Abstract
A cultured porcine pulmonary artery (PA) model was used to examine the effects of prolonged nitric oxide (NO) treatment on the response to acutely applied NO, cGMP analog, or atrial natriuretic peptide (ANP). Twenty-four-hour treatment with the NO donor (Z)-1-[N-(2-aminoethyl)-N-(2-ammonioethyl)amino]diazen-1-ium-1,2-diolate (DETA-NO) resulted in >10-fold decrease in the response to acutely applied DETA-NO. In parallel with this, the relaxant response to acutely applied cGMP analog, β-phenyl-1,N2-etheno-8-bromoguanosine-3′,5′-cyclic monophosphorothioate, Sp isomer (Sp-8-Br-PET-cGMPS), and ANP decreased. The reduction in ANP responsiveness in PA was not associated with a reduction in cGMP levels evoked by 10−6 M ANP. Twenty-four hours in culture and treatment with DETA-NO decreased total cGMP-dependent protein kinase (cGKI) mRNA level compared with that in freshly prepared PA (1.05 ± 0.12, 0.42 ± 0.08, and 0.11 ± 0.01 amol/μg, respectively). Total cGKI protein levels were decreased to a lesser extent by 24 h in culture and further decreased by 24-h DETA-NO treatment compared with that in freshly prepared PA (361 ± 33, 272 ± 20, and 238 ± 25 ng/mg total protein, respectively). Maximal cGMP-stimulated phosphotransferase activity was reduced in 24-h cultured and DETA-NO-treated PA (986 ± 84, 815 ± 81, and 549 ± 78 pmol Pi·min−1·mg soluble protein−1), but the cGMP concentration resulting in 50% of maximal phosphotransferase activity was not. cGKI specific activity (maximal cGMP-activated phosphotransferase activity/ng cGKI) was significantly reduced in PA treated with DETA-NO for 24 h compared with freshly prepared and 24-h cultured PA (1.95 ± 0.22, 2.64 ± 0.25, and 2.85 ± 0.28 pmol Pi·min−1·ng cGKI−1, respectively). We conclude that prolonged NO treatment induces decreased acute NO responsiveness in PA in part by decreasing cGMP sensitivity. It does so by decreasing both cGKI expression and cGKI specific activity.
Keywords: guanosine 3′,5′-cyclic monophosphate; guanosine 3′,5′-cyclic monophosphate-dependent protein kinase
the nitric oxide (NO) signaling system plays a significant role in the regulation of vascular smooth muscle tone and, thus, of systemic hemodynamics. Multiple factors regulate this system, including NO production and bioavailability, cGMP production by soluble guanylyl cyclase (sGC), cGMP breakdown by phosphodiesterases (PDE), and transduction of the cGMP signal by smooth muscle cGMP-dependent protein kinases (cGKI) and their associated protein targets involved in decreasing myoplasmic calcium (12, 19, 28) and myofilament calcium sensitivity (44). Acute, short-lived increases in NO transiently activate this system, resulting in short-term vascular smooth muscle relaxation (21). The effects of prolonged increases in NO, as may occur with polymicrobial sepsis or prolonged NO treatment (38), on NO signaling in vascular smooth muscle are less well established but may play a role in the pathophysiology of pulmonary hypertension associated with sepsis (31).
In a well-characterized cultured porcine pulmonary artery (PA) preparation, prolonged NO exposure caused hyporesponsiveness to acutely applied NO (37). Prolonged NO treatment causes decreased sGC expression and activity, a finding consistent with previous findings in animals with endotoxic shock (51) and in transgenic mice overexpressing endothelial nitric oxide synthase (NOS) (50). In addition, prolonged NO treatment decreases sGC specific activity, a finding consistent with the recent finding that S-nitrosylation of this enzyme decreases its specific activity (41). The effects of prolonged NO exposure on cGMP sensitivity and cGKI expression and activity are less well understood in fully differentiated, functionally intact vascular smooth muscle. cGKI expression decreases significantly over time in cultured vascular smooth muscle cells (VSMC) (13), yet it is unclear how phenotypically similar such cultured cells are to contractile phenotype VSMC (1, 14). Nonetheless, the finding that prolonged treatment of cultured VSMC with nitrovasodilators decreases cGKI expression (43) suggests a possible role for NO hyporesponsiveness downstream of NO-activated cGMP production, specifically decreased cGMP sensitivity. Prolonged NO and cGMP treatment-mediated downregulation in cGMP sensitivity and cGKI expression in VSMC is further supported by the finding that sGC and cGKI reciprocally regulate one another's level of expression (3). Prolonged NO and cGMP treatment decreases cGMP sensitivity and cGKI expression in lamb pulmonary vein, verifying a significant role for this downstream system in modulating NO responsiveness in unambiguously contractile VSMC (17). Reduced cGMP sensitivity following prolonged exposure to NO was attributed exclusively to reduced cGKI activity on the basis of reduced expression.
An additional mechanism that may account for decreased cGMP sensitivity following prolonged NO treatment is decreased cGKI specific activity. A difference in cGKI specific activity accounts, in part, for differences in NO and cGMP responsiveness between PA and airway smooth muscle (45). Diabetes in rabbits (7) and hyperglycemia in VSMC (29) result in decreased cGKI activity, an effect that involves NADPH oxidase-derived reactive oxygen species and may involve both reduced cGKI expression and cGKI specific activity. The recent finding that cGKIα is susceptible to oxidative modification of specific thiols provides a possible mechanism by which NO and other forms of oxidative stress might regulate cGKI specific activity independent of phosphorylation or cGMP (5). The effect of prolonged NO treatment on cGKI expression and enzyme specific activity, however, has yet to be quantitatively assessed in either cultured VSMC or vascular smooth muscle in native vessels.
In the present studies, a cultured PA preparation was used to test the hypothesis that prolonged NO treatment induces NO hyporesponsiveness in PA in part by reducing cGMP sensitivity via reductions in both cGKI expression and specific activity.
MATERIALS AND METHODS
Tissue preparation.
After Institutional Animal Care and Use Committee approval, pigs (domestic crossbred, weight 35–77 kg) were anesthetized with intravenous pentobarbital (100 mg/kg) and exsanguinated by bilateral transection of the carotid arteries. The lungs were excised and immersed in chilled physiological salt solution (PSS) with a composition of (in mM) 110.5 NaCl, 25.7 NaHCO3, 5.6 dextrose, 3.4 KCl, 2.4 CaCl2, 1.2 KH2PO4, and 0.8 MgSO4. Third-generation PA was dissected from the lung parenchyma, cut into rings, and cleaned of adventitia under microscopic observation, and the endothelium was removed by gentle rubbing of the luminal surface with a moist cotton swab. For isometric force measurements, PA strips of 0.1- to 0.2-mm width, 1-cm length, and 0.2- to 0.3-mg wet weight were prepared. Third-generation PA was used in these studies because of ease of preparation, better definition of myocyte orientation, and the amenability of the tissue size to biochemical studies.
Organ culture.
In organ culture experiments, endothelium-denuded PA rings were placed in 10 ml of minimum essential medium (MEM) with Earle's salts and l-glutamine (Invitrogen, Carlsbad, CA), with 100 U penicillin and 100 μg streptomycin/ml at 37°C in a humidified 5% CO-95% air incubator (Forma Scientific) as previously described (37). When appropriate, rings were incubated with 1 mM (Z)-1-[N-(2-aminoethyl)-N-(2-ammonioethyl)amino]diazen-1-ium-1,2-diolate (DETA-NO) for 24 h. DETA-NO was selected since it slowly releases authentic NO (23), giving rise to stable relaxations and NO concentrations at a given DETA-NO concentration (37), and does not require biotransformation to release NO. The highest concentration of DETA-NO used in these studies results in a solution NO ≈1 μM that is stable for 24 h at 37°C (37).
Mechanical measurements.
Strips were suspended in 5-ml tissue baths filled with PSS (37°C) aerated with 94% O2-6% CO2, pH 7.4. One end of the strips was anchored to a metal hook at the bottom of the tissue bath; the other end was attached to a calibrated force transducer (model FT03D, Grass Instruments/Astro-Med, West Warwick, RI). During a 3-h equilibration period, the strips were repeatedly contracted isometrically with 40 mM KCl and then relaxed. The length of the strips was increased after each contraction-relaxation cycle until active force was maximal (optimal length). All strips were subsequently maintained at optimal length. Relaxed PA strips were then contracted with norepinephrine (1 μM), and the absence of endothelium was verified by failure of acetylcholine (1 μM) to cause relaxation. Strips were then relaxed again until commencement of a study. Before concentration-response studies, all strips were incubated with 10 μM indomethacin to prevent the formation of prostanoids. In previous studies, contraction of PA during incubation with indomethacin had no effect on tissue cGMP (cGMPi) (22).
Concentration-response curves.
Concentration-response curves were performed with PA strips that were freshly prepared, 24-h cultured, and 24-h cultured + 1 mM DETA-NO. A phenylephrine concentration sufficient to result in a submaximal contraction was used in all cases. This was determined by initially contracting the strip to maximal isometric force with phenylephrine and then reducing the bath phenylephrine to ≈330 nM, which resulted in isometric force 50 ± 10% of the maximal value. In separate sets of experiments, cumulative responses to DETA-NO, membrane-permeant, PDE-resistant cGKI agonist β-phenyl-1,N2-etheno-8-bromoguanosine-3′,5′-cyclic monophosphorothioate, Sp isomer (Sp-8-Br-PET-cGMPS; Biolog Life Science Institute, Bremen, Germany), atrial natriuretic peptide (ANP), forskolin, and membrane-permeant, PDE-resistant cAMP-dependent protein kinase agonist 5,6-dichloro-1-β-d-ribofuranosylbenzimidazole-3′,5′-cyclic monophosphorothioate, Sp isomer (Sp-5,6-DCl-cBIMPS) were determined. The specified agent was accumulatively added to the PA strip in concentrations ranging from 10−9 to 10−4 M. When DMSO was required as the solvent (Sp-8-Br-PET-cGMPS, Sp-5,6-DCl-cBIMPS, and forskolin) for the agent, a control strip was submaximally contracted with phenylephrine and treated with a volume of DMSO equal to that used in the concentration-response curve determination for the poorly water-soluble agent. Each n in the data refers to data obtained from a different animal.
cGMP measurements.
PA strips for these studies were either freshly prepared, 24-h cultured, or 24-h cultured + 1 mM DETA-NO. They were then washed extensively with PSS and were placed in 37°C baths containing PSS aerated with 94% O2-6% CO2, pH 7.4. In the presence of 500 μM 3-isobutyl-1-methylxanthine (IBMX), a membrane-permeant PDE inhibitor, PA strips were incubated with or without 1 μM ANP for 1 min. The PA strips were then frozen with liquid nitrogen and stored in a −70°C freezer. Strips were then homogenized, and the soluble extract was assayed for cGMP with a commercially available RIA kit (Amersham Biosciences, Piscataway, NJ) as previously described (36). The protein concentration in the tissue homogenate was determined by the Lowry method, with bovine serum albumin (BSA) dissolved in 1 N NaOH as the standard. cGMPi was expressed in picomoles per milligram of protein.
Total RNA preparation.
Total RNA was prepared from RNAlater (Ambion, Austin, TX)-treated PA tissue by extraction from tissue samples ground under liquid nitrogen with guanidinium isothiocyanate and subsequent sedimentation through CsCl (10). PA samples used in these experiments were freshly prepared, 24-h cultured, and 24-h cultured with 1 mM DETA-NO. After precipitation of total RNA with isopropanol, washing with ethanol, and suspension of the resultant total RNA in water, RNA quality was assessed by electrophoresis on an agarose gel. Only samples in which the ratio of 28S to 18S ribosomal RNA band intensity (in arbitrary intensity units) was at least >1.2, with little evidence of laddering below or between these bands, were studied. The optical density (OD) 260 nm/280 nm was >1.8 for these samples, and OD 260 nm was used to quantitate the amount of total RNA obtained from a sample with the relation OD 260 nm = 1 at 40 μg/ml RNA. cDNA from PA was prepared from the total RNA samples with avian myeloblastosis virus reverse transcriptase (Clontech, Mountain View, CA) and was stored at −20°C until use in cGKI mRNA expression measurements.
Measurement of cGKI mRNA expression.
Total cGKI mRNA levels were measured with the LightCycler Real-Time PCR system (Roche Applied Science, Indianapolis, IN) and the SYBR Green indicator method (47). cDNA samples from PA for real-time PCR quantitation were obtained as follows. Six micrograms of total RNA from a PA sample was subjected to reverse transcription (RT). One microliter of the resulting 41-μl RT product was combined with the SYBR Green Master Mix (Roche Applied Science) containing the cGKI primers. Standards for quantitation were 0.001, 0.01, 0.1, 1.0, and 10 amol of the full-length cGKIα sequence (45) (accession no. DQ119109) subcloned into a pET28 expression vector (Novagen, San Diego, CA). Sense (1930–1956) and antisense (2158–2133) primers encompass exons 16, 17, and 18, a region cGKIα and cGKIβ have in common, thus providing a measurement of total cGKI. A crossover point was selected at the midpoint of the light amplitude curve. A plot of cycle number at crossover versus log concentration of standard was obtained and fit with a linear regression analysis. Sample mRNA concentration was quantitated by interpolation. Preliminary experiments were used to establish standard concentrations such that sample concentrations would be approximately in the midrange of the standard. PCR efficiency for both the standard and the sample was 2.0. cGKI mRNA levels are reported as attomoles per microgram of total RNA.
Measurement of cGKI protein concentration.
The full-length cGKIα sequence was inserted into a pAcHLT baculovirus transfer vector with a (His)6 tag on the NH2-terminal end (BD Biosciences, Pharmingen, San Diego, CA). Sf 21 cells were transfected with baculovirus containing this transfer vector and raised in 1 liter of medium. The cell pellet was lysed in the presence of protease inhibitor cocktail (P8849, Sigma-Aldrich, St. Louis, MO). After centrifugation, the supernatant was purified with a Ni Sepharose High Performance column (Amersham Biosciences, Pittsburgh, PA) with 0.5 M imidazole elution. (His)6-cGKIα was quantitated with the amido black staining method (35), with BSA (0.125–2 μg) serving as the protein standard. In a previous study using the same study design, we (37) demonstrated equal protein loading using SDS-PAGE and zinc staining to quantitate actin and myosin bands.
As previously described (45), freshly prepared, 24-h cultured, and 24-h cultured + 1 mM DETA-NO PA strips were flash-frozen by rapid immersion in liquid nitrogen and stored at −70°C until proteins were to be extracted. They were then placed in a chilled mortar on dry ice and pulverized, and the resulting powder was suspended in extraction buffer composed of (in mM) 10 KH2PO4, pH 7.0, 1 dithiothreitol (DTT), 1 EDTA, 1 phenylmethylsulfonyl fluoride, and 5 NaF, with 1 μg/ml leupeptin and 1 μg/ml pepstatin A. The sample was centrifuged at 4,000 g for 10 min to pellet insoluble material. Protein concentration in the extraction buffer supernatant was determined with the Bradford method (2). Soluble protein (2.0 μg) from the tissue extracts was loaded into wells, and 0.1, 0.3, 0.67, 1, 1.67, and 3 ng of (His)6-cGKIα were loaded into adjacent lanes to generate a standard curve. Proteins were separated by electrophoresis in SDS-7.5% polyacrylamide precast minigels (Bio-Rad) with Tris-glycine-SDS buffer. The proteins were transferred to a polyvinylidene difluoride (PVDF) membrane for 45 min at 100 V, and the membrane was subsequently washed with 10 mM Tris-buffered saline containing 5% (wt/vol) BSA for 15 min (25°C). The membrane was then treated overnight with Tris-buffered saline containing 0.2% Tween 20 and 1:20,000 dilution of cGKI polyclonal rabbit antibody directed against the COOH terminus shared by both cGKIα and cGKIβ (StressGen Biotechnology, San Diego, CA). After washing, the membrane was treated with 1:10,000 anti-rabbit horseradish peroxidase-conjugated IgG antibody (Santa Cruz Biotechnology, Santa Cruz, CA) for 30 min and then washed again. Membranes were then treated with SuperSignal West Dura Extended Duration Substrate (Pierce, Rockford, IL) and imaged for chemiluminescent signal with an EC3 charge-coupled device camera imaging system (UVP, Upland, CA). The intensities of sample and standard bands were determined with LabWorks version 4.6 software (UVP). The standard data were best fit with a nonlinear regression curve, and the equation for this curve and the sample band intensity were used to determine sample concentration, as previously described (45). cGKI standard concentrations were selected such that the sample band intensity was in the midrange of the standard curve.
Measurement of cGKI activity.
cGKI activity in homogenate from freshly prepared, 24-h cultured, and 24-h cultured + 1 mM DETA-NO PA was determined by measuring phosphate transfer from ATP to a cGK-selective substrate, bovine lung cGMP-binding cGK-specific PDE NH2-terminal peptide (BPDEtide; RKISASEFDRPLR) (11). As previously described (45), PA strips were frozen in liquid nitrogen and pulverized while on dry ice. The resulting powder was suspended in ice-cold homogenization buffer comprised of (in mM) 10 sodium phosphate, pH 7.0, 1 EDTA, 1 DTT, 250 sucrose, and 1 phenylmethylsulfonyl fluoride, with 1 μg/ml pepstatin A and 1 μg/ml leupeptin for 30 min. The suspension was then centrifuged for 20 min at 12,000 g and 4°C. The supernatant was saved, and the protein concentration was determined with the Bradford assay. For phosphotransferase activity measurements, 10 μl of the ∼1 mg/ml tissue extract was added to 50 μl of phosphotransferase assay mixture comprised of (in mM) 0.2 ATP, 10 magnesium acetate, 20 Tris, pH 7.4, 0.5 IBMX, 10 DTT, and 10 sodium fluoride, with 1 μM PKI5-24 and ∼250 cpm/pmol [γ-32P]ATP, with 60 μM BPDEtide as the cGK-selective substrate. The cGMP concentration ([cGMP]) in the assay mixture was varied from 0.01 to 100 μM in half-log steps. One micromolar synthetic peptide inhibitor of cAMP-dependent protein kinase (9) PKI5-24 (TTYADFIASGRTGRRNAIHD) was also added to the reaction mixture. After 10-min reaction time at 30°C, the reaction was terminated by transfer of 25 μl of the assay mixture to a 1-in.-square section of Whatman P-81 phosphocellulose. The phosphocellulose sections were washed in 800 ml of 75 mM phosphoric acid three times and then once with 95% ethanol. Dried phosphocellulose sections were placed in scintillation vials and counted by Cerenkov radiation on a Beckman LS6000IC counter. All data were obtained in triplicate and averaged. Calculated phosphotransferase activity was reported as picomoles of Pi transferred per minute per milligram of protein and picomoles of Pi transferred per minute per nanogram of cGKI in the tissue extract used in the assay. Using this method, we previously demonstrated (45) that the phosphotransferase activity is attributable to cGKI activation.
Materials.
DETA-NO was purchased from Alexis Biochemical (Ann Arbor, MI). [2,8-3H]cGMP and [γ-32P]ATP were from DuPont NEN. BPDEtide and PKI5-24 were synthesized by the Mayo Protein Core Facility. Unless otherwise specified in the text, all other drugs and chemicals were purchased from Sigma Chemical (St. Louis, MO). All drugs and chemicals were dissolved in distilled water, except for Sp-8-Br-PET-cGMPS, Sp-5,6-DCl-cBIMPS, and forskolin, which were dissolved in 50% DMSO in water.
Statistical analysis.
Data are expressed as means ± SE; n represents the number of pigs. The effects of ANP on isometric force and cGMPi were assessed by repeated-measures ANOVA with post hoc analysis by the Student-Newman-Keuls method. cGKI mRNA expression, cGKI protein expression, and phosphotransferase activities between treatment groups were compared by unpaired Student's t-test. P values <0.05 were considered statistically significant. Concentration-response curves were compared by nonlinear regression analysis as described by Meddings et al. (32). In this method, force (F) at any concentration of drug (C) is given by the equation F = FmC/(EC50 + C), where Fm represents the maximal isometric force and EC50 represents the concentration that produces half-maximal isometric force for that drug. Nonlinear regression analysis was used to fit values of Fm and EC50 to data for F and C for each condition studied. This method allows comparison of curves to determine whether they are significantly different and whether this overall difference can be attributed to differences in Fm, EC50, or both parameters. A P value <0.05 was considered statistically significant.
RESULTS
Physiological studies.
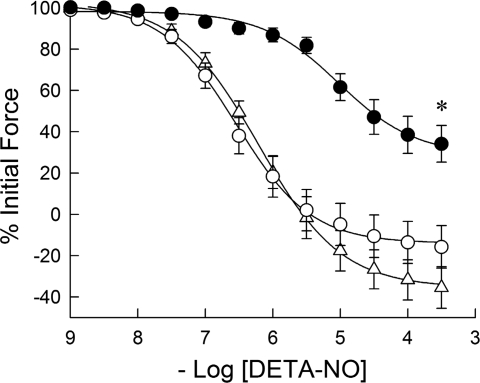
The effect of culture and of 24-h exposure to 1 mM DETA-NO on the isometric force response of PA to acutely applied DETA-NO after a phenylephrine-induced contraction at ∼50% of maximal isometric force is shown in Fig. 1. The pEC50 values for freshly prepared and 24-h cultured PA without and with 24 h DETA-NO treatment were 6.21 ± 0.18, 6.51 ± 0.15, and 5.07 ± 0.16, with a significant difference (P < 0.001) between DETA-NO-treated PA and the other treatment groups. In addition, the maximal relaxation was significantly decreased in the DETA-NO-treated group.
Fig. 1.
Effects of organ culture and nitric oxide (NO) donor (Z)-1-[N-(2-aminoethyl)-N-(2-ammonioethyl)amino]diazen-1-ium-1,2-diolate (DETA-NO) on the relaxant effect of acutely applied DETA-NO to porcine pulmonary artery (PA) submaximally contracted with phenylephrine. The concentration responses to acutely applied DETA-NO in freshly prepared (▵) and 24-h cultured (○) PA were not significantly different. The concentration response to acutely applied DETA-NO was significantly right shifted and the maximal extent of relaxation reduced in PA cultured with 1 mM DETA-NO for 24 h (•). Data are means ± SE; n = 6. *Concentration-response curve is significantly different (P < 0.01) from that for freshly prepared and 24-h cultured PA.
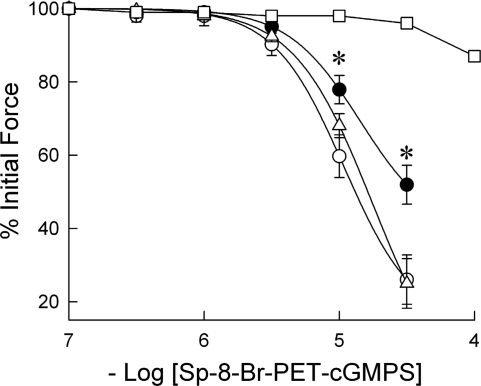
The effect of culture and of prolonged exposure to DETA-NO on the isometric force response of PA to acutely applied membrane-permeant, PDE-resistant cGKI agonist Sp-8-Br-PET-cGMPS is shown in Fig. 2. Relaxations to this agent took >20 min to reach a stable value (data not shown). A control set of experiments used volumes of the solvent (DMSO) used to dissolve the cGMP analog equivalent to those added in the concentration-response curve. A significant DMSO-mediated relaxation was observed (13 ± 1.2%) when the volume of DMSO was equivalent to that required to attain a 10−4 M Sp-8-Br-PET-cGMPS bath concentration. The concentration response to Sp-8-Br-PET-cGMPS was therefore carried out to only 3.16 × 10−5 M, to eliminate confounding solvent-mediated relaxation. Twenty-four hours in culture had no effect on Sp-8-Br-PET-cGMPS-induced relaxation compared with that obtained in freshly prepared PA, and 75% relaxation was attained at the highest concentration. Twenty-four-hour culture in the presence of DETA-NO significantly (P < 0.01) decreased relaxation to 10−5 and 3.16 × 10−5 M Sp-8-Br-PET-cGMPS, compared with that obtained in both freshly prepared and 24-h cultured PA, with 48 ± 5.3% relaxation attained at the highest concentration.
Fig. 2.
Effect of organ culture and NO donor DETA-NO on the relaxant effect of acutely applied membrane-permeant, phosphodiesterase-resistant cGMP-dependent protein kinase (cGKI) agonist β-phenyl-1,N2-etheno-8-bromoguanosine-3′,5′-cyclic monophosphorothioate, Sp isomer (Sp-8-Br-PET-cGMPS) to porcine PA submaximally contracted with phenylephrine. The relaxant response to acutely applied Sp-8-Br-PET-cGMPS in freshly prepared (▵) and 24-h cultured (○) PA was not significantly different but was significantly decreased (•) in PA cultured with 1 mM DETA-NO for 24 h. Relaxation to an equal volume of DMSO, in which the Sp-8-Br-PET-cGMPS was dissolved, is also shown (□). Data are means ± SE; n = 6. *Significantly different (P < 0.01) from response observed in both freshly prepared and 24-h cultured PA.
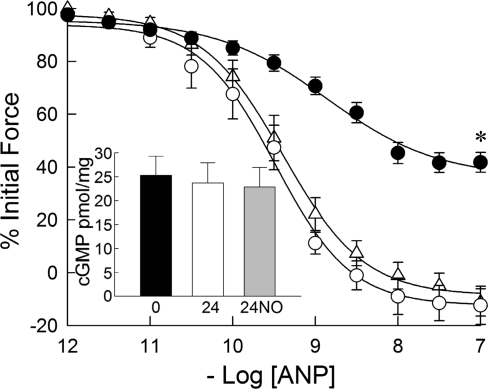
Relaxation to the particulate guanylyl cyclase agonist ANP was not significantly different between freshly prepared and 24-h cultured PA, with pEC50 of 9.48 ± 0.09 and 9.39 ± 0.10, respectively (Fig. 3). Relaxation to ANP was significantly decreased in PA treated with DETA-NO for 24 h, with pEC50 = 8.86 ± 0.07, and maximal relaxation was also significantly decreased (P < 0.01) compared with freshly prepared and 24-h cultured PA. Tissue cGMP levels after treatment with 10−6 M ANP in the presence of IBMX (Fig. 3, inset) were not significantly different between freshly prepared and 24-cultured PA without and with 24-h DETA-NO treatment (25.3 ± 3.9, 23.7 ± 4.2, and 22.9 ± 4.1 pmol/mg protein, respectively).
Fig. 3.
Effects of organ culture and NO donor DETA-NO on the relaxant effect of acutely applied atrial natriuretic peptide (ANP) to porcine PA submaximally contracted with phenylephrine. The concentration responses to acutely applied ANP in freshly prepared (▵) and 24-h cultured (○) PA were not significantly different. The concentration response to acutely applied ANP was significantly right shifted and the maximal extent of relaxation reduced in PA cultured with DETA-NO for 24 h (•). cGMP levels (pmol/mg tissue) obtained in PA rings treated with 1 μM ANP for 1 min in the presence of 500 μM 3-isobutyl-1-methylxanthine were not significantly different in freshly prepared (0) and 24-h cultured PA without (24) or with (24NO) 1 mM DETA-NO (inset). Data are means ± SE; n = 6. *Concentration-response curve is significantly different from that for freshly prepared and 24-h cultured PA (P < 0.05).
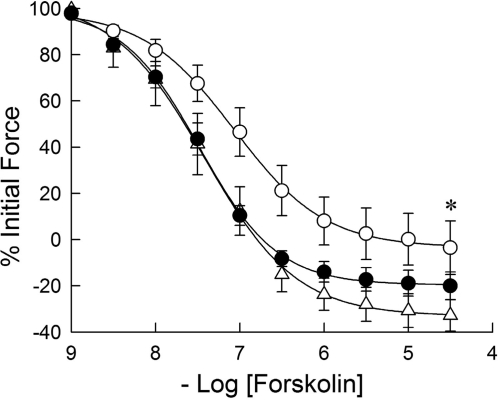
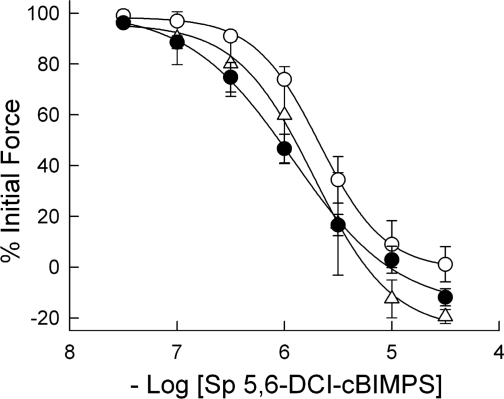
Relaxation to the adenylyl cyclase activator forskolin (Fig. 4) was decreased after 24 h in culture compared with freshly prepared PA, with pEC50 7.07 ± 0.12 vs. 7.48 ± 0.09 (P < 0.01). Twenty-four-hour DETA-NO-treated PA had relaxation characteristics indistinguishable from those in freshly prepared PA but was significantly more forskolin sensitive than 24-h cultured PA, with pEC50 7.42 ± 0.13. Relaxation to a membrane-permeant, PDE-resistant cAMP-dependent protein kinase agonist, Sp-5,6-DCl-cBIMPS, was unchanged by 24 h in culture without or with 24 h DETA-NO treatment (Fig. 5). For both of these agents, volumes of DMSO equivalent to those used in adding them to the tissue baths over the entire concentration range resulted in no PA relaxation (see Fig. 2).
Fig. 4.
Effects of organ culture and NO donor DETA-NO on the relaxant effect of acutely applied forskolin to porcine PA submaximally contracted with phenylephrine. The concentration response to acutely applied forskolin in 24-h cultured PA (○) was significantly right shifted and the maximal extent of relaxation reduced compared with that obtained in freshly prepared PA (▵). The concentration response to acutely applied forskolin in PA treated with DETA-NO for 24 h (•) was significantly left shifted and greater in extent than in PA cultured for 24 h without DETA-NO, but was not significantly different from that observed in freshly prepared PA. Data are means ± SE; n = 6. *Concentration-response curve is significantly different from that for freshly prepared and 24-h DETA-NO treated PA (P < 0.05).
Fig. 5.
Effects of organ culture and NO donor DETA-NO on the relaxant effect of acutely applied 5,6-dichloro-1-β-d-ribofuranosylbenzimidazole-3′,5′-cyclic monophosphorothioate, Sp isomer (Sp-5,6-DCl-cBIMPS), a membrane-permeant, phosphodiesterase-resistant cAMP-dependent protein kinase agonist, to porcine PA submaximally contracted with phenylephrine. The concentration responses to acutely applied Sp-5,6-DCl-cBIMPS in freshly prepared (▵), 24-h cultured (○), and 24-h cultured with DETA-NO (•) PA were not significantly different. Data are means ± SE; n = 6.
Biochemical studies.
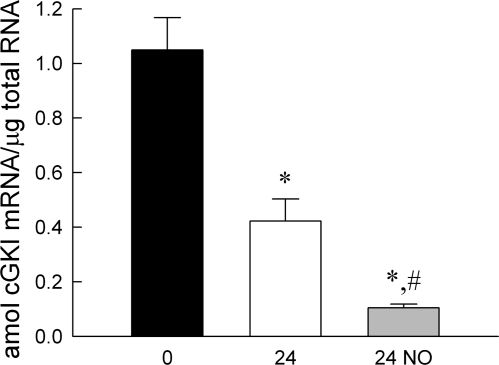
The total cGKI mRNA level (Fig. 6) in freshly prepared PA was 1.05 ± 0.12 amol/μg total RNA. This decreased significantly (P < 0.01) after 24 h in culture and was further decreased (P < 0.01) by 24-h treatment with DETA-NO, to 0.42 ± 0.08 and 0.11 ± 0.01 amol/μg total RNA, respectively.
Fig. 6.
Effects of organ culture and NO donor DETA-NO on cGKI mRNA expression in porcine pulmonary artery. 0, 24 and 24 NO, total RNA for determining mRNA obtained from PA freshly prepared, 24-h cultured, and 24-h cultured in presence of DETA-NO, respectively. Results are quantitative with cGKI subcloned into an expression vector as the standard in real-time PCR measurements. Units are attomoles of cGKI mRNA per microgram of total RNA. Significant differences: from *freshly prepared (*P < 0.01) and #24-h cultured (P < 0.01) conditions.
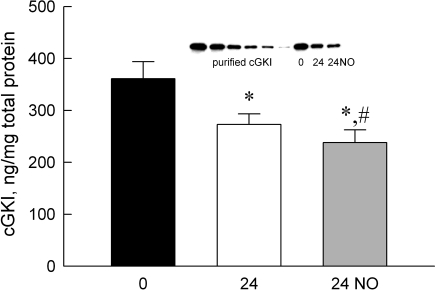
The total cGKI protein levels are shown in Fig. 7. The inset shows a representative immunoblot with known amounts of purified recombinant cGKI applied (see materials and methods) and with 1 μg of total soluble fraction protein loaded onto the lane for each of the indicated treatment groups. The total cGKI protein level in freshly prepared PA was 361 ± 33 ng/mg soluble protein. Total cGKI protein level in PA decreased significantly (P < 0.01) after 24 h in culture and was further decreased (P < 0.05) by 24-h treatment with DETA-NO, to 272 ± 20 and 238 ± 25 ng/mg, respectively.
Fig. 7.
cGKI protein levels in the soluble fraction of tissue homogenates from porcine PA with a quantitative immunoblotting method. Inset: representative immunoblot using known amounts of recombinant, purified cGKI to create a standard curve and 1 μg of homogenate from freshly prepared (0) and PA cultured for 24 h without (24) and with (24 NO) DETA-NO. cGKI levels are decreased in PA at 24 h and are further reduced at 24 h in the presence of DETA-NO. *Different from freshly prepared, #different from 24 h (P < 0.05). Data are means ± SE; n = 6.
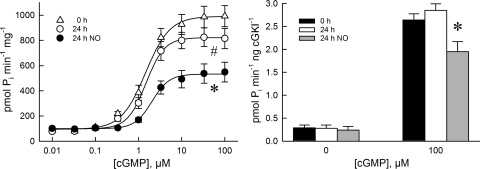
Phosphotransferase activity in the absence of added cGMP (Fig. 8, left) was not significantly different between freshly prepared, 24-h cultured, and 24-h DETA-NO treated PA (102 ± 17, 89 ± 11, and 79 ± 15 pmol·min−1·mg total protein−1, respectively). Phosphotransferase activity increased with [cGMP] and attained maximal activation (apparent cGKI Vmax) at 100 μM cGMP. Apparent cGKI Vmax was significantly decreased in 24-h cultured PA (P < 0.05) and further decreased (P < 0.01) in 24-h DETA-NO-treated PA compared with freshly prepared PA (986 ± 84, 815 ± 81, and 549 ± 78 pmol·min−1·mg−1 soluble protein, respectively). The [cGMP] at which phosphotransferase activity was 50% of maximal (apparent KA for cGMP) was 1.85 ± 0.33, 2.01 ± 0.22, and 2.18 ± 0.25 μM for freshly prepared, 24-h cultured and 24-h DETA-NO-treated PA, respectively. When the phosphotransferase activity was normalized to the amount of cGKI protein present in the sample (defined as cGKI specific activity in this study), there was no significant difference in cGKI specific activity in the absence of added cGMP between freshly prepared, 24-h cultured, and 24-h DETA-NO-treated PA (Fig. 8, right). cGKI specific activities at apparent Vmax for freshly prepared and 24-h cultured PA, 2.64 ± 0.25 and 2.85 ± 0.28 pmol·min−1·ng cGKI−1, respectively, were not significantly different. cGKI specific activity in 24-h DETA-NO-treated PA, however, was significantly decreased to 1.95 ± 0.22 pmol·min−1·ng cGKI−1 (P < 0.05).
Fig. 8.
Effects of organ culture and NO donor DETA-NO on cGMP-activated phosphotransferase activity measurements in the soluble fraction of homogenates prepared from porcine PA. The maximal cGMP-activated phosphotransferase activity (apparent Vmax) was decreased relative to that obtained from freshly prepared PA (▵) in 24-h cultured PA (○) and was further decreased in PA cultured for 24 h with DETA-NO (•). The cGMP concentration ([cGMP]) at which 50% of Vmax (apparent cGMP KA) was attained did not differ significantly between the different treatment groups. Specific activity (phosphotransferase activity/ng cGKI present in homogenate) was measured with [cGMP] 0 and 100 μM (right). 0 h, 24 h, and 24 h NO, freshly prepared and 24 h cultured without and with DETA-NO. Maximal cGMP-activated phosphotransferase specific activity measured was the same in freshly prepared and 24-h cultured PA but was significantly reduced in PA cultured for 24 h with DETA-NO. Data are means ± SE; n = 6. *Different (P < 0.05) from freshly prepared and 24-h cultured PA.
DISCUSSION
The significant findings of this study are that prolonged exposure of porcine PA to an NO donor reduces responsiveness to both acutely applied NO and ANP in part by decreasing cGMP sensitivity. The reduction in cGMP sensitivity was associated with a reduction in cGKI mRNA, cGKI protein levels, and cGMP-activated phosphotransferase activity. Prolonged exposure to NO also reduced cGMP-activated phosphotransferase activity per unit of cGKI protein in porcine PA. This suggests a form of cGKI activity regulation beyond that associated with cGMP and the level of cGKI expression in PA vascular smooth muscle.
Cultured vessels have proven useful to characterize smooth muscle responsiveness (8), including studies of NO-mediated responses (25). We previously demonstrated (37) that the cultured PA model maintains normal DNA, total RNA, actin, and myosin protein levels, specific force, sensitivity to an α-adrenergic receptor agonist, and sensitivity to NO donor-induced relaxation after agonist-induced submaximal contraction after 48 h in culture. DETA-NO, an NO donor that spontaneously releases authentic NO in buffered solutions with a t1/2 ≈ 24 h (23), was used in these studies. Confirming these NO release kinetics, we observed that 1 mM DETA-NO resulted in ∼1 μM NO for 24 h in MEM culture medium (37). DETA-NO thus minimizes the role of variable NO release kinetics and bioconversion in the induction and assessment of NO-induced NO hyporesponsiveness.
cGMP sensitivity.
Within 24 h, exposure to DETA-NO significantly reduced the responsiveness of cultured PA to an acutely applied NO donor. We previously demonstrated (37) that this was associated with a reduction in cGMP production due to decreased sGC expression, activity, and specific activity. It remained possible, however, that part of the observed change in NO responsiveness was accounted for by a reduction in cGMP sensitivity. Establishing a relationship between tissue steady-state cGMP level (i.e., that obtained in the absence of PDE inhibitors) and extent of relaxation as a measure of cGMP sensitivity was not possible in that study, since 90% relaxation was obtained without a measurable increase in cGMP. cGMP sensitivity was probed in the present study in two distinct ways. In the first, a membrane-permeant, PDE-resistant cGMP analog, Sp-8-Br-PET-cGMPS (6), was used to activate cGKI and to relax PA submaximally contracted with the α-adrenergic receptor agonist phenylephrine. cGMP sensitivity was unchanged by 24 h in culture but was significantly reduced by 24-h exposure to NO. A full concentration-response curve was not possible with this agent because of significant relaxant effects by the diluent, DMSO, at the surprisingly high cGMP analog concentrations required to attain relaxation (EC50 ∼10−5 M). This reduced cGMP sensitivity predicts that cGMP from a second, NO-independent particulate guanylyl cyclase, natriuretic peptide receptor (18), should also be decreased after prolonged NO exposure. As with the cGMP analog, 24 h in culture resulted in no significant change in the response to natriuretic peptide receptor agonist ANP, whereas 24 h treatment with DETA-NO resulted in a significant increase in EC50 and a reduction in maximal relaxation. The finding that tissue cGMP levels in response to 10−6 M ANP were not significantly different in control, 24-h cultured, and 24-h DETA-NO-treated PA suggests that the shift in the ANP response is not due to decreased ANP-induced cGMP production. The tissue cGMP levels were, however, measured in the presence of IBMX to estimate particulate guanylyl cyclase activity; it is possible that the shift in the ANP response is due to a previously described effect (30) possibly involving cGMP-cGKI-mediated activation of PDE V (33). Our previous finding (37) that PDE activity is not changed after 24-h DETA-NO treatment suggests that this is an unlikely explanation, and the two methods used therefore both support a prolonged NO exposure-mediated reduction in cGMP sensitivity in porcine PA. This is consistent with a previous finding obtained in lamb pulmonary vein with another cGMP analog, in which 24-h treatment with NO resulted in a significant reduction in cGMP sensitivity (17).
Reduced cGMP production due to decreased sGC activity/specific activity (37) and the decreased cGMP sensitivity reported here thus both play a significant and independent role in decreased NO responsiveness following prolonged NO treatment in PA. The relative contribution of each to NO-induced NO hyporesponsiveness cannot, however, be quantitatively assessed from these results.
While the cultured PA model demonstrated no change in maximum specific force after prolonged treatment with NO, a significant right shift was observed in the phenylephrine response curve, suggesting the possibility that prolonged NO may interfere with other receptor-mediated signaling (37). In addition, cross-activation between the cAMP and cGMP signaling systems (40, 49) raises the possibility that prolonged NO might exert effects on cAMP/cAMP-dependent protein kinase responses in PA. In the present study, 24 h in culture and 24-h DETA-NO treatment had no significant effect on the relaxant response in PA to a membrane-permeant, PDE-resistant cAMP analog. In contrast to this, 24 h in culture resulted in a significant decrease in response to forskolin, an adenylyl cyclase activator. Twenty-four-hour treatment with DETA-NO normalized this response compared with freshly prepared PA. Decreased forskolin responsiveness in cultured coronary artery has been observed previously in KCl-contracted coronary arteries (46), although the authors did not, in our opinion, fully interpret their data, and is consistent with the increase in cAMP PDE activity we reported in 24-h cultured PA (37). The sensitization to forskolin observed in PA treated with NO for 24 h relative to 24-h cultured PA is similar to that reported in gut smooth muscle after acute application of sodium nitroprusside (34). This was mechanistically attributed to PDE3 inhibition and may explain the present findings, although cAMP PDE activity levels were not altered by 24-h treatment with NO (37). Taken in conjunction with the previous finding of no DETA-NO-related change in specific force or actin or myosin expression, the results indicate that prolonged DETA-NO treatment does not result in a general change in smooth muscle phenotype in this cultured porcine PA model.
cGKI expression.
Previous studies have qualitatively demonstrated a reduction in cGKI mRNA expression related to prolonged exposure to NO donors in cultured VSMC (43) and in cultured pulmonary veins (17). cGKI expression in cultured VSMC decreases independent of exogenous NO for reasons that are not completely clear but depends on cell density (13) and may be modulated by cytokines (4). The effect of organ culture itself on cGKI expression was not reported in a study of cultured pulmonary veins (17). Total cGKI mRNA expression was measured in the present study, since cGMP sensitivity presumably reflects an integrated response by both isoforms. cGKI mRNA levels in 24-h cultured PA decreased >60%. The possibility that this reduction in cGKI mRNA expression in PA is due to cGMP or NOS activity is unlikely in the absence of endothelium on the cultured PA and given the previous finding that there was no measurable inducible NOS found in PA by immunoblotting after up to 48 h in culture (37) in the presence or absence of DETA-NO. The level of cGKI mRNA in freshly prepared PA measured in this study is somewhat lower than previously reported but remains in the 1–2 amol/μg total RNA range (45). In the present study real-time PCR with vectors containing the full-length cGKIα sequence as a standard was used. The plasmid was isolated in large quantities and relatively free of spectroscopically confounding contaminants and may thus be more accurately quantitated with absorbance. The cGKI mRNA quantitation results are susceptible to variability in the efficiency in the RT step for the tissue samples, which the standards do not undergo. The relatively low variability in mRNA measurements suggests that this is not a major concern, but the possibility of a consistent systematic error associated with the RT remains. Nonetheless, the results provide a precise lower limit for the amount of cGKI mRNA present in PA. Prolonged NO resulted in a further significant reduction in cGKI mRNA expression. If the 24-h cultured PA was used as the standard, 24-h DETA-NO resulted in a 75% reduction in cGKI mRNA expression. The mechanism by which this reduction occurred was not the subject of this study, but the results are compatible with a previously reported cGMP-associated mechanism (17, 43).
cGKI levels are reportedly ∼0.1 μM in a variety of tissues (20, 24). More specifically, total cGKI protein expression has been quantitated in cultured VSMC (13, 43), in rat lung tissue (43), and in porcine PA and trachea (45). The cGKI protein level in freshly prepared PA obtained in the present study, 360 ng/mg, was in reasonable agreement with those previously reported in PA using a commercially available cGKI standard (45) and in rat lung tissue (43), 550 and ∼500 ng/mg total protein, respectively. cGKI protein expression decreased in PA after 24 h in culture, but to a lesser extent than the reduction in cGKI mRNA, 25% versus 60%. A further reduction in cGKI protein was observed after 24-h DETA-NO treatment, but this was again much less than the NO-mediated reduction in cGKI mRNA, a 34% reduction versus a 90% reduction. The NO-mediated reduction in cGKI protein expression reported in the present study is similar to that obtained in rat lung after 4-day exposure to isosorbide dinitrate (43), demonstrating that this cultured PA preparation provides results similar to those obtained in vivo with a different class of NO donor. The difference between the quantitative cGKI mRNA and protein level results suggests that caution must be exercised when attempting to use mRNA findings for anything other than a qualitative indicator of cGKI protein expression. Possible explanations for the difference between the effects of culture and DETA-NO on cGKI mRNA and protein levels are a greater mRNA turnover relative to that for protein or a transcriptionally mediated reduction in mRNA production, as previously indicated (43). On the basis of present results, an estimated lower limit for the t1/2 for cGKI protein turnover is >24 h.
cGKI activity.
The cGMP-activated phosphotransferase activity assay used in this study (11) almost exclusively reflects cGKI activity (45) and will hereafter be described as cGKI activity. In freshly prepared PA, maximal cGKI activity (Vmax) was similar to that reported in our previous study, ∼1,000 pmol·min−1·mg total protein−1. Two alternatively spliced cGKI isoforms, cGKIα and cGKIβ, are present in vascular smooth muscle, but their relative quantities are not precisely known (20, 27, 48). cGKI activity increased as expected with [cGMP], with maximal activity attained at 100 μM cGMP. The cGMP KA for PA homogenate was ∼2 μM, which is significantly greater than observed for purified recombinant cGKIα (39). This suggests either that the majority of the cGKI in porcine PA is cGKIβ or that cGKI is regulated differently when intracellular than in its purified form. This is a distinct possibility because of extensive cGKI binding to its primary phosphorylation targets in the more complex myoplasmic environment.
As with cGKI mRNA levels, we deliberately chose to measure total cGKI protein levels since cGKI activity reflects that of both cGKI isoforms. When normalized to the amount of total cGKI present, the cGKI specific activity in freshly prepared PA was >2 pmol·min−1·ng cGKI−1, a result similar to that seen in isolated, purified cGKI (∼3 pmol·min−1·ng cGKI−1) (39, 48) and of the same order of magnitude as previously reported in porcine PA (45). The discrepancy between the present results and those obtained from freshly prepared ovine fetal pulmonary artery and vein, in which the total cGMP-activated phosphotransferase activity was an order of magnitude lower (16, 17), may be due to a difference in the tissue homogenate preparation or a lower cGKI level in ovine PA. The similarity in cGKI specific activity reported here and that of purified cGKI suggests little role for tissue homogenate phosphatase activity in the phosphorylation state of BPDEtide substrate under the assay conditions used. In addition, this agreement in specific activity between tissue homogenate and purified cGKI specific activity provides compelling support for the validity of the quantitative immunoblotting methods used in these studies.
As expected, cGMP-activated phosphotransferase activity in 24-h cultured PA decreased in parallel with the reduction in the cGKI protein levels. The reduction in cGKI activity was not, however, associated with a reduction in either NO responsiveness or cGMP and ANP sensitivity. This suggests greater complexity in the relationship between cGKI protein levels/enzyme activity and cGMP sensitivity than a simple linear correlation and may indicate that under normal circumstances there is more than enough cGKI present to functionally transduce cGMP signals. The cGMP KA was unchanged by either 24 h in culture or treatment with DETA-NO, suggesting no change in the distribution of cGKI isoforms transducing cGMP signals. There was no reduction in cGKI specific activity observed in 24-h cultured PA, which indicates that the reduction in total activity was fully accounted for by a reduction in cGKI expression. The effect of 24-h vessel culture on cGKI mRNA, protein, and enzyme activity levels has not been reported previously. The mechanism by which cGKI mRNA and protein expression are decreased remains unclear and will be examined in future studies.
After 24-h treatment with DETA-NO, a significant reduction in both total and specific cGKI activity was observed when compared with those obtained in either freshly prepared or 24-h cultured PA. Such an effect would not be readily evident in the absence of quantitative cGKI protein level measurements. The reduction in cGKI specific activity following NO treatment suggests an effect either on the enzyme itself or on factors regulating its activity, since the assay conditions eliminate a role for PDE modulation of cGMP levels. In the absence of well-characterized additional cGKI regulatory factors, the results support the possibility of an NO-associated modification of enzyme activity. Several enzyme modifications regulating cGKI specific activity are known, including autophosphorylation (15, 42) and, more recently, oxidative modification of specific thiols on cGKI (5, 26). While these specific mechanisms may not account for the effect of prolonged NO treatment on specific activity observed in this study, a significant proportion of the reduction in cGMP sensitivity is nonetheless due to reduced cGKI specific activity.
In summary, the present study used a cultured vessel preparation to demonstrate that both NO responsiveness and cGMP sensitivity were reduced in porcine PA after prolonged exposure to NO. The reduction in cGMP sensitivity was corroborated by a reduction in ANP-mediated relaxation. Twenty-four-hour treatment of PA with NO did not decrease cAMP-mediated signaling, suggesting a degree of specificity to the NO-cGMP signaling system. The NO-induced reduction in cGMP sensitivity was associated with a reduction in the expression and activity of the major vascular smooth muscle cGMP transducing enzyme, cGKI. However, only some of the prolonged NO treatment-induced reduction in cGKI activity could be accounted for by reduced cGKI protein levels, because there was evidence for a significant reduction in the cGKI specific activity or units of activity normalized to the amount of protein in the sample. We conclude that both reduced cGKI expression and specific activity may play a significant role in NO hyporesponsiveness under conditions of nitrosative stress.
GRANTS
This study was supported in part by National Heart, Lung, and Blood Institute Grant HL-69968 (W. J. Perkins).
Acknowledgments
We thank Kathy Street and Susan Kost for expert technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Boerth NJ, Dey NB, Cornwell TL, Lincoln TM. Cyclic GMP-dependent protein kinase regulates vascular smooth muscle cell phenotype. J Vasc Res 34: 245–259, 1997. [DOI] [PubMed] [Google Scholar]
- 2.Bradford MM A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976. [DOI] [PubMed] [Google Scholar]
- 3.Browner NC, Dey NB, Bloch KD, Lincoln TM. Regulation of cGMP-dependent protein kinase expression by soluble guanylyl cyclase in vascular smooth muscle cells. J Biol Chem 279: 46631–46636, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Browner NC, Sellak H, Lincoln TM. Downregulation of cGMP-dependent protein kinase expression by inflammatory cytokines in vascular smooth muscle cells. Am J Physiol Cell Physiol 287: C88–C96, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Burgoyne JR, Madhani M, Cuello F, Charles RL, Brennan JP, Schroder E, Browning DD, Eaton P. Cysteine redox sensor in PKGIa enables oxidant-induced activation. Science 317: 1393–1397, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Butt E, Pohler D, Genieser HG, Huggins JP, Bucher B. Inhibition of cyclic GMP-dependent protein kinase-mediated effects by (Rp)-8-bromo-PET-cyclic GMPS. Br J Pharmacol 116: 3110–3116, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang S, Hypolite JA, Velez M, Changolkar A, Wein AJ, Chacko S, DiSanto ME. Downregulation of cGMP-dependent protein kinase-1 activity in the corpus cavernosum smooth muscle of diabetic rabbits. Am J Physiol Regul Integr Comp Physiol 287: R950–R960, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Chen L, Xin X, Eckhart AD, Yang N, Faber JE. Regulation of vascular smooth muscle growth by alpha1-adrenoreceptor subtypes in vitro and in situ. J Biol Chem 270: 30980–30988, 1995. [DOI] [PubMed] [Google Scholar]
- 9.Cheng HC, Kemp BE, Pearson RB, Smith AJ, Misconi L, Van Patten SM, Walsh DA. A potent synthetic peptide inhibitor of the cAMP-dependent protein kinase. J Biol Chem 261: 989–992, 1986. [PubMed] [Google Scholar]
- 10.Chirgwin JM, Przybyla AE, MacDonald RJ, Rutter WJ. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry 18: 5294–5299, 1979. [DOI] [PubMed] [Google Scholar]
- 11.Collins SP, Uhler MD. Cyclic AMP- and cyclic GMP-dependent protein kinases differ in their regulation of cyclic AMP response element-dependent gene transcription. J Biol Chem 274: 8391–8404, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Cornwell TL, Lincoln TM. Regulation of intracellular Ca2+ levels in cultured vascular smooth muscle cells. Reduction of Ca2+ by atriopeptin and 8-bromo-cyclic GMP is mediated by cyclic GMP-dependent protein kinase. J Biol Chem 264: 1146–1155, 1989. [PubMed] [Google Scholar]
- 13.Cornwell TL, Soff GA, Traynor AE, Lincoln TM. Regulation of the expression of cyclic GMP-dependent protein kinase by cell density in vascular smooth muscle cells. J Vasc Res 31: 330–337, 1994. [DOI] [PubMed] [Google Scholar]
- 14.Dey NB, Foley KF, Lincoln TM, Dostmann WR. Inhibition of cGMP-dependent protein kinase reverses phenotypic modulation of vascular smooth muscle cells. J Cardiovasc Pharmacol 45: 404–413, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Francis SH, Poteet-Smith C, Busch JL, Richie-Jannetta R, Corbin JD. Mechanisms of autoinhibition in cyclic nucleotide-dependent protein kinases. Front Biosci 7: d580–d592, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Gao Y, Dhanakoti S, Trevino EM, Sander FC, Portugal AM, Usha Raj J. Effect of oxygen on cyclic GMP-dependent protein kinase-mediated relaxation in ovine fetal pulmonary arteries and veins. Am J Physiol Lung Cell Mol Physiol 285: L611–L618, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Gao Y, Dhanakoti S, Trevino EM, Wang X, Sander FC, Portugal AD, Usha Raj J. Role of cGMP-dependent protein kinase in development of tolerance to nitric oxide in pulmonary veins of newborn lambs. Am J Physiol Lung Cell Mol Physiol 286: L786–L792, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Garbers DL, Chrisman TD, Wiegn P, Katafuchi T, Albanesi JP, Bielinski V, Barylko B, Redfield MM, Burnett JC Jr. Membrane guanylyl cyclase receptors: an update. Trends Endocrinol Metab 17: 251–258, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geiselhoringer A, Werner M, Sigl K, Smital P, Worner R, Acheo L, Stieber J, Weinmeister P, Feil R, Feil S, Wegener J, Hofmann F, Schlossmann J. IRAG is essential for relaxation of receptor-triggered smooth muscle contraction by cGMP kinase. EMBO J 23: 4222–4231, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hofmann F The biology of cyclic GMP-dependent protein kinases. J Biol Chem 280: 1–4, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci USA 84: 9265–9269, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones KA, Lorenz RR, Morimoto N, Sieck GC, Warner DO. Halothane reduces force and intracellular Ca2+ in airway smooth muscle independently of cyclic nucleotides. Am J Physiol Lung Cell Mol Physiol 268: L166–L172, 1995. [DOI] [PubMed] [Google Scholar]
- 23.Keefer LK, Nims RW, Davies KM, Wink DA. “NONOates” (1-substituted diazen-1-ium-1,2-diolates) as nitric oxide donors: convenient nitric oxide dosage forms. Methods Enzymol 268: 281–293, 1996. [DOI] [PubMed] [Google Scholar]
- 24.Keilbach A, Ruth P, Hofmann F. Detection of cGMP dependent protein kinase isozymes by specific antibodies. Eur J Biochem 208: 467–473, 1992. [DOI] [PubMed] [Google Scholar]
- 25.Kullo IJ, Mozes G, Schwartz RS, Gloviczki P, Tsutsui M, Katusic ZS, O'Brien T. Enhanced endothelium-dependent relaxations after gene transfer of recombinant endothelial nitric oxide synthase to rabbit carotid arteries. Hypertension 30: 314–320, 1997. [DOI] [PubMed] [Google Scholar]
- 26.Landgraf W, Regulla S, Meyer HE, Hofmann F. Oxidation of cysteines activates cGMP-dependent protein kinase. J Biol Chem 266: 16305–16311, 1991. [PubMed] [Google Scholar]
- 27.Landgraf W, Ruth P, Keilbach A, May B, Welling A, Hofmann F. Cyclic GMP-dependent protein kinase and smooth muscle relaxation. J Cardiovasc Pharmacol 20: S18–S22, 1992. [PubMed] [Google Scholar]
- 28.Lincoln TM, Cornwell TL, Taylor AE. cGMP-dependent protein kinase mediates the reduction of Ca2+ by cAMP in vascular smooth muscle cells. Am J Physiol Cell Physiol 258: C399–C407, 1990. [DOI] [PubMed] [Google Scholar]
- 29.Liu S, Ma XY, Gong MC, Shi LH, Lincoln T, Wang SX. Glucose down-regulation of cGMP-dependent protein kinase I expression in vascular smooth muscle cells involves NAD(P)H oxidase-derived reactive oxygen species. Free Radic Biol Med 42: 852–863, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Madhani M, Scotland RS, MacAllister RJ, Hobbs AJ. Vascular natriuretic peptide receptor-linked particulate guanylate cyclases are modulated by nitric oxide-cyclic GMP signalling. Br J Pharmacol 139: 1289–1296, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manktelow C, Bigatello LM, Hess D, Hurford WE. Physiologic determinants of the response to inhaled nitric oxide in patients with acute respiratory distress syndrome. Anesthesiology 87: 297–307, 1997. [DOI] [PubMed] [Google Scholar]
- 32.Meddings JB, Scott RB, Fick GH. Analysis and comparison of sigmoidal curves: application to dose-response data. Am J Physiol Gastrointest Liver Physiol 257: G982–G989, 1989. [DOI] [PubMed] [Google Scholar]
- 33.Mullershausen F, Friebe A, Feil R, Thompson WJ, Hofmann F, Koesling D. Direct activation of PDE5 by cGMP: long-term effects within NO/cGMP signaling. J Cell Biol 160: 719–727, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murthy KS, Zhou H, Makhlouf GM. PKA-dependent activation of PDE3A and PDE4 and inhibition of adenylyl cyclase V/VI in smooth muscle. Am J Physiol Cell Physiol 282: C508–C517, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Penheiter AR, Bajzer Z, Filoteo AG, Thorogate R, Torok K, Caride AJ. A model for the activation of plasma membrane calcium pump isoform 4b by calmodulin. Biochemistry 42: 12115–12124, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Perkins WJ, Pabelick C, Warner DO, Jones KA. cGMP-independent mechanism of airway smooth muscle relaxation induced by S-nitrosoglutathione. Am J Physiol Cell Physiol 275: C468–C474, 1998. [DOI] [PubMed] [Google Scholar]
- 37.Perkins WJ, Taniguchi M, Warner DO, Chini EN, Jones KA. Reduction in soluble guanylyl cyclase-specific activity following prolonged treatment of porcine pulmonary artery with nitric oxide. Am J Physiol Lung Cell Mol Physiol 293: L84–L95, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Roos CM, Frank DU, Xue C, Johns RA, Rich GF. Chronic inhaled nitric oxide: effects on pulmonary vascular endothelial function and pathology in rats. J Appl Physiol 80: 252–260, 1996. [DOI] [PubMed] [Google Scholar]
- 39.Ruth P, Landgraf W, Keilbach A, May B, Egleme C, Hofmann F. The activation of expressed cGMP-dependent protein kinase isozymes Ialpha and Ibeta is determined by the different amino-termini. Eur J Biochem 202: 1339–1344, 1991. [DOI] [PubMed] [Google Scholar]
- 40.Sausbier M, Schubert R, Voigt V, Hirneiss C, Pfeifer A, Korth M, Kleppisch T, Ruth P, Hofmann F. Mechanisms of NO/cGMP-dependent vasorelaxation. Circ Res 87: 825–830, 2000. [DOI] [PubMed] [Google Scholar]
- 41.Sayed N, Baskaran P, Ma X, van den Akker F, Beuve A. Desensitization of soluble guanylyl cyclase, the NO receptor, by S-nitrosylation. Proc Natl Acad Sci USA 104: 12312–12317, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith JA, Francis SH, Walsh KA, Kumar S, Corbin JD. Autophosphorylation of type Ibeta cGMP-dependent protein kinase increases basal catalytic activity and enhances allosteric activation by cGMP or cAMP. J Biol Chem 271: 20756–20762, 1996. [DOI] [PubMed] [Google Scholar]
- 43.Soff GA, Cornwell TL, Cundiff DL, Gately S, Lincoln TM. Smooth muscle cell expression of type I cyclic GMP-dependent protein kinase is suppressed by continuous exposure to nitrovasodilators, theophylline, cyclic GMP, and cyclic AMP. J Clin Invest 100: 2580–2587, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Surks HK, Mochizuki N, Kasai Y, Georgescu SP, Tang KM, Ito M, Lincoln TM, Mendelsohn ME. Regulation of myosin phosphatase by a specific interaction with cGMP-dependent protein kinase Ialpha. Science 286: 1583–1587, 1999. [DOI] [PubMed] [Google Scholar]
- 45.Taniguchi M, Kwak YL, Jones KA, Warner DO, Perkins WJ. Nitric oxide sensitivity in pulmonary artery and airway smooth muscle: a possible role for cGMP responsiveness. Am J Physiol Lung Cell Mol Physiol 290: L1018–L1027, 2006. [DOI] [PubMed] [Google Scholar]
- 46.Thorne GD, Shimizu S, Paul RJ. Hypoxic vasodilation in porcine coronary artery is preferentially inhibited by organ culture. Am J Physiol Cell Physiol 281: C24–C32, 2001. [DOI] [PubMed] [Google Scholar]
- 47.Wittwer CT, Herrmann MG, Moss AA, Rasmussen RP. Continuous fluorescence monitoring of rapid cycle DNA amplification. Biotechniques 22: 130–131, 134–138, 1997. [DOI] [PubMed] [Google Scholar]
- 48.Wolfe L, Corbin JD, Francis SH. Characterization of a novel isozyme of cGMP-dependent protein kinase from bovine aorta. J Biol Chem 264: 7734–7741, 1989. [PubMed] [Google Scholar]
- 49.Worner R, Lukowski R, Hofmann F, Wegener JW. cGMP signals mainly through cAMP kinase in permeabilized murine aorta. Am J Physiol Heart Circ Physiol 292: H237–H244, 2007. [DOI] [PubMed] [Google Scholar]
- 50.Yamashita T, Kawashima S, Ohashi Y, Ozaki M, Rikitake Y, Inoue N, Hirata K, Akita H, Yokoyama M. Mechanisms of reduced nitric oxide/cGMP-mediated vasorelaxation in transgenic mice overexpressing endothelial nitric oxide synthase. Hypertension 36: 97–102, 2000. [DOI] [PubMed] [Google Scholar]
- 51.Yamashita T, Kawashima S, Ohashi Y, Ozaki M, Ueyama T, Ishida T, Inoue N, Hirata K, Akita H, Yokoyama M. Resistance to endotoxin shock in transgenic mice overexpressing endothelial nitric oxide synthase. Circulation 101: 931–937, 2000. [DOI] [PubMed] [Google Scholar]