as well as those of others, TUNEL in situ of lung sections from WT F4 normoxia-maintained mice showed the expected low levels of DNA damage (10, 39). However, levels of DNA strand breaks in normoxia-maintained terc−/− F4 lung were moderately high. To quantitate the differences in levels of DNA damage, TUNEL-labeled sections were observed microscopically and the number of fluorescein-stained, TUNEL-positive cells was counted per microscopic field at ×20. Non-airway-containing fields were counted in sections of the left lobe of each lung. To normalize for the lower number of nuclei present in terc−/− F4 sections, data were calculated as a percentage of the number of TUNEL-positive nuclei per total DAPI-positive nuclei in each field (Fig. 6B). The percentage of TUNEL-positive cells in sections from WT F4 animals was 2.80% (SE ±2.4), while terc−/− F4 sections exhibited a mean of 30.95% TUNEL-positive cells (SE ±5.19). By Student's t-test, where n = 3 for each sample, this difference was highly significant (P = 0.0044). To determine whether damage caused by base oxidation was also present in normoxia-maintained WT terc−/− F4 lung, sections were stained with a fluorescein-labeled antibody to the 8-OHdG adduct. The resulting data are presented in Fig. 6C, top. The section from WT F4 lung shows no 8-OHdG-positive cells, although an examination of multiple sections did show infrequent positive staining. However, a small number of 8-OHdG-positive cells were routinely observed in tissue from terc−/− F4 lung, often in patches, as shown in Fig. 6C, top right (green arrows). Since both TUNEL and 8-OHdG analysis showed that a chronic, low level of damaged and potentially dying cells was present in normoxia-maintained terc−/− F4 lung tissue, we attempted to identify these cells. Immunohistochemical analysis was performed with double staining for T1a or SP-C and 8-OHdG. Data from representative sections are presented in Fig. 6C, middle and bottom. Figure 6C, middle right, shows that 8-OHdG could be detected in some T1a-positive cells in terc−/− F4 lung (green arrows), indicating the presence of oxidative DNA damage in the AEC1 population of these animals. Likewise, the Fig. 6C, bottom right, shows that some 8-OHdG-positive cells were also positive for SP-C expression (yellow arrows). We noted that OHdG staining in SP-C-positive cells was often cytoplasmic, indicating oxidation in the mitochondrial compartment, as has been observed by examination of lung epithelium under acute hyperoxic conditions (54). In keeping with the observation that some 8-OHdG expression occurs in the AEC1 population, we found that in the SP-C/8-OHdG double-stained sections, 8-OHdG adducts could be found in non-SP-C-positive cells (green arrows). In addition, non-8-OHdG-positive AEC2 were also observed (red arrows). Together, these data indicate that while a portion of the cells containing oxidized DNA were AEC2, not all AEC2 were affected and not all affected cells were AEC2. 8-OHdG immunohistochemical analyses were quantitated by counting the number of 8-OHdG-positive cells per left lobe section in six sections obtained from two individual animals for each strain. These data are shown in Fig. 6D. The number of 8-OHdG-positive cells in normoxic WT F4 lung was consistently low, with a mean of 0.67 cells (SE ±0.41) observed per section. This number rose significantly in normoxic terc−/− F4 lung, to a mean of 6.83 cells (SE ±1.66). By Student's t-test, this difference was significant (P = 0.0042).
Fig. 6.
terc−/− F4 lung tissue exhibits markers for DNA damage under normoxic conditions. A: TUNEL in situ of normoxia-maintained WT F4 and terc−/− F4 samples. Lung tissue samples were subjected to TUNEL to detect cells carrying DNA strand breaks. FITC-labeled dUTP incorporated into damaged DNA appears green, while tissue cell nuclei were stained blue with DAPI. Sections were observed at ×20. B: quantitation of TUNEL in situ of normoxia-maintained WT F4 and terc−/− F4 samples. TUNEL-labeled sections were observed microscopically, and the number of TUNEL-positive cells was counted per total number of cells (by DAPI) in each microscopic field at ×20. Fields were counted from sections obtained the left lobe of each lung. For each sample, n = 3. *P = 0.0044 by Student's t-test. C: 8-oxoguanine (8-OHdG, 8-oxo-dG) immunohistochemical analysis of normoxia-maintained WT F4 and terc−/− F4 samples and colocalization with SP-C expression. Top: lung tissue from WT F4 and terc−/− F4 mice maintained in normoxia was analyzed for 8-OHdG expression by immunohistochemistry. Green arrows indicate brightly staining OHdG-positive cells. Middle: immunohistochemistry for 8-OHdG was combined with staining for T1a expression. T1a-positive and 8-OHdG double-positive cells are indicated by green arrows. Bottom: immunohistochemistry for 8-OHdG was combined with staining for SP-C expression. SP-C-positive cells and OHdG-positive cells are indicated by red and green arrows, respectively. Yellow arrows point to red, SP-C-positive cells with cytoplasmic expression (green) of 8-OHdG. Sections were observed at ×40. D: quantitation of 8-OHdG in situ of normoxia-maintained WT F4 and terc−/− F4 samples. 8-OHdG-labeled sections were observed microscopically, and the number of OHdG-positive cells was counted per whole left lobe section. Six sections from 2 individual animals were analyzed for each sample (n = 6). *P = 0.0042 by Student's t-test.
Elevated expression of activated form of stress signaling pathway kinase SAPK/JNK and its substrate c-jun can be detected in AEC2 isolated from telomerase-null mice maintained in normoxia.
Because some portion of the SP-C-positive AEC2 population exhibits DNA damage in terc−/− F4 mice, we wished to determine whether apoptotic and stress markers could also be found in these cells. Because we also wished to correlate level of damage with telomere length in a quantitative manner, fresh AEC2 isolates from normoxia-maintained WT F4 and terc−/− F2, F3, and F4, all cohorts with established telomere lengths, were analyzed. First, fresh cell isolates were subjected to Western blotting. After normalization of loading by probing for β-actin, expression of the stress signal transducing kinase SAPK/JNK was analyzed. Blots representative of the results of three separate experiments are shown in Fig. 7. No change was observed in expression of SAPK/JNK at the protein level in telomerase-null samples from any generation compared with WT (Fig. 7). However, when the same blot was stripped and reprobed with an antibody to phosphorylated, and therefore activated, SAPK/JNK (Thr183/Tyr185), a clear difference was observed. In normoxia, no activation of SAPK/JNK by phosphorylation was observed in WT F4 AEC2, while the AEC2 isolated from all generations of terc−/− lung showed SAPK/JNK activation. In addition, the activation of one specific downstream target of the SAPK/JNK pathway, the transcription factor c-Jun, was also analyzed. No phosphorylated c-Jun (Ser63) was observed in WT samples, but the protein was phosphorylated to a moderate degree in all terc−/− samples. The level of phosphorylated c-Jun increased in correlation with telomere length, with the highest expression observed in terc−/− F4. By densitometric scanning no significant difference was detected in the level of phosphorylated SAPK/JNK, and although the level of phosphorylated c-Jun expression did increase with decreasing telomere length, the differences among terc−/− generations, when expression was normalized to actin and quantitated by densitometric scanning, was not significant (not shown).
Fig. 7.
AEC2 isolated from normoxia-maintained terc−/− F2, F3, and F4 lung show that stress response signaling is exclusively activated in the stress-activated protein kinase (SAPK)/c-Jun NH2-terminal kinase (JNK) pathway. AEC2 freshly isolated from normoxia-maintained WT and terc−/− lungs were analyzed for protein expression by Western blotting. Data presented are representative of expression in AEC2 isolated from 3 separate animals from each generation (WT F4, terc−/− F2, F3, and F4). Equality of loading was determined by probing for β-actin expression. A: SAPK/JNK is phosphorylated and therefore activated to phosphorylate its downstream target, c-Jun, in normoxia-maintained terc−/− AEC2. The level of expression of stress signaling kinase proteins SAPK/JNK is similar in WT F4 AEC2 and all terc−/− samples. Phosphorylation, and therefore activation, of SAPK/JNK in normoxic AEC2 only occurs in terc−/− AEC2. No significant difference in the level activation was observed among terc−/− generations by densitometric scanning (not shown). Phosphorylation of the JNK downstream target, c-Jun, at Ser63 occurs only in terc−/− AEC2. Although the level of phosphorylation in terc−/− AEC2 trended higher with increasing generation and decreasing telomere length, the difference among terc−/− samples was not significant by densitometric analysis (not shown). B: the stress-activated protein kinase p38K is expressed but not activated in normoxia-maintained terc−/− AEC2. Blots for WT F4 and terc−/− AEC2 from 3 generations showed no expression of p38K in WT AEC2 but elevated expression in all normoxic terc−/− samples. Probing for p38K phosphorylated at Thr180/Tyr182 showed no activation of the kinase in either WT F4 or terc−/− samples. Specificity of the P-p38K antibody was confirmed by inclusion of a positive control sample (+).
Figure 7B shows that in the case of the p38K stress-activated signaling pathway, expression of p38K protein was significantly elevated in all terc−/− AEC2 samples compared with WT. However, it was not phosphorylated, and therefore not activated, in either WT or telomerase-null AEC2 in normoxia. [A positive control sample for phospho-p38K (Thr180/Tyr182) was included in these analyses to confirm antibody activity and specificity.] These data are consistent with observations by others that the SAPK/JNK pathway is activated by sustained and chronic stress, including oxidative stress, while the p38K pathway is more often activated in response to acute stress (46). Thus even maintenance in normoxia, in the absence of acute challenge, appears to induce signaling in a subpopulation of AEC2, which in turn results in the elevated numbers of TUNEL- and 8-OHdG-positive cells observed in situ.
Markers for DNA damage, apoptosis, and oxidative stress can be detected in AEC2 isolated from telomerase-null mice.
To determine whether activated SAPK/JNK signaling and elevated TUNEL and 8-OHdG staining in telomerase-null AEC2 correlated with expression of specific apoptosis- and/or stress-related markers, fresh AEC2 isolates from normoxia-maintained WT F4 and terc−/− F2, F3, and F4, all cohorts with established telomere lengths, were analyzed. First, fresh cell isolates were subjected to Western blotting. After normalization of loading by probing for β-actin expression, the level of expression of downstream apoptotic markers caspases-3 and -6, as well as the apoptotic agonist Bax, were analyzed. A representative set of blots from three separate experiments is shown in Fig. 8A. Essentially none of these apoptotic/stress markers could be observed in AEC2 isolated from WT F4 animals, although a low level of uncleaved caspase-3 was present in those cells. In contrast, AEC2 from terc−/− F2, F3, and F4 animals all showed expression of cleaved and uncleaved caspase-3, caspase-6, and Bax. The expression of the oxidative stress marker HSP-25 was also elevated in terc−/− AEC2, even though these cells were isolated from animals maintained under normoxic conditions. Although expression of cleaved and uncleaved caspase-3 and HSP-25 trended toward higher levels from terc−/− generations F2 to F4, the mean level of expression of these proteins, as well as that of caspase-6 and Bax, did not differ significantly from each other by densitometric analysis of multiple blots (not shown).
Fig. 8.
AEC2 isolated from normoxia-maintained terc−/− F2, F3, and F4 lung exhibit altered expression of markers for DNA damage, oxidative stress, and apoptosis. A: expression of markers for apoptosis and stress in AEC2 isolated from normoxia-maintained terc−/− lung. AEC2 freshly isolated from normoxia-maintained WT and terc−/− lung were analyzed for protein expression by Western blotting. Data presented are representative of expression in AEC2 isolated from 3–5 separate animals from each generation (WT F4, terc−/− F2, F3, and F4). Equality of loading was determined by probing for β-actin expression. The levels of expression of apoptotic markers caspase-3 (uncleaved and cleaved), caspase-6, apoptotic agonist Bax, and stress marker heat shock protein (HSP)-25 were then analyzed. B: TUNEL FACS analysis of AEC2 isolated from normoxia-maintained WT and terc−/− lung. Fresh, uncultured AEC2 isolated from WT F4 and terc−/− F2, F3, and F4 lung were fixed and analyzed by FACS to ascertain level of TUNEL. A representative experiment from 3 repetitions is shown. C: quantitation of TUNEL-positive AEC2 isolated from normoxia-maintained WT and terc−/− lung. Data from 3 separate experiments were analyzed, and the mean numbers of TUNEL-positive AEC2 were compared. In WT F4 animals maintained in normoxia, mean % of TUNEL-positive cells was 2.82% (SE ±1.38). Under the same conditions, mean % of TUNEL-positive AEC2 in terc−/− F2 fresh isolates increased to 11.1% (SE ±2.15). In terc−/− F3 isolates, % was 16.91% (SE ±0.57), while % in terc−/− F4 AEC2 was 19.9% (SE ±4.04). By Spearman nonparametric rank correlation analysis of the difference between WT F4 and terc−/− samples, *P = 0.001. For differences among terc−/− samples as a group, **P = 0.011. D: correlation of telomere length to the level of TUNEL present in AEC2 isolated from normoxia-maintained WT and terc−/− lung. Mean % of AEC2 that were TUNEL positive from each generation were correlated with mean telomere length. Regression analysis gave an R2 value of 0.9631.
Western blotting utilizes whole cell lysates, in which small differences in protein expression may not be detectable or significant. To quantitate the level of DNA damage in AEC2 and determine whether generational differences could be detected at the single-cell level, freshly isolated cells were subjected to TUNEL and analyzed by FACS. These data, presented in Fig. 8B, show that elevated numbers of TUNEL-positive cells are present in telomerase-null lung from F2, F3, and F4 generations of mice, and that the percentage of these cells increases successively with each generation. Mean TUNEL levels in AEC2 from the terc−/− animals were low, but still significant, rising from 2.82% in WT F4 cells to 11.1% in terc−/− F2 and up to 19.9% in terc−/− F4. As can be seen in Fig. 8C, quantitation of TUNEL-positive percentages from three separate animals for each generation and analysis by Spearman nonparametric rank correlation showed a highly significant difference between WT F4 and the terc−/− animals as a group (P = 0.001). In addition, the trend in increased TUNEL in terc−/− as a group, by the same method of analysis, was also significant (P = 0.011). To determine the correlation between TUNEL levels in isolated AEC2 and telomere length, mean values for both were plotted against each other (Fig. 8D). This correlation was very strong, with an R2 value of 0.9631.
DISCUSSION
Compromise of telomerase activity directly affects telomere length and integrity and, in turn, has been implicated in whole animal, whole organ, and cellular function. In normally aging animals, shortened telomeres are markers for cells that have become both vulnerable to damage and inefficient at regeneration and injury repair (6). In humans, mutations in the terc gene have been shown to underlie the rare DC syndromes as well as certain hematologic disorders (43, 63, 65), conditions that feature failure and possible exhaustion of stem/progenitor cell populations. Recently, studies have shown that development of human adult-onset idiopathic pulmonary fibrosis (IPF) correlates with mutations in both tert and terc genes and that shortening of telomeres increases human risk for IPF (3, 4), although these studies have so far not been borne out in a tert-null mouse model (40). However, multiple studies in mice and humans have shown that altered telomerase function and shortening telomeres can have a significant impact on cellular integrity, particularly in the development of both chromosomal and mitochondrial DNA damage due to oxidative stress (13, 23, 41, 44). Our present study shows that this same phenotype can be observed in the lungs of inbred, late-generation terc−/− mice carrying acutely shortened telomeres.
AEC2 are known targets for oxidative stress, both in vivo and in culture (10, 66, 68). Because we see an increasing level of DNA damage in AEC2 with decreasing telomere length, it may be that simply shortening telomeres creates enough chromosomal instability to render cells vulnerable to oxidative damage. This may also underlie the activation of SAPK/JNK and the appearance of apoptosis and stress markers in AEC2 from all generations of terc−/− animals, indicating that even reduction of telomere length by half (from ∼40 kb to ∼20 kb in F2) can have an impact on AEC2 integrity. In the present study, although the telomeres of even fourth-generation terc−/− mice were observed to be, on average, longer than those of average human telomeres (∼12 kb vs. ∼10 kb), the drastic shortening of terc−/− telomeres alone may contribute to murine chromosomal instability, such that cell stress and DNA damage events are eventually triggered. This phenomenon has been observed in both murine and human models, where even the relatively minor reductions in telomere lengths due to aging precipitate chronic cell damage (58). Telomere length itself has also been shown to have an intrinsic impact on the response to chronic and acute oxidative stress in a manner that is exquisitely sensitive to changes in telomere length and is species independent (53, 56, 59).
A chronic level of SAPK/JNK signaling, but no activation of the p38K pathway, is consistent with the low level of DNA damage/apoptotic marker expression observed in terc−/− AEC2 and observations by others that SAPK/JNK is more often activated by chronic stress, while the p38K pathway is induced by acute stress. However, notable exceptions have also been observed (46). The presence of activated SAPK/JNK signaling across terc−/− generations is significant in light of recent studies that have shown that JNK is a critical factor in the onset of pulmonary cell damage that occurs as a result of ischemia-reperfusion injury, hyperoxia, and ventilator-induced injury. Elimination of JNK via genetic knockdown ameliorates the damage caused by these injuries (19, 32, 49). The differential activation of the SAPK/JNK and p38K pathways in normoxic AEC2 indicates divergent signaling upstream of both. Further investigation of upstream kinases and receptors may provide insight into the stimulus for terc−/− AEC2 stress signaling in a normoxic environment.
Because telomerase itself is knocked down in all terc−/− generations, the appearance of stress markers in these animals may also be due to the absence of a necessary activity of telomerase in AEC2 apart from telomere maintenance. Evidence from a number of studies supports a role for telomerase in protection of cellular integrity in addition to a role in telomere maintenance. Possible mechanisms include transport of TERT to mitochondria, where it inhibits the proapoptotic factor Bax and promotes the function of the apoptotic antagonist Bcl-2 (1, 16, 18, 44, 60). This mitochondrial function of TERT is intriguing in light of previous studies that showed that the JNK signaling pathway can be stimulated by signals from damaged mitochondria (20). In addition, our immunohistological observations of terc−/− F4 lung occasionally showed 8-OHdG adducts in the cytoplasm of AEC2, presumably at the site of mitochondrial DNA damage, a documented consequence of both normal aging and telomerase knockdown. However, it must be noted that these observations, and indeed the observation of an increased number of TUNEL-positive AEC2, were made in a relatively small percentage of the total population, even in terc−/− F4 animals. The reason for damage in a discrete population of cells is unknown, but it may indicate focal areas of increased ROS and/or inflammatory cytokines or reflect a subpopulation that is particularly susceptible to low-level environmental stress.
We previously described (21, 39, 52) a specific pattern of telomerase expression and activity induced in whole lung and in AEC2 isolated from wild-type animals exposed to, and recovering from, acute hyperoxia. However, those same studies show that telomerase activity in normoxia-maintained animals is normally quite low. In the absence of data on a positive role for telomerase other than telomere maintenance in the lung under normoxic conditions, we cannot say at this time whether the main cause of the chronic alveolar simplification, collagen depletion, apparent mild lung injury, and loss of AEC2 we observe in late-generation terc−/− mice is due to shortened telomeres alone or to an inability to upregulate telomerase in response to mild but cumulative normoxic stress. Thus what role telomerase itself might play under these baseline conditions, in the absence of any repair stimulus, is unknown. It should be noted that while functional telomerase is absent across all terc−/− generations tested and extremely low in wild-type controls, significant differences among generations were still noted at the level of whole lung and cellular compromise, particularly with regard to DNA damage as analyzed at the single-cell level by TUNEL FACS. Thus the impact of shortening telomere length alone on pulmonary and AEC2 integrity appears to be substantial. Figure 9 presents a possible mechanism for our observations of increasing DNA damage in terc−/− AEC2 carrying progressively shortened telomeres while maintained in normoxia. We hypothesize that in WT AEC2, either the normoxic environment does not induce stress signaling or minor stress events are modulated by both translocation of TERT to mitochondria and the stabilizing influence of normal-length telomeres. In contrast, because terc−/− AEC2 cannot respond to minor stress events by TERT translocation, they are vulnerable to mitochondrial damage, which feeds back to upregulate stress signaling. These effects may be amplified in a dose-dependent manner in terc−/− AEC2 by chronic instability induced by shortened telomeres. Thus terc−/− AEC2 may be both prone to internally induced stress signaling and/or more sensitive to normoxic stress levels, which results in activated SAPK/JNK signaling to the point where a threshold level of cellular and DNA damage leads to apoptosis. The increase in DNA strand breaks that occurs in direct proportion to the decrease in telomere length may be due to an increase in chromosomal stability that triggers internal stress signaling or by another, as yet to be determined mechanism the intensity of which strongly correlates with telomere length. Thus we speculate that while the absence of telomerase inclines terc−/− cells to stress sensitivity, it is the drastic shortening of telomeres that precipitates DNA, and presumably cellular, damage.
Fig. 9.
Schematic for possible mechanisms that underlie the differences observed in cellular integrity of WT AEC2 vs. terc−/− AEC2. In WT AEC2 either the normoxic environment does not induce stress signaling or minor stress events are modulated by both translocation of TERT to mitochondria and the stabilizing influence of normal-length telomeres. Both events feed back to inhibit the JNK signaling cascade, resulting in quiescence and maintenance of cellular integrity. In terc−/− AEC2 cells cannot respond to minor stress events by TERT translocation. Any resulting mitochondrial damage feeds back to upregulate stress signaling. These effects may be amplified in a dose-dependent manner by chronic instability induced by shortened telomeres, leading to loss of cellular integrity, the level of which correlates with telomere length.
Proper telomere length has a documented impact on the stem and progenitor cell populations of a variety of organs (33, 41, 48). It has been postulated that AEC2 are, or contain, the progenitor population for the distal lung epithelium (51). Previously, we showed (52) that the AEC2 total population is indeed heterogeneous, with at least four different subpopulations that can be differentiated based on their response to hyperoxic injury, telomerase activity, and expression of E-cadherin. Kim and colleagues (35) characterized a unique population of distal lung epithelial cells, the bronchioalveolar stem cells, which reside at bronchioalveolar junctions and express markers for both alveolar and distal airway cells. At this point, we do not know what effect knockdown of telomerase and/or telomere shortening has on any of these populations, although CCSP-positive airway cells in the terc−/− mice used in our study appeared normal by morphology. However, the reduction in AEC2 observed in our terc−/− F4 population could indicate that the progenitor population for these cells is affected by telomere shortening. This hypothesis may well describe the other phenomena observed in our terc−/− model, with its chronic damage and alveolar simplification. Although it is beyond the scope of the present study, we are currently analyzing the impact of telomerase knockdown on whole lung and AEC2 recovery from hyperoxia- and hydrogen peroxide-induced oxidative stress, in an attempt to determine how telomerase and telomere dysfunction might impair lung stem/progenitor cells when challenged.
Since the terc−/− mutation is global, we presume telomere shortening has an impact on nonepithelial lung cell types, which could play both direct and indirect roles in AEC2 compromise and the development of the terc−/− lung phenotype. The observed reduction in collagen synthesis in terc−/− F4 animals is presumably due to downregulation of lung fibroblast activity. A critical impact on fibroblast function has been reported by others using a telomerase-null model (40). In addition, changes in endothelial and immunologic cell populations could also affect whole lung integrity and response to insult. Because all these cellular compartments must coordinate in a functional manner to maintain lung health even under normoxic conditions, we acknowledge that the alterations observed in the epithelium of distal lung may be only a part of the underlying cause of altered terc−/− lung morphology and AEC2 integrity. In addition, terc−/− mice, even in later generations, appear grossly normal and do not exhibit outward signs of pulmonary impairment. However, decreased absolute numbers of AEC2 in terc−/− distal lung, along with alveolar simplification and an increased level of epithelial cell DNA damage, could have serious consequences for survival of any increased oxidative challenge. Alterations in cellularity, alveolar size, and proper AEC2 function and surfactant production are strongly associated with the debilitation seen in aging lungs (34, 62), in lung injury (8, 66), and with neonatal respiratory failure and developmental dysfunction (15). In addition, the evidence of chronic SAPK/JNK signaling, which is a well-documented response to the presence of TNF-α as well as oxidative stress, may indicate that a chronic, low level of inflammation exists in these animals. Indeed, activation of all MAPK pathways, with specific pathways activated in specific cell types, has been observed in patients with IPF (67). These findings indicate a potential mechanism for the increased propensity of telomerase-null human patients to develop fibrosis, the acute nature of which has been recently been linked to telomere length (3). An examination of terc−/− lung for altered surfactant expression, evidence of increased inflammation, and susceptibility to fibrosis is currently under way.
In conclusion, the data presented here show that the proper maintenance of telomere length is associated with maintenance of overall lung health even under normoxic conditions. Knockdown of telomerase activity results in accelerated telomere shortening and has a notable impact on lung structural and cellular compartments. Some of these changes, particularly at the level of DNA damage in terc−/− AEC2, correlate strongly with telomere length. A chronic level of stress signaling and apoptotic/stress marker expression, even in normoxia, indicate that telomerase-null lung cells carrying acutely shortened telomeres may be primed for further, more serious injury at a rate far greater than that observed for wild-type lung.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grant 2R01-HL-65352-7 to B. Driscoll.
Acknowledgments
The authors thank Dr. Fred Dorey of the Statistics Core of Childrens Hospital Los Angeles for assistance with data analysis.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Ahmed S, Passos JF, Birket MJ, Beckmann T, Brings S, Peters H, Birch-Machin MA, von Zglinicki T, Saretzki G. Telomerase does not counteract telomere shortening but protects mitochondrial function under oxidative stress. J Cell Sci 121: 1046–1053, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Albertine KH, Plopper CG. DNA oxidation or apoptosis: will the real culprit of DNA damage in hyperoxic lung injury please stand up? Am J Respir Cell Mol Biol 26: 381–383, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Alder JK, Chen JJ, Lancaster L, Danoff S, Su SC, Cogan JD, Vulto I, Xie M, Qi X, Tuder RM, Phillips JA 3rd, Lansdorp PM, Loyd JE, Armanios MY. Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc Natl Acad Sci USA 105: 13051–13056, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armanios MY, Chen JJ, Cogan JD, Alder JK, Ingersoll RG, Markin C, Lawson WE, Xie M, Vulto I, Phillips JA 3rd, Lansdorp PM, Greider CW, Loyd JE. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med 356: 1317–1326, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Atexier C POT of gold: modeling dyskeratosis congenita in the mouse. Genes Dev 22: 1731–1736, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aubert G, Lansdorp PM. Telomeres and aging. Physiol Rev 88: 557–579, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Barker GF, Manzo ND, Cotich KL, Shone RK, Waxman AB. DNA damage induced by hyperoxia: quantitation and correlation with lung injury. Am J Respir Cell Mol Biol 35: 277–288, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blasco MA, Lee HW, Hande MP, Samper E, Lansdorp PM, DePinho RA, Greider CW. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell 91: 25–34, 1997. [DOI] [PubMed] [Google Scholar]
- 10.Buckley S, Driscoll B, Anderson KD, Warburton D. Apoptosis and DNA damage in AEC2 cultured from hyperoxic rats. Am J Physiol Lung Cell Mol Physiol 274: L714–L720, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Butterfield DA, Howard BJ, Yatin S, Allen KL, Carney JM. Free radical oxidation of brain proteins in accelerated senescence and its modulation by N-tert-butyl-alpha-phenylnitrone. Proc Natl Acad Sci USA 94: 674–678, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Canela A, Vera E, Klatt P, Blasco MA. High-throughput telomere length quantification by FISH and its application to human population studies. Proc Natl Acad Sci USA 104: 5300–5305, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan SW, Blackburn EH. New ways not to make ends meet: telomerase, DNA damage proteins and heterochromatin. Oncogene 21: 553–563, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Chen Z, Chintagari NR, Guo Y, Bhaskaran M, Chen J, Gao L, Jin N, Weng T, Liu L. Gene expression of rat alveolar type II cells during hyperoxia exposure and early recovery. Free Radic Biol Med 43: 628–642, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clark JC, Wert SE, Bachurski CJ, Stahlman MT, Stripp BR, Weaver TE, Whitsett JA. Targeted disruption of the surfactant protein B gene disrupts surfactant homeostasis, causing respiratory failure in newborn mice. Proc Natl Acad Sci USA 92: 7794–7798, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cong YS, Wright WE, Shay JW. Human telomerase and its regulation. Microbiol Mol Biol Rev 66: 407–425, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cong Y, Shay JW. Actions of human telomerase beyond telomeres. Cell Res 18: 725–732, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Del Bufalo D, Rizzo A, Trisciuoglio D, Cardinali G, Torrisi MR, Zangemeister-Wittke U, Zupi G, Biroccio A. Involvement of hTERT in apoptosis induced by interference with Bcl-2 expression and function. Cell Death Differ 12: 1429–1438, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Dolinay T, Wu W, Kaminski N, Ifedigbo E, Kaynar AM, Szilasi M, Watkins SC, Ryter SW, Hoetzel A, Choi AM. Mitogen-activated protein kinases regulate susceptibility to ventilator-induced lung injury. PLoS ONE 3: e1601, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dougherty CJ, Kubianski LA, Frazier DP, Li H, Xiong WC, Bishopric NH, Webster KA. Mitochondrial signals initiate the activation of c-Jun N-terminal kinase (JNK) by hypoxia-reoxygenation. FASEB J 18: 1060–1070, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Driscoll B, Buckley S, Bui KC, Anderson KD, Warburton D. Telomerase in alveolar epithelial development and repair. Am J Physiol Lung Cell Mol Physiol 279: L1191–L1198, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci USA 101: 17312–17315, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flores I, Cayuela ML, Blasco MA. Effects of telomerase and telomere length on epidermal stem cell behavior. Science 309: 1253–1256, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Flores I, Benetti R, Blasco MA. Telomerase regulation and stem cell behaviour. Curr Opin Cell Biol 18: 254–260, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Garcia CK, Wright WE, Shay JW. Human diseases of telomerase dysfunction: insights into tissue aging. Nucleic Acids Res 35: 7406–7416, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldman F, Bouarich R, Kulkarni S, Freeman S, Du HY, Harrington L, Mason PJ, Londono-Vallejo A. The effect of TERC haploinsufficiency on the inheritance of telomere length. Proc Natl Acad Sci USA 102: 17119–17124, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris JB, Chang LY, Crapo JD. Rat lung alveolar type I epithelial cell injury and response to hyperoxia. Am J Respir Cell Mol Biol 4: 115–125, 1991. [DOI] [PubMed] [Google Scholar]
- 28.Hemann MT, Rudolph KL, Strong MA, DePinho RA, Chin L, Greider CW. Telomere dysfunction triggers developmentally regulated germ cell apoptosis. Mol Biol Cell 12: 2023–2030, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herrera E, Samper E, Blasco MA. Telomere shortening in mTR−/− embryos is associated with failure to close the neural tube. EMBO J 18: 1172–1181, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hosokawa M, Fujisawa H, Ax S, Zahn-Daimler G, Zahn RK. Age-associated DNA damage is accelerated in the senescence-accelerated mice. Mech Ageing Dev 118: 61–70, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Hultdin M, Gronlund E, Norrback K, Eriksson-Lindstrom E, Just T, Roos G. Telomere analysis by fluorescence in situ hybridization and flow cytometry. Nucleic Acids Res 26: 3651–3656, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishii M, Suzuki Y, Takeshita K, Miyao N, Kudo H, Hiraoka R, Nishio K, Sato N, Naoki K, Aoki T, Yamaguchi K. Inhibition of c-Jun NH2-terminal kinase activity improves ischemia/reperfusion injury in rat lungs. J Immunol 172: 2569–2577, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Ju Z, Jiang H, Jaworski M, Rathinam C, Gompf A, Klein C, Trumpp A, Rudolph KL. Telomere dysfunction induces environmental alterations limiting hematopoietic stem cell function and engraftment. Nat Med 13: 742–747, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Kawakami M, Paul JL, Thurlbeck WM. The effect of age on lung structure in male BALB/cNNia inbred mice. Am J Anat 170: 1–21, 1984. [DOI] [PubMed] [Google Scholar]
- 35.Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 121: 823–835, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Kurz DJ, Decary S, Hong Y, Trivier E, Akhmedov A, Erusalimsky JD. Chronic oxidative stress compromises telomere integrity and accelerates the onset of senescence in human endothelial cells. J Cell Sci 117: 2417–2426, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Lansdorp PM Self-renewal of stem cells. Biol Blood Marrow Transplant 3: 171–178, 1997. [PubMed] [Google Scholar]
- 38.Lechel A, Manns MP, Rudolph KL. Telomeres and telomerase: new targets for the treatment of liver cirrhosis and hepatocellular carcinoma. J Hepatol 41: 491–497, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Lee J, Reddy R, Barsky L, Weinberg K, Driscoll B. Contribution of proliferation and DNA damage repair to alveolar epithelial type 2 cell recovery from hyperoxia. Am J Physiol Lung Cell Mol Physiol 290: L685–L694, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Liu T, Chung MJ, Ullenbruch M, Yu H, Jin H, Hu B, Choi YY, Ishikawa F, Phan SH. Telomerase activity is required for bleomycin-induced pulmonary fibrosis in mice. J Clin Invest 117: 3800–3809, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luiten RM, Pene J, Yssel H, Spits H. Ectopic hTERT expression extends the life span of human CD4+ helper and regulatory T-cell clones and confers resistance to oxidative stress-induced apoptosis. Blood 101: 4512–4519, 2003. [DOI] [PubMed] [Google Scholar]
- 42.Luna LG Manual of Histological Staining Methods of the Armed Forces Institute of Pathology. New York: McGraw-Hill, 1968, p. 79.
- 43.Marrone A, Stevens D, Vulliamy T, Dokal I, Mason PJ. Heterozygous telomerase RNA mutations found in dyskeratosis congenita and aplastic anemia reduce telomerase activity via haploinsufficiency. Blood 104: 3936–3942, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Massard C, Zermati Y, Pauleau AL, Larochette N, Métivier D, Sabatier L, Kroemer G, Soria JC. hTERT: a novel endogenous inhibitor of the mitochondrial cell death pathway. Oncogene 25: 4505–4514, 2006. [DOI] [PubMed] [Google Scholar]
- 45.Masutomi K, Possemato R, Wong JM, Currier JL, Tothova Z, Manola JB, Ganesan S, Lansdorp PM, Collins K, Hahn WC. The telomerase reverse transcriptase regulates chromatin state and DNA damage responses. Proc Natl Acad Sci USA 102: 8222–8227, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsuzawa A, Ichijo H. Molecular mechanisms of the decision between life and death: regulation of apoptosis by apoptosis signal-regulating kinase 1. J Biochem (Tokyo) 130: 1–8, 2001. [DOI] [PubMed] [Google Scholar]
- 47.Mondello C, Scovassi AI. Telomeres, telomerase, apoptosis. Biochem Cell Biol 82: 498–507, 2004. [DOI] [PubMed] [Google Scholar]
- 48.Morrison SJ, Prowse KR, Ho P, Weissman IL. Telomerase activity in hematopoietic cells is associated with self-renewal potential. Immunity 5: 207–216, 1996. [DOI] [PubMed] [Google Scholar]
- 49.Morse D, Otterbein LE, Watkins S, Alber S, Zhou Z, Flavell RA, Davis RJ, Choi AM. Deficiency in the c-Jun NH2-terminal kinase signaling pathway confers susceptibility to hyperoxic lung injury in mice. Am J Physiol Lung Cell Mol Physiol 285: L250–L257, 2003. [DOI] [PubMed] [Google Scholar]
- 50.O'Reilly MA DNA damage and cell cycle checkpoints in hyperoxic lung injury: braking to facilitate repair. Am J Physiol Lung Cell Mol Physiol 281: L291–L305, 2001. [DOI] [PubMed] [Google Scholar]
- 51.Rawlins EL, Hogan BL. Epithelial stem cells of the lung: privileged few or opportunities for many? Development 133: 2455–2465, 2006. [DOI] [PubMed] [Google Scholar]
- 52.Reddy R, Buckley S, Doerken M, Barsky L, Weinberg K, Anderson KD, Warburton D, Driscoll B. Isolation of a putative progenitor subpopulation of alveolar epithelial type 2 cells. Am J Physiol Lung Cell Mol Physiol 286: L658–L667, 2004. [DOI] [PubMed] [Google Scholar]
- 53.Richter T, von Zglinicki T. A continuous correlation between oxidative stress and telomere shortening in fibroblasts. Exp Gerontol 42: 1039–1042, 2007. [DOI] [PubMed] [Google Scholar]
- 54.Roper JM, Mazzatti DJ, Watkins RH, Maniscalco WM, Keng PC, O'Reilly MA. In vivo exposure to hyperoxia induces DNA damage in a population of alveolar type II epithelial cells. Am J Physiol Lung Cell Mol Physiol 286: L1045–L1054, 2004. [DOI] [PubMed] [Google Scholar]
- 55.Rossi DJ, Bryder D, Seita J, Nussenzweig A, Hoeijmakers J, Weissman IL. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature 447: 725–729, 2007. [DOI] [PubMed] [Google Scholar]
- 56.Rubio MA, Davalos AR, Campisi J. Telomere length mediates the effects of telomerase on the cellular response to genotoxic stress. Exp Cell Res 298: 17–27, 2004. [DOI] [PubMed] [Google Scholar]
- 57.Rudolph KL, Chang S, Lee HW, Blasco M, Gottlieb GJ, Greider C, DePinho RA. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell 96: 701–712, 1999. [DOI] [PubMed] [Google Scholar]
- 58.Ruzankina Y, Asare A, Brown EJ. Replicative stress, stem cells and aging. Mech Ageing Dev 129: 460–466, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Samper E, Flores JM, Blasco MA. Restoration of telomerase activity rescues chromosomal instability and premature aging in Terc−/− mice with short telomeres. EMBO Rep 2: 800–807, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Santos JH, Meyer JN, Van Houten B. Mitochondrial localization of telomerase as a determinant for hydrogen peroxide-induced mitochondrial DNA damage and apoptosis. Hum Mol Genet 15: 1757–1768, 2006. [DOI] [PubMed] [Google Scholar]
- 61.Satyanarayana A, Wiemann SU, Buer J, Lauber J, Dittmar KE, Wustefeld T, Blasco MA, Manns MP. Telomere shortening impairs organ regeneration by inhibiting cell cycle re-entry of a subpopulation of cells. EMBO J 22: 4003–4013, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vincent R, Adamson IY. Cellular kinetics in the lungs of aging Fischer 344 rats after acute exposure to ozone. Am J Pathol 146: 1008–1016, 1995. [PMC free article] [PubMed] [Google Scholar]
- 63.Vulliamy TJ, Marrone A, Knight SW, Walne A, Mason PJ, Dokal I. Mutations in dyskeratosis congenita: their impact on telomere length and the diversity of clinical presentation. Blood 107: 2680–2685, 2006. [DOI] [PubMed] [Google Scholar]
- 64.Vulliamy T, Beswick R, Kirwan M, Marrone A, Digweed M, Walne A, Dokal I. Mutations in the telomerase component NHP2 cause the premature ageing syndrome dyskeratosis congenita. Proc Natl Acad Sci USA 105: 8073–8078, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wong JM, Collins K. Telomerase RNA level limits telomere maintenance in X-linked dyskeratosis congenita. Genes Dev 20: 2848–2858, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yee M, Vitiello PF, Roper JM, Staversky RJ, Wright TW, McGrath-Morrow SA, Maniscalco WM, Finkelstein JN, O'Reilly MA. Type II epithelial cells are critical target for hyperoxia-mediated impairment of postnatal lung development. Am J Physiol Lung Cell Mol Physiol 291: L1101–L1111, 2006. [DOI] [PubMed] [Google Scholar]
- 67.Yoshida K, Kuwano K, Hagimoto N, Watanabe K, Matsuba T, Fujita M, Inoshima I, Hara N. MAP kinase activation and apoptosis in lung tissues from patients with idiopathic pulmonary fibrosis. J Pathol 198: 388–396, 2002. [DOI] [PubMed] [Google Scholar]
- 68.Zaher TE, Miller EJ, Morrow DM, Javdan M, Mantell LL. Hyperoxia-induced signal transduction pathways in pulmonary epithelial cells. Free Radic Biol Med 42: 897–908, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]