Abstract
Smurf2 is an E3 ubiquitin ligase that targets TGF-β receptor activated Smad2 and Smad3 for the proteasome in primary articular chondrocytes, thus stimulating their hypertrophic differentiation. Comparatively, how Smurf2 functions in growth plate chondrocytes in a developing long bone is an open question. In this study, we measured the mRNA levels of endogenous Smurf2 and type X collagen in chick growth plate at different embryonic stages to monitor the correlation between the level of Smurf2 expression and chondrocyte maturational stage. We found that high levels of Smurf2 were associated with the differentiative and proliferative stages, while Smurf2 levels were thereafter decreased as the chondrocytes matured toward hypertrophy. In addition, we injected Smurf2-RCAS into chick wing buds at HH stage 20–23 and examined how the ectopic overexpression of Smurf2 in condensing chondrogenic mesenchymeaffects the subsequent process of chondrocyte maturation and ossification during embryonic development. Histological analysis showed that overexpression of Smurf2 in a developing wing bud accelerated chondrocyte maturation and endochondral ossification, which may result from a decrease in TGF-β signaling in the infected chondrocytes with Smurf2-RCAS.
Keywords: Smurf2, TGF-β signaling, chondrocyte, embryonic development, endochondral ossification
INTRODUCTION
The process of endochondral ossification consists of multiple stages. The first stage is the condensation of mesenchymal cells, which subsequently differentiate into chondrocytes and produce cartilage matrix proteins including type 2 collagen (Col2a1) and Aggrecan, and form cartilage rudiments. In the second stage, chondrocytes undergo proliferation and maturation into hypertrophy, and express hypertrophic chondrocyte specific marker, type X collagen (ColX). Finally, calcification of matrix and invasion of blood vessels takes place, hypertrophic chondrocytes undergo apoptosis, and cartilage is removed and replaced by bone.
During the long bone lengthening through endochondral ossification, chondrocyte proliferation and maturation are tightly regulated by several growth factors such as Indian hedgehog (Ihh), parathyroid hormone-related protein (PTHrP), and members of the TGF-β superfamily. TGF-β exists in three isoforms, TGF-β1, -β2, and -β3, and has diverse functions in the regulation of chondrocyte differentiation and maturation. TGF-β induces chondrogenesis and Col2a1 expression in limb bud cartilage and mesenchymal stem cell culture.1–3 On the other hand, TGF-β is a potent inhibitor of chondrocyte maturation, evidenced in part by accelerated maturational progression when TGF-β signaling is reduced.4,5 Ihh is expressed by prehypertrophic chondrocytes and coordinates with PTHrP expressed by periarticular chondrocytes to form a negative feedback loop regulating the growth and differentiation of chondrocytes.6 TGF-β2, expressed in the perichondrium, mediates the effect of Ihh on the expression or acts upstream of PTHrP in this feedback loop.7 In addition, PTHrP may also act downstream of TGF-β, mediating its inhibitory effect on chondrocyte hypertrophic differentiation.8
TGF-β signaling is regulated at various levels. The expression level of TGF-β in the chondrocytes of the growth plate does not always correlate with the levels of its biologically active polypeptide in the matrix environment due to post-transcriptional mechanisms including regulation and activation of TGF-β latent forms by vitamin D3 and matrix metalloproteinases (MMPs).9–11 In addition, TGF-β intracellular signaling is modulated by the expression levels of TGF-β receptors (type II and type I)and TGF-β receptor regulated Smads (Smad2 and Smad3). Furthermore, the TGF-β signaling cascade within the cells is regulated by intracellular signals such as inhibitory Smads and Smurfs. For example, Smad7 exerts an inhibitory function through binding to the TGF-β type I receptor and prevention of recruitment and phosphorylation of Smad3.12 Smad7 also acts as an adaptor to promote Smurf2-mediated ubiquitination and degradation of the TGF-β type I receptor.13 Although Smurf2 ubiquitinates both BMP and TGF-β activated Smads for degradation in mammalian cells,14,15 we found that Smurf2 specifically targets TGF-β receptor activated Smads for ubiquitination and degradation in primary articular chondrocytes, and leads to advanced cell maturation due to loss of TGF-β signaling.16 However, how Smurf2 affects chondrocyte maturation during embryonic development is totally unknown. In this study, we monitored the expression levels of endogenous Smurf2 in chondrocytes during embryonic development and overexpressed Smurf2 in a developing chick wing bud, and found: (1) the expression levels of Smurf2 are associated with chondrocyte stage during embryonic development, and (2) ectopic overexpression of Smurf2 in chick wing buds accelerated cartilagematuration and endochondral ossification.
METHODS
Chondrocyte Isolation and Cell Culture
Chondrocytes were isolated from chick tibia growth plates and sterna at Hamburger-Hamilton (HH) stage 43 (17th day of incubation) as described previously.17–20 Briefly, tibiotarsi were dissected. The bone shell and the tarsus region were separated from the cartilaginous growth plate and discarded. Proliferating chondrocytes were isolated from the proliferative zone of the growth plate and caudal and cephalic chondrocytes were isolated from the caudal part and cephalic part of the sterna, respectively. Cells were cultured with Ham’s F-12 medium (GIBCO) containing 10% FBS and 50 U penicillin/streptomycin.
Real-Time RT-PCR
Total RNA was isolated from tibial growth plates, sterna, and from the cultured cells with the RNeasy mini kit (Qiagen). Real-time RT-PCR was performed as previously described.21 Data from each sample were normalized to GAPDH expression. The primers used for real-time RTPCR were: (1) GAPDH: 5′-ACCACAGTCCAT GCCA TCAC-3′ (forward) and 5′-TCCACCACCCTGTTGCT GTA-3′ (reverse)22; (2) ColX: 5′-ATTGCCAG GGATGAAGGGACATAG-3′ (forward) and 5′-AGG TATTCCTGAAGGTCCTCTTGG-3′ (reverse); and (3) Smurf2: 5′-AAAGCAGGGAGCTGGTTTTCTG G-3′ (forward) and 5′-GACTGC CCACATGTGGCACCGTT-3′ (reverse).
Infection of Chondrocytes and Immunocytochemistry
A Smurf2-expressing construct (Smurf2-RCAS [replication competent avian sarcoma retrovirus]) was generated by cloning a flag-tagged human Smurf2 cDNA, supplied by Dr. Jeffrey Wrana, into an RCAS via ClaI enzyme sites. The method used for transfection of chick fibroblasts with retrovirus and harvest of retrovirus-containing medium was described previously.23,24 Sternal chondrocytes were then infected with a thin layer of medium containing Smurf2-RCAS, and the expression of Smurf2 in the infected cells was examined by immunocytochemistry as described previously.20 Briefly, chondrocytes were incubated with the first monoclonal antibodies antiflag (M2; Sigma) overnight at 4°C. The secondary antibody used was rhodamine-conjugated donkey antimouse IgG (H + L) (Jackson ImmunoResearch Laboratories). Slides were mounted in 95% glycerol in PBS.
Luciferase Assay
Sternal chondrocytes were infected with Smurf2-RCAS and transfected with the TGF-β -responsive promoter construct p3TP-lux (contains multiple TGF-β responsive elements) using Superfect (Qiagen). The SV40 renilla-luc plasmid was cotransfected to serve as an internal control as previously described.25 Three hours after transfection, cultures were treated with 5 ng/mL TGF-β1 (Bachem). Forty hours later, cells were lysed and extracts were prepared using the Dual Luciferase Assay System (Promega) as directed by the manufacturer.
Morphological Analysis of Wing Buds
An established method using infected chick embryo fibroblasts with RCAS virus was used to infect the developing wing buds.24,26 Briefly, fibroblasts were infected with Smurf2-RCAS-containing medium. Cells were passed three times, and then pelleted, stained with 0.01% nile blue sulfate, and approximately 1,000 cells (0.1 µL) were injected into the right wing buds at stage 20–23.24,27 Wing buds were harvested at stage 30–34, and stainedwith Alcian blue (stain cartilage in blue) and alizarin red (stain mineralized matrix in red). More than 50 wing buds were injected with Smurf2-RCAS, and 40 wing buds were injected with RCAS. Approximately 30 wing buds infected with Smurf2-RCAS and 25 wing buds infected with RCAS were processed for morphological analysis.
RESULTS
Smurf2 Is Differentially Regulated during Chondrocyte Differentiation and Proliferation in Developing Embryos
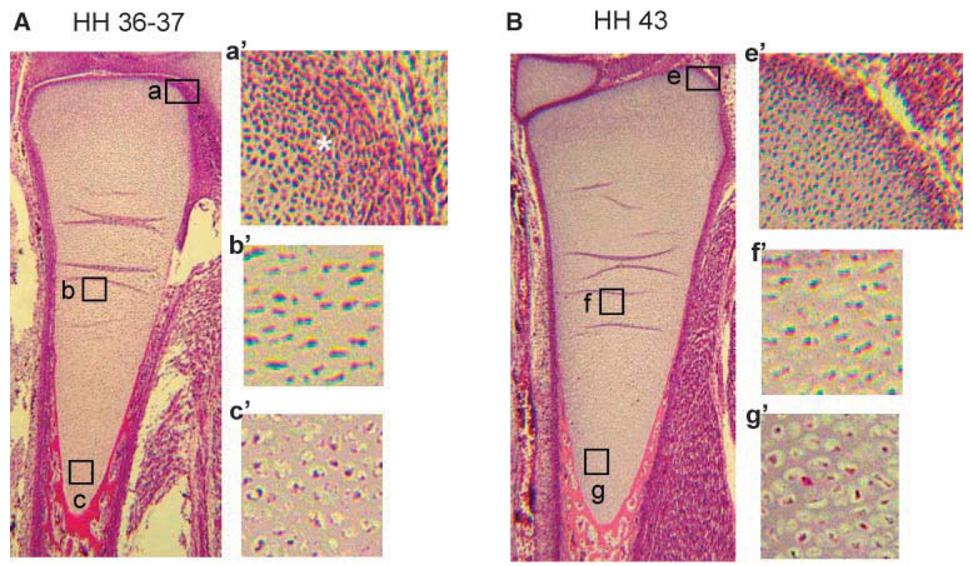
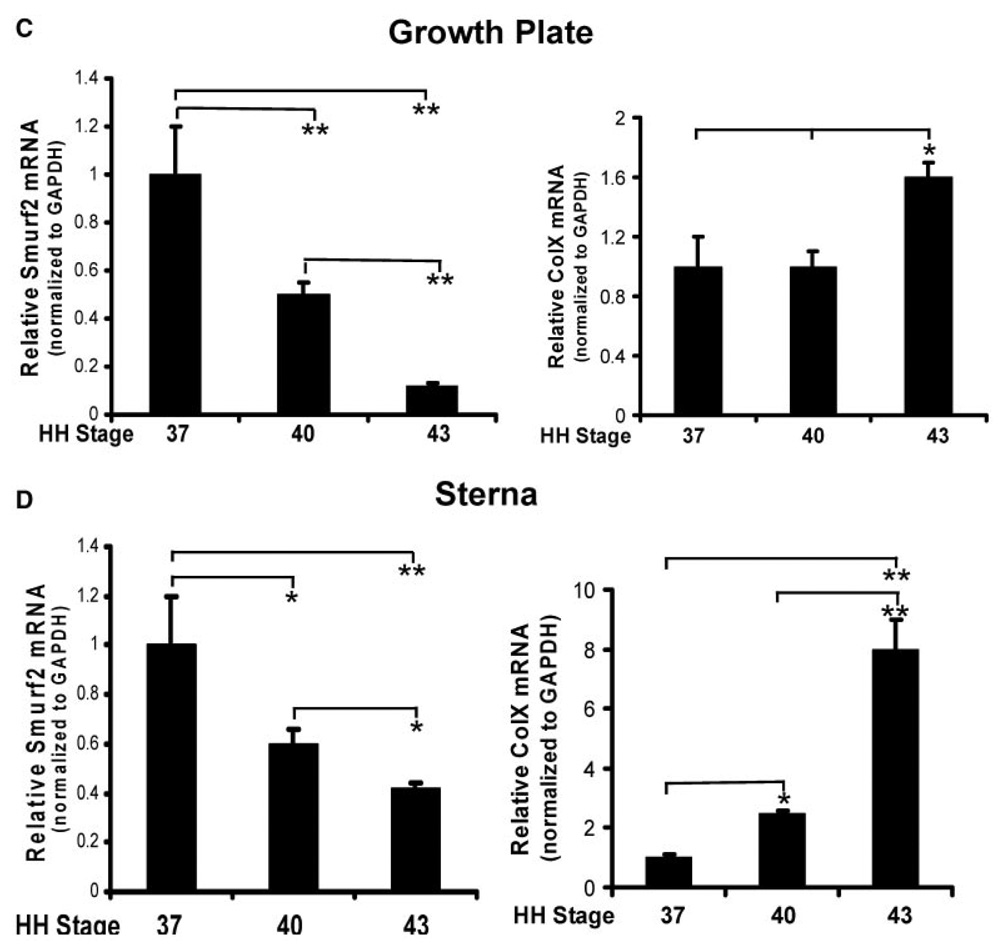
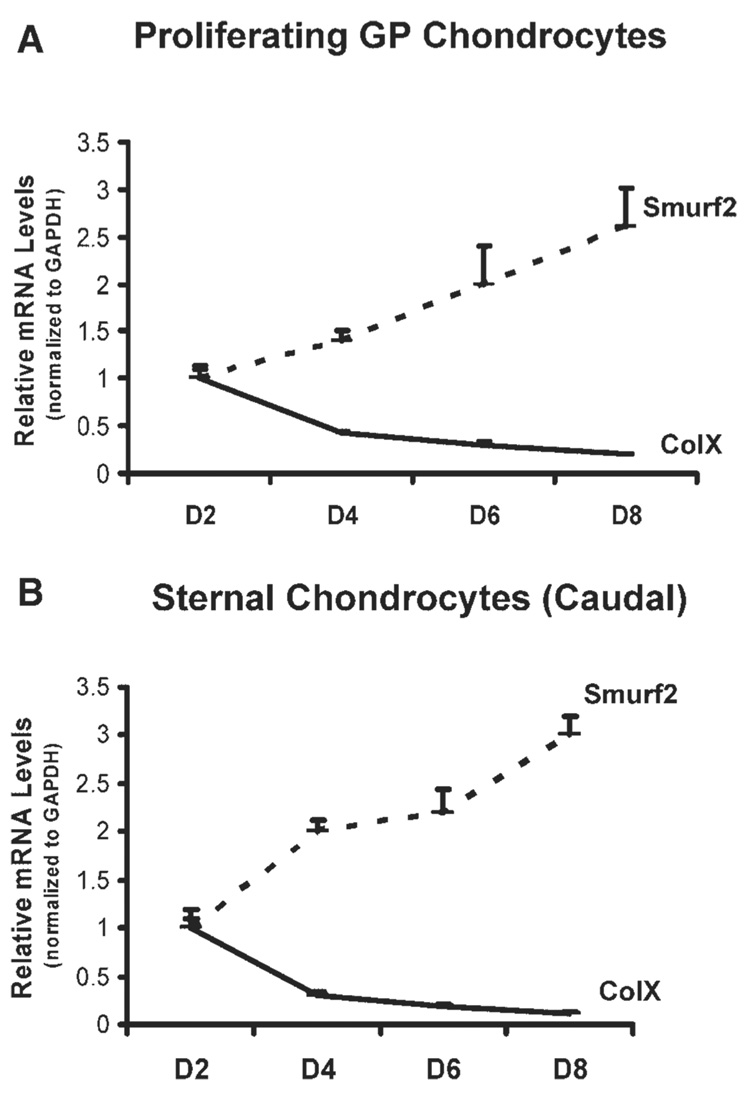
Our previous study has shown that overexpression of Smurf2 in primary chondrocytes stimulated chondrocyte maturation by inhibition of TGF-β signaling.16 To understand whether the expression levels of endogenous Smurf2 are associated with chondrocyte maturational stage in vivo, we examined the mRNA levels of Smurf2 and ColX in growth plate and sterna during embryonic development. Embryonic tibial growth plate and sternae were isolated from chick embryos at 11.5, 15.5, and 17.5 days, representing HH stages 37, 40, and 43, respectively.18 Hematoxylin and eosin staining of limb buds at stages 37 and 43 revealed several remarkable differences between them (Fig. 1A,B). Firstly, the end region of the growth plate of stage 37 limbs was condensed mesen-chyme in which the cells were undergoing chondrocyte differentiation (Fig. 1A, asterisk in a′), while in a similar region at stage 43, the condensed tissue was only in the margin of the growth plate (Fig. 1B, e′). This suggests that more differentiating chondrocytes were in the growth plate of stage 37 than stage 43 embryos. Second, the differentiated chondrocytes in the growth plate of stage 37 embryos were proliferating (Fig. 1A, b and b′), and only a small number of cells were maturing into hypertrophic chondrocytes (Fig. 1A, c and c′). However, in the growth plate of stage 43, the majority of chondrocytes were proliferating and maturing into hypertrophic chondrocytes (Fig. 1B, f, f′, g, g′). This suggests that more differentiating and proliferating chondrocytes were in the growth plate of stage 37 than stage 43 embryos and more maturing chondrocytes were in the growth plate of stage 43 than stage 37 embryos. Finally, chondrocytes in the hypertrophic zone of the growth plate at stage 43 were round and enlarged (hypertrophic), while chondrocytes in similar regions at stage 37 were maturing toward the hypertrophic stage (Fig. 1A,B, compare g′ to c′). Consistent with the histological analysis of the growth plate at stages 37 and 43, the transcription levels of ColX were relatively lower in the growth plate at stage 37 than at stage 43 (Fig. 1C, right panel). In contrast to the expression pattern of ColX in the growth plate, the mRNA levels of Smurf2 were much higher in the growth plate at stage 37 than at stage 43 (Fig. 1C, left panel). The expression pattern of ColX and Smurf2 in the embryonic sternae was similar to that the growth plate (Fig. 1D). The gene expression data, along with the histological evidence, suggest that high levels of Smurf2 are associated with chondrocyte differentiation and proliferation during embryonic development. In addition, the expression pattern of ColX and Smurf2 in the primary chondrocyte cultures was similar to that in the embryonic chondrocytes. Specifically, chondrocytes, released from the caudal portion of the sternae and from the proliferative zone of growth plate, reversed to an earlier differentiation stage (retro-differentiation) for reinitiation of chondrocyte proliferation and reestablishment of a matrix environment at the first week of culture.11,19 While the chondrocytes were undergoing differentiation and proliferation, the mRNA levels of ColX were decreased, however Smurf2 was increased (Fig. 2), suggesting that Smurf2 is required for chondrocyte proliferation and differentiation in vitro.
Figure 1. Up-regulation of Smurf2 in chondrocytes at early stage of embryonic development.
(A,B) Morphology of ulna growth plate at HH stage 36–37 (A) and stage 43 (B) stained with hematoxylin and eosin (H&E). a′, b′, and c′ are the enlarged regions of a, b, and c in (A). e′, f′, and g′ are the enlarged regions of e, f, and g in (B). (C,D) Relative expression levels of Smurf2 and ColX in growth plate (C) and sterna (D). Total RNA was extracted from the growth plate and sternum at HH stages 37, 40, and 43. The mRNA levels were determined by real time RT-PCR. Error bars represent the standard deviation of the mean of the results (n = 4). *p < 0.05, **p < 0.01.
Figure 2.
(A,B) Relative expression levels of Smurf2 and ColX in growth plate (GP) chondrocytes (A) and sternal chondrocytes (B). Growth plate chondrocytes and sternal chondrocytes were cultured at 37°C for 2, 4, 6, and 8 days. Total RNA was extracted from the cells and the mRNA levels of Smurf2 and ColX were determined by real time RT-PCR. Error bars represent the standard deviation of the mean of the results (n = 3).
TGF-β Activates Smurf2 Transcription in Chondrocytes
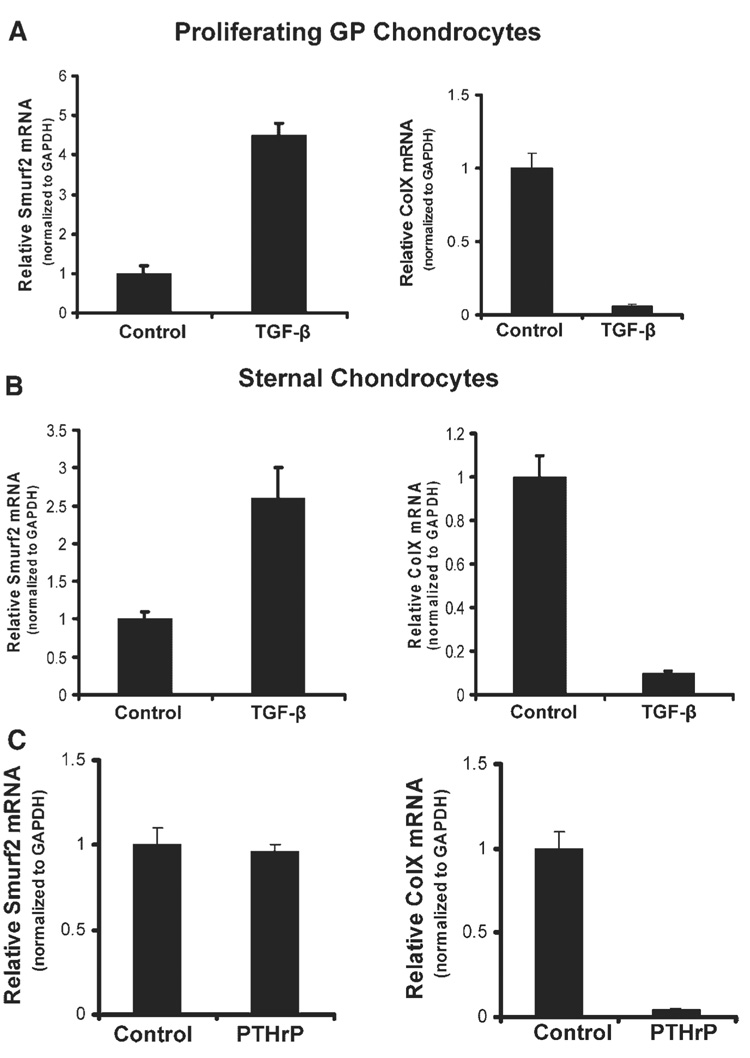
As mentioned, TGF-β signaling promotes chondrocyte differentiation and proliferation during chondrogenesis, and inhibits chondrocyte maturation.1,2,28,29 The up-regulation of Smurf2 gene expression in the differentiating and proliferating chondrocytes occurred coincidentally with the repression of the ColX gene. Therefore, TGF-β signaling could have been responsible for the upregulation of Smurf2 in the chondrocytes. To test this hypothesis, we treated caudal and cephalic chondrocytes from sternae and proliferating chondrocytes from growth plate with TGF-β, and examined the mRNA levels of Smurf2 and ColX. As expected, Smurf2 mRNA levels were dramatically up-regulated in the TGF-β-treated cells (Fig. 3A,B, left panel) in which ColX gene expression was repressed coincidentally (Fig. 3A,B, right panel). These data suggest that the activation of Smurf2 and repression of ColX by TGF-β occurred at the same time in the primary chondrocytes. Previous studies showed that TGF-β inhibition of chondrocyte maturation might be through PTHrP.7,8 Therefore, we examined whether the effect of TGF-β on Smurf2 expression was PTHrP-dependent. PTHrP dramatically inhibited ColX gene expression in chondrocytes (Fig. 3C, right panel); however, no significant difference in the mRNA levels of Smurf2 could be detected between the cells treated with and without PTHrP (Fig. 3C, left panel), indicating that TGFβ-mediated up-regulation of Smurf2 expression is PTHrP independent.
Figure 3. Stimulation of Smurf2 expression by TGF-β in chondrocytes is PTHrP-independent.
(A,B) TGF-β stimulates Smurf2 and inhibits ColX expression in chondrocytes from growth plates (A) and sterna (B). (C) PTHrP inhibits ColX but not Smurf2 expression in growth plate chondrocytes. Chondrocytes were cultured in the presence or absence of PTHrP for 2 days, and the mRNA levels of Smurf2 and ColX were determined by real time RT-PCR. Error bars represent the standard deviation of the mean of the results (n = 4).
Overexpression of Smurf2 Accelerates Chondrocyte Maturation and Endochondral Ossification
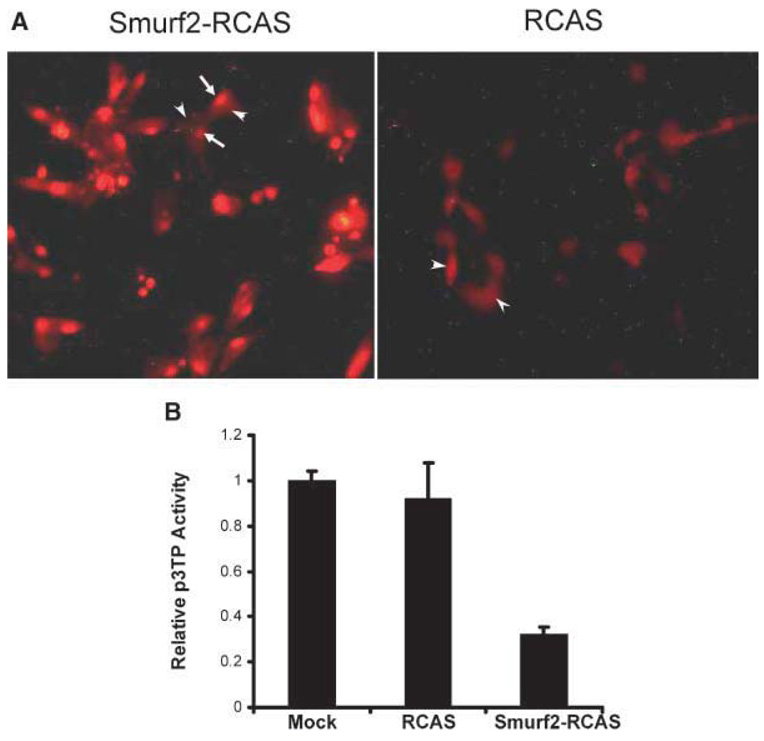
To understand how Smurf2 regulates endochondral ossification during embryonic development, we cloned the human flag-Smurf2 cDNA into RCAS, and injected the Smurf2-RCAS into developing chick wing buds at stage 20–23. Before performing the injection, we first examined the expression and function of Smurf2 in the caudal sternal chondrocytes infected with Smurf2-RCAS. Immunostaining of the infected cells with an antiflag antibody revealed that Smurf2 protein was detected mainly in the nucleus (Fig. 4A, arrows) but not in the cytoplasm (Fig. 4A, arrowheads) of chondrocytes. This signal is specific, because only background was detected in the chondrocytes infected with the empty RCAS (Fig. 4A, RCAS, arrowheads). To examine whether overexpression of Smurf2 in growth plate chondrocytes blocked TGF-β signaling as was seen in articular chondrocytes,16 we performed the p3TPLux assay. We found that the reporter activity in the chondrocytes infected with Smurf2-RCAS was decreased 70% compared to cells infected with the empty RCAS or the mock group (Fig. 4B). These results indicated: (1) growth plate chondrocytes infected with Smurf2-RCAS expressed high levels of Smurf2 protein in the nucleus, and (2) the overexpressed Smurf2 in the chondrocytes blocked TGF-β signaling, consistent with the previous finding in the articular chondrocytes.16
Figure 4. Smurf2 accelerates endochondral ossification.
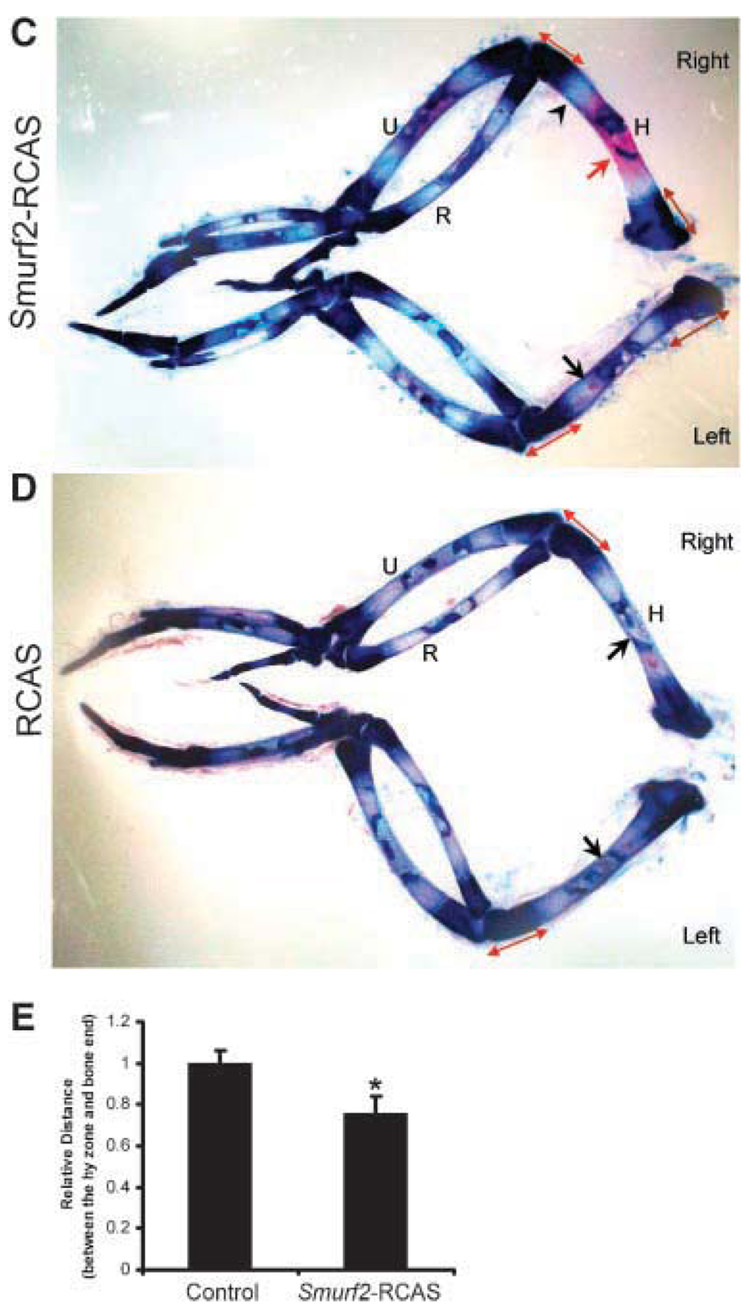
(A) Smurf2 is overexpressed in chondrocytes infected with Smurf2-RCAS. Arrows indicate the accumulation of flag-tagged Smurf2 protein in the nucleus of the chondrocytes stained with a rhodamine-coupled antiflag antibody (left). Arrowheads indicate the background in the chondrocytes infected with Smurf2-RCAS (left), or empty RCAS (right). (B) Overexpression of Smurf2 in chondrocytes inhibits TGF-β signaling. Chondrocytes infected with Smurf2-RCAS (Smurf2-RCAS), RCAS (RCAS), and vehicle without any of the virus (Mock) were transfected with p3TP-Lux. Luciferase activity was determined after 40 h. (C,D) Injection of chick wing buds with Smurf2-RCAS accelerated cartilage maturation and endochondral ossification. Smurf2-RCAS (C) or RCAS(D) was injected into the right wing bud at HH stage 20–23, and the left wing buds were uninfected controls. Wing buds were harvested at HH stage 30–34, and stained with Alcian blue and Alizarin red. Red arrow indicates an expanded ossification domain in the humerus infected with Smurf2-RCAS; black arrows indicate small patches of ossification in the control humerus. Double arrowhead lines indicate the distance between the hypertrophic domain and the articular surface. An arrowhead indicates the hypertrophic zone. H, humerus; R, radius; U, ulna. (E) Quantitative analysis of the distance between the hypertrophic (hy) zone and the ends of long bones. Error bars represent the standard deviation of the mean of the results (n = 4). *p < 0.05.
Finally, we examined the effect of Smurf2 on cartilage maturation and ossification in developing wing buds by Alcian blue and alizarin red staining. Alcian blue and alizarin red staining revealed that overexpression of Smurf2 stimulated chondrocyte maturation and ossification. Specifically, by stage 30–34, extensive mineralization had occurred in the center of infected right humeri with Smurf2-RCAS (Fig. 4C, right, arrow), whereas only a very small patch of mineralization was observed in the uninfected left control limbs (Fig. 4C, left, arrow) or in limbs infected with empty RCAS (Fig. 4D, right, arrow). These data indicated that ectopic over-expression of Smurf2 in developing wing buds stimulated endochondral ossification. In addition, the distance between the hypertrophic domain (Fig. 4C, bright region indicated by an arrowhead) and the ends of humerus was reduced about 18% in size in the limbs infected with Smurf2-RCAS compared to that of uninfected ones and the ones infected with RCAS (Fig. 4C–E), indicating that ectopic overexpression of Smurf2 in developing wing buds accelerates chondrocyte maturation.30 No overall difference could be observed between the limbs infected with RCAS and uninfected limbs (Fig. 4D), suggesting that the morphological changes in the limbs infected with Smurf2-RCAS are not artifactual.
DISCUSSION
Regulation of TGFβ Signaling
TGF-β signaling is regulated at multiple levels. TGF-β molecules are synthesized and secreted as latent forms, and need either vitamin D3 or MMPs to activate the latent forms to become biologically active peptides.9,10,31,32 During embryonic development, the latent form of TGF-β is normally expressed in chondrocytes with abundant expression of Col210; however, the chondrocytes producing vitamin D3 and MMPs, which are used to activate the latent forms, are not exactly the same cells as that for secreting latent TGF-β.10,31,32 It appears that the expression levels of TGF-β are not consistent with the levels of biologically active TGF-β peptides in some chondrocyte populations in certain biological situations.9,10 More importantly, once TGF-β binds to its receptors, the phosphorylated receptor activates not only the Smad pathway but also MAPK pathway.33 Thus, it is a major over-simplification to evaluate TGF-β signaling solely according to the expression levels of TGF-β or Smads. Given the fact that TGF-β signaling stimulates chondrogenesis and inhibits chondrocyte maturation, and although we do not have direct evidence showing that up-regulation of Smurf2 in the early stage of embryonic chondrocytes is due to relatively higher levels of TGF-β signaling, the up-regulation of Smurf2 in these immature chondrocytes occurred coincidentally with the inhibition of ColX gene expression. This suggests that the expression levels of Smurf2 in chondrocytes are correlated with TGF-β signaling. This hypothesis is further confirmed by a dramatic stimulation of Smurf2 expression in chondrocytes treated with TGF-β. Although one study suggested that TGF-β-mediated up-regulation of Smurf2 expression in human sarcoma cells was through a Smad-independent PI3 kinase pathway,34 we continue to study the molecular mechanisms underlying regulation of Smurf2 expression by TGF-β in chondrocytes. Based on the previous study that said that Smurf2 specifically targets TGF-β receptor activated Smads in chondrocytes and inhibits TGF-β signaling, the up-regulation of Smurf2 by TGF-β in the embryonic chondrocytes may represent an auto-inhibitory feed back mechanism for TGF-β signaling in vivo.
Smurf2 Accelerates Chondrocyte Maturation and Ossification
During embryonic development, Smurf2 expression levels are high in the immature chondrocytes and associated with chondrocyte differentiation and proliferation; however, Smurf2 levels are down-regulated as chondrocytes mature. To understand how Smurf2 regulates chondrocyte maturation, we infected developing wing buds with Smurf2-RCAS at HH stage 20–23, and examined the morphological changes after 1 week incubation. Because Smurf2-RCAS is propagated in avian proliferating cells, the proliferating chondrocytes and subsequent maturing chondrocytes in the infected wing buds theoretically have relatively higher levels of Smurf2 than chondrocytes from other zones. This pattern of Smurf2 overexpression in the infected wing buds with Smurf2-RCAS allowed us to study the pathological effect of Smurf2 on the fate of proliferating and maturing chondrocytes. Alcian blue and alizarin red staining showed that chondrocyte maturation and ossification are advanced in the wing buds infected with Smurf2-RCAS, which may be due to loss of TGF-β signaling in the infected cells. The inhibitory effect of TGF-β signaling on chondrocyte maturation is essential for proper bone development.5,7,8 Thus, the Smurf2-TGF-β feedback loop may be critical to ensure fine control of TGF-β signaling output, so that the rate of chondrocyte maturation is carefully regulated during embryonic endochondral development.
ACKNOWLEDGMENTS
This research was supported by NIH RO1AR045700 (R. N. Rosier), NIH/IAMS, P50 AR054041 (R. N. Rosier), and the Aircast Foundation (Q. Wu). The authors thank Dr. Cristin M. Ferguson for her technical support.
Footnotes
Published online in Wiley InterScience (www.interscience.wiley.com).
REFERENCES
- 1.Kulyk WM, Rodgers BJ, Greer K, et al. Promotion of embryonic chick limb cartilage differentiation by transforming growth factor-beta. Dev Biol. 1989;135:424–430. doi: 10.1016/0012-1606(89)90191-7. [DOI] [PubMed] [Google Scholar]
- 2.Sekiya I, Vuoristo JT, Larson BL, et al. In vitro cartilage formation by human adult stem cells from bone marrow stroma defines the sequence of cellular and molecular events during chondrogenesis. Proc Natl Acad Sci USA. 2002;99:4397–4402. doi: 10.1073/pnas.052716199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chimal-Monroy J, Díaz de León L. Differential effects of transforming growth factors beta 1,beta 2, beta 3 and beta 5 on chondrogenesis in mouse limb bud mesenchymal cells. Int J Dev Biol. 1997;41:91–102. [PubMed] [Google Scholar]
- 4.Serra R, Johnson M, Filvaroff EH, et al. Expression of a truncated, kinase-defective TGF-beta type II receptor in mouse skeletal tissue promotes terminal chondrocyte differentiation and osteoarthritis. J Cell Biol. 1997;39:541–552. doi: 10.1083/jcb.139.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang X, Chen L, Xu X, et al. TGF-beta/Smad3 signals repress chondrocyte hypertrophic differentiation and are required for maintaining articular cartilage. J Cell Biol. 2001;153:35–46. doi: 10.1083/jcb.153.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vortkamp A, Lee K, Lanske B, et al. Regulation of rate of cartilage differentiation by Indian hedgehog and PTH-related protein. Science. 1996;273:613–622. doi: 10.1126/science.273.5275.613. [DOI] [PubMed] [Google Scholar]
- 7.Alvarez J, Sohn P, Zeng X, et al. TGFbeta2 mediates the effects of hedgehog on hypertrophic differentiation and PTHrP expression. Development. 2002;129:1913–1924. doi: 10.1242/dev.129.8.1913. [DOI] [PubMed] [Google Scholar]
- 8.Serra R, Karaplis A, Sohn P. Parathyroid hormone-related peptide (PTHrP)-dependent and -independent effects of transforming growth factor beta (TGF-beta) on endochondral bone formation. J Cell Biol. 1999;145:783–794. doi: 10.1083/jcb.145.4.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts AB, Sporn MB. The transforming growth factor βs. In: Sporn MB, Roberts AB, editors. Peptide Growth Factors and their Receptors—Handbook of Experimental Pathology. Heidelberg: Springer-Verlag; 1990. pp. 419–472. [Google Scholar]
- 10.Millan FA, Denhez F, Kondaiah P, et al. Embryonic gene expression patterns of TGF beta 1, beta 2 and beta 3 suggest different developmental functions in vivo. Development. 1991;111:131–143. doi: 10.1242/dev.111.1.131. [DOI] [PubMed] [Google Scholar]
- 11.Pedrozo HA, Schwartz Z, Gomez R, et al. Growth plate chondrocytes store latent transforming growth factor (TGF)-beta 1 in their matrix through latent TGF-beta 1 binding protein-1. J Cell Physiol. 1998;177:343–354. doi: 10.1002/(SICI)1097-4652(199811)177:2<343::AID-JCP16>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi H, Abdollah S, Qiu Y, et al. The MAD-related protein Smad7 associates with the TGFbeta receptor and functions as an antagonist of TGFbeta signaling. Cell. 1997;89:1165–1173. doi: 10.1016/s0092-8674(00)80303-7. [DOI] [PubMed] [Google Scholar]
- 13.Kavsak P, Rasmussen RK, Causing CG, et al. Smad7 binds to Smurf2 to form an E3 ubiquitin ligase that targets the TGF beta receptor for degradation. Mol Cell. 2000;6:1365–1375. doi: 10.1016/s1097-2765(00)00134-9. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Chang C, Gehling DJ. Regulation of Smad degradation and activity by Smurf2, an E3 ubiquitin ligase. Proc Natl Acad Sci USA. 2001;98:974–979. doi: 10.1073/pnas.98.3.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin X, Liang M, Feng XH. Smurf2 is a ubiquitin E3 ligase mediating proteasome-dependent degradation of Smad2 in transforming growth factor-beta signaling. J Biol Chem. 2000;275:36818–36822. doi: 10.1074/jbc.C000580200. [DOI] [PubMed] [Google Scholar]
- 16.Zuscik MJ, Baden JF, Wu Q, et al. 5-azacytidine alters TGF-beta and BMP signaling and induces maturation in articular chondrocytes. J Cell Biochem. 2004;92:316–331. doi: 10.1002/jcb.20050. [DOI] [PubMed] [Google Scholar]
- 17.Schmid TM, Conrad HE. Metabolism of low molecular weight collagen by chondrocytes obtained from histologically distinct zones of the chick embryo tibiotarsus. J Biol Chem. 1982;257:12451–12457. [PubMed] [Google Scholar]
- 18.Sanes JR. On the republication of the Hamburger-Hamilton stage series. Dev Dyn. 1992;195:229–230. doi: 10.1002/aja.1001950403. [DOI] [PubMed] [Google Scholar]
- 19.Chen Q, Johnson DM, Haudenschild DR, et al. Progression and recapitulation of the chondrocyte differentiation program: cartilage matrix protein is a marker for cartilage maturation. Dev Biol. 1995;172:293–306. doi: 10.1006/dbio.1995.0024. [DOI] [PubMed] [Google Scholar]
- 20.Wu QQ, Chen Q. Mechanoregulation of chondrocyte proliferation, maturation, and hypertrophy: ion-channel dependent transduction of matrix deformation signals. Exp Cell Res. 2000;256:383–391. doi: 10.1006/excr.2000.4847. [DOI] [PubMed] [Google Scholar]
- 21.Wittwer CT, Herrmann MG, Moss AA, et al. Rasmussen RP Continuous fluorescence monitoring of rapid cycle DNA amplification. Biotechniques. 1997;22:130–134. doi: 10.2144/97221bi01. [DOI] [PubMed] [Google Scholar]
- 22.Wu Q, Zhang Y, Chen Q, et al. Indian hedgehog is an essential component of mechanotransduction complex to stimulate chondrocyte proliferation. J Biol Chem. 2001;276:35290–35296. doi: 10.1074/jbc.M101055200. [DOI] [PubMed] [Google Scholar]
- 23.Zhang D, Schwarz EM, Rosier RN, et al. ALK2 functions as a BMP type I receptor and induces Indian hedgehog in chondrocytes during skeletal development. J Bone Miner Res. 2003;18:1593–1604. doi: 10.1359/jbmr.2003.18.9.1593. [DOI] [PubMed] [Google Scholar]
- 24.Ferguson CM, Schwarz EM, Reynolds PR, et al. Smad2 and 3 mediate transforming growth factor-beta1-induced inhibition of chondrocyte maturation. Endocrinology. 2000;41:4728–4735. doi: 10.1210/endo.141.12.7848. [DOI] [PubMed] [Google Scholar]
- 25.Riddle RD, Johnson RL, Laufer E, et al. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell. 1993;75:1401–1416. doi: 10.1016/0092-8674(93)90626-2. [DOI] [PubMed] [Google Scholar]
- 26.Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryos. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- 27.Crabb ID, O’Keefe RJ, Puzas JE, et al. Synergistic effect of transforming growth factor beta and fibroblast growth factor on DNA synthesis in chick growth plate chondrocytes. J Bone Miner Res. 1990;5:1105–1112. doi: 10.1002/jbmr.5650051103. [DOI] [PubMed] [Google Scholar]
- 28.Leonard CM, Fuld HM, Frenz DA, et al. Role of transforming growth factor-beta in chondrogenic pattern formation in the embryonic limb: stimulation of mesenchymal condensation and fibronectin gene expression by exogenous TGF-beta and evidence for endogenous TGFbeta-like activity. Dev Biol. 1991;145:99–109. doi: 10.1016/0012-1606(91)90216-p. [DOI] [PubMed] [Google Scholar]
- 29.Minina E, Wenzel HM, Kreschel C, et al. BMP and Ihh/PTHrP signaling interact to coordinate chondrocyte proliferation and differentiation. Development. 2001;128:4523–4534. doi: 10.1242/dev.128.22.4523. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz Z, Brooks B, Swain L, et al. Production of 1,25-dihydroxyvitamin D3 and 24,25-dihydroxyvitamin D3 by growth zone and resting zone chondrocytes is dependent on cell maturation and is regulated by hormones and growth factors. Endocrinology. 1992;130:2495–2504. doi: 10.1210/endo.130.5.1572278. [DOI] [PubMed] [Google Scholar]
- 31.Boyan BD, Schwartz Z, Park-Snyder S, et al. Latent transforming growth factor-beta is produced by chondrocytes and activated by extracellular matrix vesicles upon exposure to 1, 25-(OH)2 D3. J Biol Chem. 1994;269:28374–28381. [PubMed] [Google Scholar]
- 32.Heldin CH, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 33.Ohashi N, Yamamoto T, Uchida C, et al. Transcriptional induction of Smurf2 ubiquitin ligase by TGF-beta. FEBS. 2005;579:2557–2563. doi: 10.1016/j.febslet.2005.03.069. [DOI] [PubMed] [Google Scholar]
- 34.Mello MA, Tuan RS. Effects of TGF-beta1 and triiodothyronine on cartilage maturation: in vitro analysis using long-term high-density micromass cultures of chick embryonic limb mesenchymal cells. J Orthop Res. 2006;24:2095–2105. doi: 10.1002/jor.20233. [DOI] [PubMed] [Google Scholar]