Introduction
At any given time, depression affects approximately 10% of the population and can present itself at all ages1. The World Health Organization (WHO) ranked it the most disabling medical condition 2 in part because of its relapsing and chronic nature3.
The etiology of depression is not known and the syndrome lacks universal biomarkers 4. Apoptosis may be implicated in the pathophysiology of depression 5 although this issue is still debated6. Apoptosis is characterized by structural changes that ultimately lead to the disintegration of the cell. The process is activated by a group of cysteine proteases known as caspases. BCL-2 and related members of this family of proteins regulate the release of cytochrome c from mitochondria into the cytoplasm, which in turn mediates the mitochondrial pathway of apoptosis. Interestingly 3 established antidepressant drug therapies, fluoxetine 7, moclobemide 8 and desipramine 9 upregulate BCL-2 in purified mitochondria and in isolated neural stem cells, respectively. It is not clear however if this upregulation of BCL-2 plays a critical role in their antidepressant effects. Einat et al 10 tested BCL-2 heterozygous mice in large open field, elevated plus-maze, emergence test, black/white box and the FST. They demonstrated a clear increase in anxiety-like behaviors in mice with reduced mitochondrial BCL-2 levels. They did not observe any effects on depressive-like behaviors (FST) nor general locomotion levels.
Ceramide is a membrane sphingolipid and a key regulator of apoptosis, in part through its action on lysosomes and mitochondrial pathways affecting the BCL-2 family. Ceramide occupies a central place in sphingolipid biosynthesis and metabolism. Inhibition of ceramidase (CDase) will increase levels of endogenous ceramide which in turn will modulate BCL-2 levels. The recent availability of CDase inhibitors presented a unique opportunity to determine in vivo the role of the apoptotic pathway in learned helplessness.
In the present study, we investigated the relation between hippocampal BCL-2 expression and behavioral measures of learned helplessness. We were interested whether an in-vivo modulation of hippocampal BCL-2 would lead to similar reports by Einat et al 10 with respect to immobility in the forced swim test. We hypothesized that LCL385, a pro-apoptotic compound and an inhibitor of acid ceramidase (anti-CDase), 11 will lead to a drop in BCL-2 levels. We chose DMI as an active antidepressant control knowing that it also exerts overlapping effects on sphingomyelin and ceramide metabolism as the anti-ceramidases 12.
Methods
Animals
Forty males Sprague Dawley (Charles River Laboratories, Wilmington, MA) took part in this experiment. All animals were handled for 2–5 days to allow for appropriate acclimatization and randomized into one of 5 groups (www.randomization.com). At the beginning of the experiment, rats weighed between 250 and 300 g and were housed in separate cages in an animal room with constant temperature (21 ± 1 °C) and 12 hours light/dark cycle (lights on/off at 6 am/6 pm), with free access to food and water. All experiments were performed in the early to mid phase of the light cycle under standard room fluorescent lights. The American Association for the Accreditation of Laboratory Animal Care approved the animal facility. All housing and behavioral procedures conform to the Principles of Laboratory Animal Care issued by the National Institutes of Health, with local laws and regulations, and were approved by the Institutional Animal Care and Use Committee in MUSC.
Behavioral Experiments
After acclimatization, all animals performed individually a 15 min FST in tanks of 25 cm in diameter and 45 cm in height filled with tap water at about 25°C (± 1)13. At the end of each session, animals were taken out of water, dried with a towel and placed back in their respective cage. The water was replaced after each session. The 15 min FST session (commonly referred to as ‘baseline stressor’) was followed by 3 daily doses of either LCL385, desipramine (DMI) or saline (SAL). Seventy-two hours from baseline, animals performed a 6 min FST to assess behavioral despair. All sessions were performed between 10 a.m. and 12 p.m. and were videotaped from the side and later scored by an expert rater masked to conditions and hypotheses. Behavioral measures were sampled at 5 second intervals for the duration of the test: (1) immobility - floating in the water without struggling, and making only those movements necessary to keep the head above water; (2) swimming - making active swimming motions more than necessary to keep the head above water; moving around in the cylinder; (3) climbing - making active movements with the forepaws in and out of the water, usually directed against the walls.
Drug
DMI was purchased from Sigma-Aldrich (St. Louis, MO). LCL385 ((1R, 2R)-2-N-(tetradecylamino)-1-phenyl-1, 3-propandiol hydrochloride 14) was synthesized by Lipidomics Core at MUSC. Unpublished preliminary work in our laboratory suggested that LCL385 lethal dose was 50mg/kg. In the absence of any published behavioral reports pertaining to this compound, we chose a parametric dosing design. The administration schedule followed drug regimens previously reported to be effective antidepressants dosing for DMI 15,16. Animals received LCL385 (10mg/kg, 20mg/kg or 30mg/kg), DMI (20mg/kg) or SAL over 72 hours. Doses were freshly prepared and dispensed in equivalent volumes (5ml/kg) in 3 intra-peritoneal injections. All compounds were dissolved in water containing 6.7% dimethyl sulfoxide (DMSO). The last dose was given 30 min prior to behavioral test and approximately 1 hour before euthanesia.
BCL-2 western blotting
Within 30 minutes of completing the 6 min FST, rats were euthanized after deep anesthesia with isoflurane. This method is consistent with the recommendations of the Panel on Euthanasia of the American Veterinary Medical Association. Brain hippocampus tissues were homogenized in phosphate buffer saline (PBS) supplemented with 1% NP-40, 0.5% sodium deoxycholate, protease inhibitor cocktail (1 tablet/10ml, Roche Diagnostics, Indianapolis, IN), and 1% SDS by sonicating for 30 seconds in an ice bath. The supernatant was collected following centrifugation at 13,000 rpm for 15 min at 4 °C. The protein concentrations in the supernatant were determined by a BCA protein assay kit from Pierce. The protein samples were separated by 12.5 % SDS-polyacrylamide gel electrophoresis, and transferred to a blotting membrane. The membrane was incubated with antibodies against BCL-2 (1:200, Lab Vision, Fremont, CA), and β-actin (1:2500, Sigma) overnight at 4 °C, and then incubated with horseradish peroxidase conjugated secondary antibody for 1 h at room temperature. Finally, the blots were developed using western blot reagents (SuperSignal West Pico, Pierce). Semi-quantitative analysis of BCL-2 expression levels was performed using the computer program ImageJ when β-actin was adopted as the internal standard.
Data analysis
Descriptive statistics were generated from all primary data and were tested for homogeneity of variance. An analysis of variance (ANOVA) was performed on each primary outcome measure with post-hoc statistics (Tukey HSD). (JMP statistical software package). Significance level was set at p<0.05.
Results
Out of 40 animals, behavioral data from 2 were eliminated because their FST were not collected on tape. Behavioral measures and BCL-2/β-actin means and standard deviations are listed in table 1. There were no significant differences across all 3 doses of LCL385 on any of the behavioral measures or on BCL-2/ β-actin ratio in hippocampus (df=23; F>0.59; p>0.11).
Table 1.
Basic descriptive statistics per group including sample size, means and standard deviations
| Condition | Sample Size | BCL-2/Β-actin | Immobilization | Swimming | Climbing | Diving |
|---|---|---|---|---|---|---|
| SAL | 8* | 1.31 (0.57) | 38.57 (4.82) | 27.86 (6.54) | 5.57 (6.55) | 0 |
| DMI | 8* | 1.15 (0.53) | 22.43 (15.66) | 34 (13.66) | 15.43 (7.46) | 0.14 (0.38) |
| LCL385 10mg/kg | 8◊ | 0.69 (0.39) | 36.75 (11.27) | 30.12 (8.76) | 5.12 (3.91) | 0 |
| LCL385 20mg/kg | 8◊ | 0.54 (0.23) | 39.62 (2.87) | 24.5 (4.87) | 7.87 (3.76) | 0 |
| LCL385 30mg/kg | 8◊ | 0.69 (0.35) | 32.62(7.48) | 33.37 (9.78) | 5.87 (3.98) | 0.12 (0.35) |
Only N=7 for FST behavioral data analysis
Non-significant differences across LCL385 groups (10, 20 and 30 mg/kg)
Behavioral Effects on FST
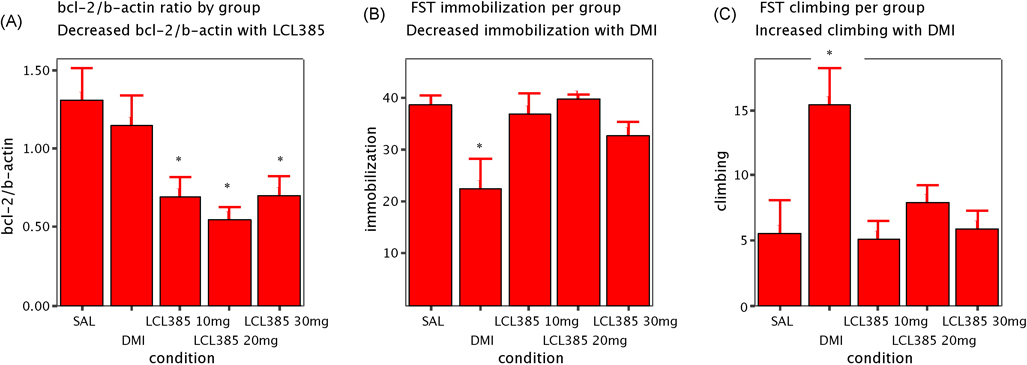
There were significant differences across all 5 conditions (SAL; DMI and LCL385 10mg/kg, LCL385 20mg/kg and LCL385 30mg/kg ) on immobility (df=37, F=3.94; p=0.01) (figure 1, A, table 2). As expected, DMI significantly reduced immobility compared to placebo (p=0.024). DMI was significantly different from LCL385 10mg/kg (p=0.045) and LCL385 20mg/kg (p=0.011). DMI showed no difference compared to LCL385 30mg/kg (p=0.25). There were also significant differences across all 5 conditions on climbing (df=37, F=4.8; p=0.004) (figure 1, B). DMI significantly increases climbing compared to placebo (p=0.011) and LCL385 10mg/kg (p=0.005) and LCL385 30mg/kg (p=0.011). DMI showed a trend for significance compared to LCL385 20mg/kg (p=0.063).
Figure 1.
Means and standard errors of hippocampal BCL-2/ Β-actin ratio (A), immobilization scores (B) and climbing scores (over 6 min FST) (C) by group (SAL, DMI and LCL385).
Table 2.
Statistical comparisons between and across groups. Significant level set at p= 0.05
| Sum of Squares | df | Mean Square | F | Sig. | |
|---|---|---|---|---|---|
| BCL-2/β-actin Between Groups | 3.498 | 4 | 0.875 | 4.82 | .003 |
| Within Groups | 6.364 | 35 | .182 | ||
| Total | 9.862 | 39 | |||
| immobilization Between Groups | 1411.032 | 4 | 352.758 | 3.945 | .01 |
| Within Groups | 2950.679 | 33 | 89.415 | ||
| Total | 4361.711 | 37 | |||
| swimming Between Groups | 477.1563 | 4 | 119.289 | 1.432 | .24 |
| Within Groups | 2749.607 | 33 | 83.321 | ||
| Total | 3226.763 | 37 | |||
| climbing Between Groups | 528.999 | 4 | 132.250 | 4.806 | 0.004 |
| Within Groups | 908.054 | 33 | 27.517 | ||
| Total | 1437.053 | 37 | |||
| diving Between Groups | .163 | 4 | .041 | .774 | .550 |
| Within Groups | 1.732 | 33 | .052 | ||
| Total | 1.895 | 37 |
There were no differences between LCL385 and placebo on either immobility (p>0.74) or climbing (p.0.93).
There were no significant differences in swimming across all 5 conditions (df=37, F=1.43, p=0.24).
BCL-2/ Β-actin in hippocampus
There was an overall significant effect of group on BCL-2/ β-actin in hippocampus (df=39; F=4.81; p=0.003). All 3 doses of LCL385 significantly decreased BCL-2/ β-actin in hippocampus when given in 3 injections over 72h compared to placebo (10mg/kg p=0.048; 20mg/kg p=0.008 and 30mg/kg p=0.05). Only 20mg/kg showed a trend for difference from DMI (p=0.056). Conversely, DMI given in 3 injections over 72h did not show significant differences in BCL-2 β-actin compared to placebo (p=0.44).
Discussion
To our knowledge, this is the first investigation of an inhibitor of acid CDase and the behavioral response to stress in rats. Despite reported similarities between LCL385 and the tricyclic antidepressant desipramine on ceramide metabolism, the inhibitor of acid CDase significantly reduces BCL-2 expression in the hippocampus but does not exert any depressant effect as tested. Conversely, a 3-day administration regimen of DMI does not increase the anti-apoptotic marker.
Ceramide is a membrane sphingolipid and is a key regulator of apoptosis. It binds to cathepsin D in lysosome and translocates to mitochondria upon agonist stimulation resulting in cytochrome c release and activation of downstream caspases. The regulation of ceramide levels is controlled by the activities of enzymes that either synthesize or catabolize it. CDase controls the biosynthesis of sphingosine from ceramide whereas sphingomyelinase controls the biosynthesis of ceramide from sphingomyelin. These products have opposite biological actions. Three types of CDases have been described to date and classified. These enzymes can be regulated by platelet derived growth factor, tumor necrosis factor (TNF) or nitric oxide (NO)17. DMI also inhibits CDase 12. In addition, DMI induces intracellular proteolytic degradation of acid sphingomyelinase but not other lysosomal enzymes, and accordingly it has been widely used in the literature as a specific acid sphingomyelinase inhibitor 18. It can block cell killing induced by cytotoxic agents 19. It is for these reasons we chose DMI as the active antidepressant control in our study.
Mitochondrial function and BCL-2 expression may be associated with mood and anxiety disorders20. Mitochondria are known to regulate cellular energy production, and intracellular Ca++ levels which in turns plays a critical role in apoptosis. BCL-2 family is known to have either pro or anti-apoptotic properties but BCL-2 in particular plays a critical role in the survival of the cell. Such a role has been also suggested by studies demonstrating the direct effect of certain antidepressant drugs on BCL-2 expression21. As noted earlier though, Einat et al did not observe depressive-like behaviors in their BCL-2 heterozygous mice when tested with the FST. Interestingly, null mutants exhibited major physiological abnormalities. Our study replicates these findings by reverting to the administration of LCL385 compound to reduce BCL-2 expression and not employing a heterozygous rodent model. DMI’s complex action on sphingolipid metabolism may explain the differences seen with LCL385 in behavioral and anti-apoptosis measures. In our study, DMI shows a trend towards a total decrease in BCL-2/Β-actin ratios but not equal to LCL385. These results are unlike what has been previously reported with DMI in situ9.
Notwithstanding a careful choice in the active control compound, and the parametric dosing of LCL385, our study has limitations. Animals were tested with only one behavioral test. The FST has been predominantly used as an antidepressant screen but has its disadvantages which constrain our results4. We chose it for its high degree of pharmacological validity as evidenced by its sensitivity to major classes of antidepressant drugs15 22. The FST has also been extensively used to document ‘prodepressant-like’ behavior in stressed rats under various conditions23 24 25 26. In addition, in this study we chose to focus on one anatomical region and one apoptosis marker. This restricted region of interest approach may be preferable for prospective hypothesis testing but could mask broader effects of DMI or anti-CDase on other critical regions in modulating stress-related behaviors. Future studies should include in addition to the hippocampus, the frontal and cingulate lobes, the amygdala and possibly mid-brain monoanimes nuclei.
In summary, we have shown that reducing an anti-apoptotic protein (BCL-2) levels in the hippocampus is not associated with significant depressive-like behaviors in rats. DMI and LCL385 anti-CDase showed divergent effects and argue for a complex interaction between behavioral anti-depressant effects and neuroprotection.
Acknowledgment
This project was funded by K08 MH070615-01A1 (ZN) and through general funds from the Department of Cell Biology, the Department of Neurosciences, the Laboratory of Drug Disposition & Pharmacogenetics, the Brain Stimulation Laboratory and the Mood Disorders Program at MUSC. The authors would like to thank Bill Carlezon and Irvin Lucki for their input on the behavioral paradigms, Saeed Elojeimy for discussing LCL385 early behavioral effects and lethal doses and Aram Parsegian for scoring the FST.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of general psychiatry. 2005;62(6):593. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 2.Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet. 1997;349(9063):1436. doi: 10.1016/S0140-6736(96)07495-8. [DOI] [PubMed] [Google Scholar]
- 3.Greden JF. The burden of recurrent depression: causes, consequences, and future prospects. The Journal of clinical psychiatry. 2001;62 Suppl 22:5. [PubMed] [Google Scholar]
- 4.Nestler EJ, Gould E, Manji H, Buncan M, Duman RS, Greshenfeld HK, Hen R, Koester S, Lederhendler I, Meaney M, Robbins T, Winsky L, Zalcman S. Preclinical models: status of basic research in depression. Biological psychiatry. 2002;52(6):503. doi: 10.1016/s0006-3223(02)01405-1. [DOI] [PubMed] [Google Scholar]
- 5.Lucassen PJ, Vollmann-Honsdorf GK, Gleisberg M, Czeh B, De Kloet ER, Fuchs E. Chronic psychosocial stress differentially affects apoptosis in hippocampal subregions and cortex of the adult tree shrew. The European journal of neuroscience. 2001;14(1):161. doi: 10.1046/j.0953-816x.2001.01629.x. [DOI] [PubMed] [Google Scholar]; Manji HK, Duman RS. Impairments of neuroplasticity and cellular resilience in severe mood disorders: implications for the development of novel therapeutics. Psychopharmacology bulletin. 2001;35(2):5. [PubMed] [Google Scholar]
- 6.Lucassen PJ, Heine VM, Muller MB, van der Beek EM, Wiegant VM, De Kloet ER, Joels M, Fuchs E, Swaab DF, Czeh B. Stress, depression and hippocampal apoptosis. CNS Neurol Disord Drug Targets. 2006;5(5):531. doi: 10.2174/187152706778559273. [DOI] [PubMed] [Google Scholar]
- 7.Nahon E, Israelson A, Abu-Hamad S, Varda SB. Fluoxetine (Prozac) interaction with the mitochondrial voltage-dependent anion channel and protection against apoptotic cell death. FEBS letters. 2005;579(22):5105. doi: 10.1016/j.febslet.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 8.Chiou SH, Ku HH, Tsai TH, Lin HL, Chen LH, Chien CS, Ho LL, Lee CH, Chang YL. Moclobemide upregulated Bcl-2 expression and induced neural stem cell differentiation into serotoninergic neuron via extracellular-regulated kinase pathway. British journal of pharmacology. 2006;148(5):587. doi: 10.1038/sj.bjp.0706766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang YY, Peng CH, Yang YP, Wu CC, Hsu WM, Wang HJ, Chan KH, Chou YP, Chen SJ, Chang YL. Desipramine activated Bcl-2 expression and inhibited lipopolysaccharide-induced apoptosis in hippocampus-derived adult neural stem cells. J Pharmacol Sci. 2007;104(1):61. doi: 10.1254/jphs.fp0061255. [DOI] [PubMed] [Google Scholar]
- 10.Einat H, Yuan P, Manji HK. Increased anxiety-like behaviors and mitochondrial dysfunction in mice with targeted mutation of the Bcl-2 gene: further support for the involvement of mitochondrial function in anxiety disorders. Behavioural brain research. 2005;165(2):172. doi: 10.1016/j.bbr.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 11.Holman DH, Turner LS, El-Zawahry A, Elojeimy S, Liu X, Bielawski J, Szulc ZM, Norris K, Zeidan YH, Hannun YA, Bielawska A, Norris JS. Lysosomotropic acid ceramidase inhibitor induces apoptosis in prostate cancer cells. Can. Chemother. Pharmacol. 2007 doi: 10.1007/s00280-007-0465-0. In Press. [DOI] [PubMed] [Google Scholar]
- 12.Elojeimy S, Holman DH, Liu X, El-Zawahry A, Villani M, Cheng JC, Mahdy A, Zeidan Y, Bielwaska A, Hannun YA, Norris JS. New insights on the use of desipramine as an inhibitor for acid ceramidase. FEBS letters. 2006;580(19):4751. doi: 10.1016/j.febslet.2006.07.071. [DOI] [PubMed] [Google Scholar]
- 13.Porsolt RD. Behavioral despair; Abstracts, International Conference on New Directions in Affective Disorders, S; 1991. [Google Scholar]
- 14.Szulc ZM, Mayroo N, Bai A, Bielawski J, Norris J, Hannun YA, Bielawska A. Novel Analogs of D-e-MAPP and B13. Part 1. Synthesis and Biological Evaluation as Potential Anticancer Agents. Bioorg. Med. Chem. 2007 doi: 10.1016/j.bmc.2007.08.033. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Detke MJ, Johnson J, Lucki I. Acute and chronic antidepressant drug treatment in the rat forced swimming test model of depression. Exp Clin Psychopharmacol. 1997;5(2):107. doi: 10.1037//1064-1297.5.2.107. [DOI] [PubMed] [Google Scholar]
- 16.Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev. 2005;29(4–5):547. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Hannun YA, Luberto C, Argraves KM. Enzymes of sphingolipid metabolism: from modular to integrative signaling. Biochemistry. 2001;40(16):4893. doi: 10.1021/bi002836k. [DOI] [PubMed] [Google Scholar]
- 18.Hurwitz R, Ferlinz K, Sandhoff K. The tricyclic antidepressant desipramine causes proteolytic degradation of lysosomal sphingomyelinase in human fibroblasts. Biological chemistry Hoppe-Seyler. 1994;375(7):447. doi: 10.1515/bchm3.1994.375.7.447. [DOI] [PubMed] [Google Scholar]
- 19.Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nature reviews. 2004;4(8):604. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- 20.Manji HK, Drevets WC, Charney DS. The cellular neurobiology of depression. Nat Med. 2001;7(5):541. doi: 10.1038/87865. [DOI] [PubMed] [Google Scholar]
- 21.Lucassen PJ, Fuchs E, Czeh B. Antidepressant treatment with tianeptine reduces apoptosis in the hippocampal dentate gyrus and temporal cortex. Biological psychiatry. 2004;55(8):789. doi: 10.1016/j.biopsych.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 22.Cryan JF, Page ME, Lucki I. Differential behavioral effects of the antidepressants reboxetine, fluoxetine, and moclobemide in a modified forced swim test following chronic treatment. Psychopharmacology (Berl) 2005;182(3):335. doi: 10.1007/s00213-005-0093-5. [DOI] [PubMed] [Google Scholar]
- 23.Cancela LM, Rossi S, Molina VA. Effect of different restraint schedules on the immobility in the forced swim test: modulation by an opiate mechanism. Brain research bulletin. 1991;26(5):671. doi: 10.1016/0361-9230(91)90159-h. [DOI] [PubMed] [Google Scholar]
- 24.Strekalova T, Spanagel R, Bartsch D, Henn FA, Gass P. Stress-induced anhedonia in mice is associated with deficits in forced swimming and exploration. Neuropsychopharmacology. 2004;29(11):2007. doi: 10.1038/sj.npp.1300532. [DOI] [PubMed] [Google Scholar]
- 25.Wann BP, Bah TM, Boucher M, Courtemanche J, Le Marec N, Rousseau G, Godbout R. Vulnerability for apoptosis in the limbic system after myocardial infarction in rats: a possible model for human postinfarct major depression. J Psychiatry Neurosci. 2007;32(1):11. [PMC free article] [PubMed] [Google Scholar]
- 26.Kohen R, Neumaier JF, Hamblin MW, Edwards E. Congenitally learned helpless rats show abnormalities in intracellular signaling. Biological psychiatry. 2003;53(6):520. doi: 10.1016/s0006-3223(02)01503-2. [DOI] [PubMed] [Google Scholar]