Abstract
Several lines of evidence indicate that depletion of glutathione (GSH), a critical thiol antioxidant, is associated with the pathogenesis of idiopathic pulmonary fibrosis (IPF). However, GSH synthesis depends on the amino acid cysteine (Cys), and relatively little is known about the regulation of Cys in fibrosis. Cys and its disulfide, cystine (CySS), constitute the most abundant low-molecular weight thiol/disulfide redox couple in the plasma, and the Cys/CySS redox state (Eh Cys/CySS) is oxidized in association with age and smoking, known risk factors for IPF. Furthermore, oxidized Eh Cys/CySS in the culture media of lung fibroblasts stimulates proliferation and expression of transitional matrix components. The present study was undertaken to determine whether bleomycin-induced lung fibrosis is associated with a decrease in Cys and/or an oxidation of the Cys/CySS redox state and to determine whether these changes were associated with changes in Eh GSH/glutathione disulfide (GSSG). We observed distinct effects on plasma GSH and Cys redox systems during the progression of bleomycin-induced lung injury. Plasma Eh GSH/GSSG was selectively oxidized during the proinflammatory phase, whereas oxidation of Eh Cys/CySS occurred at the fibrotic phase. In the epithelial lining fluid, oxidation of Eh Cys/CySS was due to decreased food intake. Thus the data show that decreased precursor availability and enhanced oxidation of Cys each contribute to the oxidation of extracellular Cys/CySS redox state in bleomycin-induced lung fibrosis.
Keywords: idiopathic pulmonary fibrosis, oxidative stress, glutathione, diet
idiopathic pulmonary fibrosis (IPF) is a progressive fibrotic disorder characterized by structural alterations in the lung parenchyma due, in part, to excessive fibroblast proliferation and deposition of extracellular matrix components such as collagen and fibronectin (13). Several lines of evidence indicate that perturbations in glutathione (GSH) homeostasis are related to the pathogenesis of IPF. Observations in IPF patients demonstrate a marked decrease in epithelial lining fluid (ELF) GSH levels (8, 39). Additionally, in vitro studies show that fibroblast proliferation is suppressed at optimal extracellular GSH levels, and inhibition of GSH synthesis stimulates transforming growth factor-β1 (TGF-β1)-mediated collagen synthesis (9, 30). Together, these data support a mechanistic role for altered GSH homeostasis in the fibrotic process. However, GSH synthesis depends on the amino acid cysteine (Cys), and relatively little is known about the regulation of Cys in fibrosis.
Cys, its disulfide, cystine (CySS), GSH, and glutathione disulfide (GSSG) comprise the major low-molecular weight thiol/disulfide redox control systems in mammals (22). These control systems are compartmentalized; GSH/GSSG provides control mechanisms within cells, whereas Cys/CySS predominates in the extracellular fluid. Recent advances in redox signaling mechanisms have revealed that the redox states (Eh) of these couples are not in equilibrium with each other and have distinct regulatory functions (20, 21, 26). We have shown that oxidized Eh Cys/CySS in the culture media of lung fibroblasts stimulates proliferation and expression of transitional matrix components such as fibronectin, and this occurs in the absence of apparent changes to the GSH pool (40). Additionally, Eh Cys/CySS is oxidized in association with age and smoking, known risk factors for IPF (27, 34). Thus oxidation of Cys/CySS redox state may represent a hitherto unidentified pathogenic mechanism in lung fibrosis.
The purpose of the present study was to determine whether bleomycin-induced fibrosis is associated with a decrease in Cys and/or an oxidation of the Cys/CySS redox state and to determine whether these changes were associated with changes in Eh GSH/GSSG. Mice received bleomycin intratracheally, and GSH and Cys redox states were measured at time points known to correlate with the proinflammatory and profibrotic phases of lung injury. To control for the effect of food intake on Cys concentrations, responses in bleomycin-treated mice were compared with pair-fed, saline-treated controls. We observed distinct effects on plasma GSH and Cys redox systems during the progression of bleomycin-induced lung injury. Plasma Eh GSH/GSSG was selectively oxidized during the proinflammatory phase, whereas oxidation of Eh Cys/CySS occurred at the fibrotic phase. Interestingly, Eh Cys/CySS in the ELF was substantially more oxidized than the plasma pool and was due, entirely, to bleomycin-related decrease in food intake. Thus the data show that decreased precursor availability and enhanced oxidation of Cys each contribute to the oxidation of extracellular Cys/CySS redox state in bleomycin-induced lung fibrosis.
METHODS
Materials.
All chemicals were purchased from Sigma (St. Louis, MO) except where indicated. Distilled, deionized water was used for analytical purposes. HPLC quality solvents were used for HPLC.
Experimental animals and pair feeding.
Experiments were conducted using 10- to 14-wk-old, female C57BL/6J mice (The Jackson Laboratories, Bar Harbor, ME). Mice were housed individually for pair feeding and maintained on a 12:12-h light-dark cycle at the Division of Animal Resources at Emory University. All animals were fed pelleted rodent food (Test Diet 5015; LabDiet, Richmond, IN) and had free access to water. Nesting material was presented daily to each mouse to compensate for the absence of other animals (5). All experiments were initiated during the light cycle. Measurement of food intake in bleomycin-treated mice was performed by providing mice with a weighed food pellet at day 0 and manually recording weight of the remaining food the next day. Fresh, weighed food was provided daily. The amount of food ingested by bleomycin-treated mice was averaged, and this amount was provided to pair-fed PBS controls. All animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee at Emory University.
Bleomycin administration.
Bleomycin, dissolved in sterile PBS, was administered intratracheally at a dose of 3.2 U bleomycin/kg body wt under ketamine-xylazine anesthesia. Animals were killed at 1, 3, 7, and 14 days postbleomycin. Pair-fed PBS animals received intratracheal injection of PBS and were killed at corresponding time points.
Sample collection and analysis of Cys, CySS, GSH, and GSSG.
Samples were collected using a method optimized to minimize errors due to collection and processing (25). Mice were anesthetized by isoflurane inhalation (Baxter, Deerfield, IL), and blood was collected by submandibular bleeding using a 4-mm mouse bleeding lancet (Medipoint, Mineola, NY).
Because red blood cell GSH levels are ∼2 orders of magnitude greater than plasma GSH levels, 1% hemolysis can result in an increase of ∼7 μM GSH in the plasma. To minimize artificial overestimation of GSH due to hemolysis, blood was collected into a heparin-coated Eppendorf tube to inhibit coagulation. We have determined that for mouse plasma, a >0.2% hemolysis is visually detected. Consequently, samples were evaluated visually for hemolysis, and hemolyzed samples were not included in the analysis.
The collected blood (0.18 ml) was immediately transferred to 0.02 ml of preservation solution. The preservation solution included heparin, serine borate to inhibit degradation of GSH by γ-glutamyltranspeptidase, bathophenanthroline disulfonate to inhibit oxidation of GSH and Cys, and iodoacetic acid to alkylate GSH and Cys. To facilitate quantification of the thiols and disulfides, γ-glutamyl glutamate (γ-Glu-Glu) was used an internal standard (23). Samples were centrifuged at 16,000 g for 60 s to remove precipitated protein, and 0.1 ml of the supernatant was immediately transferred to an equal volume of ice-cold 10% (wt/vol) perchloric acid. Samples were immediately stored at −80°C and derivatized with dansyl chloride within 1 mo. Stability tests have shown that nonderivatized samples are stable for at least 2 mo at −80°C (25).
Bronchoalveolar lavage fluid (BALF) was obtained after mice were euthanized. Briefly, 0.6 ml of sterile PBS was instilled into the lung via a tracheal incision and withdrawn with gentle suction. This procedure took ∼10 min to complete. The collected BALF was centrifuged at 200 g for 6–7 min. Cell-free supernatant (0.15 ml) was mixed with an equal volume of ice-cold 10% (wt/vol) perchloric acid containing 20 μM γ-Glu-Glu and stored at −80°C until derivatization with iodoacetic acid and dansyl chloride.
For HPLC analysis (Gilson Medical Electronics, Middleton, WI), derivatized samples were centrifuged, and 50 μl (plasma) or 65 μl (BALF) of the aqueous layer was applied to the Supelcosil LC-NH2 column (25 cm × 4.6 mm; Supelco, Bellefonte, PA). Derivatives were separated with a sodium acetate gradient in methanol/water and detected by fluorescence (25). Concentrations of thiols and disulfides were determined by integration relative to the internal standard. Redox states (Eh) of the GSH/GSSG and Cys/CySS pools were calculated from concentrations of GSH, GSSG, Cys, and CySS in molar units with the following forms of the Nernst equation for pH 7.4: GSH/GSSG, Eh = −264 + 30 log [(GSSG)/(GSH)2]; Cys/CySS, Eh = −250 + 30 log [(CySS)/(Cys)2] (Ref. 24).
Urea measurements.
Because urea diffuses readily through the body, plasma and ELF urea concentrations are identical (41). Therefore, estimates of Cys and CySS levels in the ELF were obtained after normalization for dilution using the urea dilution factor. The urea dilution factor is equal to ureaplasma/ureaBALF, and estimation of BALF dilution using this method is considered to be reasonably accurate (41, 49). To minimize variability of recovery, dwell times for saline were maintained at less than 2 min under all experimental conditions. The concentration of urea in plasma and BALF was measured using a urea assay kit with a sensitivity range of 0.08–100 mg/dl (BioAssay Systems, Hayward, CA).
Histopathology.
Lungs were fixed by intratracheal instillation of neutral buffered formalin (10%). After further fixation overnight at room temperature, the tissue was embedded in paraffin, sectioned, and stained by hematoxylin and eosin (H&E). Masson's trichrome stain was used to detect collagen deposition. All sections were studied by light microscopy. For immunohistochemistry, fibronectin rabbit polyclonal antibody (Sigma) and anti-TGF-β1 antibody (Sigma) were used.
Cell culture.
Primary fibroblasts were isolated from the lungs of 8- to 10-wk-old wild-type C57BL/6J or from transgenic mice expressing the full-length human fibronectin promoter-luciferase reporter gene construct as previously described (48). Cells were maintained under 5% CO2 at 37°C in DMEM supplemented with 10 U/ml penicillin, 10 μg/ml streptomycin, and 10% fetal bovine serum (Mediatech, Manassas, VA) but were transferred to serum-free media for 12–24 h before experimental manipulations.
Statistical methods.
Data are presented as means + SE. Statistical analysis was done using SAS v. 9.1 (SAS Institute, Cary, NC). Analyses were performed using a generalized linear model using two-way ANOVA with time and treatment specified as the main effects and time × treatment as the interaction term. Tukey-Kramer post hoc analysis was used for all comparisons with P value < 0.05.
RESULTS
Effect of bleomycin on lung histology, food intake, and weight loss.
The pathophysiology of bleomycin-induced lung injury has been characterized in previous studies (33, 42). Endotracheal instillation of bleomycin leads to lung fibrosis and occurs in three stages. Bleomycin-induced cytotoxicity leads to apoptosis and necrosis of alveolar epithelial cells followed by an inflammatory phase that peaks at day 7 and is characterized by infiltration of neutrophils and lymphocytes in the lung. An aberrant repair and remodeling process ensues, resulting in enhanced deposition of matrix molecules such as collagen, which peaks at day 14.
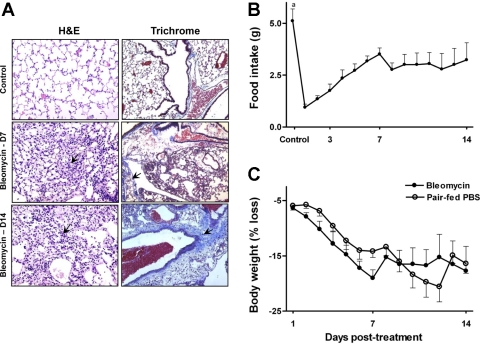
Figure 1A shows photomicrographs of lung sections from untreated controls and at days 7 and 14 after bleomycin treatment. On the left of Fig. 1, lung sections from bleomycin-treated mice stained with H&E showed a progressive loss of normal pulmonary architecture with inflammatory cell infiltration and edema at day 7. By day 14, there is a partial clearance of inflammation. On the right of Fig. 1, sections were stained with Masson's trichrome to highlight the presence of collagen. At day 7 after bleomycin, modest amounts of collagen were present along with inflammatory cells around airways. Lungs from mice at day 14 after bleomycin stained extensively for aniline blue indicating increased collagen deposition.
Fig. 1.
In A, lung sections show extensive inflammation and disruption of alveolar architecture in bleomycin-treated animals at day 7 (D7; arrow, middle left). By day 14, there is a partial clearance of inflammation, but cellular infiltrates are still visible (arrow, bottom left). Lung sections stain extensively for aniline blue day 14 after bleomycin, demonstrating extensive collagen deposition (arrow, bottom right; magnification, ×40). Food intake was measured in bleomycin-treated mice daily for 14 days (B). Food intake in pair-fed controls was restricted to amount of food consumed by bleomycin-treated animals. In C, weight loss in bleomycin-treated mice and time-matched pair-fed PBS controls is presented as percent decline in body weight compared with weight at baseline. Data points and error bars correspond to the means + SE. aP < 0.001 compared with bleomycin-treated animals. H&E, hematoxylin and eosin.
Because food intake is an important determinant of Cys homeostasis (35), we designed the study to control for food intake by pair feeding the control group to the bleomycin-treated group. Bleomycin administration significantly decreased food intake within day 1 of treatment (Fig. 1B; untreated controls, 5.11 ± 0.41 g vs. bleomycin-treated mice at day 1, 1 ± 0.1 g; P < 0.0001), and intake remained substantially decreased at all subsequent time points measured (day 7, 3.5 ± 0.3 g; day 14, 3.2 ± 0.8 g; P < 0.01 compared with untreated controls).
In Fig. 1C, decline in body weight is shown for bleomycin-treated animals and pair-fed PBS controls. There was a linear decrease in body weight from day 1 to 7 postbleomycin (percent weight loss at day 1, 6.38% ± 0.53% vs. day 7, 19.01% ± 1.5%; P < 0.001), and weight loss remained stable thereafter. No statistically significant differences in weight loss were observed between pair-fed control mice and bleomycin-treated animals (treatment effect, P = 0.144; time × treatment effect, P = 0.45), indicating that weight loss occurred as a consequence of decreased food intake.
Oxidation of plasma GSH/GSSG redox state coincides with the inflammatory phase of bleomycin-induced lung injury.
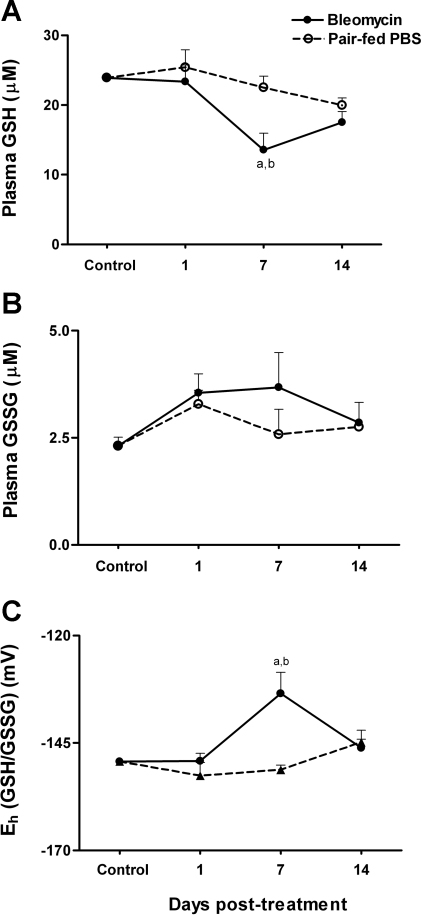
We next determined whether GSH levels are decreased after bleomycin administration and whether similar responses are observed in pair-fed controls. A significant decline in plasma GSH levels was observed at day 7 coinciding with the proinflammatory phase of lung injury (Fig. 2A; untreated controls, 24 ± 0.21 μM; pair-fed day 7, 22.5 ± 1.6 μM; bleomycin day 7, 13.5 ± 2.4 μM; P < 0.05 compared with untreated controls and pair-fed controls). The decrease in plasma GSH at day 7 caused a significant oxidation of Eh GSH/GSSG by an average of 15 mV (Fig. 2C; untreated controls, −149 ± 0.3 mV; pair-fed day 7, −151.2 ± 1.1 mV; bleomycin day 7, −133.5 ± 4.9 mV; P < 0.01 compared with untreated controls and pair-fed controls). GSH levels completely recovered by day 14, leading to normalization of Eh GSH/GSSG.
Fig. 2.
At days 1, 7, and 14, mice were killed, and plasma was collected for HPLC analysis of GSH (A) and GSSG (B). In C, the GSH/GSSG redox state (Eh GSH/GSSG) was calculated from GSH and GSSG concentrations using the Nernst equation. Data are expressed as means + SE. aValues significantly different from untreated controls. bValues significantly different from corresponding pair-fed controls.
Examination of the disulfide form, GSSG, revealed a trend for increase at days 1 and 7 after bleomycin treatment. This increase was, however, not significant (Fig. 2B; untreated controls, 2.3 ± 0.2 μM; bleomycin day 1, 3.5 ± 0.4 μM; bleomycin day 7, 3.6 ± 0.8 μM). At day 14, GSSG levels were 2.8 ± 0.5 μM. Thus, although GSH levels decreased significantly at day 7 with bleomycin treatment, no time- or treatment-related effects were observed in plasma GSSG. The lack of significant increase in GSSG indicates that the activities of the γ-glutamyltranspeptidase and dipeptidases are sufficient to remove GSSG formed. Increased activity of these enzymes could also account for the decrease in plasma GSH at day 7. Examination of plasma GSH, GSSG, and Eh GSH/GSSG in pair-fed controls revealed no significant effect of decreased food intake on plasma Eh levels.
Oxidation of plasma Cys/CySS redox state coincides with the fibrotic phase of bleomycin-induced lung injury.
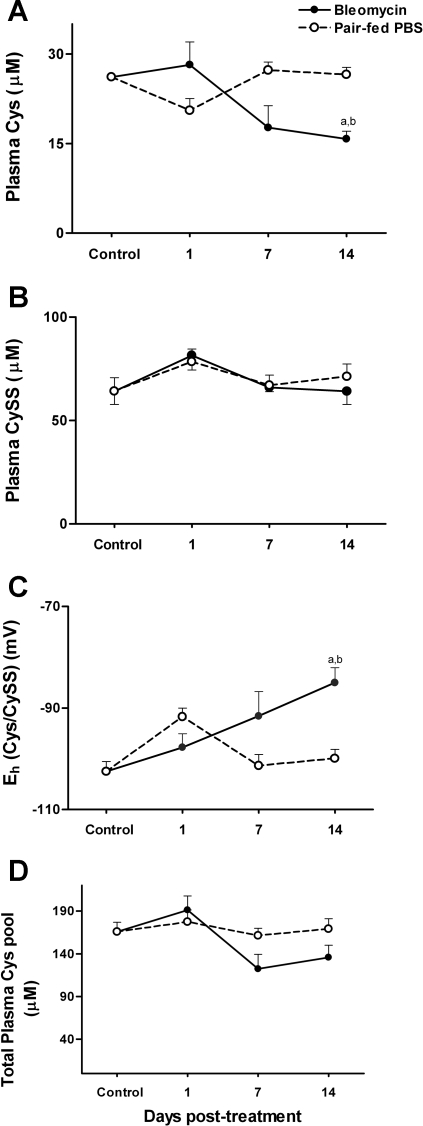
Relatively little is known about the dynamics of the plasma Cys/CySS pool in fibrosis. Cys and CySS concentrations in the plasma were measured immediately after bleomycin administration at day 1 and at the peak proinflammatory (day 7) and profibrotic phases (day 14) of lung injury. No significant differences in plasma Cys levels were observed at days 1 and 7 postbleomycin, but Cys levels dropped significantly at day 14 (untreated controls, 26.2 ± 0.08 μM; pair-fed day 14, 26.5 ± 1.2 μM; bleomycin day 14, 15.7 ± 1.3 μM; P < 0.05 compared with untreated controls and pair-fed controls; Fig. 3A). In contrast to the decrease in Cys, plasma levels of the disulfide, CySS, did not change with bleomycin treatment (Fig. 3B; untreated controls, 73 ± 5.4 μM; bleomycin day 7, 66 ± 2.2 μM; bleomycin day 14, 64 ± 6.3 μM).
Fig. 3.
At days 1, 7, and 14, mice were killed, and plasma was collected for HPLC analysis of amino acid cysteine (Cys; A) and cystine (CySS; B). In C, Eh Cys/CySS was calculated from Cys and CySS concentrations using the Nernst equation. The total Cys pool is calculated as Cys + (2 × CySS) (D). Data are expressed as means + SE. aValues significantly different from untreated controls. bValues significantly different from corresponding pair-fed controls.
Despite the relative stability of plasma CySS, the decrease in plasma Cys resulted in oxidation of Eh Cys/CySS by an average of 20 mV at day 14 (untreated controls, −102.5 ± 1.9 mV; pair-fed day 14, 100 ± 1.7 mV; bleomycin day 14, −85 ± 3 mV; P < 0.05 compared with untreated controls and pair-fed controls; Fig. 3C). In pair-fed controls, Eh Cys/CySS did not change with time except for an average 10-mV oxidation at day 1 (pair-fed controls day 1, 92 ± 1.2 mV; P < 0.05 compared with untreated controls), suggesting that oxidation of Eh Cys/CySS at day 14 was a consequence of the disease process and not related to food intake. Analysis of the total plasma Cys pool revealed no significant changes with time or treatment (Fig. 3D; time effect, P = 0.4; treatment effect, P = 0.3).
Oxidation of Eh Cys/CySS in the lung ELF is due to bleomycin-related alterations in food intake.
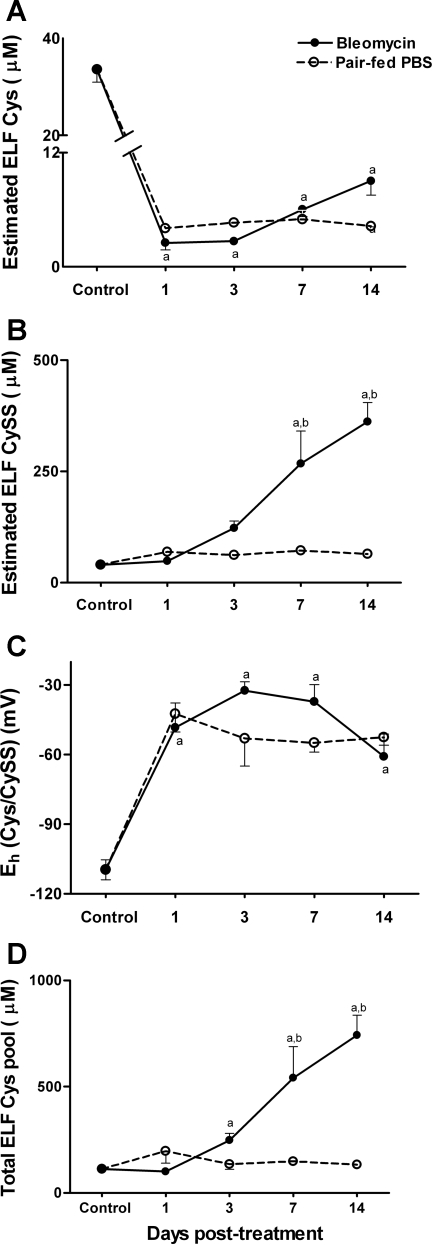
The decrease in GSH levels in ELF in the bleomycin model is well-documented (14). Because Cys is a precursor for GSH and because the plasma pool and the ELF pool are independently regulated (49), we determined whether Eh Cys/CySS was oxidized in the ELF. Levels of Cys and CySS were determined in the ELF, immediately after death, at the peak proinflammatory and profibrotic phases of bleomycin-induced lung injury. For these analyses, the ELF was collected into a preservation solution designed to prevent oxidation of Cys during storage. Levels of Cys and CySS are presented after correction for dilution using the urea dilution factor.
As shown in Fig. 4A, Cys levels in the ELF rapidly declined within 24 h after bleomycin treatment (untreated controls, 33.6 ± 2.6 μM; bleomycin day 1, 2.5 ± 0.75 μM; P < 0.0001) and remained significantly below control values at all subsequent time points measured. No statistically significant differences in Cys levels were observed between bleomycin-treated animals and pair-fed controls at any time point (treatment effect, P = 0.65). The comparable decline in Cys in both treatment groups suggests that the decline in ELF Cys is a consequence of reduced food intake.
Fig. 4.
Bronchoalveolar lavage fluid (BALF) was sampled from bleomycin-treated mice and pair-fed mice at time points shown. Levels of Cys (A) and CySS (B) in epithelial lining fluid (ELF) are shown after correction using the urea dilution factor. In C, Eh Cys/CySS was calculated from Cys and CySS concentrations using the Nernst equation. The total Cys pool is calculated as Cys + (2 × CySS) (D). Data are expressed as means + SE. aValues significantly different from untreated controls. bValues significantly different from corresponding pair-fed controls.
In contrast to Cys, levels of CySS increased after bleomycin-treatment (untreated controls, 40.4 ± 2.5 μM; bleomycin day 7, 267.8 ± 73.2 μM; bleomycin day 14, 362 ± 43 μM; P < 0.0001; Fig. 4B). CySS levels increased modestly in pair-fed controls (pair-fed day 7, 72 ± 9.4 μM; pair-fed day 14, 64.2 ± 7.2 μM; Fig. 4B). This increase was, however, not statistically significant, suggesting that the increase in ELF CySS in bleomycin-treated animals occurred as a consequence of the inflammatory and fibrotic process in response to lung injury.
Because the redox state of the Cys/CySS couple is driven by the absolute concentrations of Cys (24), Eh Cys/CySS was oxidized equally in pair-fed controls and bleomycin-treated animals (Fig. 4C; untreated controls, −108 ± 5.1 mV; bleomycin day 7, −37.2 ± 7.3 mV; bleomycin day 14, −60.9 ± 9.7 mV; pair-fed day 7, −55 ± 4.01 mV; pair-fed day 14, −52.5 ± 3.5 mV; P < 0.0001 compared with untreated controls).
Calculation of the total Cys pool [Cys + (2 × CySS)] revealed a significant increase with bleomycin treatment at day 3 (untreated controls, 113 + 6.1 μM; bleomycin day 3, 248 ± 32 μM; P < 0.05 compared with untreated controls) and days 7 and 14 (bleomycin day 7, 541.5 ± 147 μM; bleomycin day 14, 742 ± 95.2 μM; P < 0.0001 compared with untreated controls and pair-fed controls).
Thus oxidation of Eh Cys/CySS at days 7 and 14 in bleomycin-treated animals was associated with an increase in total pool size while in pair-fed controls; oxidation in Eh was associated without a major change in the total pool size. Taken together, the data show that plasma Cys/CySS is preserved in face of nutrient depletion, whereas in the lung lining fluid, nutrient deprivation induces perturbations in Cys homeostasis.
Temporal changes in lung fibrotic markers occur in bleomycin-treated mice but not in pair-fed controls.
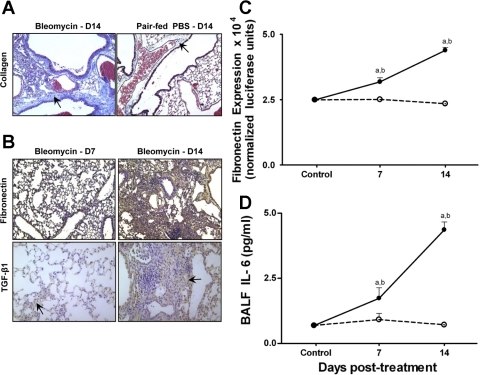
Studies in pair-fed animals suggested that diet alone could result in an oxidized Eh Cys/CySS in the ELF. Therefore, we evaluated lung sections from pair-fed mice for fibrotic changes. As shown in Fig. 5A, lung sections from pair-fed controls did not show increased staining for collagen- compared with bleomycin-treated animals at day 14. Furthermore, immunohistochemical analysis of lung sections showed no increase in fibronectin or TGF-β1 both at days 7 and 14 (data not shown). As expected, lung sections from bleomycin-treated mice demonstrated a substantial increase in staining for fibronectin and TGF-β1 at day 14 (Fig. 5B).
Fig. 5.
Lung microscopic sections showed extensive collagen deposition in bleomycin-treated animals but not pair-fed controls (arrows, A). Lung sections from bleomycin-treated mice stain extensively for fibronectin (brown, B, top right) and transforming growth factor-β1 (TGF-β1; arrow, B, bottom right) at day 14 (magnification, ×40). BALF from bleomycin-treated mice induces luciferase expression in fibroblasts transfected with a fibronectin promoter-luciferase construct (C). In D, BALF from bleomycin-treated mice show increased IL-6 levels. Data are expressed as means + SE. aValues significantly different from untreated controls. bValues significantly different from corresponding pair-fed controls.
To confirm the lack of profibrotic activity in the BAL of pair-fed controls, we examined fibronectin-inducing activity in the ELF of pair-fed vs. bleomycin-treated animals. In these analyses, ELF was not collected into a preservation solution, and therefore the observed effects are not related to Cys/CySS redox state but rather to the presence of profibrotic mediators in the ELF. Fibroblasts transfected with a fibronectin luciferase reporter construct were stimulated with lavage fluid, and fibronectin expression was determined (Fig. 5C). Fibronectin is one of the major extracellular matrix proteins involved in fibrosis and increases substantially in the fibrotic lung (29). Lavage fluid harvested from bleomycin-treated mice at days 7 and 14 significantly increased activity of the fibronectin promoter (P < 0.01 compared with untreated controls and pair-fed controls). Furthermore, fibronectin bioactivity was greater at day 14 compared with day 7 after bleomycin (P < 0.0001). ELF obtained from pair-fed controls did not induce fibronectin expression.
Measurement of the profibrotic cytokine IL-6 (50) in the BALF by ELISA revealed a significant increase in IL-6 at days 7 and 14 in bleomycin-treated animals compared with untreated and pair-fed controls (Fig. 5D; P < 0.05). In bleomycin-treated animals, IL-6 levels were greater at day 14 compared with day 7 (P < 0.01). We detected similar magnitude of differences in BALF fibronectin activity and IL-6 between bleomycin-treated animals and pair-fed controls after normalization for dilution using the urea dilution factor. Thus the data show that oxidation of Eh Cys/CySS in the ELF precedes maximal expression of collagen, fibronectin, TGF-β1, and IL-6 in bleomycin-treated animals but not pair-fed controls.
DISCUSSION
Bleomycin is widely used to model lung fibrosis in mice (33). The cytotoxicity of bleomycin is mediated by two main structural components: a bithiazole ring, which partially intercalates into the DNA helix, and pyrimidine and imidazole moieties, which bind iron and oxygen to generate free radicals (45, 50). Studies investigating the pulmonary fate of intratracheal, tritiated bleomycin show that less than 1% of the radioactivity is retained at 24 h (28). This indicates that bleomycin is rapidly metabolized in the lung. Thus the dynamics of GSH/GSSG and Cys/CySS redox states at days 7 and 14 (Figs. 2–4) represent changes arising from bleomycin-induced lung injury rather than a direct oxidative effect of bleomycin.
The oxidation of plasma Eh GSH/GSSG at day 7, coinciding with the proinflammatory phase of lung injury, can be explained by perturbations in GSH homeostasis due to inflammation. A greater partitioning of sulfur amino acids for hepatic protein synthesis compared with GSH synthesis occurs during inflammation (16) and could account for the decrease in plasma GSH at day 7. The role of GSH in the detoxification of reactive oxygen species (ROS) produced during the inflammatory response to injury could also lead to oxidation of Eh GSH/GSSG (18, 32). Interestingly, the decrease in plasma GSH at day 7 is not associated with a corresponding decrease in the precursor Cys pool. This observation is, however, not unexpected. Although Cys and GSH share a precursor-product relationship (6), biochemical studies show that GSH can serve as a precursor for Cys (31). In vivo, degradation of plasma GSH by γ-glutamyltranspeptidase in the kidney yields cysteinyl glycine, which is cleaved by dipeptidases to Cys (31). γ-Glutamyltranspeptidase is highly inducible by oxidative stress (51), and increased degradation of GSH can contribute to a decrease in plasma GSH with an associated maintenance of plasma Cys.
By day 14, GSH levels recover, leading to normalization of plasma Eh GSH/GSSG. The dynamics of the plasma GSH pool during the fibrotic phase of lung injury are in agreement with observations by Borok et al. (7) and Teramoto et al. (46) who reported that plasma GSH levels in IPF patients are not significantly different from healthy controls. Although we observed no significant changes in plasma GSSG at day 14, GSSG levels are elevated in IPF patients (46). This may be related to perturbations in GSH turnover, due to increased oxidant burden, associated with progressive fibrosis in human IPF.
In contrast to GSH, levels of Cys decrease at day 14. The decrease in plasma Cys and the associated oxidation of Eh Cys/CySS in bleomycin-treated animals, but not pair-fed controls, indicates that perturbations in Cys homeostasis occur as a consequence of the fibrotic process. TGF-β1, a key fibrotic mediator, induces production of extracellular hydrogen peroxide in endothelial cells, which could lead to oxidation of plasma Cys (11). However, plasma CySS levels do not change, suggesting that mechanisms relating to increased transport of CySS into tissues may also be involved. Indeed, studies by Sato et al. (43) and others (4) have shown the induction of specific CySS transporters in response to peroxide and other prooxidant stimuli. A role for TGF-β1-mediated transport of Cys into tissues to support increased protein synthesis cannot be excluded. In unpublished observations, we have found that TGF-β1 induces depletion of extracellular Cys and CySS in a dose-dependent manner in lung fibroblasts. Indeed, increased protein synthesis during tissue repair places a higher demand on amino acid supply (36, 47). Notwithstanding these possibilities, the decline in Cys levels resulted in a 20-mV oxidation of Eh Cys/CySS at day 14. This magnitude of oxidation is sufficient to cause a fivefold change in the ratios of reduced to oxidized forms of proteins with vicinal dithiols (23). Thus substantial redox-dependent changes could occur for plasma or membrane proteins that interact with the Cys/CySS couple.
A significant observation is the disequilibrium in the redox states of plasma Cys/CySS and GSH/GSSG at days 7 and 14. This suggests that the redox states of these major thiol/disulfide redox systems are regulated independently in the plasma, during inflammation and fibrosis. We (1) recently reported that in cells subjected to Cys deficiency, oxidation of Eh Cys/CySS occurs in the absence of changes to Eh GSH/GSSG. Similarly, Banjac and colleagues (2) found that overexpression of the CySS/glutamate antiporter, system xc−, protected lymphoma cells from apoptosis induced by GSH depletion by a mechanism relating to increase in extracellular Cys and not cellular GSH.
Interestingly, measurements of Eh Cys/CySS in the alveolar space reveal a more profound oxidation in the ELF compared with the plasma pool. Similar observations have been made in alcoholics where the Eh GSH/GSSG is oxidized by 40 mV in the lining fluid compared with a 20-mV oxidation in plasma (49). Additionally, Eh Cys/CySS in the ELF is oxidized before fibrosis.
Together with previous observations in lung fibroblasts that oxidized Eh Cys/CySS stimulates fibronectin expression via increase in TGF-β1, this indicates that oxidized ELF Eh Cys/CySS may contribute to lung fibrosis. However, ELF Eh Cys/CySS is also oxidized in pair-fed controls in the absence of fibronectin expression. These data would suggest that oxidized Eh Cys/CySS in the ELF is insufficient to promote fibrosis but can contribute to fibrosis in the setting of injury, characterized by the destruction of basement membranes and the infiltration of fibroblasts into the alveolar spaces. Thus oxidized Eh Cys/CySS may represent a predisposing state in the air space that is active only after exposure to a “second hit” that compromises alveolar barrier integrity.
Although Eh Cys/CySS is comparable between bleomycin-treated animals and pair-fed controls, an important distinction is the greater than twofold elevation in CySS levels with bleomycin treatment. The substantial increase in CySS is likely due to enhanced oxidation of extracellular Cys by ROS released from activated inflammatory cells sequestered in the air space and humoral factors such as TNF-α and TGF-β1 in the ELF (38). This elevation in CySS may be toxic to alveolar epithelial cells by mechanisms relating to oxidation of membrane and extracellular proteins and/or by increased efflux of intracellular glutamate via the xc− system (3, 20). However, despite the elevation in CySS, the comparable decline in Cys in bleomycin-treated animals and pair-fed controls leads to a similar oxidation of Eh Cys/CySS. Because Cys enters the Nernst equation as a squared term, Eh Cys/CySS is largely driven by the absolute concentrations of Cys (24). This indicates that dietary adequacy of Cys and Cys precursors is critical in maintaining the reducing capacity of the Cys/CySS redox couple in the ELF.
The decrease in Cys and GSH reserves is likely to play an important role in the inflammatory and fibrotic response to injury. Cys and GSH are well-established determinants of cytokine and growth factor production during activation of the immune system. For instance, oral pretreatment of mice with GSH and N-acetylcysteine (NAC), a Cys precursor, attenuates LPS-induced increase in TNF-α (37). Furthermore, alcohol-induced depletion of GSH is proposed to underlie the increased susceptibility of alcoholics to sepsis-induced acute lung injury (15). The mechanistic role of Eh Cys/CySS in inflammatory and fibrotic cell responses is only beginning to be elucidated. In vitro, oxidized Eh Cys/CySS stimulates adhesion of leukocytes to the pulmonary endothelium (17), sensitizes epithelial cells to apoptosis (19), induces NF-κB in endothelial cells (12), and stimulates TGF-β1 expression in lung fibroblasts (40). Thus oxidized Eh Cys/CySS can potentiate the inflammatory and fibrotic response to injury. The temporal association between oxidation of ELF Eh Cys/CySS and induction of lung fibrotic markers in bleomycin-induced injury is consistent with this possibility. Consequently, maintenance of optimal Cys and GSH reserves during inflammation and fibrosis is likely to be beneficial. Indeed, multiple animal studies have consistently shown that NAC ameliorates the inflammatory and fibrotic response to bleomycin (10, 14, 44). However, the early timing of the intervention and the lack of pharmacokinetic data on NAC complicate the interpretation of the results. Additional studies are required to confirm that the benefits of NAC in animal models relate to preservation of Cys and GSH redox systems.
In conclusion, the data show that decreased precursor availability and enhanced oxidation of Cys each contribute to the oxidation of extracellular Cys/CySS redox state in bleomycin-induced lung fibrosis. Consequently, oxidation of Eh Cys/CySS may be important in the pathogenesis of fibrosis. Further studies are needed to investigate whether preservation of Eh Cys/CySS represents a useful therapeutic target in IPF and related fibrotic disorders.
GRANTS
This work was supported by National Institutes of Health Grants ES-009047 and ES-011195 (D. P. Jones), 5K01-HL-084683-02 (M. Rojas), and K08-HL-077533-01 (A. M. Ramirez), the McKelvey Lung Transplantation Center (K. L. Brigham, A. M. Ramirez, and M. Rojas), and the Robert W. Woodruff Health Sciences Fund (J. Roman).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Anderson CL, Iyer SS, Ziegler TR, Jones DP. Control of extracellular cysteine/cystine redox state by HT-29 cells is independent of cellular glutathione. Am J Physiol Regul Integr Comp Physiol 293: R1069–R1075, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Banjac A, Perisic T, Sato H, Seiler A, Bannai S, Weiss N, Kölle P, Tschoep K, Issels RD, Daniel PT, Conrad M, Bornkamm GW. The cystine/cysteine cycle: a redox cycle regulating susceptibility versus resistance to cell death. Oncogene 27: 1618–1628, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Bannai S Transport of cystine and cysteine in mammalian cells. Biochim Biophys Acta 779: 289–306, 1984. [DOI] [PubMed] [Google Scholar]
- 4.Bannai S, Sato H, Ishii T, Taketani S. Enhancement of glutathione levels in mouse peritoneal macrophages by sodium arsenite, cadmium chloride and glucose/glucose oxidase. Biochim Biophys Acta 1092: 175–179, 1991. [DOI] [PubMed] [Google Scholar]
- 5.Belz EE, Kennell JS, Czambel RK, Rubin RT, Rhodes ME. Environmental enrichment lowers stress-responsive hormones in singly housed male and female rats. Pharmacol Biochem Behav 76: 481–486, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Blanco RA, Ziegler TR, Carlson BA, Cheng PY, Park Y, Cotsonis GA, Accardi CJ, Jones DP. Diurnal variation in glutathione and cysteine redox states in human plasma. Am J Clin Nutr 86: 1016–1023, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Borok Z, Buhl R, Grimes GJ, Bokser AD, Hubbard RC, Holroyd KJ, Roum JH, Czerski DB, Cantin AM, Crystal RG. Effect of glutathione aerosol on oxidant-antioxidant imbalance in idiopathic pulmonary fibrosis. Lancet 338: 215–216, 1991. [DOI] [PubMed] [Google Scholar]
- 8.Cantin AM, Hubbard RC, Crystal RG. Glutathione deficiency in the epithelial lining fluid of the lower respiratory tract in idiopathic pulmonary fibrosis. Am Rev Respir Dis 139: 370–372, 1989. [DOI] [PubMed] [Google Scholar]
- 9.Cantin AM, Larivee P, Begin RO. Extracellular glutathione suppresses human lung fibroblast proliferation. Am J Respir Cell Mol Biol 3: 79–85, 1990. [DOI] [PubMed] [Google Scholar]
- 10.Cortijo J, Cerda-Nicolas M, Serrano A, Bioque G, Estrela JM, Santangelo F, Esteras A, Llombart-Bosch A, Morcillo EJ. Attenuation by oral N-acetylcysteine of bleomycin-induced lung injury in rats. Eur Respir J 17: 1228–1235, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Das SK, Fanburg BL. TGF-β1 produces a “prooxidant” effect on bovine pulmonary artery endothelial cells in culture. Am J Physiol Lung Cell Mol Physiol 261: L249–L254, 1991. [DOI] [PubMed] [Google Scholar]
- 12.Go YM, Jones DP. Intracellular proatherogenic events and cell adhesion modulated by extracellular thiol/disulfide redox state. Circulation 111: 2973–2980, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Gross TJ, Hunninghake GW. Idiopathic pulmonary fibrosis. N Engl J Med 345: 517–525, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Hagiwara SI, Ishii Y, Kitamura S. Aerosolized administration of N-acetylcysteine attenuates lung fibrosis induced by bleomycin in mice. Am J Respir Crit Care Med 162: 225–231, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Holguin F, Moss I, Brown LA, Guidot DM. Chronic ethanol ingestion impairs alveolar type II cell glutathione homeostasis and function and predisposes to endotoxin-mediated acute edematous lung injury in rats. J Clin Invest 101: 761–768, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hunter EA, Grimble RF. Cysteine and methionine supplementation modulate the effect of tumor necrosis factor alpha on protein synthesis, glutathione and zinc concentration of liver and lung in rats fed a low protein diet. J Nutr 124: 2319–2328, 1994. [DOI] [PubMed] [Google Scholar]
- 17.Iyer SS, Jones DP, Brigham KL, Rojas M. Oxidation of plasma cysteine/cystine redox state in endotoxin-induced lung injury. Am J Respir Cell Mol Biol. In press. [DOI] [PMC free article] [PubMed]
- 18.Jaeschke H Enhanced sinusoidal glutathione efflux during endotoxin-induced oxidant stress in vivo. Am J Physiol Gastrointest Liver Physiol 263: G60–G68, 1992. [DOI] [PubMed] [Google Scholar]
- 19.Jiang S, Moriarty-Craige SE, Orr M, Cai J, Sternberg P Jr, Jones DP. Oxidant-induced apoptosis in human retinal pigment epithelial cells: dependence on extracellular redox state. Invest Ophthalmol Vis Sci 46: 1054–1061, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Jones DP Extracellular redox state: refining the definition of oxidative stress in aging. Rejuvenation Res 9: 169–181, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Jones DP Radical-free biology of oxidative stress. Am J Physiol Cell Physiol 295: C849–C868, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones DP Redefining oxidative stress. Antioxid Redox Signal 8: 1865–1879, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Jones DP Redox potential of GSH/GSSG couple: assay and biological significance. Methods Enzymol 348: 93–112, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Jones DP, Carlson JL, Mody VC, Cai J, Lynn MJ, Sternberg P. Redox state of glutathione in human plasma. Free Radic Biol Med 28: 625–635, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Jones DP, Carlson JL, Samiec PS, Sternberg P Jr, Mody VC Jr, Reed RL, Brown LA. Glutathione measurement in human plasma. Evaluation of sample collection, storage and derivatization conditions for analysis of dansyl derivatives by HPLC. Clin Chim Acta 275: 175–184, 1998. [DOI] [PubMed] [Google Scholar]
- 26.Jones DP, Go YM, Anderson CL, Ziegler TR, Kinkade JM Jr, Kirlin WG. Cysteine/cystine couple is a newly recognized node in the circuitry for biologic redox signaling and control. FASEB J 18: 1246–1248, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Jones DP, Mody VC Jr, Carlson JL, Lynn MJ, Sternberg P Jr. Redox analysis of human plasma allows separation of pro-oxidant events of aging from decline in antioxidant defenses. Free Radic Biol Med 33: 1290–1300, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Lazo JS, Pham ET. Pulmonary fate of [3H]bleomycin A2 in mice. J Pharmacol Exp Ther 228: 13–18, 1984. [PubMed] [Google Scholar]
- 29.Limper AH, Roman J. Fibronectin. A versatile matrix protein with roles in thoracic development, repair and infection. Chest 101: 1663–1673, 1992. [DOI] [PubMed] [Google Scholar]
- 30.Liu RM, Liu Y, Forman HJ, Olman M, Tarpey MM. Glutathione regulates transforming growth factor-β-stimulated collagen production in fibroblasts. Am J Physiol Lung Cell Mol Physiol 286: L121–L128, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Meister A Glutathione metabolism and its selective modification. J Biol Chem 263: 17205–17208, 1988. [PubMed] [Google Scholar]
- 32.Minamiyama Y, Takemura S, Koyama K, Yu H, Miyamoto M, Inoue M. Dynamic aspects of glutathione and nitric oxide metabolism in endotoxemic rats. Am J Physiol Gastrointest Liver Physiol 271: G575–G581, 1996. [DOI] [PubMed] [Google Scholar]
- 33.Moore BB, Hogaboam CM. Murine models of pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 294: L152–L160, 2008. [DOI] [PubMed] [Google Scholar]
- 34.Moriarty SE, Shah JH, Lynn M, Jiang S, Openo K, Jones DP, Sternberg P. Oxidation of glutathione and cysteine in human plasma associated with smoking. Free Radic Biol Med 35: 1582–1588, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Nkabyo YS, Gu LH, Jones DP, Ziegler TR. Thiol/disulfide redox status is oxidized in plasma and small intestinal and colonic mucosa of rats with inadequate sulfur amino acid intake. J Nutr 136: 1242–1248, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Peng X, Yan H, You Z, Wang P, Wang S. Clinical and protein metabolic efficacy of glutamine granules-supplemented enteral nutrition in severely burned patients. Burns 31: 342–346, 2005. [DOI] [PubMed] [Google Scholar]
- 37.Peristeris P, Clark BD, Gatti S, Faggioni R, Mantovani A, Mengozzi M, Orencole SF, Sironi M, Ghezzi P. N-acetylcysteine and glutathione as inhibitors of tumor necrosis factor production. Cell Immunol 140: 390–399, 1992. [DOI] [PubMed] [Google Scholar]
- 38.Rahman I, Biswas SK, Jimenez LA, Torres M, Forman HJ. Glutathione, stress responses, and redox signaling in lung inflammation. Antioxid Redox Signal 7: 42–59, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Rahman I, Skwarska E, Henry M, Davis M, O'Connor CM, FitzGerald MX, Greening A, MacNee W. Systemic and pulmonary oxidative stress in idiopathic pulmonary fibrosis. Free Radic Biol Med 27: 60–68, 1999. [DOI] [PubMed] [Google Scholar]
- 40.Ramirez A, Ramadan B, Ritzenthaler JD, Rivera HN, Jones DP, Roman J. Extracellular cysteine/cystine redox potential controls lung fibroblast proliferation and matrix expression through upregulation of transforming growth factor-β. Am J Physiol Lung Cell Mol Physiol 293: L972–L981, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Rennard SI, Basset G, Lecossier D, O'Donnell KM, Pinkston P, Martin PG, Crystal RG. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J Appl Physiol 60: 532–538, 1986. [DOI] [PubMed] [Google Scholar]
- 42.Rojas M, Xu J, Woods CR, Mora AL, Spears W, Roman J, Brigham KL. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol 33: 145–152, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sato H, Kuriyama-Matsumura K, Hashimoto T, Sasaki H, Wang H, Ishii T, Mann GE, Bannai S. Effect of oxygen on induction of the cystine transporter by bacterial lipopolysaccharide in mouse peritoneal macrophages. J Biol Chem 276: 10407–10412, 2001. [DOI] [PubMed] [Google Scholar]
- 44.Serrano-Mollar A, Closa D, Prats N, Blesa S, Martinez-Losa M, Cortijo J, Estrela JM, Morcillo EJ, Bulbena O. In vivo antioxidant treatment protects against bleomycin-induced lung damage in rats. Br J Pharmacol 138: 1037–1048, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suzuki H, Nagai K, Yamaki H, Tanaka N, Umezawa H. On the mechanism of action of bleomycin: scission of DNA strands in vitro and in vivo. J Antibiot (Tokyo) 22: 446–448, 1969. [DOI] [PubMed] [Google Scholar]
- 46.Teramoto S, Fukuchi Y, Uejima Y, Shu CY, Orimo H. Superoxide anion formation and glutathione metabolism of blood in patients with idiopathic pulmonary fibrosis. Biochem Mol Med 55: 66–70, 1995. [DOI] [PubMed] [Google Scholar]
- 47.Thomas DR Specific nutritional factors in wound healing. Adv Wound Care 10: 40–43, 1997. [PubMed] [Google Scholar]
- 48.Tomic R, Lassiter CC, Ritzenthaler JD, Rivera HN, Roman J. Anti-tissue remodeling effects of corticosteroids: fluticasone propionate inhibits fibronectin expression in fibroblasts. Chest 127: 257–265, 2005. [DOI] [PubMed] [Google Scholar]
- 49.Yeh MY, Burnham EL, Moss M, Brown LA. Chronic alcoholism alters systemic and pulmonary glutathione redox status. Am J Respir Crit Care Med 176: 270–276, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoshida M, Sakuma J, Hayashi S, Abe K, Saito I, Harada S, Sakatani M, Yamamoto S, Matsumoto N, Kaneda Y. A histologically distinctive interstitial pneumonia induced by overexpression of the interleukin 6, transforming growth factor beta 1, or platelet-derived growth factor B gene. Proc Natl Acad Sci USA 92: 9570–9574, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang H, Forman HJ, Choi J. Gamma-glutamyl transpeptidase in glutathione biosynthesis. Methods Enzymol 401: 468–483, 2005. [DOI] [PubMed] [Google Scholar]