Abstract
Background
Concerns about intimal disruption and spasm have limited enthusiasm for endoscopic radial artery harvest (ERAH), although the risk of these problems after this procedure remains uncertain. Radial artery conduits were screened intraoperatively before and after ERAH vs open harvest using catheter-based high-resolution optical coherence tomogaraphy (OCT) imaging.
Methods
Twenty-four cadavers nad 60 coronary artery bypass graft (CABG) patients scheduled to receive a RA graft underwent OCT imaging before (in situ) and after (ex vivo) open harvest or ERAH. spasm was quantified by the percentage change in luminal volume between images. Intimal disruption was classified as minor or severe depending on whether the defect was confined to branch ostia or involve the luminal surface. Histology was used to confirm OCT findings.
Results
Luminal volume significantly declined after harvest in all RAs from CABG patients, but there was no difference between groups: -43% ± 29% vs -35% ± 38% change after ERAH (n = 21) vs open harvest (n = 39; p = 0.342). Significantly more intimal injury was noted after ERAH vs open harvest (34/41 vs 9/43, intimal tears/total evaluated RAs, p < 0.0001). Most intimal injury was minor: only 2 tears involved the luminal surface of the RA (both after ERAH). Serial imaging in cadavers revealed that 86% of ostial tears occur in ERAH during the initial blunt dissection step using the endoscope.
Conclusions
Although branch injury is a pitfall of ERAH, OCT imaging documented that the quality of RA procured is acceptable and comparable with open harvest. Catheter-based OCT provides an important quality assurance tool for RA harvest.
The success of the internal mammary artery (IMA) as a bypass graft for coronary artery bypass grafting (CABG) [1] has prompted many to consider alternative arterial conduits such as the radial artery (RA). However, the RA graft has demonstrated highly variable patency at mid-term follow-up [2-5]. Because the RA is a friable vessel, meticulous tissue handling is considered a critical factor for the outcome of this conduit [6]. Therefore, differences in harvesting technique between surgical groups may explain some of the variability in the reported patency rates.
Particularly concerning is the suitability of the RA for endoscopic harvest (ERAH). Endoscopic techniques require more direct handling of the vessel than a traditional open harvest [7]. Analyses of focal vessel biopsy specimens from harvested RAs have led others to conclude that ERAH has no adverse effect on endothelial integrity [7-9]. However, traumatic harvesting technique would be expected to cause a discrete intimal tear in an otherwise normal-appearing vessel.
Our group has shown that catheter-based optical coherence tomography (OCT) provides images of the conduit at near histologic resolution, which is a far better method for detecting focal pathology within bypass conduits intraoperatively compared with histology[10]. This study used a clinical and cadaver model to evaluate OCT as a tool for intraoperative feedback on the quality of RAs procured using ERAH vs open techniques.
Material and Methods
Subject Enrollment
From June 2004 until May 2007, 72 of 653 patients that underwent isolated CABG at the University of Maryland were scheduled to receive a RA graft and were eligible for inclusion. Patients were excluded from the study for a creatinine level exceeding 2.0 mg/dL (n = 8) or refusal or inability to obtain informed consent (n = 2). After Institutional Review Board approval (protocol #H25350), 60 clinical subjects in whom the RA was considered as a conduit between March 2006 and March 2007 provided informed consent and were enrolled into a prospective observational study comparing endoscopic and open RA harvesting techniques. Exclusion criteria included abnormal oximetric Allen test [11], hemodialysis requirement, uncontrolled diabetes mellitus, or Raynaud disease. Twelve cadavers obtained within 72 hours of death from the Maryland State Anatomy Board provided additional subjects for analysis of ERAH (RA harvest performed bilaterally, n = 24 RAs).
Conduit Procurement
The RAs were harvested using endoscopic (n = 21 clinical, n = 20 cadaver) and open (n = 39 clinical, n = 4 cadaver) techniques by a single technician with more than 100 total cases of experience with ERAH and open harvest. Group assignment was nonrandomized. In all patients, a tract of saphenous vein was harvested endoscopically and evaluated by OCT as an internal control for comparison of vessel friability vs the RA. The cadaver model was used to determine the influence of the learning curve on ERAH results by comparing RA quality after harvest by 2 technicians, one with minimal (<20 clinical cases) experience vs one with extensive (>500 clinical cases) experience.
Endoscopic RA harvest was initiated by insertion of a conical dissection cannula at the wrist (VasoView6, Guidant Corp, Minneapolis, MN). The balloon on the blunt-tip trochar port was inflated with less than 5 mL of saline to establish a seal necessary to create carbon dioxide pressure within the tunnel of 10 to 12 mm Hg. Exposure of the vessel was created by blunt dissection under direct endoscopic visualization. Bipolar electrocautery (20 W) was used for branch ligation, which was confirmed by lengthwise passage of the vessel C-ring [7]. After proximal ligation (proximal stab incision), the conduit was removed, flushed with heparinized saline, and stored in a plasmalyte solution containing glyceryl trinitrate and verapamil [12]. Open harvest was performed with standard techniques [6] using clips and electrocautery for branch ligation.
Coronary Artery Bypass Graft Technique
A single surgeon performed off-pump CABG through a median sternotomy using suction-based exposure and stabilizing devices (Octopus 4.3, Medtronic Inc, Minneapolis, MN). Heparin was given at the completion of left IMA harvest (activated clotting time >300 seconds and heparin >2 IU/mL) and was reversed by half the dose of protamine calculated by heparin-protamine titration. Preoperative aspirin (325 mg/d) was continued and given within 6 hours after the operation.
Optical Coherence Tomography Imaging
Conduits were imaged by insertion of an OCT catheter (ImageWire, LightLab Imaging, Westford, MA) first into the in situ RA using an upper arm tourniquet and infusion of heparinized saline to clear blood. Examination was performed on saphenous vein and repeated on the RA ex vivo after harvest and removal of the vessel from the limb, as previously described [13].
In cadavers, additional OCT examinations were performed serially after multiple insertions of the OCT probe to rule out intimal injury from the probe itself and after sequential steps of ERAH (ie, after initial RA blunt dissection, after branch ligation using bipolar cautery, and after C-ring manipulation of the vessel).
The OCT images were analyzed by 2 technicians blinded to group assignment, with harvesting injury categorized as minor when intimal disruption was restricted to the ostia of branch points and severe when the luminal surface was involved [10]. Luminal volume of the RA was calculated from OCT data using automated imaging processing software. Spasm was quantified for clinical RAs by comparing the luminal volume before and after harvest. Intimal thickness was quantified by comparison with the media for the calculation of an intima-media thickness (IMT) ratio [14].
Histologic Examination
Biopsy specimens were obtained from cadaveric RAs and discarded portions of clinical RAs to confirm the diagnosis of vascular trauma. To exactly calibrate the OCT images against the corresponding histopathologic sections, the vessel site where the biopsy specimen was obtained was marked externally at the location of the catheter, visualized by gross examination of the infrared light at the catheter tip (13). These image-guided biopsy specimens were embedded and frozen in cutting compound (Tissue-Tek O.C.T., Redding, CA), then sectioned at 5 μm and analyzed for endothelial integrity as described [15].
Ex Vivo Radial Artery Perfusion Analysis
To simulate the effects of grafting into the coronary circulation, 10 cadaveric RAs with minor ostial intimal tears were perfused ex vivo after harvest with Hank's solution for 30 minutes using a pulsatile perfusion pump (Masterflex L/S, Cole-Parmer Instrument Co, Vernon Hills, IL), as described [16]. Flow into the RA was initiated at 50 mL/min and titrated to maintain pressure of 80 to 110 mm Hg, as monitored by a pressure transducer catheter (Mikro-Tip, Millar Instruments, Inc, Houston, TX).
Graft Patency Analysis
Blood flow was measured in each RA graft using transit time ultrasound imaging (Medistim, Inc, Oslo, Norway) before and after native artery occlusion to rule out competitive flow [17]. Radial artery patency at 1 week was determined by blinded review of computed tomography angiography (CTA). Patency was defined as any flow through the length of the graft regardless of the presence of stenosis, as described [18]. Radial artery graft spasm was defined as a patent graft with a luminal diameter less than the size of its coronary target (ie, Fitzgibbon B patency). To determine the effect of contrast enhancement differences on vessel diameter measurements, the average contrast density, expressed as Hounsfield Units (HU), was measured at the mid-portion of each of the grafts and ascending aorta using the workstation pixel-averaging tool.
Statistics
The primary end point of this study was to compare the incidence of intimal injury (ie, number of intimal tears per conduit) after ERAH vs open harvest using the Fisher exact test. The change in mean luminal volume measured from the in situ and ex vivo OCT scans, a measure of RA spasm, was compared between groups using a t test. In preliminary data using discarded RA segments, we found that ERAH was associated with a fivefold increase in minor intimal injury vs open RA harvest [10]. Therefore, 25 RAs per group were expected to provide an 80% power to detect a fourfold difference in intimal injury between groups at p = 0.05. The reproducibility of OCT interpretations was quantified by interobserver κ correlation coefficients. Baseline patient characteristics were compared between groups using the t test and Fisher exact test, as appropriate. Statistical analysis was performed using the InStat statistical package (GraphPad Software, Inc, San Diego, CA) with assistance of a bio-statistician (A. J.).
Results
Study Subjects
Cadaveric and clinical OCT examinations were performed on 84 RAs (60 clinical, 24 cadaveric) and 74 saphenous veins (all clinical) within an average examination time of 2.7 ± 0.3 minutes per conduit. Baseline characteristics and comorbidities were similar between ERAH vs open RA harvest groups for clinical patients (Table 1) and for the cadaveric groups. Follow-up of this cohort with CTA was 95% complete, with examinations excluded in 3 patients because serum creatinine levels exceeded 2.0 mg/dL.
Table 1.
Baseline Profile of the Clinical Patient Groups
| Characteristics | ERAH (n = 21) Mean ± SD or No. (%) | Open RA Harvest (n = 39) Mean ± SD or No. (%) | p Value |
|---|---|---|---|
| Age, year | 66.0 ± 11.6 | 64.4 ± 10.6 | 0.67 |
| Male sex | 14 (66) | 28 (71) | 1.00 |
| Diseased vessels, No. | 2.9 ± 0.3 | 2.8 ± 0.4 | 0.40 |
| BMI, kg/m2 | 28.2 ± 4.8 | 29.7 ± 6.0 | 0.39 |
| Hypertension | 15 (71) | 35 (90) | 0.14 |
| Diabetes mellitus | 8 (39) | 15 (38) | 1.00 |
| Dyslipidemia | 16 (76) | 31 (79) | 1.00 |
| Smoking | 17 (80) | 25 (64) | 0.13 |
| Renal failure | 0 (0) | 0 (0) | 1.00 |
| Prior CVA | 1 (5) | 2 (5) | 1.00 |
| PVD | 3 (14) | 6 (15) | 1.00 |
| Medications | |||
| ASA | 19 (90) | 32 (82) | 0.47 |
| β-blockers | 15 (71) | 35 (89) | 0.14 |
| ACE inhibitors | 10 (48) | 20 (51) | 1.00 |
| Statins | 17 (81) | 31 (79) | 1.00 |
ACE = angiotensin-converting enzyme; ASA = acetylsalicylic add; CVA = cerebrovascular acddent; ERAH = endoscopic radial artery harvest; PVD = peripheral vascular disease; RA = radial artery; SD = standard deviation.
Optical Coherence Tomography-Guided Radial Artery Conduit Selection
In most of the cases, the length of RA that was procured (13.0 ± 2.7 cm) was longer than what was required for creating the bypass graft (10.0 ± 2.5 cm). This excess length provided an opportunity to discard a selected portion of the vessel based on intraoperative OCT feedback. The distal portion was discarded in 23 of 27 RA harvests (85%); however, the identification of a large intimal tear (n = 1) or severe intimal thickening (n = 3) in the proximal RA prompted the proximal portion to be discarded in 4 vessels (15%). The average IMT ratio was significantly reduced in the portion of the RA that was grafted vs discarded (0.60 ± 0.21 vs 0.73 ± 0.32, p = 0.008), corroborating that the optimal portion of the RA was chosen.
Validation of Optical Coherence Tomography Findings
Portions of RAs diagnosed as normal vs injured by OCT imaging showed a significant difference in endothelial integrity on analysis of registered biopsy sections (normal OCT, 64% ± 20% endothelial integrity vs intimal tear by OCT, 24% ± 20% endothelial integrity, p = 0.001). On each occasion that OCT diagnosed an intimal tear in the lumen or branch ostium of a portion of RA that was available for biopsy, the OCT diagnosis was confirmed histologically (Fig 1). Identification of intimal damage was highly reproducible, with interobserver κ correlation values of 0.85 for minor trauma and 1.0 for severe trauma. The ability of OCT to resolve the intima was evidenced by a strong correlation between the IMT ratio measured by OCT vs histology (R = 0.88, p = 0.0007).
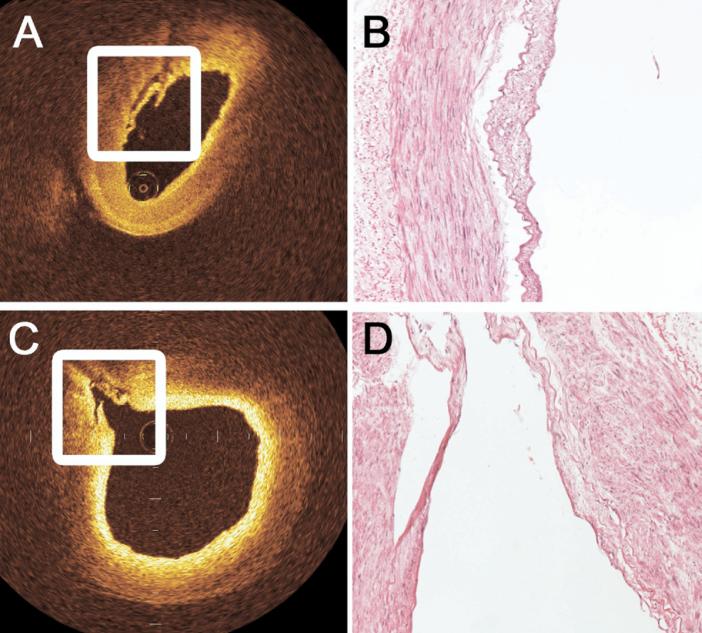
Fig 1.
Histologic confirmation of intimal injury was noted by the ex vivo optical coherence tomography (OCT) examination on (A) the luminal surface of the radial artery and (C) within the ostia of branch points exceeding 0.3 mm in diameter with thickened intima near the ostium. Image-guided biopsy specimens were obtained from areas suggested to be abnormal by OCT. As shown by these representative examples of (B) severe and (D) minor injury, registered histologic sections confirmed the diagnosis of intimal injury on every occasion and illustrate the near histologic resolution of OCT. (A: original magnification ×20, eosin stain; B: original magnification ×20, eosin stain.)
Intimal Injury After Open Versus Endoscopic Harvest
The OCT examination revealed that 31 of 39 clinical RAs (79%) harvested by an open technique showed a completely normal intimal layer, with no evidence of focal intimal trauma (Table 2). In contrast, completely normal intima was observed in only 5 of 21 (24%) of clinical RAs after ERAH (Table 2). Intimal disruption confined to the ostium of branch points was noted more frequently after ERAH vs open harvest (32 of 231 vs 23 of 427 branches involved, p = 0.0003). Tears involving the luminal surface were not found after open harvest but were noted in 2 conduits after ERAH; 1 was noted in the distal RA adjacent to the inflated balloon of the blunt trochar port, the other approximately at the mid point of the graft. The average endothelial integrity of RAs that were harvested open vs endoscopically was not significantly different (52% ± 30% vs 42% ± 28%, p = 0.213). In contrast, 74 endoscopically harvested saphenous veins showed no severe injury and ostial tears in 26 of 884 assessed branch points.
Table 2.
Harvest-Related Trauma in Clinical Radial Arteries
| OCT Imaging Characteristic | ERAH (n = 21) | Open RA Harvest (n = 39) | p Value |
|---|---|---|---|
| Intimal trauma-any type RAs, No (%) | 16 (76) | 8 (21) | <0.0001 |
| Severe luminal tears RAs, No (%) | 2 (10) | 0 (0) | 0.12 |
| Minor ostial tears RAs with any tear, No (%) | 15 (71) | 8 (21) | 0.0002 |
| Branches, No (%) | 32/231 (14) | 23/427 (5%) | 0.0003 |
| Spasm during harvest Luminal volume change, mean ± SD % | -43.8 ± 28.8 | -34.7 ± 38.0 | 0.342 |
ERAH = endoscopic radial artery harvest; OCT = optical coherence tomography; RA = radial artery.
A subgroup of 10 endoscopically harvested cadaveric RAs with minor intimal injury at the ostium of branches (45 involved branches) underwent ex vivo perfusion to determine whether the intimal injury extended onto the luminal surface. After 1 hour of perfusion at arterial pressure, none of these minor intimal tears showed any evidence of change in their imaging appearance.
Cadaver Model of Intimal Injury
The pattern of OCT-detected intimal injury was similar between cadaveric and clinical groups (Table 3). Serial OCT imaging during ERAH performed in the cadaver model revealed that insertion of the OCT imaging probe itself was not associated with RA injury on any occasion. Instead, 86% of ostial tears were found immediately after the blunt “tunneling” dissection step. The remaining ostial tears (14%) were noted immediately after the branch dissection step using the C-ring. The incidence of ostial injury differed between experienced and inexperienced harvesters (16% vs 14% injured branch ostia respectively, p = 0.843). Ostial tears were only noted in branches that had a thickened intima (ie, IMT ratio >0.6) and within branches having a diameter of less than 0.3 mm (Fig 1C, D).
Table 3.
Harvest-Related Trauma in Cadaver Radial Arteries
| OCT Imaging Characteristic | ERAH (n = 20) | Open RA Harvest (n = 4) | p Value |
|---|---|---|---|
| Intimal trauma-any type RAs, No. (%) | 18 (90) | 1 (25) | 0.018 |
| Severe luminal tears RAs, No. (%) | 3 (15) | 0 (0) | 1.00 |
| Minor ostial tears RA with any tear, No. (%) | 17 (85) | 1 (25) | 0.035 |
| RA branches, No. (%) | 44/260 (17) | 2/52 (4) | 0.017 |
ERAH = endoscopic radial artery harvest; OCT = optical coherence tomography; RA = radial artery.
Intraoperative Spasm of the Clinical Radial Artery
The luminal volume of the RA before harvest was similar for ERAH and open harvest groups and showed a significant decline after harvest in both groups from 1.06 ± 0.32 cm3 to 0.61 ± 0.21 cm3 vs from 1.12 ± 0.23 cm3 to 0.74 ± 0.34 cm3 (Fig 2). However, the degree of change between the two measurements was not significantly different between groups (43.8% ± 28.8% vs 34.7% ± 38.0% reduction in luminal volume between measurements, p = 0.342). Compared with open RA conduits, ERAH conduits showed no difference in blood flow (56 ± 31.2 mL/min endoscopic vs 63 ± 34.7 mL/min open, p = 0.443) or pulsatility index (2.5 ± 0.9 endoscopic vs 2.4 ± 0.6 open) measured intraoperatively.
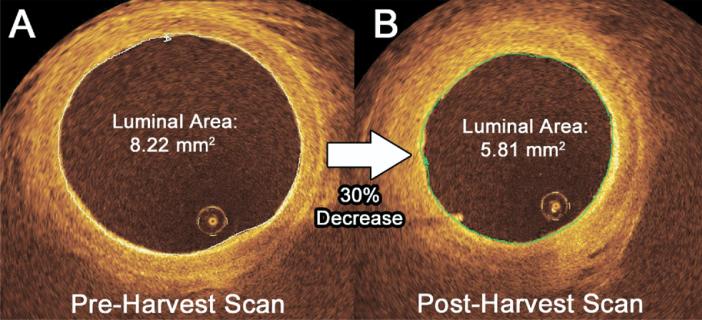
Fig 2.
Intraoperative evaluation of radial artery spasm using optical coherence tomography (OCT). Luminal diameter measurements obtained by OCT were compared for all radial arteries (RAs) at two time points: (A) in situ before harvest and (B) ex vivo after harvest. This representative example of a vessel from the endoscopic RA harvest group demonstrates a decline in the luminal area by 30% between the two measurements, suggesting spasm in response to intraoperative manipulation. The luminal volume of the RA was calculated between these time points using postprocessing software to maximize the sensitivity for detecting spasm.
Postoperative Radial Artery Spasm and Patency
Results of predischarge CTA showed complete occlusion of the graft in 1 of the RA grafts in the ERAH group and in 0 in the open harvest group. Postoperative spasm was diagnosed in 9 RAs procured endoscopically vs 10 procured by open harvest (Fig 3). Therefore, Fitzgibbon A patency was 52.3% vs 74.3% for ERAH vs open harvest (p = 0.244). Radial arteries diagnosed with postoperative spasm showed no difference in their average IMT ratio (0.28 ± 0.12 vs 0.28 ± 0.25, p = 0.938) or number of branches with ostial injury (0.24 ± 0.42 vs 0.19 ± 0.37, p = 0.635), endothelial integrity by histology (42% ± 24% vs 48% ± 30%, p = 0.433), or intraoperative flow measured by transit time ultrasound imaging (46.9 ± 30.4 vs 54.6 ± 24.2 mL/min, p = 0.287).
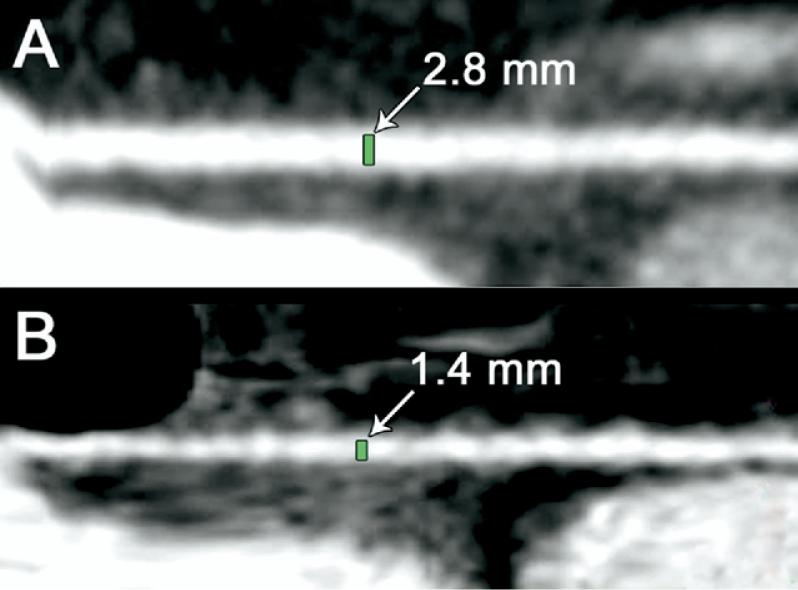
Fig 3.
Postoperative evaluation of radial artery (RA) spasm using 64-multislice detector computed tomography. Two-dimensional planar image reconstructions were obtained parallel to the long axis of the RA to directly measure the diameters of the bypass grafts. These representative examples of RA grafts were imaged on postoperative day 5 after coronary artery bypass grafting. (A) Patient with a RA graft with an unaffected diameter. (B) Radial artery graft with “string sign.”
The density of contrast opacification in the aortic root was higher than the mid point in the evaluated RA grafts (330 ± 85 vs 257 ± 67 HU, p = 0.0009), but a similar difference was noted for IMA and saphenous vein grafts. In addition, there was no significant difference in the contrast opacification within RA grafts categorized as normal vs spastic (271 ± 51 vs 244 ± 44 HU, p = 0.451).
Comment
Imaging of the RA highlights two features that make this vessel particularly challenging to harvest for use as a bypass conduit: its friability and tendency to spasm. Atherosclerosis within the RA, quantified by OCT as an IMT ratio exceeding 0.6, was shown to increase the risk of intimal injury in response to endoscopic harvest. In addition, ERAH led to a 43% decline in luminal volume of the RA despite routine intraluminal and topical application of vasodilators. Compared with qualitative indicators of spasm, such as a reduction in pulse or blood flow, luminal volume change defined by OCT provides a far more sensitive end point for quantifying the effects of surgical manipulation. In contrast, saphenous veins evaluated from this cohort appeared relatively more resistant to injury during harvest than RAs, consistent with the growing view that veins are highly suitable conduits for endoscopic harvest [19].
These data may be interpreted as suggesting that ERAH is a step back from the harvesting principles that helped to revive the RA as an effective bypass graft. However, severe intimal injury and intraoperative and postoperative spasm, the features most likely to affect the ultimate outcome of RA grafts, were not increased in RAs procured by ERAH compared with an open harvest control group. Although not statistically powered to confirm equivalence between groups, our data lend further credence to prior reports suggesting that ERAH is a safe method for RA procurement [7-9].
Despite a favorable experience with ERAH relative to the open technique, we did note a subtle pattern of intimal injury centering on the ostia of branches that was more common after endoscopic harvest. Serial OCT imaging in the cadaver model established a temporal relationship of this ostial injury to one specific step of ERAH: blunt endoscopic dissection of the RA away from subcutaneous tissues in the forearm. Although necessary for creating visualization around the vessel, this step can cause traction on RA branches leading to intimal injury at their ostia. Ostial tears were not associated with the risk of RA vasospasm either intraoperatively (defined by OCT imaging) or postoperatively (defined by CTA). In addition, these minor intimal rents did not propagate onto the luminal surface after exposure to arterial pressure in an ex vivo perfusion apparatus. Therefore, the clinical importance of this finding remains uncertain and requires further study.
Severe intimal tears or spasm of the RA were not associated with detectable abnormalities on gross appearance, leaving OCT imaging as perhaps the only reliable way for our group to have made these findings. Optical coherence tomography provides an ideal method to screen for subtle intimal injury distributed heterogeneously within the vessel [20]. Measurements of luminal volume of the RA before and after harvest provide means for quantifying spasm with a high level of precision. The applications of OCT imaging in cardiology have been hindered by the need to flush blood from the vasculature to obtain an optimal image [14]. In contrast, conduit imaging is performed on an exsanguinated segment of vessel flushed with crystalloid solution, providing images with optimal quality that correlate closely with histology, corroborating prior applications of OCT for evaluating the coronary [14] and peripheral vasculature [21]. More important, imaging can be obtained in real time on the portion of the RA that is actually used for grafting.
Our study was limited because it was not a randomized comparison of harvesting methods but a prospective investigation of conduit quality in an effort to improve practice at a single institution, a study design that has the potential to introduce bias. We controlled for this possibility by using several measures. First, the influence of the learning curve was addressed in the cadaver model by demonstrating that there was no relationship between intimal injury and experience of the harvesting technician.
Second, we analyzed end points such as intimal injury and graft spasm by using highly the sensitive methods of OCT and CTA with technicians blinded to study group assignment.
Third, we monitored closely for sources of variability that would likely confound the analyses, such as comparisons of baseline characteristics to measure selection bias, measurements of blood flow in the RA graft to rule out poor anastomotic quality, and competitive flow.
Fourth, we attempted to minimize the technical differences between the open and endoscopic procedures as much as possible, such as the use of electrocautery to assist in RA dissection during both. Although the use of the harmonic scalpel may be associated with less risk of spasm [22], it is not compatible with the VasoView endoscopic system and was therefore not used in the open cases during this comparison.
Despite these safeguards, our data should be considered “hypothesis generating” until completion of an ongoing clinical trial demonstrates whether these intimal abnormalities detected by OCT relate to the risk of RA graft failure.
In summary, our data suggest that intimal disruption and spasm of the harvested RA are sequelae of both open and endoscopic harvest that can be easily and rapidly identified by intraoperative OCT. Optical coherence tomography screening may therefore prove to be a critical tool providing feedback about surgical harvesting technique. These data support the prospective investigation of an imaging strategy that combines in situ OCT scanning to select only healthy portions of the RA for harvest and ex vivo examinations to provide feedback about conduit quality to reduce the variability of RA grafting.
Acknowledgments
Dr Poston is supported by grants from American Heart Association (Scientist Development Grant, 043518N), University of Maryland (intramural grant), Tobacco Restitution Fund at the University of Maryland, NIH (RO1 HL084080), Guidant Corporation and Joseph and Corinne C. Schwartz research grant. Supplies for OCT imaging were donated by LightLab Imaging, Inc.
Footnotes
Presented at the Basic Science Forum of the Fifty-fourth Annual Meeting of the Southern Thoracic Surgical Association, Bonita Springs, FL, Nov 7-10, 2007.
References
- 1.Goldman S, Zadina K, Moritz T, et al. Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery: results from a Department of Veterans Affairs Cooperative Study. J Am Coll Cardiol. 2004;44:2149–56. doi: 10.1016/j.jacc.2004.08.064. [DOI] [PubMed] [Google Scholar]
- 2.Tatoulis J, Royse AG, Buxton BF, et al. The radial artery in coronary surgery: a 5-year experience: clinical and angiographic results. Ann Thorac Surg. 2002;73:143–7. doi: 10.1016/s0003-4975(01)03290-8. [DOI] [PubMed] [Google Scholar]
- 3.Desai ND, Cohen EA, Naylor CD, Fremes SE. A randomized comparison of radial-artery and saphenous-vein coronary bypass grafts. N Eng J Med. 2004;351:2302–9. doi: 10.1056/NEJMoa040982. [DOI] [PubMed] [Google Scholar]
- 4.Khot UN, Friedman DT, Pettersson G, Smedira NG, Li J, Ellis SG. Radial artery bypass grafts have an increased occurrence of angiographically severe stenosis and occlusion compared with left internal mammary arteries and saphenous vein grafts. Circulation. 2004;109:2086–91. doi: 10.1161/01.CIR.0000127570.20508.5C. [DOI] [PubMed] [Google Scholar]
- 5.Buxton BF, Raman JS, Ruengsakulrach P, et al. Radial artery patency and clinical outcomes: five-year interim results of a randomized trial. J Thorac Card Surg. 2003;125:1363–71. doi: 10.1016/s0022-5223(02)73241-8. [DOI] [PubMed] [Google Scholar]
- 6.Royse AG, Royse CF, Shah P, Williams A, Kaushik S, Tatoulis J. Radial artery harvest technique, use and functional outcome. Eur J Card Surg. 1999;15:186–93. doi: 10.1016/s1010-7940(98)00311-x. [DOI] [PubMed] [Google Scholar]
- 7.Shapira OM, 2, Eskenazi BR, Hunter CT, et al. Endoscopic versus conventional radial artery harvest-is smaller better? J Card Surg. 2006;21:329–35. doi: 10.1111/j.1540-8191.2006.00266.x. [DOI] [PubMed] [Google Scholar]
- 8.Griffith GL, Allen KB, Waller BF, et al. Endoscopic and traditional saphenous vein harvest: a histologic comparison. Ann Thorac Surg. 2000;69:520–3. doi: 10.1016/s0003-4975(99)01364-8. [DOI] [PubMed] [Google Scholar]
- 9.Shapira OM, Eskenazi BR, Anter E, et al. Endoscopic versus conventional radial artery harvest for coronary artery bypass grafting: functional and histologic assessment of the conduit. J Thorac Card Surg. 2006;131:388–94. doi: 10.1016/j.jtcvs.2005.07.036. [DOI] [PubMed] [Google Scholar]
- 10.Burris NS, Schwartz K, Tang CM, et al. Catheter-based infrared light scanner as a tool to assess conduit quality in coronary artery bypass surgery. J Thorac Cardiovasc Surg. 2007;133:419–27. doi: 10.1016/j.jtcvs.2006.09.056. [DOI] [PubMed] [Google Scholar]
- 11.Barbeau GR, Arsenault F, Dugas L, Simard S, Lariviere MM. Evaluation of the ulnopalmar arterial arches with pulse oximetry and plethysmography: comparison with the Allen's test in 1010 patients. Am Heart J. 2004;147:489–93. doi: 10.1016/j.ahj.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 12.He GW, Rosenfeldt FL, Angus JA. Pharmacological relaxation of the saphenous vein during harvesting for coronary artery bypass grafting. Ann Thorac Surg. 1993;55:1210–7. doi: 10.1016/0003-4975(93)90036-h. [DOI] [PubMed] [Google Scholar]
- 13.Brown EN, Burris NS, Gu J, et al. Thinking inside the graft: applications of optical coherence tomography in coronary artery bypass grafting. J Biomed Opt. 2007;12:051704. doi: 10.1117/1.2799521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kume T, Akasaka T, Kawamoto T, et al. Assessment of coronary intima-media thickness by optical coherence tomography: comparison with intravascular ultrasound. Circ J. 2005;69:903–7. doi: 10.1253/circj.69.903. [DOI] [PubMed] [Google Scholar]
- 15.Poston RS, Gu J, Brown JM, et al. Endothelial injury and acquired aspirin resistance as promoters of regional thrombin formation and early vein graft failure after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2006;131:122–30. doi: 10.1016/j.jtcvs.2005.08.058. [DOI] [PubMed] [Google Scholar]
- 16.Stooker W, Niessen HW, Wildevuur WR, et al. Perivenous application of fibrin glue reduces early injury to the human saphenous vein graft wall in an ex vivo model. Eur J Cardiothorac Surg. 2002;21:212–7. doi: 10.1016/s1010-7940(01)01121-6. [DOI] [PubMed] [Google Scholar]
- 17.Bolotin G, Kypson A, Nifong LW, Chitwood R. A technique for evaluating competitive flow for intraoperative decision making in coronary artery surgery. Ann Thorac Surg. 2003;76:2118–120. doi: 10.1016/s0003-4975(03)00652-0. [DOI] [PubMed] [Google Scholar]
- 18.Frazier AA, Qureshi F, Read KM, Gilkeson RC, Poston RS, White CS. Coronary artery bypass grafts: assessment with multidetector CT in the early and late postoperative settings. Radiographics. 2005;25:881–96. doi: 10.1148/rg.254045151. [DOI] [PubMed] [Google Scholar]
- 19.Cheng D, Allen K, Cohn W, et al. Endoscopic vascular harvest in coronary artery bypass grafting surgery: a meta-analysis of randomized trials and controlled trials. Innovat Technol Tech Cardiothorac Vasc Surg. 2005;1:61–74. doi: 10.1097/01.gim.0000196316.48694.41. [DOI] [PubMed] [Google Scholar]
- 20.Gaudino M, Tondi P, Serricchio M, et al. Atherosclerotic involvement of the radial artery in patients with coronary artery disease and its relation with midterm radial artery graft patency and endothelial function. J Thorac Cardiovasc Surg. 2003;126:1968–71. doi: 10.1016/s0022-5223(03)01226-1. [DOI] [PubMed] [Google Scholar]
- 21.Meissner OA, Rieber J, Babaryka G, et al. Intravascular optical coherence tomography: comparison with histopathology in atherosclerotic peripheral artery specimens. J Vasc Interv Radiol. 2006;17:343–9. doi: 10.1097/01.RVI.0000195324.52104.00. [DOI] [PubMed] [Google Scholar]
- 22.Posacioglu H, Atay Y, Bulent C, Saribulbul O, Hamulu A. Easy harvest of radial artery with ultrasonically activated scalpel. Ann Thorac Surg. 1998;65:984–7. doi: 10.1016/s0003-4975(98)00059-9. [DOI] [PubMed] [Google Scholar]