Abstract
We previously demonstrated that angiotensin-(1-7) [Ang-(1-7)], which is increased in the kidney and urine during pregnancy, influences normal fluid expansion of pregnancy. These previous studies were completed by chronic administration of the Ang-(1-7) receptor antagonist D-Alanine-[Ang-(1-7)] (A-779) at a dose of 48 μg/kg/hr after the start of pregnancy (gestational days 11-19). To further explore the role of Ang-(1-7) on kidney function during early, middle, and late pregnancy, Sprague Dawley rats were chronically pretreated 8 days prior to pregnancy and throughout pregnancy (gestational days 0-19) with vehicle or A-779 at a dose of 24 μg/kg/hr. Metabolic studies were completed in virgin animals and throughout pregnancy (gestational days 4-5, 14-15, and 18-19). Chow consumption and water intake increased throughout pregnancy while the difference between intake and output (balance) was increased only at late (day 19) pregnancy with both vehicle and A-779 administration. Urine volume and urinary osmolality were significantly increased and decreased respectively throughout pregnancy in vehicle treated rats only. In late (19 day) pregnancy, A-779 administration significantly decreased chow consumption and water intake. In virgin animals, A-779 administration significantly increased urine volume, while during late pregnancy (19 day), urine volume was significantly decreased with A-779 administration. These studies using pretreatment with a lower dose of A-779 prior to pregnancy confirm results of higher dose A-779 administration after the start of pregnancy. These studies show that Ang-(1-7) produces antidiuresis in virgin rats and diuresis in late gestation. Ang-(1-7) also contributes to the enhanced water intake during pregnancy allowing maintenance of the normal volume expanded state despite diuresis.
INTRODUCTION
Normal pregnancy is a condition in which there is progressive increase of the renin-angiotensin system, specifically evidenced by an increase in circulating concentrations of angiotensinogen, renin activity, and angiotensin II (Ang II) (1-3). Our laboratory has previously demonstrated that pregnancy is also associated with increased circulating, urinary excretion, and renal concentrations of angiotensin-(1-7) [Ang-(1-7)] (4) (5). Merrill et al (3) and Valdes et al. (6) demonstrated increased plasma concentrations and urinary excretion of Ang-(1-7) during the third trimester of human pregnancy. Urinary Ang-(1-7) concentration was also shown to increase with gestation in rats (4). Renal concentration of Ang-(1-7) increased at mid (15 day) and late (19 day) gestation in rats with Ang-(1-7) being co-localized with its processing enzyme angiotensin converting enzyme 2 (ACE2) in the proximal and distal tubular cells of the inner cortex/outer medulla regions of kidneys from Sprague Dawley rats (5). Previously, the volemic condition of the animal has been shown to effect the renal actions of Ang-(1-7). Ang-(1-7) has been shown to produce diuresis in normal volemic male rats with denervated kidneys (7) and in kidneys perfused in situ with Ang-(1-7) (8). However, Santos et al. (9) (10) demonstrated that Ang-(1-7) can produce antidiuresis independently of vasopressin (AVP) in water-expanded male rats that can be accounted for by potent actions on proximal tubules (11) and inner medullary collecting ducts (9).
Recently, our laboratory explored the actions of Ang-(1-7) on kidney function in virgin female and pregnant rats by chronic infusion of a specific Ang-(1-7) receptor antagonist D-alanine-[Ang-(1-7)] (A-779) [48 μg/kg/hr] on days 11-19 of gestation. These studies uncovered that Ang-(1-7) elicits contrasting effects on renal fluid balance depending on the physiological condition of the animal producing anti-diuresis in virgin females and diuresis during pregnancy. To further explore whether prior blockade of Ang-(1-7) before pregnancy would alter the contribution of Ang-(1-7) to fluid balance, we aimed to pre-treat virgin female Sprague Dawley rats with a lower dose (24 μg/kg/hr) of A-779 prior to pregnancy and evaluate the effects of Ang-(1-7) in early, middle, and late gestation. We hypothesized that longer treatment with a lower dose of the Ang-(1-7) receptor antagonist would demonstrate similar kidney effects of Ang-(1-7) as those seen with the shorter higher dose regimen producing antidiuresis in virgin female rats and diuresis during pregnancy.
METHODS
Animals
Virgin female Sprague Dawley rats at 9 weeks of age and 14 week old males were obtained from Harlan Laboratories (Indianapolis, IN) and housed under a 12h light/dark cycle in a facility approved by the Association for Assessment and Accreditation of Laboratory Animal Care. Females were individually housed following surgical procedures and during pregnancy. All protocols were approved by the Animal Care and Use Committee of Wake Forest University School of Medicine and are in compliance with NIH guidelines.
Surgical Procedures
A 2 mL Alzet (5μL/hr) osmotic pump (Alzet Osmotic Pumps, Cupertino CA) connected to the jugular vein via PE-60 tubing was placed in the subcutaneous tissue allowing continuous intravenous infusion of vehicle or the Ang-(1-7) antagonist, D-Alanine-[Ang-(1-7)] (A-779) (Bachem, Torrance CA) at a dose of 24 μg/kg/hr for 8 days. The dose was selected based on previous publications (12;13) which demonstrated that A-779 was a specific antagonist of Ang-(1-7), and we selected half the dose used to treat animals after the start of pregnancy in our previous study (14) based on consideration of the longer duration of treatment. Female virgin rats were placed in metabolic cages following pump implantation and 24 hour metabolic studies, including water and food consumption, were conducted on treatment day 6-7. At day 7 of treatment, females were removed from metabolic cages, placed in standard cages, and mated for up to 14 days. Daily vaginal smears were obtained and stained with hematoxylin and eosin for visualization of sperm. Day 0 of pregnancy was designated as the day when sperm were found in the vaginal smear. Animals deemed pregnant were returned to metabolic cages for the remaining study duration. To allow for continuous drug administration, animals were anesthetized with isoflurane and an incision was made in the back of the neck for removal of the original osmotic pump. To supply continuous drug administration, additional osmotic pumps were connected via the incision to the original PE-60 jugular vein cannula on day 5 of mating (if needed, 11%), and days 1 or 2 and 12 of pregnancy. The length of drug administration at day 19 of pregnancy varied between 28 and 40 days based upon animal mating. 24 hour metabolic studies and food and water consumption were measured on gestational days 5, 15, and 19. 19 day pregnant animals were weighed and sacrificed by decapitation.
Biochemical measurements
Urinary sodium and potassium concentrations were measured using the Nova Biomedical automated electrolyte analyzer (Nova Biomedical, MA). Freezing point depression (Precision Systems Inc, MA) was used to determine urinary osmolality.
Statistical Analysis
Comparisons throughout pregnancy in vehicle treated rats only and A-779 treated rats only during pregnancy were analyzed by a repeated measures one-way ANOVA followed by Newman Keuls post hoc test. Comparisons at each time point (virgin, 15-day pregnant, and 19-day pregnant) between vehicle and A-779 groups were completed by unpaired Student t test. A probability of <0.05 was considered statistically significant. All values were expressed as mean ± SEM. The calculation of percent during pregnancy were calculated by assigning the vehicle treated group (either virgin or appropriate time comparison) as 100% and determining the appropriate percent increase or decrease of the value during pregnancy.
RESULTS
Table 1 shows maternal body weight and fetal characteristics with and without A-779 at 19 days of gestation. There was no effect of A-779 on maternal or fetal body weight or fetal length or number (Table 1). Vehicle and A-779 treated rats consumed increased quantities of chow (Figure 1) at early, mid, and late gestation as compared to non-pregnant controls. Administration of A-779 prior to pregnancy significantly decreased chow consumption by 83% at the 19th day gestation as compared to vehicle administration. Water consumption (Figure 1) significantly increased throughout gestation with animals consuming levels that were 186% and 177% above non-pregnant animals treated with vehicle and A-779 respectively at late gestation. A-779 administration significantly decreased water consumption by 83% and 80% respectively at 15 and 19 days of pregnancy. Urine volume (Figure 2) increased throughout gestation (y= 0.75x + 16.54, urine volume over time) in the vehicle treated rats reaching levels that were 201% higher at 19 days gestation as compared to non-pregnant controls. A-779 administration significantly increased urine volume by 126% in virgin animals and showed no further change in urine volume with pregnancy (y= 0.19 + 19.91, urinary volume over time). Urine volume (Figure 2) was significantly reduced by 76% at the 19th day of gestation by A-779 administration. The difference between intake and output reflecting balance (Figure 2) was increased at the 19th day of normal pregnancy increasing 200% and 246% respectively as compared to vehicle and A-779 treated non-pregnant animals. The difference between intake and output was not significantly altered in virgin or pregnant animals with A-779 administration. Urinary osmolality (Figure 2) showed a similar yet inverse pattern to urine volume throughout gestation with decreased (y= -17.11x + 1313, urinary osmolality over time) urinary osmolality in vehicle treated rats and no change (y= -3.08x + 1197, urinary osmolality over time) in urinary osmolality over time in pregnant animals treated with A-779. A-779 administration tended to decrease urinary osmolality in virgin animals while tending to increase urinary osmolality in 19 day pregnant animals as compared to vehicle treated time controls. Urinary Na (Table 2) did not change in either vehicle (y= 0.01x + 1.10, urinary Na over time) or A-779 (y= 0.01x + 1.38, urinary Na over time) treated animals throughout pregnancy. Urinary K (Table 2) did not change in either vehicle (y= 0.10x + 2.46, urinary K over time) or A-779 (y= 0.04x + 3.17, urinary K over time) treated animals throughout pregnancy. A-779 treatment did not alter urinary Na and K in virgin and pregnant rats as compared to vehicle treated time controls.
Table 1.
Maternal body weight, and fetus characteristics in Sprague Dawley rats treated with vehicle and A-779.
| 19d-Pregnant | ||
|---|---|---|
| Vehicle | A-779 | |
| Maternal Body Weight (g) | 376 ± 5.6 | 363 ± 9.8 |
| Fetal number | 14.9 ± 0.8 | 14.5 ± 0.7 |
| Fetal Weight (g) | 2.33 ± 0.07 | 2.36 ± 0.10 |
| Fetal Length (cm) | 2.88 ± 0.02 | 2.89 ± 0.04 |
Values are expressed as mean ± SEM. Differences between treatment groups at the 19th day of pregnancy were compared using an unpaired student’s t-test. n=7-8/group.
Figure 1.
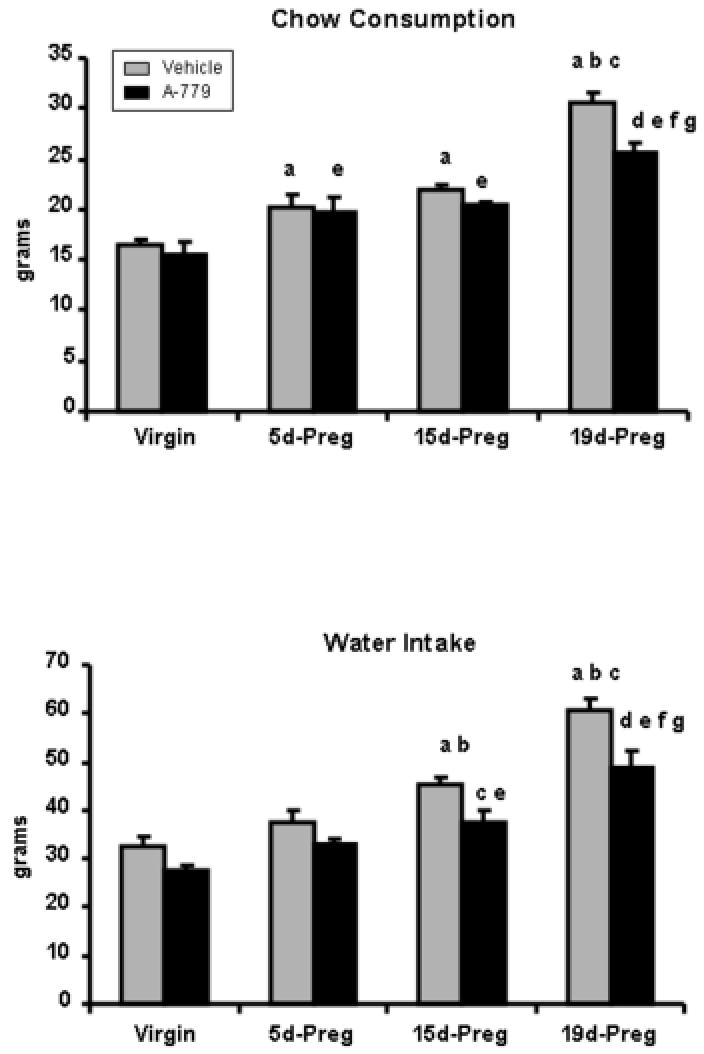
Chow consumption and water intake in virgin, 5d-Preg, 15d-Preg, and 19d-Preg Sprague Dawley rats treated with vehicle (gray bars) or A-779 (black bars). During gestation, water intake and chow consumption were increased. A-779 significantly decreased chow consumption at day 19 of pregnancy and water intake at days 15 and 19 of pregnancy. Values are expressed as mean ± SEM. Differences between the means were evaluated by a one-way ANOVA with Newman-Keuls post-hoc test throughout gestation in each treatment group. Differences between groups at each time point during pregnancy were compared using an unpaired student’s t-test. n=4-6 per group. a p<0.05 vs virgin vehicle; b p<0.05 vs 5d-Preg vehicle; c p<0.05 vs 15d-Preg vehicle; d p<0.05 vs 19d-Preg vehicle; e p<0.05 vs virgin A-779; f p< 0.05 vs 5d-Preg A-779; g p<0.05 vs 15d-Preg A-779.
Figure 2.
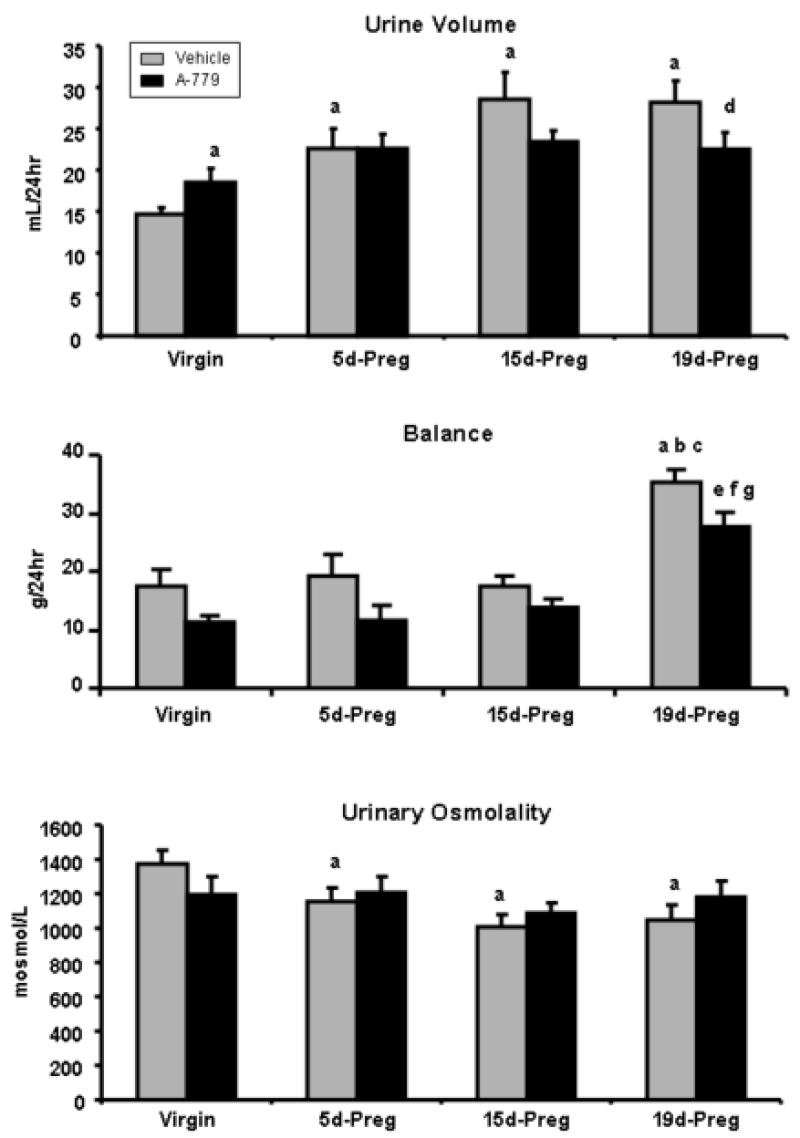
Urine volume, balance, and urinary osmolality in virgin, 5d-Preg, 15d-Preg, and 19d-Preg Sprague Dawley rats treated with vehicle (gray bars) or A-779 (black bars). During gestation, urine volume and balance were increased while urinary osmolality was decreased. In virgin animals, A-779 administration increased urine volume while A-779 decreased urine volume in 19d-Preg animals. Values are expressed as mean ± SEM. Differences between the means were evaluated as previously described in Figure 1. n=4-10 per group. a p<0.05 vs virgin vehicle; b p<0.05 vs 5d-Preg vehicle; c p<0.05 vs 15d-Preg vehicle; d p<0.05 vs 19d-Preg vehicle; e p<0.05 vs virgin A-779; f p< 0.05 vs 5d-Preg A-779; g p<0.05 vs 15d-Preg A-779.
Table 2.
Urinary electrolytes in virgin and pregnant (days 5, 15, and 19) Sprague Dawley rats treated with vehicle or A-779.
| Virgin | 5d-Pregnant | 15d-Pregnant | 19d-Pregnant | |||||
|---|---|---|---|---|---|---|---|---|
| Vehicle | A-779 | Vehicle | A-779 | Vehicle | A-779 | Vehicle | A-779 | |
| Urinary Na (mmol/24hr) | 1.05 ±0.13 | 1.26 ± 0.13 | 1.29 ± 0.19 | 1.51 ± 0.14 | 1.25 ± 0.15 | 1.29 ± 0.10 | 1.40 ± 0.12 | 1.19 ± 0.12 |
| Urinary K (mmol/24hr) | 2.42 ± 0.25 | 2.99 ± 0.28 | 3.10 ± 0.38 | 3.64 ± 0.33 | 3.62 ± 0.31 | 3.68 ± 0.19 | 4.57 ± 0.24 | 3.94 ± 0.42 |
Values are expressed as mean ± SEM. Differences between the means were evaluated by a one-way ANOVA with Newman-Keuls post-hoc test throughout gestation in each treatment group. Differences between treatment groups at the 19th day of pregnancy were compared using an unpaired student’s t-test. n=8-10/group.
DISCUSSION
The present study confirms our previous demonstration (14) of the effects of endogenous Ang-(1-7) by use of its antagonist A-779 on fluid regulation and electrolytes. Under the experimental design of pretreatment of animals prior to pregnancy with A-779, Ang-(1-7) elicits contrasting effects on urine volume depending upon the animal’s physiological state producing diuresis in late gestation and antidiuresis in virgin females. These changes in urinary volume were associated with small changes in urinary osmolality although not significant such that Ang-(1-7) tended to decrease urinary osmolality in late gestation and increase urinary osmolality in virgin females. These studies also demonstrate additional effects of Ang-(1-7) through increased water intake and chow consumption in late gestation that was not present in virgin animals. Ang-(1-7) showed no effect on urinary electrolytes or fetus characteristics during pregnancy under this lengthened duration of treatment with the Ang-(1-7) antagonist A-779.
This study was unique in design as compared to our previous publication (14) by lowering the dose and increasing the duration of A-779 administration. The current study pretreated female rats prior to pregnancy with A-779 (24μg/kg/hr) such that 19 day pregnant animals received 27-40 days of continuous A-779 administration. This contrasts with our previous design which treated rats continuously with A-779 (48μg/kg/hr) from days 11 through 19 of gestation only. Results were similar under the respective experimental designs with Ang-(1-7) producing diuresis associated with a tendency or a significant decrease in urinary osmolality in late gestation and antidiuresis associated with a tendency or a significant increase in urinary osmolality in virgin females. These findings in both studies suggested that primary role of Ang-(1-7) shifts from fluid conservation in virgin females to fluid loss during pregnancy with no change in urinary electrolyte excretion suggesting an aquaretic role of Ang-(1-7).
Until our recent publication (14), the actions of Ang-(1-7) on the female kidney and during pregnancy had been unknown. In males, Ang-(1-7) had previously been shown to have diuretic and natiuretic properties in male Sprague Dawley rats that were independent of renal blood flow and glomerular filtration rates (8) (7). These diuretic actions of Ang-(1-7) were in contrast to a potent antidiuretic effect of the peptide in volume expanded male rats (10) and in male Wistar and spontaneously hypertensive rats (SHR) treated chronically with A-779 (12). The specificity of the Ang-(1-7) effects were demonstrated in mas receptor knockout mice where the antidiuretic effect of Ang-(1-7) after acute water load was lost (15). The current study demonstrates again that Ang-(1-7) can have differing fluid regulating actions depending on the physiological state of the animal producing antidiuresis in virgin female animals and diuresis in late gestation.
The present study also demonstrates that water intake is significantly decreased with A-779 administration confirming our previous report which suggests that Ang-(1-7) can serve as a dipsogen, contributing significantly to the enhanced water intake in late gestation. Ang-(1-7) is generally not regarded as a dipsogen (16). This later characteristic of Ang-(1-7) is confirmed in our virgin animals as the effects of Ang-(1-7) are restricted to the kidney with no change in water intake. The suggestion that Ang-(1-7) has a central component on fluid intake during pregnancy warrants further study.
Pregnancy is associated with hormonal, biochemical, hemodynamic, and renal changes including increased plasma volume (PV) by 50%, increased cardiac output (CO) by 30-40% despite normal or decreased blood pressure, increased glomerular filtration rate (GFR), and resetting of osmoreceptors. The increase in thirst by Ang-(1-7) during pregnancy which we report is consistent with a lowering of the osmotic threshold for thirst and vasopressin (AVP) secretion that is associated with normal pregnancy (17) (18;19). The increased urinary volume excretion which we report in the current and previous publications appears to also be an overall consequence of pregnancy as demonstrated by metabolic studies in goats, sheep, and Sprague Dawley, and Long-Evans rats (18) (4;20). Thus, there must be tight regulation between the increases in water intake and urinary excretion such that PV can expand normally.
While not examined specifically in this present study due to the paired experimental design, the increase in water intake and diuresis by Ang-(1-7) in late gestation in the present study suggests similar fluid regulating mechanisms to those described in our previous publication (14). These associated mechanisms include a resetting and further leftward shift to higher levels of AVP for every level of osmolality which is already decreased with pregnancy by 8-10 mosm/kg (17). An increase in circulating AVP can compensate for factors that increase diuresis such that normal expansion of PV can occur. Additionally, kidney water channels including aquaporin 1 (AQP1) and aquaporin 2 (AQP2) have been shown to be involved in water retention. AQP1 is an AVP independent water channel highly expressed in the descending thin limb and proximal tubule. Previously, we showed that the Ang-(1-7) can mediate the diuresis seen in pregnancy by down-regulation of the AQP1 water channel without a change in the collecting duct AVP sensitive water channel AQP2 (14). While aquaretic changes without alterations in collecting duct permeability are not typical and AQP2 has previously been shown to increase in the kidney medullary papillae during pregnancy (21), AQP1 knockout mice are unable to concentrate their urine appropriately in response to water deprivation and show decreased transepithelial osmotic water permeability in isolated proximal tubules (22). These previous studies suggest that the Ang-(1-7) mediated diuresis in pregnancy could result from mechanistic changes involving AVP, AQP1, and AQP2 which all deserve further study to determine their exact role in water reabsorption during pregnancy.
Unlike other antihypertensive agents including the angiotenin converting enzyme (ACE) inhibitors, use of the Ang-(1-7) receptor antagonist, A-779, does not appear to have detrimental effects on fetal characteristics including the fetal size, length, or number or on maternal body weight in late gestation. Previous studies have shown that placentas perfused with the ACE inhibitors enalapril and temocapril demonstrated that both drugs can cross the human placenta in similar quantities in maternal-fetal directions (23) contributing to the contraindication of such drugs in pregnancy. Infants exposed to ACE inhibitors have been shown to be at increased risked for malformations of the cardiovascular system and the central nervous system even when ACE inhibitors are administered in the first trimester of pregnancy (24). Angiotensin II (Ang II) is formed from Angiotensin I (Ang I) by ACE and is important in early embryologic development of the heart, kidney, and brain (25) and impaired proliferation of fetal smooth-muscle cells in the ductus arteriosus (26). Thus, any agent that could potentially cross the placenta and block the actions of Ang II could be detrimental to normal fetal development. In the ovine fetus, infusion of Ang-(1-7) or the receptor antagonist A-779 to the fetus directly did not have any effect on blood pressure or renal function (27). This finding is consistent with current results of more chronic administration of A-779 with no fetal change.
In the setting of increased renal expression of Ang-(1-7) during pregnancy (5), the current study supports our previous report that endogenous blockade of Ang-(1-7) actions produce contrasting effects on renal fluid balance depending upon if the animal is pregnant or not. Even when the experimental design is altered such that there is a longer duration of a lower dose of the Ang-(1-7) receptor antagonist, A-779, the renal actions of Ang-(1-7) remain the same producing anti-diuresis in virgin female animals and diuresis in late gestation. The increased water intake associated with the increase in diuresis in late gestation contributes to the normal plasma volume expansion of pregnancy. These studies demonstrate that Ang-(1-7) is an important factor in mediation of normal fluid expansion during pregnancy.
Acknowledgments
This work was supported in part by grants from NIH/NHLBI (HL51952) (HL56973), NIH/NICHD(HD42631), Sigma Xi, the Scientific Research Society, and a venture grant from Wake Forest University School of Medicine. An unrestricted grant from the Unifi Corporation (Greensboro, NC) and the Farley-Hudson Foundation (Jacksonville, NC) is also acknowledged. J. Joyner was supported in part by training grants from the US Department of Health and Human Services (GM63485) obtained by the molecular medicine graduate program at Wake Forest University and from the Mid-Atlantic American Heart Association (AHA061547U).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.August P, Lenz T, Ales KL, Druzin ML, Edersheim TG, Hutson JM, et al. Longitudinal study of the renin-angiotensin-aldosterone system in hypertensive pregnant women: Deviations related to the development of superimposed preeclampsia. Am J Obstetr Gynecol. 1990;163:1612–21. doi: 10.1016/0002-9378(90)90639-o. [DOI] [PubMed] [Google Scholar]
- 2.Brown MA, Wang J, Whitworth JA. The renin-angiotensin-aldosterone system in pre-eclampsia. 56. Vol. 19. Clinical & Experimental Hypertension; New York: 1997. pp. 713–26. [DOI] [PubMed] [Google Scholar]
- 3.Merrill DC, Karoly M, Chen K, Ferrario CM, Brosnihan KB. Angiotensin-(1-7) in normal and preeclamptic pregnancy. Endocrine. 2002;18:239–45. doi: 10.1385/ENDO:18:3:239. [DOI] [PubMed] [Google Scholar]
- 4.Neves LAA, Williams AF, Averill DB, Ferrario CM, Walkup MP, Brosnihan KB. Pregnancy enhances the Angiotensin (Ang)-(1-7) vasodilator response in mesenteric arteries and increases the renal concentration and urinary excretion of Ang-(1-7) Endocrinology. 2003;14:3338–43. doi: 10.1210/en.2003-0009. [DOI] [PubMed] [Google Scholar]
- 5.Joyner J, Neves LA, Granger JP, Alexander BT, Merrill DC, Chappell MC, et al. Temporal-spatial expression of angiotensin-(1-7) and angiotensin converting enzyme 2 in the kidney of normal and hypertensive pregnant rats. Am J Physiol Regul Integr Comp Physiol. 2007;239:R169–R177. doi: 10.1152/ajpregu.00387.2006. [DOI] [PubMed] [Google Scholar]
- 6.Valdes G, Germain AM, Corthorn J, Berrios C, Foradori AC, Ferrario CM, et al. Urinary vasodilator and vasoconstrictor angiotensins during menstrual cycle, pregnancy, and lactation. Endocrine. 2001;16(2):117–22. doi: 10.1385/ENDO:16:2:117. [DOI] [PubMed] [Google Scholar]
- 7.Handa RK, Ferrario CM, Strandhoy JW. Renal actions of angiotensin-(1-7) in vivo and in vitro studies. Am J Physiol. 1996;270:F141–F147. doi: 10.1152/ajprenal.1996.270.1.F141. [DOI] [PubMed] [Google Scholar]
- 8.DelliPizzi A, Hilchey SD, Bell-Quilley CP. Natriuretic action of angiotensin (1-7) Br J Pharmacol. 1994;111:1–3. doi: 10.1111/j.1476-5381.1994.tb14014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santos RAS, Silva ACS, Magaldi AJ, Khosla MC, Ceasr KR, Passaglio KT, et al. Evidence for a physiological role of angiotensin-(1-7) in the control of hydroelectrolyte balance. Hypertension. 1996;27:875–84. doi: 10.1161/01.hyp.27.4.875. [DOI] [PubMed] [Google Scholar]
- 10.Santos RAS, Baracho NCV. Angiotensin-(1-7) is a potent antidiuretic peptide in rats. Braz J Med Biol Res. 1992;25:651–4. [PubMed] [Google Scholar]
- 11.Garcia NH, Garvin JL. Angiotensin 1-7 has a biphasic effect on fluid absorption in the proximal straight tubule. J Am Soc Nephrol. 1994;5:1133–8. doi: 10.1681/ASN.V541133. [DOI] [PubMed] [Google Scholar]
- 12.Simoes e Silva AC, Bello APC, Baracho NCV, Khosla MC, Santos RAS. Diuresis and natriuresis produced by long term administration of a selective angiotensin-(1-7) antagonist in normotensive and hypertensive rats. Regulatory Peptides. 1998;74:177–84. doi: 10.1016/s0167-0115(98)00038-x. [DOI] [PubMed] [Google Scholar]
- 13.Sampaio WO, Nascimento AAS, Santos RA. Regulation of cardiovascular signaling by Kinins and products of similar converting enzyme systems. Systemic and regional hemodynamic effects of angiotensin-(1-7) in rats. Am J Physiol Heart Circ Physiol. 2003;284:H1985–H1994. doi: 10.1152/ajpheart.01145.2002. [DOI] [PubMed] [Google Scholar]
- 14.Joyner J, Neves LA, Stovall K, Ferrario CM, Brosnihan KB. Angiotensin-(1-7) serves as an aquaretic by increasing water intake and diuresis in association with downregulation of aquaporin-1 during pregnancy in rats. Am J Physiol Regul Integr Comp Physiol. 2008;294(3):R1073–R1080. doi: 10.1152/ajpregu.00572.2007. [DOI] [PubMed] [Google Scholar]
- 15.Santos RAS, Simoes e Silva AC, Maric C, Silva DM, Machado RP, de Bul I, et al. Angiotensin-(1-7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci USA. 2003;100(14):8258–63. doi: 10.1073/pnas.1432869100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fitzsimons JT. The effect on drinking of peptide precursors and of shorter chain peptide fragments of angiotensin II injected into the rat’s diencephalon. J Physiol. 1971;214:295–303. doi: 10.1113/jphysiol.1971.sp009433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Durr JA, Stamoutsos B, Lindheimer MD. Osmoregulation during pregnancy in the rat. J Clin Invest. 1981;68:337–46. doi: 10.1172/JCI110261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barron WM, Durr J, Stamoutsos B, Lindheimer MD. Osmoregulation and vasopressin secretion during pregnancy in Brattleboro rats. Am J Physiol. 1985;248(1Pt 2):R29–R37. doi: 10.1152/ajpregu.1985.248.1.R29. [DOI] [PubMed] [Google Scholar]
- 19.Lindheimer MD, Barron WM, Durr J, Davison JM. Water homeostasis and vasopressin release during rodent and human gestation. Am J Kid Dis. 1987;IX:270–5. doi: 10.1016/s0272-6386(87)80121-x. [DOI] [PubMed] [Google Scholar]
- 20.Olsson K. Pregnancy -- a challenge to water balance. News Physiol Sci. 1986;1:131–4. [Google Scholar]
- 21.Ohara M, Martin PY, Xu DL, St JJ, Pattison TA, Kim JK, et al. Upregulation of aquaporin 2 water channel expression in pregnant rats. J Clin Invest. 1998;101(5):1076–83. doi: 10.1172/JCI649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schnermann J, Chou CL, Ma T, Traynor T, Knepper MA, Verkman AS. Defective proximal tubular fluid reabsorption in transgenic aquaporin-1 null mice. Proc Natl Acad Sci U S A. 1998;95(16):9660–4. doi: 10.1073/pnas.95.16.9660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reisenberger K, Egarter C, Sternberger B, Eckenberger P, Eberle E, Weissenbacher ER. Placental passage of angiotensin-converting enzyme inhibitors. Am J Obstet Gynecol. 1996;174(5):1450–5. doi: 10.1016/s0002-9378(96)70587-2. [DOI] [PubMed] [Google Scholar]
- 24.Cooper WO, Hernandez-Diaz S, Arbogast PG, Dudley JA, Dyer S, Gideon PS, et al. Major congenital malformations after first-trimester exposure to ACE inhibitors. N Engl J Med. 2006;354(23):2443–51. doi: 10.1056/NEJMoa055202. [DOI] [PubMed] [Google Scholar]
- 25.Hu F, Morrissey P, Yao J, Xu Z. Development of AT1 and AT2 receptors in the ovine fetal brain. Dev Brain Res. 2004;150:51–61. doi: 10.1016/j.devbrainres.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 26.Burrell JH, Hegarty BD, McMullen JR, Lumbers ER. Effects of gestation on ovine fetal and maternal angiotensin receptor subtypes in the heart and major blood vessels. Exp Physiol. 2001;86(1):71–82. doi: 10.1113/eph8602075. [DOI] [PubMed] [Google Scholar]
- 27.Moritz KM, Campbell DJ, Wintour EM. Angiotensin-(1-7) in the ovine fetus. American Journal of Physiology - Regulatory Integrative & Comparative Physiology. 2001;280(2):R404–R409. doi: 10.1152/ajpregu.2001.280.2.R404. [DOI] [PubMed] [Google Scholar]


