Abstract
Background and Aims
Molecular markers of pancreatic neoplasia could aid in the evaluation of suspicious pancreatic lesions where cytology is non-diagnostic. We evaluated the utility of detecting and measuring aberrantly methylated DNA as markers of pancreatic and other periampullary cancers.
Methods
Methylation analysis was performed on endoscopically-obtained brush samples from the biliary and pancreatic duct from 130 individuals with biliary tract strictures: 41 with pancreatic ductal adenocarcinoma, 10 with biliary tract cancers, 13 with other periampullary neoplasms, and 66 with non-neoplastic strictures including 27 with primary sclerosing cholangitis and 39 with other benign strictures. Brush DNA concentrations of methylated Cyclin D2, NPTX2, and TFPI2 promoter DNA were measured by real-time quantitative MSP (QMSP). Conventional MSP was also performed using a 5 gene panel.
Results
QMSP could accurately distinguish patients with pancreatic cancer and other periampullary cancers from those with benign periampullary disease: 73.2% of patients with pancreatic ductal adenocarcinoma had at least one gene positive for methylation by QMSP (defined as ≥1% TFPI-2 DNA and ≥3% methylated NPTX2 and Cyclin D2 DNA) in their brush samples compared to 80% of patients with a biliary tract cancer and only 13.6% of patients with a benign stricture (p<0.001). Cytology had 19.5% sensitivity, and 100% specificity. QMSP had significantly better overall diagnostic accuracy than both cytology and MSP.
Conclusions
The detection and quantification of aberrantly methylated DNA in endoscopic brush samples is a promising tool to differentiate benign from malignant biliary strictures.
Keywords: DNA methylation, pancreatic cancer, Methylation specific PCR, cholangiocarcinoma, primary sclerosing cholangitis, ERCP, brush cytology
INTRODUCTION
Periampullary cancers often present as biliary tract strictures but diagnosing these strictures can be difficult. Early diagnosis is important because survival is best for patients diagnosed with early-stage disease. Pancreatic ductal adenocarcinoma is the 4th leading cause of cancer death in the USA and has the lowest survival rate for any solid cancer (~2%) 1. Cholangiocarcinomas have somewhat better overall survival 2; depending on the location of the cancer 5-year survival rates of 27–60% are achievable for patients with the smallest tumors 3, 4. Improvements in imaging have facilitated the diagnosis of periampullary cancers 5, 6. For example, in expert hands, endoscopic ultrasound outperforms thin-slice multi-detector pancreatic protocol CT for identifying pancreatic masses 5–7, particularly small lesions and benign neoplasms. Cytology or biopsy is usually required to establish a diagnosis. Brushing biliary tract strictures during ERCP is safe and simple and cellular yields are usually better than with fine needle aspiration (FNA) cytology 8, 9, which is best used to sample masses identified by endoscopic ultrasound 6. Indeed, the sensitivity of EUS-FNA for diagnosing of pancreatic masses is ~80% 5, 6. In contrast, ERCP brush cytologyof has only modest sensitivity (<50%)10 and may have lower sensitivity than other cytological specimens 8, 11–14. Digital image analysis (DIA) of cytology specimens to help classify cells as diploid or aneuploid is of only modest benefit. In one study, DIA of pancreaticobiliary cytology had a sensitivity and specificity of 48% and 92%, respectively 12. For this reason, molecular markers are being evaluated for their potential utility to diagnose pancreaticobiliary lesions.
Many genetic, epigenetic and protein alterations arise during pancreaticobiliary tumorigenesis15 The types of markers that have been commonly evaluated as diagnostic assays are mutations, chromosomal gains and losses and DNA methylation alterations. The most common genetic alterations of pancreatic ductal adenocarcinomas include oncogene (KRAS), and suppressor gene mutations (p16, p53, SMAD4)16. ~90% of pancreatic adenocarcinomas harbor mutant KRAS and can be detected using mutation-specific assays, but it is not specific, although quantification may help 16 17. Pancreatic cancers also have extensive transcriptomic18, 19 and proteomic20 alterations, but these alterations have not yet yielded diagnostic markers. Chromosomal gains and losses are common in pancreatic and biliary cancers21–24: Their detection by fluorescence in-situ hybridization modestly improves the prediction of cancer in biliary brushings 10 12. Another approach involves microdissecting suspicious cells to detect chromosomal losses using microsatellite markers25, 26. Because non-invasive neoplasms such as IPMNs undergo chromosomal losses, this approach is probably better at distinguishing neoplastic from non-neoplastic lesions, rather than cancer from benign neoplasms 23.
The detection of aberrant DNA methylation is a promising marker strategy for diagnosing periampullary cancer. Promoter methylation, a common mechanism for silencing genes during tumorigenesis, is readily detected using methylation-specific PCR (MSP). Numerous genes are aberrantly methylated and silenced in pancreatic cancer and rarely methylated in non-neoplastic pancreas, including TFPI2, NPTX2, Cyclin D2, FOXE1 and others 27–33, and this methylation is detectable in pancreatic fluids 29, 34 35. In this study, we examine the diagnostic performance of MSP and quantitative MSP (QMSP) assays on brush cytology specimens obtained during ERCP from patients undergoing diagnostic evaluation.
METHODS
Patients and Samples
Endoscopic brush samples were collected for cytology and DNA methylation analysis from 130 patients with biliary tract strictures either from within the biliary (n=118) or pancreatic duct (n=4) or both (n=8). The samples were obtained at the time of ERCP as part of clinical research protocols approved by The Cleveland Clinic Institutional Review Board. Brush samples were obtained in duplicate, one for cytology and one for marker analysis with the order determined by a closed envelope randomization scheme. Brush samples for methylation analysis were placed in 95% alcohol and immediately stored in a −80oC freezer for later batched analysis. Brushings were collected from 5 groups of patients with strictures (see table 1). A cancer diagnosis was determined by histological or cytological or imaging criteria. In addition to ERCP, patients with a bile duct stricture underwent abdominal spiral CT and/or MRI scan. The absence of cancer was based on clinical evaluation and follow-up of one or more years. Cytology specimens underwent DNA methylation analysis without knowledge of the clinical diagnosis.
Table 1.
Patient demographic profiles
| Disease group | n | mean age (S.D)yrs | gender (M,F) |
|---|---|---|---|
| Pancreatic ductal adenocarcinoma | 41 | 67.4 (12.0) | 28,13 |
| Biliary tract adenocarcinoma | 10 | 67.0 (13.8) | 6,4 |
| Other periampullary malignancies | 13 | 60.5 (12.4) | 7,6 |
| Primary sclerosing cholangitis (PSC)-associated strictures | 27 | 55.2 (16.4) | 19,10 |
| Non-PSC-associated benign strictures | 39 | 56.7 (18.2) | 12,27 |
Bisulfite Treatment and Methylation Specific PCR
DNA was extracted from brush samples and bisulfite-modified as previously described32. One microliter (~20ng) of bisulfite-treated DNA was PCR amplified with RDA buffer (67mM Tris pH 8.8, 16mM (NH4)2SO4, 10mM β-Mercaptoethanol, 1 μg/μl BSA). PCR conditions were: 95°C for 2 min; 45 cycles of 95°C for 20s, 58–62°C for 20s, and 72°C for 30s; and (c) a final extension of 4 min at 72°C. Primer sequences are listed in Table 2.
Table 2.
Primers and Probes used in this study
| Primers for MSP | |
| Methylated Forward | |
| RPRM | 5′-GCG AGT GAG CGT TTA GTT C-3′ |
| SARP2 | 5′-GTC GGG GCG TAT TTA GTT C-3′ |
| DAB1 | 5′-TAG AGG CGC GAT TGT AAG TC-3′ |
| TFPI2 | 5′-TTT CGT ATA AAG CGG GTA TTC-3′ |
| NPTX2 | 5′-GAA AGG GCG CGC GGA TTC-3′ |
| Unmethylated Forward | |
| RPRM | 5′-TTG TGA GTG AGT GTT TAG TTT G-3′ |
| SARP2 | 5′-GGG TGT ATT TAG TTT GTA GTG-3′ |
| DAB1 | 5′-TTA GAG GTG TGA TTG TAA GTT G-3′ |
| TFPI2 | 5′-GGA TGT TTG TTT TGT ATA AAG TG-3′ |
| NPTX2 | 5′-AAG AAA GGG TGT GTG GAT TTG-3′ |
| QMSP primers | |
| FOXE1 | 5′-TCG TAG GGT TGG AGA TTT AC-3′ |
| CCD2 | 5′-ACG TTT AGC GTA GAT ATT TC-3′ |
| B-Actin | 5′-TGG TGA TGG AGG AGG TTT AGT AAG T-3′ |
| Primers for NPTX2 and TFPI-2 were the same as the MSP primers | |
| Probes for QMSP | |
| CCD2 | 5′-6FAM-CCG CCC AAC GAC CAC GCA AAA AAA ACC CG-TAMRA-3′ |
| TFPI-2 | 5′-6FAM-CGA AAA AAC GCC TAA CGA AAA AAA AT-TAMRA-3′ |
| NPTX2 | 5′-6FAM-CGC GAA ACA AAA ATC TCC TAC CG-TAMRA -3′ |
| FOXE1 | 5′- 6FAM- ACG CGA ACC CAA ACG AAA CGA C -TAMRA -3′ |
| B-Actin | 5′-6FAM-ACC ACC ACC CAA CAC ACA ATA ACA AAC ACA-TAMRA-3′ |
Quantitative Methylation Analysis
DNA templates were amplified by fluorescence-based quantitative real-time methylation-specific PCR (QMSP)36. Primers and probes were designed to amplify specifically bisulfite-converted promoter DNA of NPTX2, Cyclin D2, TFPI2, FOXE1, and β-actin (used as the internal reference gene to quantify modified DNA levels in a sample)(Table 2). QMSP was performed using the AB 7300 (Applied Biosystems, Foster City, CA). QMSP was performed using Quantitect PCR reagents (Qiagen); conditions were 60 cycles of 95°C for 15s, 60°C for 30s. Methylated DNA levels were quantified using serially-diluted bisulfite-modified completely methylated DNA. The ratio of the level of methylated DNA to modified DNA from the β-actin quantification yielded the percentage of methylated DNA in a sample.
The intra-assay variation of the QMSP assays was determined by performing the same QMSP assay 6 times. The intra-assay variation using 50ng, 5ng and 0.5ng of input DNA of the CCD2 QMSP was 1.5%, 2.3% and 11.5%, respectively. Similarly, for NPTX2 it was 1.3%, 1.3% and 2%, respectively and for TFPI-2, it was 1.8%, 1.6% and 28%. An estimate of the lower limit of sensitivity of each QMSP was determined by assaying 20ng of modified DNA containing methylated DNA concentrations of 10%, 5%, 1%, 0.5% and 0% for each QMSP assay in triplicate for each concentration (2ng, 1ng, 0.2ng, 0.1ng and 0ng of input DNA). All 3 QMSP assays could reliably detect methylation at the 0.1 ng concentration.
Statistics
DNA methylation profiles associated with pancreatic ductal adenocarcinoma and biliary tract cancers were compared to strictures from patients with non-neoplastic strictures. The non-neoplastic group was also subdivided into the PSC vs. the non-PSC benign biliary strictures. The optimal methylated DNA concentration cut-off was determined for its diagnostic utility. The sensitivity and specificity was calculated for each of the markers and marker panels. Cytology was considered positive if a definitive diagnosis was made from the cytological analysis. In addition, we also examined the accuracy of cytology when suspicious results were also categorized as a positive for malignancy. The majority of patients (n=118) had brushings from the one duct. Patients who had samples from both ducts were considered to have a positive QMSP result if either sample was positive. Separate comparisons were made for the conventional MSP results and quantitative MSP results. The sensitivity, specificity and overall accuracy of the MSP vs. the QMSP assays and the QMSP vs. cytology results were compared by comparing the 95% confidence interval (CI) for each measurement and differences in the proportion of methylated genes and the probability of having one or more genes methylated between diagnostic groups was compared by Chi-squared test. The average number of positive methylation tests among the genes in the panels out of all the possible positive methylation tests in each group was compared using Student’s t-test. The relationship of methylation with age was determined using Pearson’s correlation coefficient. A two-tailed P value of less than 0.05 was considered statistically significant. Statistical analysis was performed using the Excel statistics software (Microsoft, Redmond, WA), STATA version 8.2 software.
RESULTS
Patients
The demographic profiles of the patients that underwent methylation analysis by QMSP are listed in Table 1. The patients with strictures due to pancreatic and biliary adenocarcinomas were significantly older than the patients with benign strictures (p<0.01). However, the age range of controls was similar to that of cases and differences in age did not explain the differences in the methylation profiles of cancer patients vs. controls (see Figure 1 and 2).
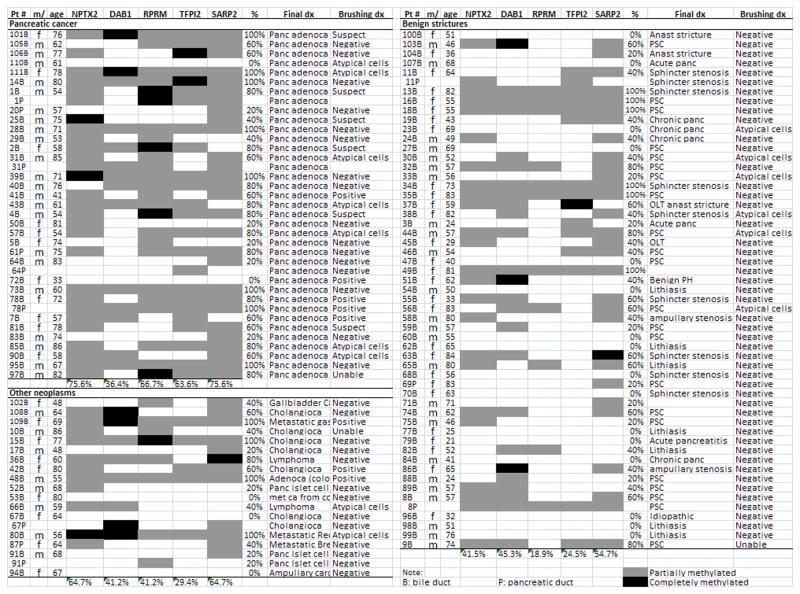
Figure 1.
Methylation analysis of endoscopic brush samples by MSP.
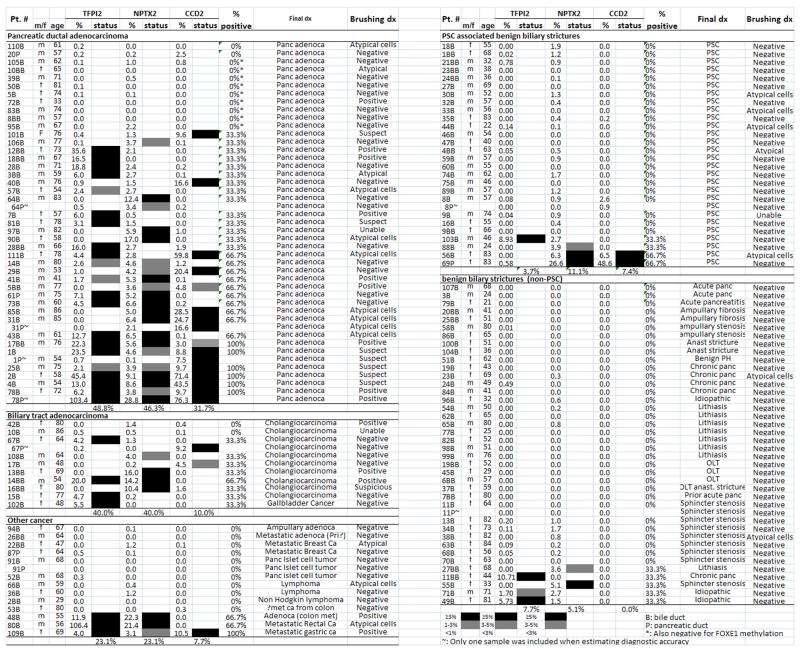
Figure 2.
DNA methylation in endoscopic brush samples determined by QMSP.
DNA methylation analysis of brush samples using conventional MSP
We first selected a panel of genes that we have previously found to be commonly methylated in pancreatic cancer tissues, but rarely in normal pancreata 29, 37, 38. We tested 5 such genes (NPTX2, DAB1, RPRM, TFPI2 and SARP2) for aberrant methylation in brush samples using conventional MSP in 104 patient samples (see Table 3). We had previously tested TFPI2 and NPTX2 in pancreatic juice samples 35, but not in biliary tract brush samples. There were significantly more genes methylated in the endoscopic brush samples of patients with pancreatic cancer and other periampullary cancers than in samples from patients with non-neoplastic periampullary conditions (Figure 1 and Table 3). The methylation of some genes showed more discrimination for pancreatic ductal adenocarcinoma than others: NPTX2 [sensitivity 75.6% (95% CI:59–87%) and specificity 58.5% (41–73%)] TFPI-2 [sensitivity 63.6% (47–78%) and specificity 75.5% (62–85.1%)] and RPRM [sensitivity 66.6% (50–80%) and specificity 81% (68–91%)], while SARP2 and DAB1 provided no significant diagnostic discrimination. Overall, the mean percentage of positive methylated genes in the gene panel of patients with pancreatic adenocarcinoma (63.6±31.0% of all genes in the group) was significantly higher than in the non-neoplastic controls (37.0±33.4%, P<0.001). Within the disease control group, the mean percentage of methylated genes was higher in the PSC group than in the non-PSC benign stricture group (48.4±34% vs. 28.0±30%, p=0.029). Combining MSP markers to optimize sensitivity and specificity did not significantly improve accuracy. For example, setting a threshold of 2 positives of the best 3 markers (NPTX2, TFPI-2 and RPRM) as a positive MSP panel yielded a sensitivity of 52% (35–68%) and a specificity of 76.5% (62–85%), which was not significantly more accurate than using a single MSP marker. Furthermore, none of the MSP markers achieved 80% specificity. We therefore evaluated the role of QMSP, modified our marker panel and included an additional 36 patient samples.
Table 3.
Methylation analysis of endoscopic brush samples by MSP
| Group | N | % methylated genes § (mean±SD%) | % Patients with methylation* |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ≥ 1 gene | ≥ 2genes | ≥ 3 genes | ≥ 4 genes | |||||||
| N | % | N | % | N | % | N | % | |||
| Pancreas Cancer | 33 | 63.6±31.0† | 31 | 93.9‡ | 26 | 78.8£ | 24 | 72.7† | 17 | 51.5† |
| Other malignancy | 17 | 48.2±36.8 | 14 | 82.4 | 11 | 64.7 | 7 | 41.2 | 5 | 29.4 |
| Benign | 53 | 37.0±33.4 | 37 | 69.8 | 29 | 54.7 | 17 | 32.1 | 9 | 17.0 |
Note:
Chi-square test Five genes (NPTX2, DAB1, RPRM, TFPI-2 and SARP2)
t-test
p<0.001
p<0.01
p<0.05
DNA methylation analysis of endoscopic brush samples using QMSP
Since the conventional MSP assays detect but do not quantify methylation levels, we used QMSP to quantify concentrations of 3 methylated genes TFPI-2, NPTX2 and cyclin D2 to help improve their diagnostic utility. The conventional MSP assays for NPTX2 and TFPI-2 were the most discriminating in the brush sample analysis and these markers as well as cyclin D2 had performed well in pancreatic juice analysis in a previous study 35. Endoscopic brush samples from patients with pancreatic ductal adenocarcinoma usually had methylated NPTX-2 and Cyclin D2 concentrations of ≥3% and methylated TFPI-2 concentrations of ≥1% and these levels were detected in (<10% of the disease controls). This concentration of methylation was thus chosen as the cut-off for calling a QMSP result “positive for methylation”. The 3 QMSP assays had sensitivities for pancreatic ductal adenocarcinoma ranging from 32 to 49%, specificities from 89 to 100% and accuracies of 75 to 90% depending on the comparison group (Table 6). The specificity of the NPTX2 (92.4%, 95% CI:83–97%) and TFPI-2 (93.9%, CI:85.4%–98%) QMSP assays were significantly higher than their corresponding MSP assays, but sensitivities were not significant different.
Table 6.
Diagnostic sensitivity, specificity and accuracy of QMSP vs. cytology
| Group | Test | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| QMSP | TFPI-2 | NPTX2 | CCD2 | ≥1 gene | |||||||||
| Sens (CI) |
Spec (CI) |
Acc (CI) |
Sens (CI) |
Spec (CI) |
Acc (CI) |
Sens (CI) |
Spec (CI) |
Acc (CI) |
Sens (CI) |
Spec (CI) |
Acc (CI) |
||
| Pancreatic adenocarcinoma | 41 | 49 (34–64) |
93 (85–98) |
77 (68–94) |
46 (32–61) |
92 (83–97) |
75 (66–32) |
32 (20–47) |
97 (90–99) |
72 (63–80) |
73.2 ( 58–84)a |
86.4 (7–24) |
81 (73–88) i |
| Biliary tract cancer | 10 | 40 (17–69) |
93 (85–98) |
86 (78–93) |
40 (17–69) |
92 (83–97) |
86 (77–92) |
10 (2–40) |
97 (90–99) |
86 (77–92) |
80 (49–94)b |
86.4 (7–24) |
86 (76–92) |
| Other cancer | 13 | 23 (8–52) |
93 (85–98) |
82 (72–89) |
23 (8–52) |
92 (83–97) |
82 (72–89) |
8 (1–33) |
97 (90–99) |
84 (74–90) |
23 (8–52) |
86.4 (7–24) |
76 (65–85) |
| Cytology | Cytology 1 | Cytology 2 | Cytology 3 | ≥ gene ± Cytology 1 | |||||||||
| Pancreatic adenocarcinoma | 41 | 19.5 (14–34)c |
100 (95–100) |
69 (60–77)g |
32 (20–47) |
100 (95–100) |
76 (67–83) |
51 (36–66) |
91 (82–96) |
76 (67–83) |
76 (61–76)e |
86.4 (7–24) |
82 (74–88) h |
| Biliary tract cancer | 10 | 30 (11–60)d |
100 (95–100) |
92 (84–96) |
40 (17–69) |
100 (95–100) |
92 (84–96) |
40 (17–69) |
91 (82–96) |
84 (74–91) |
90 (60–96)f |
86.4 (7–24) |
88 (79–94) |
| Other cancer | 13 | 15 (4–42) |
100 (95–100) |
86 (77–92) |
15 (4–42) |
100 (95–100) |
86 (77–92) |
38 (18–64) |
91 (82–96) |
78 (68–86) |
23 (8–52) |
86.4 (7–24) |
82 (72–89) |
Note: Sensitivity (Sens), specificity (Spec) and accuracy (Acc) are expressed as percentages. CI=95% confidence Interval
χ2 test p<0.0001(a vs c) p<0.05 (b vs d, g vs h. g vs i) p>0.05 (a vs e, b vs f)
cytology 1: diagnostic of cancer, cytology 2: diagnostic or suspicious, cytology 3: diagnostic, suspicious or atypical
Thirty of 41 (73.2%, CI,58–84%) patients with pancreatic adenocarcinoma but only 9 of 66 (13.6%, CI,7–24%) disease controls had methylation of 1 or more genes in their endoscopic brush samples (P <0.001 Chi-Square). Within the disease control group, 4 of 27 patients (14.8%) with and 5 of 39 (12.8%) without PSC-associated strictures had one or more genes positive by QMSP (Figure 2). The high sensitivity of the QMSP panel also applied to patients with biliary tract cancers (80%, p<0.001 compared to the disease control group). QMSP was significantly more sensitive than cytology (19.5% more sensitive for pancreatic adenocarcinoma and 30% for other periampullary cancers). The overall accuracy of QMSP (using a cut-off of 1 or more positive genes) was statistically significantly better than cytology as a test for pancreatic cancer (X2=4.24, p<0.05). The superior accuracy of QMSP over cytology was also evident when pancreatic and biliary brush samples from the same patient were analyzed as independent samples (p<0.001). The overall diagnostic accuracy of combined QMSP and cytology was significantly better than the accuracy of diagnostic cytology alone, but combining QMSP and cytology was no more accurate than QMSP alone (Table 6). For the primary analysis of the diagnostic yield of cytology we considered only samples with a definite cytological diagnosis of cancer as positive. However, we found that if a cytological diagnosis of suspicious for cancer was also considered as a positive cytology test, the accuracy of cytology improved such that the overall accuracy of cytology was no longer significantly different to QMSP (X2=1.72, p>0.1)(Table 6).
Another measure of the difference in methylation between the pancreatic ductal adenocarcinoma brushings and those from disease controls is revealed by comparing the mean percentage of genes that were positive for methylation by QMSP (≥3% concentration of methylated DNA) out of all the possible results in the endoscopic brushings. Patients with pancreatic ductal adenocarcinoma had significantly more positive QMSP results than the benign disease controls (43.2±34.2% of all the QMSP results were positive vs. 5.6±15.0% of the disease control results, P<0.001)(Table 5). To determine if we could increase the sensitivity of our QMSP panel, we next analyzed the 9 brush samples from patients with pancreatic cancer that were unmethylated for the 3 QMSP marker panel with a 4th marker, FOXE1. Methylated FOXE1 is prevalent in pancreatic ductal adenocarcinomas and our QMSP assay for methylated FOXE1 had similar sensitivity and specificity for pancreatic cancer in pancreatic juice samples to that of NPTX2, Cyclin D2 and TFPI-2 35. These 9 brush samples amplified ample levels of the bisulfite modified B-actin DNA, but none were positive for FOXE1 methylation. The lack of detectable methylation with the 4 QMSP assays in these 9 samples suggests either that these samples did not have measureable cancer DNA, or perhaps these cancers had distinct DNA methylation patterns such that the primary cancer did not harbor methylation of any of these 4 genes, or both. Indeed, the MSP assays, which have a lower limit of detection, also failed to detect methylation in many of these samples.
Table 5.
Accuracy of the QMSP marker panel in endoscopic brush samples
| Group | N | % methylated genes§ (mean±SD%) | % Patients with QMSP positive samples* |
|||
|---|---|---|---|---|---|---|
| ≥ 1 gene | ≥ 2 genes | |||||
| N | % | N | % | |||
| Panc adenoca | 41 | 43.2±34.2† | 30 | 73.2† | 16 | 39.0† |
| Biliary tract cancer | 10 | 30.0±18.9‡ | 8 | 80.0† | 1 | 10.0 |
| Other cancer | 13 | 16.7±34.0 | 3 | 23.1 | 3 | 23.1£ |
| Benign | 66 | 5.6±15.0 | 9 | 13.6 | 2 | 3.0 |
| PSC | 27 | 7.4 ±19.2 | 4 | 14.8 | 2 | 7.4 |
| Non-PSC | 39 | 4.6±11.7 | 5 | 12.8 | 0 | 0.0 |
Note:
Chi test (three genes: TFPI2, NPTX2 and CCD2)
T-test
p<0.001
p<0.005
p<0.05
The use of other cut-offs of methylated DNA concentrations to decide if a test was positive or negative provided less optimal discrimination between the cancer and non-cancer samples. For example, the use of a 1% concentration of methylated NPTX2 and Cyclin D2 DNA and 0.5% for TFPI-2 to indicate a positive gene test increased the sensitivity of the test panel (1 or genes positive) to 78% for identifying patients with pancreatic ductal adenocarcinoma but decreased the specificity among the disease controls to 74%. But using these lower cut-offs for methylated DNA concentrations, the PSC group was more likely to have one or more a positive QMSP methylation tests than the non-PSC disease controls (41% vs. 15%, P<0.05). However, there was no significant difference in the performance of the QMSP assays within the benign stricture group between those with PSC and those without PSC using the higher methylation concentration cut-offs that were used to distinguish the adenocarcinoma strictures from non-neoplastic strictures (Figure 2 and Table 4).
Table 4.
Gene Methylation by QMSP in endoscopic brush samples
| Patient Group | N | % Patients with methylation
|
||||
|---|---|---|---|---|---|---|
| TFPI-2 | NPTX2 | CCD2 | ≥1 gene | ≥2 genes | ||
| Panc adenocarcinoma | 41 | 48.8 | 46.3 | 31.7 | 73.2 | 39.0 |
| Biliary tract cancer | 10 | 40.0 | 40.0 | 10.0 | 80.0 | 10.0 |
| Other cancer | 13 | 23.1 | 23.1 | 7.7 | 23.1 | 23.1 |
| Benign | 66 | 6.1 | 7.6 | 3.0 | 13.6 | 3.0 |
| PSC | 27 | 3.7 | 11.1 | 7.4 | 14.8 | 7.4 |
| Non-PSC | 39 | 7.7 | 5.1 | 0.0 | 12.8 | 0.0 |
DISCUSSION
In this study we demonstrate the diagnostic utility of quantifying aberrantly methylated DNA concentrations in endoscopic brush samples of biliary tract strictures. Using a 3-gene QMSP panel, 73.2% of patients with pancreatic adenocarcinoma had positive methylation in 1 or more genes, compared to only 13.6% of individuals with non-neoplastic conditions. The sensitivity of the 3-gene panel for patients with biliary tract cancers was similar, with 80% of patients having at least 1 positive result in the 3 QMSP assays evaluated, whereas methylation was only occasionally detected in brush samples from patients with other periampullary neoplasms. This is consistent with our methylated gene panel having been selected after analyzing pancreatic adenocarcinomas for aberrant hypermethylation and is likely to be specific for these cancers compared to cancers from other sites.
It is possible that adding other genes to the QMSP panel would improve the diagnostic sensitivity with only small changes in specificity, because the markers we tested have been previously found to be highly specific, being rarely detected in normal pancreatic tissues and present in majority of primary pancreatic ductal adenocarcinomas (Cyclin D2, ~65%)37, (TFPI-2, 73% 38 and NPTX2, 98%) 29). Including additional genes in our marker panel could probably also increase the number of samples that had 2 or more positive genes and provide further specificity. It is also probable that some of the samples did not contain sufficient concentrations of cancer DNA. The brush samples were obtained by experienced endoscopists who sampled the strictures in a standard fashion in the way samples are obtained for cytology, but given the poor diagnostic yield of cytology in this setting, a problem that is likely to be related to the highly scirrous nature of pancreatic ductal adenocarcinomas, sample adequacy is likely to be a limiting factor in the molecular analysis of these samples.
The accuracy of our QMSP panel suggests that it could also be used on FNA samples of solid and cystic lesions of the pancreas and periampullary region although these samples generally have fewer cells than brush cytology specimens. We also found evidence that patients with PSC-related strictures had a higher prevalence of low-level methylation (≤1% methylated DNA) by QMSP (40.7%) than those with other benign strictures (15.4%), but no difference \ in methylation when the higher cut-off of methylated DNA concentration was used. It is not known if this higher prevalence of aberrant methylation is the result of early dysplasia arising in the setting of PSC or an increase in aberrant DNA methylation due to PSC alone. Some investigators have found that chronic inflammation is associated with an increase in DNA methylation 39. We also found low-level methylation in benign pancreaticobiliary lesions by MSP and to a lesser extent QMSP. Overall, our QMSP assays could better discriminate between disease groups than MSP. Conventional MSP assays detect very low-levels of methylation in endoscopic brush samples, below levels detectable by QMSP and low-level DNA methylation is present in normal tissues and for some genes its prevalence increases with age 37.
The accuracy of our QMSP panel for differentiating neoplastic vs. non-neoplastic strictures results compare favorably to other markers such as mutant KRAS or telomerase, that have been used to differentiate benign from malignant pancreatic diseases 40, 19 These findings in brush samples complement previous results using QMSP markers in pancreatic juice samples 35. Ultimately, a combination of highly specific markers may provide the best diagnostic utility. Newer assays that can detect low concentrations of these mutations in pancreatic juice 41, and novel assays and technologies are likely to improve the detection of low concentrations of mutant DNA for cancer diagnosis in the future.
A molecular marker panel will need to achieve high accuracy to be useful in clinical practice. Cytology remains the gold standard for cancer diagnosis, but an accurate marker panel such as our QMSP panel that has been extensively validated could aid cytology in establishing a diagnosis of cancer. A positive test from an accurate molecular marker panel in the right clinical setting would provide a high posterior probability of cancer. Often a cytological or pathological diagnosis would still be necessary, but a positive QMSP marker test could encourage efforts to repeat sampling to obtain a cytological or histological diagnosis. DNA methylation analysis of cytology specimens is not yet performed clinically, but is within the capability of a molecular diagnostic lab. QMSP analysis could be performed on alcohol-fixed cytology specimens as alcohol-fixed samples retain good DNA quality. Further evaluation of the utility of QMSP analysis of pancreaticobiliary samples is required before these markers can be used in clinical practice. Such evaluation could include testing the utility of other aberrantly methylated genes and evaluating patients whose workup is inconclusive for cancer who would potentially benefit most from such a marker panel.
Ultimately, if molecular markers such as aberrantly methylated genes can be used to help diagnose periampullary cancer, these markers could also potentially be useful in identifying microscopic preinvasive neoplastic disease, such as PanINs or biliary dysplasia. Detecting PanINs is particularly important for patients with an inherited predisposition to pancreatic and other periampullary cancers 42. Detecting biliary dysplasia is an important need for patients with long-standing primary sclerosing cholangitis. Indeed, a panel of QMSP markers are being measured in the pancreatic juice of patients undergoing screening because of an increased risk of developing pancreatic cancer 43. The “CAPS” (Cancer of the Pancreas Screening) screening protocols utilize endoscopic ultrasound (EUS), MRI/MRCP, CT, pancreatic juice analysis and genetic counseling and have detected and treated pre-invasive pancreatic neoplasms in some individuals 44, 45. The resected pancreata of many of the high-risk individuals contain PanINs 44, 45, raising the possibility that subtle EUS and ERCP abnormalities found in some of these individuals reflect the presence of PanIN 46, 47. The ability to reliably detect and quantify PanIN using molecular assays in high-risk individuals would help identify individuals needing more surveillance to detect advanced pancreatic neoplasia, and could also open up the option of enrolling affected individuals in chemoprevention trials.
In summary, we find that with a 3-gene QMSP panel it is possible to differentiate patients with benign versus malignant endoscopic brush samples with high accuracy. Further studies evaluating the diagnostic utility of QMSP analysis in this setting are likely to be productive.
Dr. Goggins has licensing agreements with Oncomethylome Sciences who wish to develop into commercial products several of the methylated genes used in this study.
Acknowledgments
Grant support: Supported by the NCI grants Specialized Programs of Research Excellence in Gastrointestinal Malignancies (CA62924), and the Michael Rolfe Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Murray T, Ward E, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 2.DeOliveira ML, Cunningham SC, Cameron JL, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245:755–62. doi: 10.1097/01.sla.0000251366.62632.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Furukawa H, Okada S, Saisho H, et al. Clinicopathologic features of small pancreatic adenocarcinoma. A collective study Cancer. 1996;78:986–90. doi: 10.1002/(SICI)1097-0142(19960901)78:5<986::AID-CNCR7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 4.Cleary SP, Gryfe R, Guindi M, et al. Prognostic factors in resected pancreatic adenocarcinoma: analysis of actual 5-year survivors. J Am Coll Surg. 2004;198:722–31. doi: 10.1016/j.jamcollsurg.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Agarwal B, Abu-Hamda E, Molke KL, et al. Endoscopic ultrasound-guided fine needle aspiration and multidetector spiral CT in the diagnosis of pancreatic cancer. Am J Gastroenterol. 2004;99:844–50. doi: 10.1111/j.1572-0241.2004.04177.x. [DOI] [PubMed] [Google Scholar]
- 6.Harewood GC, Wiersema MJ. Endosonography-guided fine needle aspiration biopsy in the evaluation of pancreatic masses. Am J Gastroenterol. 2002;97:1386–91. doi: 10.1111/j.1572-0241.2002.05777.x. [DOI] [PubMed] [Google Scholar]
- 7.Canto MI, Goggins M, Hruban RH, et al. Screening for early pancreatic neoplasia in high-risk individuals: a prospective controlled study. Clin Gastroenterol Hepatol. 2006;4:766–81. doi: 10.1016/j.cgh.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Papachristou GI, Smyrk TC, Baron TH. Endoscopic retrograde cholangiopancreatography tissue sampling: when and how? Clin Gastroenterol Hepatol. 2007;5:783–90. doi: 10.1016/j.cgh.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 9.Vandervoort J, Soetikno RM, Montes H, et al. Accuracy and complication rate of brush cytology from bile duct versus pancreatic duct. Gastrointest Endosc. 1999;49:322–7. doi: 10.1016/s0016-5107(99)70008-8. [DOI] [PubMed] [Google Scholar]
- 10.Kipp BR, Stadheim LM, Halling SA, et al. A comparison of routine cytology and fluorescence in situ hybridization for the detection of malignant bile duct strictures. Am J Gastroenterol. 2004;99:1675–81. doi: 10.1111/j.1572-0241.2004.30281.x. [DOI] [PubMed] [Google Scholar]
- 11.Moreno Luna LE, Kipp B, Halling KC, et al. Advanced cytologic techniques for the detection of malignant pancreatobiliary strictures. Gastroenterology. 2006;131:1064–72. doi: 10.1053/j.gastro.2006.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barr Fritcher EG, Kipp BR, Slezak JM, et al. Correlating routine cytology, quantitative nuclear morphometry by digital image analysis, and genetic alterations by fluorescence in situ hybridization to assess the sensitivity of cytology for detecting pancreatobiliary tract malignancy. Am J Clin Pathol. 2007;128:272–9. doi: 10.1309/BC6DY755Q3T5W9EE. [DOI] [PubMed] [Google Scholar]
- 13.Harewood GC, Baron TH, Stadheim LM, et al. Prospective, blinded assessment of factors influencing the accuracy of biliary cytology interpretation. Am J Gastroenterol. 2004;99:1464–9. doi: 10.1111/j.1572-0241.2004.30845.x. [DOI] [PubMed] [Google Scholar]
- 14.Lee JG. Brush cytology and the diagnosis of pancreaticobiliary malignancy during ERCP. Gastrointest Endosc. 2006;63:78–80. doi: 10.1016/j.gie.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 15.Maitra A, Kern SE, Hruban RH. Molecular pathogenesis of pancreatic cancer. Best Pract Res Clin Gastroenterol. 2006;20:211–26. doi: 10.1016/j.bpg.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Goggins M. Molecular Markers of Early Pancreatic Cancer. J Clin Oncol. 2005;23:4524–31. doi: 10.1200/JCO.2005.19.711. [DOI] [PubMed] [Google Scholar]
- 17.Shi C, Eshleman SH, Jones D, et al. LigAmp for sensitive detection of single-nucleotide differences. Nat Methods. 2004;1:141–7. doi: 10.1038/nmeth713. Epub 2004 Oct 21. [DOI] [PubMed] [Google Scholar]
- 18.Iacobuzio-Donahue CA, Ashfaq R, Maitra A, et al. Highly expressed genes in pancreatic ductal adenocarcinomas: a comprehensive characterization and comparison of the transcription profiles obtained from three major technologies. Cancer Res. 2003;63:8614–22. [PubMed] [Google Scholar]
- 19.Logsdon CD, Simeone DM, Binkley C, et al. Molecular profiling of pancreatic adenocarcinoma and chronic pancreatitis identifies multiple genes differentially regulated in pancreatic cancer. Cancer Res. 2003;63:2649–57. [PubMed] [Google Scholar]
- 20.Gronborg M, Kristiansen TZ, Iwahori A, et al. Biomarker discovery from pancreatic cancer secretome using a differential proteomic approach. Mol Cell Proteomics. 2006;5:157–71. doi: 10.1074/mcp.M500178-MCP200. [DOI] [PubMed] [Google Scholar]
- 21.Iacobuzio-Donahue CA, van der Heijden MS, Baumgartner MR, et al. Large-scale allelotype of pancreaticobiliary carcinoma provides quantitative estimates of genome-wide allelic loss. Cancer Res. 2004;64:871–5. doi: 10.1158/0008-5472.can-03-2756. [DOI] [PubMed] [Google Scholar]
- 22.Calhoun ES, Hucl T, Gallmeier E, et al. Identifying Allelic Loss and Homozygous Deletions in Pancreatic Cancer without Matched Normals Using High-Density Single-Nucleotide Polymorphism Arrays. Cancer Res. 2006;66:7920–8. doi: 10.1158/0008-5472.CAN-06-0721. [DOI] [PubMed] [Google Scholar]
- 23.Abe T, Fukushima N, Brune K, Boehm C, Sato N, Matsubayashi H, Canto M, Petersen GM, Hruban RH, Goggins M. Genome wide allelotypes of familial pancreatic adenocarcinomas and familial and sporadic intraductal papillary mucinous neoplasms. Clin Cancer Res. 2007;13:6019–25. doi: 10.1158/1078-0432.CCR-07-0471. [DOI] [PubMed] [Google Scholar]
- 24.Walter K, Omura N, Hong SM, et al. Pancreatic cancer associated fibroblasts display normal allelotypes. Cancer Biol Ther. 2008 doi: 10.4161/cbt.7.6.5869. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khalid A, Pal R, Sasatomi E, et al. Use of microsatellite marker loss of heterozygosity in accurate diagnosis of pancreaticobiliary malignancy from brush cytology samples. Gut. 2004;53:1860–5. doi: 10.1136/gut.2004.039784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khalid A, Nodit L, Zahid M, et al. Endoscopic ultrasound fine needle aspirate DNA analysis to differentiate malignant and benign pancreatic masses. Am J Gastroenterol. 2006;101:2493–500. doi: 10.1111/j.1572-0241.2006.00740.x. [DOI] [PubMed] [Google Scholar]
- 27.Matsubayashi H, Sato N, Fukushima N, et al. Methylation of cyclin D2 is observed frequently in pancreatic cancer but is also an age-related phenomenon in gastrointestinal tissues. Clin Cancer Res. 2003;9:1446–52. [PubMed] [Google Scholar]
- 28.Ueki T, Toyota M, Sohn T, et al. Hypermethylation of multiple genes in pancreatic adenocarcinoma. Cancer Res. 2000;60:1835–9. [PubMed] [Google Scholar]
- 29.Sato N, Fukushima N, Maitra A, et al. Discovery of novel targets for aberrant methylation in pancreatic carcinoma using high-throughput microarrays. Cancer Res. 2003;63:3735–42. [PubMed] [Google Scholar]
- 30.Sato N, Matsubayashi H, Abe T, Fukushima N, Goggins M. Epigenetic downregulation of CDKN1C/p57KIP2 in intraductal papillary mucinous neoplasms of the pancreas by gene expression profiling. Clin Cancer Res. 2005;11:4681–8. doi: 10.1158/1078-0432.CCR-04-2471. [DOI] [PubMed] [Google Scholar]
- 31.Sato N, Goggins M. The Role of Epigenetic Alterations in Pancreatic Cancer. J Hepatobiliary Pancreat Surg. 2006;13:286–95. doi: 10.1007/s00534-005-1057-1. [DOI] [PubMed] [Google Scholar]
- 32.Omura M, Li C-P, Li A, et al. Genome-wide profiling of methylated promoters in pancreatic adenocarcinoma. Cancer Biol Ther. 2008 doi: 10.4161/cbt.7.7.6208. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sato N, Fukushima N, Chang R, et al. Differential and epigenetic gene expression profiling identifies frequent disruption of the RELN pathway in pancreatic cancers. Gastroenterology. 2006;130:548–65. doi: 10.1053/j.gastro.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 34.Fukushima N, Walter KM, Ueki T, et al. Diagnosing pancreatic cancer using methylation specific PCR analysis of pancreatic juice. Cancer Biol Ther. 2003;2:78–83. doi: 10.4161/cbt.183. [DOI] [PubMed] [Google Scholar]
- 35.Matsubayashi H, Canto M, Sato N, et al. DNA methylation alterations in the pancreatic juice of patients with suspected pancreatic disease. Cancer Res. 2006;66:1208–17. doi: 10.1158/0008-5472.CAN-05-2664. [DOI] [PubMed] [Google Scholar]
- 36.Eads CA, Danenberg KD, Kawakami K, et al. MethyLight: a high-throughput assay to measure DNA methylation. Nucleic Acids Res. 2000;28:E32. doi: 10.1093/nar/28.8.e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsubayashi H, Sato N, Fukushima N, et al. Methylation of cyclin D2 is observed frequently in pancreatic cancer but is also an age-related phenomenon in gastrointestinal tissues. Clin Cancer Res. 2003;9:1446–52. [PubMed] [Google Scholar]
- 38.Sato N, Parker AR, Fukushima N, et al. Epigenetic inactivation of TFPI-2 as a common mechanism associated with growth and invasion of pancreatic ductal adenocarcinoma. Oncogene. 2005;24:850–8. doi: 10.1038/sj.onc.1208050. [DOI] [PubMed] [Google Scholar]
- 39.Issa JP, Ahuja N, Toyota M, et al. Accelerated age-related CpG island methylation in ulcerative colitis. Cancer Res. 2001;61:3573–7. [PubMed] [Google Scholar]
- 40.Ohuchida K, Mizumoto K, Ogura Y, et al. Quantitative assessment of telomerase activity and human telomerase reverse transcriptase messenger RNA levels in pancreatic juice samples for the diagnosis of pancreatic cancer. Clin Cancer Res. 2005;11:2285–92. doi: 10.1158/1078-0432.CCR-04-1581. [DOI] [PubMed] [Google Scholar]
- 41.Bian Y, Matsubayashi H, Pin-Li C, et al. Detecting Low-Abundance p16 and p53 Mutations in Pancreatic Juice Using a Novel Assay: Heteroduplex Analysis of Limiting Dilution PCRs. Cancer Biol Ther. 2006;5:1392–9. doi: 10.4161/cbt.5.10.3453. [DOI] [PubMed] [Google Scholar]
- 42.Hruban R, Klein A, Eshleman J, Axilbund JE, Goggins M. Familial Pancreatic Cancer. Expert Review in Gastroenterology and Hepatology. 2007 doi: 10.1586/17474124.1.1.81. in press. [DOI] [PubMed] [Google Scholar]
- 43.Klein AP, Brune KA, Petersen GM, et al. Prospective risk of pancreatic cancer in familial pancreatic cancer kindreds. Cancer Res. 2004;64:2634–8. doi: 10.1158/0008-5472.can-03-3823. [DOI] [PubMed] [Google Scholar]
- 44.Canto MI, Goggins M, Yeo CJ, et al. Screening for pancreatic neoplasia in high-risk individuals: an EUS-based approach. Clin Gastroenterol Hepatol. 2004;2:606–21. doi: 10.1016/s1542-3565(04)00244-7. [DOI] [PubMed] [Google Scholar]
- 45.Brentnall TA, Bronner MP, Byrd DR, et al. Early diagnosis and treatment of pancreatic dysplasia in patients with a family history of pancreatic cancer. Ann Intern Med. 1999;131:247–55. doi: 10.7326/0003-4819-131-4-199908170-00003. [DOI] [PubMed] [Google Scholar]
- 46.Rajan E, Clain JE, Levy MJ, et al. Age-related changes in the pancreas identified by EUS: A prospective evaluation. Gastrointest Endosc. 2005;61:401–6. doi: 10.1016/s0016-5107(04)02758-0. [DOI] [PubMed] [Google Scholar]
- 47.Hastier P, Buckley MJ, Dumas R, et al. A study of the effect of age on pancreatic duct morphology. Gastrointest Endosc. 1998;48:53–7. doi: 10.1016/s0016-5107(98)70129-4. [DOI] [PubMed] [Google Scholar]