Abstract
The hormones insulin and leptin have been demonstrated to act in the central nervous system (CNS) as regulators of energy homeostasis at medial hypothalamic sites. In a previous review, we described new research demonstrating that, in addition to these direct homeostatic actions at the hypothalamus, CNS circuitry that subserves reward and motivation is also a direct and an indirect target for insulin and leptin action. Specifically, insulin and leptin can decrease food reward behaviors and modulate the function of neurotransmitter systems and neural circuitry that mediate food reward, i.e., midbrain dopamine and opioidergic pathways. Here we summarize new behavioral, systems, and cellular evidence in support of this hypothesis and in the context of research into the homeostatic roles of both hormones in the CNS. We discuss some current issues in the field that should provide additional insight into this hypothetical model. The understanding of neuroendocrine modulation of food reward, as well as food reward modulation by diet and obesity, may point to new directions for therapeutic approaches to overeating or eating disorders.
Keywords: motivation, food intake, dopamine
recent data suggest that the peripheral regulators of energy balance, leptin and insulin, may play important roles in the occurrence of behaviors typically classified as nonhomeostatic. For example, central insulin and leptin are sufficient to reduce operant responding for palatable foods and to attenuate food-induced conditioned place preferences (CPPs) independent of their effects to regulate energy balance. Since findings such as these have generated much interest in the potential roles of central leptin and insulin signaling in brain reward pathways, we believe it is instructive to begin with a consideration of such findings in a larger historical context. In fact, the observation that energy balance or deprivation state can significantly influence behavioral responses to obtain food or the reward associated with food is many decades old. In one early theoretical conceptualization, Hull et al. (98, 99) proposed that responding or motivation could be explained by sER = sHr × D × K, where sEr represents the reaction potential or motivation of a behavior, sHr represents the habit strength or number of experiences, D represents drive or hours of deprivation, and K represents the value, reward, or hedonic quality of the food to be obtained. Hull et al. reasoned that the expression of a behavior was a direct function not only of learning and experience but, also, the “need” state and the “value” of the food available. As an example, one might train a rat such that pressing a lever delivers a food pellet. After the rat learns the task, the number of presses on any given trial would be significantly increased by food depriving the rat and/or increasing the incentive and hedonic properties of the pellet (such as would be the case with high-fat high-sugar food pellets). Along these lines, Ferster and Skinner (64) observed that response rates on different schedules of reinforcement in pigeons were significantly increased by restriction of their body weight. In an early series of studies, Ferster and Skinner observed a direct inverse relationship between the birds' body weight and their rates of responding for food pellets.
Food restriction of experimental animals in these and other similar studies was often imposed to increase the occurrence of the behaviors that the experimenters sought to study. In many cases, rats, mice, and other experimental animals are regularly food deprived, so that the behavior, neurobiology, and genetics of learning and memory can be studied using food reward paradigms. In this way, the relationship between energy balance and “reward” or “reinforcement” has been well characterized (31, 34), although for reasons largely unrelated to that which now generates much interest and enthusiasm: the idea that the modern epidemic of obesity may be in part related to reward and hedonic mechanisms and that failure of regulatory systems might be related to dysregulations of reward systems. The historical lesson is that food deprivation or negative energy balance promotes responding for foods and other reinforcements. Coupled with our current knowledge that negative energy balance leads to low levels of circulating leptin and insulin, this leads to the hypothesis that low levels of these hormones might be associated with increased responding to obtain rewards. It also suggests the corollary hypothesis that increased leptin and insulin might be sufficient to attenuate responding for reward. In fact, recent and historical data are consistent with both hypotheses and are summarized below. An intriguing speculation based on the formulation of Hull et al. (98, 99) is that signals that reflect energy balance would be included in the variable of “drive” (D) and, as such, would be predicted to act by altering the gain of other factors determining motivation.
In 2003, we published a review exploring “a new CNS role for adiposity signals” (65). Since then, a number of studies that 1) confirm the actions of insulin and leptin to decrease food reward and the motivation to feed and 2) have begun to explore cellular and central nervous system (CNS) circuitry-related mechanisms have been carried out. Here, we provide an update of the field and critical discussion of newly developing questions. An early and continued research focus has been on the actions of these two hormones at the medial hypothalamus, which historically has been identified as a major player in the CNS regulation of metabolism, energy balance, and caloric intake in terms of physiological need. Because the behavioral, cellular, and molecular actions of insulin and leptin at the medial hypothalamus have been well studied, this material is summarized briefly. We refer the reader to recent reviews, including our 2003 review, which provide more detailed discussion and historical references on this topic (1, 11–13, 65, 96, 167, 194), which has provided the groundwork for studies assessing food reward regulation.
INSULIN AND LEPTIN: ADIPOSITY SIGNALING IN THE CNS
In 1979, it was demonstrated in nonhuman primates that insulin infused into the CNS caused a significant decline in the animals' food intake and body weight (193). This observation was made in the context of a contemporary model: that circulating humoral factors could regulate individual meal size, as well as food intake and body weight over a longer time course (47, 124, 141, 177). Porte and Woods (153) proposed that insulin served as an “adiposity signal” and completes a negative-feedback loop that links the behavior of feeding with the size of adipose stores. Many studies over the intervening decades have essentially validated this basic concept (2–4, 25, 38, 125). In the mid-1990s, the candidate adiposity signal and adipose hormone leptin was identified (197) and has been well characterized as a regulator of energy homeostasis.
As reviewed earlier (65), two critical issues needed to be addressed to argue for a role for adiposity signals in modulating any aspect of CNS function. The first issue is the need for circulating signals to have access to CNS circuitry. The presence of insulin in the CNS was reported in 1979 (88), and many studies established that the predominant amount of insulin in the CNS can be accounted for by receptor-mediated transport into the CNS (46, 52, 53, 79, 106, 115, 164, 165). Although intermittent reports have suggested that insulin can be synthesized locally in the developed CNS, quantities appear to be negligible, particularly compared with the affinity of the receptor for insulin. The relationship between CNS and plasma levels of insulin is saturable (nonlinear), consistent with a receptor-mediated transport process. In the 1990s, the adipose hormone leptin was identified, and knowledge was rapidly acquired that it (similarly) could be transported by multiple mechanisms into the CNS (7, 10, 92, 109, 121, 123, 137). Relative levels of leptin and insulin in the cerebrospinal fluid are decreased in association with obesity (8, 9, 30, 108, 162, 163, 178). The functional implication is that, in circumstances of chronic hyperinsulinemia and hyperleptinemia, such as obesity, less adiposity signaling would be available to the CNS.
The second basic issue relates to the presence of insulin and leptin receptors in the CNS. Receptors for insulin (48, 87, 113, 181, 187, 195) and leptin (60, 119) are widely expressed throughout the CNS. Extensive research has established that the medial hypothalamus, a key center for the regulation of energy homeostasis and coordination of metabolic events, is a major target for insulin and leptin action (12, 127, 142, 167). Other CNS sites and neural systems are targets for insulin and leptin action (73, 81, 86, 126). Studies utilizing antisense oligonucleotides against the insulin receptor or conditional localized knockout of the insulin receptor, aided our understanding of the have to contribution of the brain insulin receptor to energy and glucose homeostasis (27, 116, 145, 146). The leptin receptor, similarly extensively expressed, is present as different splice-variant isoforms in the CNS, with the “signaling” form OBRb having the major role in leptin action. The obese db/db mouse and Zucker fa/fa rat represent naturally occurring “knockouts” (41) of the leptin receptor, and recent use of receptor constructs with modifications in signaling capability validates the importance of CNS leptin action in energy homeostasis.
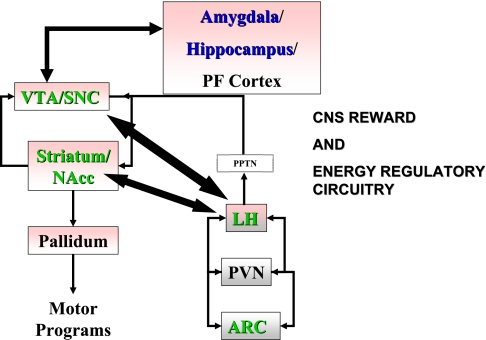
Leptin and insulin have multiple effects on energy homeostasis that depend on the activation of key hypothalamic nuclei and peptides to regulate energy balance (103). Among the most extensively studied mediators are neuropeptide Y (42, 43, 166, 173, 188), proopiomelanocortin (POMC) and its product α-melanocyte-stimulating hormone, and the melanocortin antagonist agouti-related peptide (AgRP) (for reviews see Refs. 14, 135, 168, and 194). POMC and AgRP are selectively expressed in neurons of the arcuate nucleus (ARC) colocalized with receptors for insulin and leptin, and they represent endogenous circuitry capable of regulating food intake (40, 128, 136). Genetic deletion of the critical melanocortin-4 receptor recapitulates the obese phenotype of leptin-deficient mice, including obesity (100), and selective ablation of the AgRP neurons in adult mice causes severe starvation and death (82). Leptin and insulin increase expression of the agonist α-melanocyte-stimulating hormone and decrease expression of AgRP (for review see Ref. 14). Collectively, these data suggest that leptin and insulin act on ARC melanocortin (AgRP and POMC) neurons to regulate food intake and energy balance (Fig. 1).
Fig. 1.
Energy regulatory (gray) and reward circuitry (rose) and major synaptic connections. Green labels highlight targets of insulin and leptin; blue labels highlight targets of insulin (not determined for leptin). CNS, central nervous system; PPTN, pedunculopontine tegmental nucleus; PVN, paraventricular nucleus; PF, prefrontal; VTA, ventral tegmental area; SNC substantia nigra pars compacta; NAcc, nucleus accumbens; LH, lateral hypothalamus; ARC, arcuate nucleus.
Recent work has also elucidated several other key players in the regulation of food intake and body weight that are likely to mediate (directly or indirectly) the effects of leptin and insulin. Among these, orexin-A and melanin-concentrating hormone are expressed in the lateral hypothalamus (LH) (26, 131, 151). Both are orexigenic: in mice lacking either peptide or its key receptors, metabolic rates and ingestive behavior are altered. Intriguingly, recent data suggest that orexin-A may be an important factor in the effects of drugs of abuse. Orexin antagonists blunt the behavioral response to cocaine and other psychostimulants and may be important for the rewarding effects of food as well (44; for reviews see Refs. 24 and 91).
Much has been learned about intracellular signaling by insulin and leptin in the CNS from studies in the medial hypothalamus. Initial studies validated that signaling for the CNS insulin receptor is comparable to postreceptor mechanisms in peripheral target tissues. That is, the receptor is an autophosphorylating tyrosine kinase, and its activation leads not only to tyrosine phosphorylation of other proteins, including the key signaling moiety insulin receptor substrate, but also to a cascade of additional phosphorylation events, including activation of the phosphatidylinositol 3 (PI3)-kinase pathway (83) and phosphorylation of Akt/PKB (89, 90, 112, 143, 171). The leptin receptor, upon leptin binding, can similarly initiate insulin receptor substrate phosphorylation and activation of the PI3-kinase pathway (144). However, the receptor does not have intrinsic tyrosine kinase activity; thus JAK-STAT signaling is a critical initial event, leading to transcriptional events and, ultimately, to the generation of suppressor of cytokine signaling-3, which provides negative feedback on leptin signaling (119, 157). Recently, activation of the mammalian target of rapamycin pathway in medial hypothalamic neurons has been reported, and early studies suggest that activation of this pathway is correlated with anorexigenic and metabolic homeostasis events (49). Collectively, these studies support the concept that insulin and leptin can mediate intracellular events related to ingestive behavior and caloric homeostasis via parallel and unique intracellular pathways (36, 37, 152). They provide correlative or direct evidence of the importance of these signals in mediating in vivo events. A current research emphasis is on differential effects of insulin and leptin, in terms of time course of action, intracellular mechanisms, and effects on glucose homeostasis vs. energy balance, within the hypothalamus. This detailed physiological information will contribute to insights into pathophysiology. For example, in metabolic circumstances in which plasma insulin or leptin levels are low (starvation and reduced adiposity), signaling would be decreased and drive for food intake would be increased. Obesity (excessive adiposity) would represent a pathophysiological state in which adiposity signals are decreased in relative or absolute amount in the CNS or there is direct CNS resistance to their action (35, 45, 54, 104, 138, 184). In summary, 30 years of research have confirmed the overall hypothesis that hormones that reflect the size of adipose and calorie stores can act directly within the CNS to provide negative feedback on food intake and modulate energy utilization. As discussed below, this research has paved the way for studies of insulin and leptin signaling within reward circuitry.
INSULIN, LEPTIN, AND FOOD REWARD
A current extension of this research focuses on how environmental factors such as diet composition can interact with the adiposity signal-CNS feedback loop to modulate the effectiveness of adiposity signals. In 1988, Arase and colleagues (6) observed that a high-fat diet resulted in an impairment in the action of insulin, given directly into the CNS, to decrease body weight in rats. This finding was subsequently replicated (39), and a similar observation was made for leptin (120). Extrapolation of these observations might suggest a loss of the effectiveness of endogenous adiposity signals in the CNS to provide feedback signaling. Data collected by the Centers for Disease Control document a pervasive increase of obesity in adults across the United States in the 1990s and 2000s (93, 129, 130) and a high incidence of obesity in the pediatric age group as well (114), interpreted as a significant environmental influence over the neural circuitry associated with the physiological maintenance of energy homeostasis. The epidemiological finding also emphasizes that attention should be focused on additional CNS circuitry that is directly or indirectly connected with hypothalamic circuitry to modulate feeding behavior.
One obvious target for study is the CNS circuitry that mediates motivation and reward. Components of this circuitry are activated with, and contribute to, complex behaviors such as food seeking and food intake (16, 18, 19; see below). This circuitry includes portions of the cerebral cortex, hippocampus, and amygdala and the striatonigral pathway, which is implicated in transposing motivational aspects of stimuli into motor responses, as well as in hedonic evaluation of the stimulus and associative learning (61, 101, 105, 150, 156, 158, 189, 191). As discussed below, the major neurotransmitter pathways associated with motivation and hedonics are mesolimbic dopamine (DA) and certain CNS opioid pathways. In terms of neural connectivity, the hypothalamus is linked to the “motivational circuitry” of the CNS, and numerous mono- and multisynaptic pathways between different components of the limbic circuitry and the hypothalamus have been identified (19, 20, 179). The anatomic and functional relevance of this circuitry to food intake is discussed in recent reviews (16, 110, 111). Insulin and leptin receptors are expressed throughout the limbic forebrain, including the hippocampus, the amygdala, and the LH/zona incerta area (69, 118, 119). This provides one rationale for exploring the potential that the limbic forebrain may itself be a direct target for insulin or leptin.
Food intake can be driven by energy demands, i.e., “homeostatic” feeding. However, food intake can also be driven by the palatability or pleasure associated with eating a preferred food, “nonhomeostatic” feeding (18–20). The palatability of a particular food source is assumed to be related to the flavor and taste of that food, and high-fat diets are generally considered more palatable than low-fat diets, inasmuch as they are preferentially overconsumed. In humans, individual differences exist in the reinforcing value of food, with a stronger preference for diets high in fat and carbohydrates displayed by obese than by nonobese individuals (57–59).
Berridge and colleagues provided a conceptual framework for the consideration of food reward, proposing that there is “wanting” of food (or another stimulus) and “liking” of food (16). The behavior associated with nonhomeostatic feeding is in part regulated by the mesolimbic DA system (18), and within this system, the neurotransmitter DA plays a substantial role in the regulation of food reward. They identified nucleus accumbens (NAc) DA projections as central to wanting. The NAc represents a functionally specialized subregion of the striatum, which is a critical anatomic component of CNS reward circuitry, with the extensive projection of DA neurons from the midbrain ventral tegmental area (VTA) and associated DA cell groups (substantia nigra) (101, 102). Activation of these midbrain DA neurons has been implicated in the motivating, rewarding, reinforcing, and incentive salience properties of natural stimuli such as food and water, as well as drugs of abuse (16, 18, 155, 161, 176, 192). The neural mechanisms of food reward are believed to be similar, if not identical, to drug rewards (29, 111). There is also the possibility that dysregulation of those circuits may adversely affect body weight regulation. For example, studies in humans (183) and animals (21) suggest that changes in central DA may contribute to the development of obesity. Furthermore, some human studies report a decreased propensity to engage in the use of recreational drugs and a decreased frequency of substance abuse disorders in obese individuals (172, 185). One implication of these findings is that obesity is capable of altering processes within the endogenous reward system of the brain.
Behavioral paradigms evaluating reward and food reward provide evidence for a significant effect of metabolic status on performance in these paradigms and support the idea that insulin and leptin may play a role in modulating reward function in the CNS (74). Food restriction or fasting paradigms (in which endogenous insulin and leptin are decreased) enhance the addictive or reinforcing properties of drugs of abuse, as found in drug self-administration and relapse to drug-taking paradigms; intraventricular leptin can reverse food deprivation-induced relapse to heroin self-administration (34, 169, 170). Measurement of DA levels in NAc interstitial fluid has shown a greater DA response to food reward in food-deprived than in ad libitum-fed rats (190). Data from the experimental approach of brain self-stimulation have shown that food restriction shifts the dose-response curve for self-stimulation in some perifornical hypothalamus sites to the left, such that weaker electrical current, which normally would not support sustained self-stimulation activity at these sites, becomes efficacious when animals are maintained on a food-restriction paradigm (31, 32). Intraventricular leptin administration shifts the dose-response curve for self-stimulation in food restriction-sensitive perifornical hypothalamic sites to the right (i.e., reverses the effect of food restriction), and comparable data demonstrate that administration of insulin into the brain of food-restricted or ad libitum-feeding rats increases the threshold current needed to sustain LH self-stimulation, reversing the decreased threshold observed with fasting and elevating the threshold above its “free-feeding” level (33, 76). This evidence, although limited, suggests that insulin and leptin may play a major role in mediating the effects of altered metabolic status on reward paradigms in general.
Additionally, several studies implicate insulin and leptin in food reward. In addition to normal feeding, DA activity has been implicated in behavioral paradigms that evaluate different aspects of reward or motivation: acute licking of palatable solutions (50, 160), self-administration (102), and CPP (148). Sipols and colleagues (175) demonstrated suppression of acute sucrose licking (intraventricular insulin) and Figlewicz et al. demonstrated decreased food-CPP (intraventricular insulin or leptin) (66) and sucrose self-administration (intraventricular insulin or leptin) (68) in rats fed chow ad libitum. In these studies, doses of insulin and leptin were subthreshold for effects on chronic food intake or body weight and effects were observed very acutely (within minutes). Hommel and colleagues (95) and Morton and colleagues (134) demonstrated that direct administration of leptin into the VTA decreases chow intake in ad libitum-feeding rats. Taken together, the results of these four different types of behavioral paradigms— self-administration, lick rate task, CPP, and free feeding of the baseline chow diet—demonstrate that insulin and leptin, across a concentration range from fasting to free-feeding to elevated (as would occur postprandially) levels, are able to modify behaviors that reflect acute and learned reward evaluation independent of their action(s) to regulate body adiposity. Whether the rapid and chronic effects of insulin and leptin are mediated via the same circuitry and the same neural mechanisms remains to be elucidated. Furthermore, different anatomic loci, including the LH, appear to be targets for insulin- and leptin-induced suppression of food reward (33, 76, 118).
Studies from Figlewicz and colleagues focused on insulin and, more recently, leptin effects on the midbrain DA neurons at the cellular and behavioral levels. Insulin receptors have been identified in the VTA and the striatum in previous anatomic studies using receptor autoradiography and receptor immunocytochemistry approaches (181, 187), and expression of insulin and leptin receptor mRNA in the substantia nigra has been reported (60). We have localized insulin and leptin receptors on midbrain DA neurons, including those of the VTA, as well as medial and lateral substantia nigra (69). The presence of functional receptors has been confirmed by Fulton et al. (75) and Hommel et al. (95). Thus this critical motivational circuitry has the potential to serve as a direct target for adiposity signals. Recent studies have shown that administration of insulin and leptin directly into the VTA increases PI3-kinase activity (71). Furthermore, leptin (administered peripherally, intraventricularly, or directly into the VTA) results in an increase in JAK-STAT phosphorylation, which is critical for the effect of leptin in the VTA to decrease chow feeding (134). The synaptic or neural mechanisms that underlie insulin and leptin effects on food reward remain to be elucidated. We identified one potential cellular target for insulin action, the DA reuptake transporter (DAT), which inactivates DA signaling by transporting DA back into the DA nerve terminal from the synapse (107). The synthesis and the activity or synaptic concentrations of the DAT can be regulated by intracellular signaling systems, including PI3-kinase (77, 182). We have observed in vivo and in vitro effects of insulin on expression and activity of the DAT (72, 149). Insulin can increase mRNA levels and synaptic activity of the DAT. The functional implication of this increased DAT activity could be an increase in clearance of DA from the synapse and, hence, decreased DA signaling. This would be consistent with an action of insulin to decrease the rewarding aspect of food. Comparable cellular studies to identify regulatory proteins in DA neurons that are targets of leptin have not been done, although there is evidence that leptin regulates DA release and the electrical activity of DA neurons (95).
As mentioned above with respect to energy homeostasis, some effects of insulin and leptin are mediated through secondary hypothalamic peptide effector systems, including melanocortins and orexin-A. For example, melanocortin receptors (MC3R and MC4R) are also expressed in brain regions implicated in addictive behavior (5), and pharmacological studies have outlined functional roles for these receptors in the modulation of drug-taking behavior. Antagonism of these receptors in the NAc inhibits operant responding for cocaine, whereas central agonism of this system augments amphetamine-induced behaviors (97). As discussed elsewhere, orexin neurons exhibit diverse projections in the CNS to sites including the VTA (62). Orexin-expressing neurons of the LH have μ-opioid receptors, and the molecular physiology of these neurons is altered with morphine administration or withdrawal, emphasizing their role in CNS reward circuitry (78). Orexinergic projections signal specifically on a majority of DA neurons to activate the mesolimbic pathway (198), and VTA neuron populations express both orexin receptor subtypes (122). Exogenous orexin can increase VTA dopaminergic neuron firing rates. A specific role for endogenous orexin action in the VTA on reward-seeking behavior is implied in the findings in rats that 1) an orexin antagonist could block the reinstatement of an extinguished place preference for morphine and 2) intra-VTA orexin-A was sufficient to reinstate the place preference (84). Additional evidence from studies of genetic models demonstrates the inability of orexin-deficient mice to form morphine-CPPs (140). Orexin action in the LH also appears necessary for the acquisition and expression of morphine-induced CPP (85). Finally, Borgland et al. (23) reported that intra-VTA administration of an orexin antagonist effectively prevents behavioral sensitization and neurophysiological changes that typify chronic cocaine use. The important point is that, to the degree that peripheral adiposity signals may affect reward function, they are likely to do so in part through these critical effector peptides. One important consequence of this could be that neither insulin nor leptin would have to act directly on VTA or NAc cells to exert regulatory control. Rather, as an additional mechanism, they could activate (or inactivate) effector systems in hypothalamic neurons that, in turn, project to the reward circuitry. This notion is supported by a recent study evaluating the specific CNS targets of intraventricular insulin to suppress food reward: the effect of insulin to decrease sucrose self-administration was found to be due to action at the ARC (67).
INSULIN, LEPTIN, AND PALATABILITY
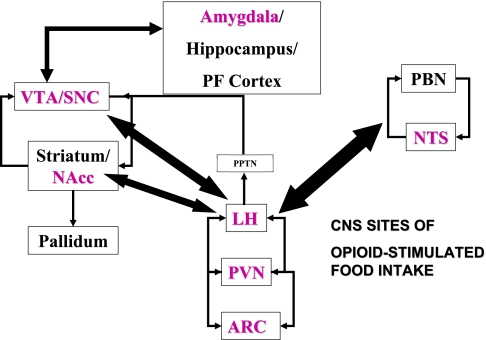
Palatability influences the amount and the type of food that is ingested (15–17). For example, it is well known that even noncaloric solutions, if they are made to taste sweet, will elicit drinking behavior in sated rats (28). Several hypothalamic peptides project to CNS areas important for taste processing (nucleus of the solitary tract) and hedonics and reward, including the VTA, NAc, and substantia nigra. As discussed above, this system is a direct target of insulin and leptin and suggests one mechanism whereby adiposity signals might modulate palatability. Berridge and colleagues (16–18) suggested that “liking” of foods is mediated in the CNS by endogenous opioids. Leptin and insulin, which simulate a state of satiation, have been found to regulate the hedonic qualities of brain self-stimulation (76), for which CNS opioidergic signaling has been implicated (32). Glass and colleagues (80) and others documented the role for opioid peptides to preferentially enhance intake of palatable food over less palatable food (rat chow), although this is somewhat dependent on the opioid receptor populations being targeted. Thus some opioid-receptive sites appear linked predominantly to palatability and some to caloric homeostasis (22, 147, 196). Whether adiposity signals can blunt palatability-induced feeding has been evaluated to a limited extent. Sipols and colleagues (174) reported that intraventricular insulin could blunt the ability of dynorphin to stimulate sucrose pellet intake and also synergize with a subthreshold dose of the dynorphin antagonist nor-binaltorphimine to decrease baseline intake of sucrose pellets (Fig. 2). This would be consistent with an action at the medial hypothalamus, where dynorphin receptors have been localized. Additionally, Figlewicz et al. (71) reported a direct action of insulin and leptin at the VTA to reverse μ-opioid-stimulated sucrose feeding. Although this study did not evaluate palatability per se, rats were tested immediately after feeding, and thus sucrose intake would not reflect caloric need but, it is presumed, would be correlated with liking of the sucrose. Given the identification in human eating disorders of a role for μ-opioids (57), such studies have potential clinical relevance. This is underscored by the recent report that leptin treatment of two obese leptin-deficient patients was sufficient to reduce food intake, reduce self-report ratings of preference for images of food, and reduce neural activity in the striatum (63).
Fig. 2.
Food intake, energy regulatory, and reward circuitry. Magenta labels highlight CNS sites in which opioid administration stimulates food intake. PBN, parabrachial nucleus; NTS, solitary tract nucleus.
Table 1 summarizes the effects of insulin and leptin on reward behaviors. Studies that have focused specifically on reward behavior linked with food or feeding stimulated within reward circuitry have not evaluated the possible generalization of insulin or leptin effects on other types of reward (e.g., drugs of abuse). However, the generalized effect of food restriction on drug seeking (34) and the study of Shalev et al. (170) demonstrating leptin reversal of food restriction-induced heroin relapse suggest that insulin, leptin, and other signals of metabolic status (e.g., ghrelin) may act by modulating dopaminergic or opioidergic function.
Table 1.
Effects of insulin or leptin on reward behaviors
| Behavior | Insulin (Route) | Leptin (Route) | Reference No. |
|---|---|---|---|
| Brain self-stimulation | Decrease (icv) | Decrease (icv) | 33, 76 |
| Relapse to heroin seeking | Not determined | Decrease (icv) | 170 |
| Acute sucrose licking | Decrease (icv) | Not determined | 175 |
| Food-conditioned place preference | Decrease (icv) | Decrease (icv, sc) | 66, 70 |
| Sucrose self-administration | Decrease (icv, ARC) | Decrease (icv) | 67, 68 |
| Acute chow intake (4–24 h) | Decrease (icv) | Decrease (icv, VTA) | 2, 3, 75, 95, 134 |
| Opioid-stimulated sucrose intake | Decrease (icv, VTA) | Decrease (VTA) | 67, 174 |
ARC, arcuate nucleus; icv, intracerebroventricularly; sc, subcutaneously; VTA, ventral tegmental area.
Finally, there are extremely acute effects (within minutes) and longer-acting effects, such as, the effect of intra-VTA leptin on 24-h chow intake (not yet examined for insulin). This is consistent with observations of acute and chronic leptin action on energy-regulatory circuitry and its function. The very acute effects of insulin, whether observed in fasted/food-restricted or free-feeding rats, support the possibility that, within the context of a meal or eating bout of, for example, 30-min duration (which would correspond to increased insulin access to the CNS), prandial insulin elevation may curtail meal size by impacting on the rewarding aspects of food. There are no data evaluating interaction or synergy of insulin and leptin on food reward, and such studies seem warranted.
INSULIN, LEPTIN, AND FOOD REWARD IN MODELS OF RESISTANCE OR OBESITY
Although the data described above were obtained from studies of nonobese rats and, for the most part, rats eating a conventional (low-fat) chow diet ad libitum, recent studies evaluating reward function and circuitry in animals that are significantly food restricted, chronically maintained on a high-fat diet, or obese suggest that disordered energy homeostasis has a significant impact on the regulation of food reward and CNS DA activity. For example, duration and severity of food restriction have opposite effects on DA release and DA turnover within the NAc (154, 190). Similarly, in the genetically obese ob/ob mouse, which lacks functional leptin, DA turnover in the NAc is decreased (75), and releasable DA stores are decreased (159) as well. In contrast, intraventricular leptin has been shown to decrease basal and food-stimulated DA release in the NAc in nonobese rats (117) and directly decrease DA neuronal activation (95). In terms of behavior, 5 wk of exposure to a moderately high-fat diet that did not induce frank obesity has been shown to increase sucrose self-administration and block the ability of intraventricular insulin or leptin to suppress it (68). In contrast, rats fed a high-fat diet for an extended period of time display decreased sucrose self-administration (51). In that series of studies, rats were maintained on a high-fat diet for ∼3 mo before undergoing training for sucrose self-administration: one group was fed the high-fat diet ad libitum, and a separate control group was pair fed the high-fat diet to match the kilocalories consumed by a low-fat control-fed group. The pair-fed group did not exhibit a significant increase in body mass or adiposity, but both groups of rats consuming the high-fat diet had elevated plasma (and presumptively, CNS) levels of leptin, and both groups exhibited attenuated sucrose self-administration relative to controls. One obvious interpretation of this finding is that the rats compared high-fat diets and sucrose, and, in effect, access to the high-fat diet in the home cage decreased the perceived reward value of sucrose. To assess whether this difference in responding was due solely to such a “contrast effect” for food, conditioned responding for amphetamine was also assessed. Amphetamine injection conditioned a place preference only in rats maintained on a low-fat diet. Consistent with this, DA turnover in the NAc was substantially decreased in rats maintained on a high-fat, relative to a low-fat, diet. Thus it appears that additional mechanism(s) for an interaction between chronic access to a high-fat diet and brain reward circuitry must be invoked. It seems reasonable to propose that impaired DA release underlies the altered reward behaviors in rats fed a high-fat diet, a hypothesis that can be readily evaluated experimentally.
A common factor among these models—chronic food restriction, diet-induced obesity, and genetic obesity—is an absence of normal leptin and insulin action. Even short-term access to a high-fat diet can inhibit central leptin or insulin signaling in the CNS. For example, previous research has suggested that access to a high-fat diet for as few as 3 days is sufficient to attenuate the central effects of insulin and leptin on food intake and glucose homeostasis (104, 132, 137, 139, 152, 184). These findings suggest that dietary dysregulation of leptin and insulin effects on reward would not be solely due to hedonic contrast, in general, or food reward, specifically. Intriguingly, studies from other model systems suggest that insulin (133, 180) and leptin (186) may have trophic effects on DA neurons; hence, models of extreme starvation, insulin and leptin resistance, or obesity may result in impaired DA neuronal function and signaling. Studies in the DA-deficient mouse model suggest that other CNS circuitry may be recruited to mediate reward function, in the absence of functional DA circuitry; for example, Hnasko and colleagues (94) demonstrated that serotonergic pathways mediate cocaine reward in this model. Although the limitations and specific parameters of the models cited here need to be taken into account, it nonetheless should be considered that states of very low insulin and leptin action would result in a low, or anhedonic, mood and decreased motivation. We suggest that insulin and leptin effects on reward circuitry and function would be bimodal, such that low “permissive” concentrations of insulin and leptin sustain dopaminergic neuronal viability and intermediate physiological levels suppress reward function but elevated levels, linked to resistance and metabolic pathophysiology, lead to impaired dopaminergic function. Whether other CNS systems could take over reward function in the context of this pathophysiology is purely conjecture. Careful evaluation of this theory might provide links between obesity and common psychiatric disorders such as eating disorders, depression, and drug addiction. It also has implications for the possible efficacy, or lack thereof, of CNS-targeted therapeutics for obesity. Thus, in Hull's equation, if the variable D has a dopaminergic component that is blunted in obese conditions, then evaluation of the regulation of the variable “habit strength” and its neuropharmacological underpinnings may prove a more fruitful line of research for therapeutic approaches.
CONCLUSION
Studies over the past decade confirm and extend the historic finding that food deprivation or restriction sensitizes brain reward circuitry and function. This review has focused on studies of insulin and leptin function. Newer studies, not reviewed here, suggest that the “hormone of need” ghrelin has predictably opposing effects. Studies in models of pathophysiology are also revealing new dimensions to the modulation of reward function. Understanding of new findings should shed considerable light on the contribution of nonhomeostatic feeding to obesity in contemporary societies that offer abundant, affordable, highly palatable foods (55, 56). Regardless of the specific outcomes of future studies, it seems reasonably clear that the neurobiological controls of eating (especially nonhomeostatic food intake) are tightly linked to the well-established circuits underlying other kinds of reward. Additionally, elucidation of the roles of leptin and insulin in the regulation of these systems may offer key insights into the evolution and function of these circuits and their control of behavior. Finally, a clearer understanding of the function of these circuits may, in fact, help us answer difficult theoretical questions: What exactly is reward? What makes something rewarding? Identification of the precise neural mechanisms would (and will) allow us to abandon the hypothetical constructs to nostalgia and, instead, formulate predictive descriptions of behavior based on specific physiological variables, essentially redefining Hull's equation. This understanding may have broad impact on the development of pharmacotherapies for obesity, as well as treatments for psychiatric disorders and drug abuse.
GRANTS
This work is supported by a Career Scientist Award from the Department of Veterans Affairs and National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-40963 (D. P. Figlewicz, Lattemann) and DK-066223 (S. C. Benoit).
REFERENCES
- 1.Ahima RS, Osei SY. Molecular regulation of eating behavior: new insights and prospects for therapeutic strategies. Trends Mol Med 7: 205–213, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Air EL, Benoit SC, Clegg DJ, Seeley RJ, Woods SC. Insulin and leptin combine additively to reduce food intake and body weight in rats. Endocrinology 143: 2449–2452, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Air EL, Benoit SC, Smith KAB, Clegg DJ, Woods SC. Acute third ventricular administration of insulin decreases food intake in two paradigms. Pharmacol Biochem Behav 72: 423–429, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Air EL, Strowski MZ, Benoit SC, Conarello SL, Salituro GM, Guan XM, Liu K, Woods SC, Zhang BB. Small molecule insulin mimetics reduce food intake and body weight and prevent development of obesity. Nat Med 8: 179–183, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Alvaro JD, Tatro JB, Quillan JM, Fogliano M, Eisenhard M, Lerner MR, Nestler EJ, Duman RS. Morphine down regulates melanocortin-4 receptor expression in brain regions that mediate opiate addiction. Mol Pharmacol 3: 583–591, 1996. [PubMed] [Google Scholar]
- 6.Arase K, Fisler JS, Shargill NS, York DA, Bray GA. Intracerebroventricular infusions of 3-OHB and insulin in a rat model of dietary obesity. Am J Physiol Regul Integr Comp Physiol 255: R974–R981, 1988. [DOI] [PubMed] [Google Scholar]
- 7.Banks W, Clever C, Farrell C. Partial saturation and regional variation in the blood-to-brain transport of leptin in normal-weight mice. Am J Physiol Endocrinol Metab 278: E1158–E1165, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Banks W, DiPalma C, Farrell C. Impaired transport of leptin across the blood-brain barrier in obesity. Peptides 20: 1341–1345, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Banks W, King B, Rossiter K, Olson R, Olson G, Kastin A. Obesity-inducing lesions of the central nervous system alter leptin uptake by the blood-brain barrier. Life Sci 69: 2765–2773, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Banks W, Niehoff M, Martin D, Farrell C. Leptin transport across the blood-brain barrier of the Koletsky rat is not mediated by a product of the leptin receptor gene. Brain Res 950: 130–136, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Barsh GS, Schwartz MW. Genetic approaches to studying energy balance: perception and integration. Nat Rev 3: 589–600, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Baskin DG, Figlewicz Lattemann D, Seeley RJ, Woods SC, Porte D Jr, Schwartz MW. Insulin and leptin: dual adiposity signals to the brain for the regulation of food intake and body weight. Brain Res 848: 114–123, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Beck B Neuropeptides and obesity. Nutrition 16: 916–923, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Benoit SC, Schwartz MW, Baskin DG, Woods SC, Seeley RJ. CNS melanocortin system involvement in the regulation of food intake and body weight. Horm Behav 37: 299–308, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Berridge K Modulation of taste affect by hunger, caloric satiety, and sensory-specific satiety in the rat. Appetite 16: 103–120, 1991. [DOI] [PubMed] [Google Scholar]
- 16.Berridge KC Food reward: brain substrates of wanting and liking. Neurosci Biobehav Rev 20: 1–25, 1996. [DOI] [PubMed] [Google Scholar]
- 17.Berridge K, Grill H. Alternating ingestive and aversive consummatory responses suggest a two-dimensional analysis of palatability in rats. Behav Neurosci 97: 563–573, 1983. [DOI] [PubMed] [Google Scholar]
- 18.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev 28: 309–369, 1998. [DOI] [PubMed] [Google Scholar]
- 19.Berthoud HR Neural control of appetite: cross-talk between homeostatic and non-homeostatic systems. Appetite 43: 315–317, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Berthoud HR Interactions between the “cognitive” and “metabolic” brain in the control of food intake. Physiol Behav 91: 486–498, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Bina KG, Cincotta AH. Dopaminergic agonists normalize elevated hypothalamic neuropeptide Y and corticotropin-releasing hormone, body weight gain, and hyperglycemia in ob/ob mice. Neuroendocrinology 71: 68–78, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Bodnar RJ Endogenous opioids and feeding behavior: a 30-year historical perspective. Peptides 25: 697–725, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Borgland SL, Taha SA, Sarti G, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron 49: 589–601, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Boutrel B, de Lecea L. Addiction and arousal: the hypocretin connection. Physiol Behav 93: 947–951, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brief DJ, Davis JD. Reduction of food intake and body weight by chronic intraventricular insulin infusion. Brain Res Bull 12: 571–575, 1984. [DOI] [PubMed] [Google Scholar]
- 26.Broberger C, deLecea L, Sutcliffe JG, Hokfelt T. Hypocretin/orexin- and melanin-concentrating hormone-expressing cells form distinct populations in the rodent lateral hypothalamus: relationship to the neuropeptide Y and agouti gene-related protein systems. J Comp Neurol 402: 460–474, 1998. [PubMed] [Google Scholar]
- 27.Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Muller-Wieland D, Kahn CR. Role of brain insulin receptor in control of body weight and reproduction. Science 289: 2122–2125, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Capaldi ED, Hunter MJ, Lyn SA. Conditioning with taste as the CS in conditioned flavor preference learning. Anim Learning Behav 25: 427–436, 1997. [Google Scholar]
- 29.Carelli RM Nucleus accumbens cell firing during goal-directed behaviors for cocaine vs. “natural” reinforcement. Physiol Behav 76: 379–387, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Caro J, Kolaczynski J, Nyce M, Ohannesian J, Opentanova I, Goldman W, Lynn R, Zhang P, Sinha M, Considine R. Decreased cerebrospinal-fluid/serum leptin ratio in obesity: a possible mechanism for leptin resistance. Lancet 348: 159–161, 1996. [DOI] [PubMed] [Google Scholar]
- 31.Carr KD Augmentation of drug reward by chronic food restriction: behavioral evidence and underlying mechanisms. Physiol Behav 76: 353–364, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Carr KD Feeding, drug abuse, and the sensitization of reward by metabolic need. Neurochem Res 21: 1455–1467, 1996. [DOI] [PubMed] [Google Scholar]
- 33.Carr KD, Kim GY, Cabeza de Vaca S. Hypoinsulinemia may mediate the lowering of self-stimulation thresholds by food restriction and streptozotocin-induced diabetes. Brain Res 863: 160–168, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Carroll ME, Meisch RA. Increased drug-reinforced behavior due to food deprivation. Adv Behav Pharmacol 4: 47–88, 1984. [Google Scholar]
- 35.Carvalheira JBC, Ribeiro EB, Guimaraes RB, Telles MM, Torsoni M, Gontijo JAR, Velloso LA, Saad MJA. Selective impairment to insulin signaling in the hypothalamus of obese Zucker rats. Diabetologia 46: 1629–1640, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Carvalheira JB, Torsoni MA, Ueno M, Amaral ME, Araujo EP, Velloso LA, Gontijo JA, Saad MJ. Cross-talk between the insulin and leptin signaling systems in hypothalamus. Obes Res 13: 48–57, 2005. [DOI] [PubMed] [Google Scholar]
- 37.Carvalheira JBC, Siloto RMP, Ignacchitti I, Brenelli SL, Carvalho CRO, Leite A, Velloso LA, Gontijo JAR, Saad MJA. Insulin modulates leptin-induced STAT3 activation in rat hypothalamus. FEBS Lett 500: 119–124, 2001. [DOI] [PubMed] [Google Scholar]
- 38.Chavez M, Kaiyala K, Madden LJ, Schwartz MW, Woods SC. Intraventricular insulin and the level of maintained body weight in rats. Behav Neurosci 109: 528–531, 1995. [DOI] [PubMed] [Google Scholar]
- 39.Chavez M, Riedy CA, Van Dijk GV, Woods SC. Central insulin and macronutrient intake in the rat. Am J Physiol Regul Integr Comp Physiol 271: R727–R731, 1996. [DOI] [PubMed] [Google Scholar]
- 40.Cheung CC, Clifton DK, Steiner RA. Proopiomelanocortin neurons are direct targets for leptin in the hypothalamus. Endocrinology 138: 4489–4492, 1997. [DOI] [PubMed] [Google Scholar]
- 41.Chua SC, Chung WK, Wu-Peng XS, Zhang Y, Liu SM, Tartaglia L, Leibel RL. Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor. Science 271: 994–996, 1996. [DOI] [PubMed] [Google Scholar]
- 42.Clark JT, Kalra PS, Crowley WR, Kalra SP. Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology 115: 427–428, 1984. [DOI] [PubMed] [Google Scholar]
- 43.Clark JT, Kalra PS, Kalra SP. Neuropeptide Y stimulates feeding but inhibits sexual behavior in rats. Endocrinology 117: 2435–2442, 1985. [DOI] [PubMed] [Google Scholar]
- 44.Clegg DJ, Air EL, Woods SC, Seeley RJ. Eating elicited by orexin-A, but not melanin-concentrating hormone, is opioid mediated. Endocrinology 143: 2995–3000, 2002. [DOI] [PubMed] [Google Scholar]
- 45.Clegg DJ, Benoit SC, Reed JA, Woods SC, Dunn-Meynell A, Levin BE. Reduced anorexic effects of insulin in obesity-prone rats fed a moderate-fat diet. Am J Physiol Regul Integr Comp Physiol 288: R981–R986, 2005. [DOI] [PubMed] [Google Scholar]
- 46.Coker GT, Studelska D, Harmon S, Burke W, O'Malley KL. Analysis of tyrosine hydroxylase and insulin transcripts in human neuroendocrine tissues. Mol Brain Res 8: 93–98, 1990. [DOI] [PubMed] [Google Scholar]
- 47.Coleman DL, Hummel KP. Effects of parabiosis of normal with genetically diabetic mice. Am J Physiol 217: 1298–1304, 1969. [DOI] [PubMed] [Google Scholar]
- 48.Corp ES, Woods SC, Porte D Jr, Dorsa DM, Figlewicz DP, Baskin DG. Localization of 125I-insulin binding sites in the rat hypothalamus by quantitative autoradiography. Neurosci Lett 70: 17–22, 1986. [DOI] [PubMed] [Google Scholar]
- 49.Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ. Hypothalamic mTOR signaling regulates food intake. Science 312: 927–930, 2006. [DOI] [PubMed] [Google Scholar]
- 50.Davis JD, Smith GP. Analysis of the rhythmic tongue movements of rats ingesting maltose and sucrose solutions. Behav Neurosci 106: 217–228, 1992. [PubMed] [Google Scholar]
- 51.Davis JF, Tracy AL, Schurdak JD, Tschop MH, Lipton JW, Clegg DJ, Benoit SC. Exposure to elevated levels of dietary fat attenuates psychostimulant reward and mesolimbic DA turnover in the rat. Behav Neurosci. In press. [DOI] [PMC free article] [PubMed]
- 52.Dernovsek K, Bar R, Ginsberg B, Lioubin M. Processing of cell-bound insulin by capillary and macrovascular endothelial cells in culture. J Clin Endocrinol Metab 58: 761–763, 1984. [DOI] [PubMed] [Google Scholar]
- 53.Dernovsek KD, Bar BS. Rapid transport of biologically intact insulin through cultured endothelial cells. Am J Physiol Endocrinol Metab 248: E244–E251, 1985. [DOI] [PubMed] [Google Scholar]
- 54.De Souza CT, Araujo EP, Bordin S, Ashimine R, Zollner RL, Boschero AC, Saad MJ, Velloso LA. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology 146: 4192–4199, 2005. [DOI] [PubMed] [Google Scholar]
- 55.Drewnowski A Energy density, palatability, and satiety: implications for weight control. Nutr Rev 56: 347–353, 1998. [DOI] [PubMed] [Google Scholar]
- 56.Drewnowski A Fat and sugar: an economic analysis. J Nutr 133: 838S–840S, 2003. [DOI] [PubMed] [Google Scholar]
- 57.Drewnowski A, Krahn DD, Demitrack MA, Nairn K, Gosnell BA. Taste responses and preferences for sweet high-fat foods: evidence for opioid involvement. Physiol Behav 51: 371–379, 1992. [DOI] [PubMed] [Google Scholar]
- 58.Drewnowski A, Kurth C, Holden-Wiltse J, Saari J. Food preferences in human obesity: carbohydrates versus fats. Appetite 18: 207–221, 1992. [DOI] [PubMed] [Google Scholar]
- 59.Drewnowski A, Popkin BM. The nutrition transition: new trends in the global diet. Nutr Rev 55: 31–43, 1997. [DOI] [PubMed] [Google Scholar]
- 60.Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB. Distribution of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol 395: 535–547, 1998. [PubMed] [Google Scholar]
- 61.Everitt BJ, Parkinson JA, Olmstead MC, Arroyo M, Robledo P, Robbins TW. Associative processes in addiction and reward. The role of amygdala-ventral striatal subsystems. Ann NY Acad Sci 877: 412–438, 1999. [DOI] [PubMed] [Google Scholar]
- 62.Fadel J, Deutch AY. Anatomical substrates of orexin-dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neuroscience 111: 379–387, 2002. [DOI] [PubMed] [Google Scholar]
- 63.Farooqi IS, Bullmore E, Keogh J, Gillard J, O'Rahilly S, Fletcher PC. Leptin regulates striatal regions and human eating behavior. Science 317: 1355, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ferster CB, Skinner BF. Schedules of Reinforcement. New York: Appelton-Century-Crofts, 1957.
- 65.Figlewicz DP Adiposity signals and food reward: expanding the CNS roles of insulin and leptin. Am J Physiol Regul Integr Comp Physiol 284: R882–R892, 2003. [DOI] [PubMed] [Google Scholar]
- 66.Figlewicz DP, Bennett J, Evans SB, Kaiyala K, Sipols AJ, Benoit SC. Intraventricular insulin and leptin reverse place preference conditioned with high fat diet in rats. Behav Neurosci 118: 479–487, 2004. [DOI] [PubMed] [Google Scholar]
- 67.Figlewicz DP, Bennett JL, Aliakbari S, Zavosh A, Sipols AJ. Insulin acts at different CNS sites to decrease acute sucrose intake and sucrose self-administration in rats. Am J Physiol Regul Integr Comp Physiol 295: R388–R394, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Figlewicz DP, Bennett JL, Naleid AM, Davis C, Grimm JW. Intraventricular insulin and leptin decrease sucrose self-administration in rats. Physiol Behav 89: 611–616, 2006. [DOI] [PubMed] [Google Scholar]
- 69.Figlewicz DP, Evans SB, Murphy J, Hoen M, Baskin DG. Expression of receptors for insulin and leptin in the ventral tegmental area/substantia nigra (VTA/SN) of the rat. Brain Res 964: 107–115, 2003. [DOI] [PubMed] [Google Scholar]
- 70.Figlewicz DP, Higgins MS, Ng-Evans SB, Havel PJ. Leptin reverses sucrose-conditioned place preference in food-restricted rats. Physiol Behav 73: 229–234, 2001. [DOI] [PubMed] [Google Scholar]
- 71.Figlewicz DP, Naleid AM, Sipols AJ. Modulation of food reward by adiposity signals. Physiol Behav 91: 473–478, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Figlewicz DP, Szot P, Chavez M, Woods SC, Veith RC. Intraventricular insulin increases dopaminergic transporter mRNA in rat VTA/substantia nigra. Brain Res 644: 331–334, 1994. [DOI] [PubMed] [Google Scholar]
- 73.Figlewicz D, Szot P, Greenwood MRC. Insulin stimulates inositol incorporation in hippocampus of lean but not obese Zucker rats. Physiol Behav 47: 325–330, 1990. [DOI] [PubMed] [Google Scholar]
- 74.Figlewicz DP, Woods SC. Adiposity signals and brain reward mechanisms. Trends Pharmacol Sci 21: 235–236, 2000. [DOI] [PubMed] [Google Scholar]
- 75.Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, Maratos-Flier E, Flier JS. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron 51: 811–822, 2006. [DOI] [PubMed] [Google Scholar]
- 76.Fulton S, Woodside B, Shizgal P. Modulation of brain reward circuitry by leptin. Science 287: 125–128, 2000. [DOI] [PubMed] [Google Scholar]
- 77.Garcia BG, Wei Y, Moron JA, Lin RZ, Javitch JA, Galli A. Akt is essential for insulin modulation of amphetamine-induced human dopamine transporter cell-surface distribution. Mol Pharmacol 68: 102–109, 2005. [DOI] [PubMed] [Google Scholar]
- 78.Georgescu D, Zachariou V, Barrot M, Mieda M, Willie JT, Eisch AJ, Yanagisawa M, Nestler EJ, DiLeone RJ. Involvement of the lateral hypothalamic peptide orexin in morphine dependence and withdrawal. J Neurosci 23: 3106–3111, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Giddings SJ, Chirgwin J, Permutt M. Evaluation of rat insulin messenger RNA in pancreatic and extrapancreatic tissues. Diabetologia 27: 343–347, 1985. [DOI] [PubMed] [Google Scholar]
- 80.Glass MJ, Billington CJ, Levine AS. Opioids and food intake: distributed functional neural pathways? Neuropeptides 33: 360–368, 1999. [DOI] [PubMed] [Google Scholar]
- 81.Grill HJ, Schwartz MW, Kaplan JM, Foxhall JS, Breininger J, Baskin DG. Evidence that the caudal brainstem is a target for the inhibitory effect of leptin on food intake. Endocrinology 143: 239–246, 2002. [DOI] [PubMed] [Google Scholar]
- 82.Gropp E, Shanabrough M, Borok E, Xu AW, Janoschek R, Buch T, Plum L, Balthasar N, Hampel B, Waisman A, Barsh GS, Horvath TL, Brüning JC. Agouti-related peptide-expressing neurons are mandatory for feeding. Nat Neurosci 8: 1289–1291, 2005. [DOI] [PubMed] [Google Scholar]
- 83.Hadari YR, Tzahar E, Nadiv O, Rothenberg P, Roberts C Jr, LeRoith D, Yarden Y, Zick Y. Insulin and insulinomimetic agents induce activation of phosphatidylinositol 3′-kinase upon its association with pp185 (IRS-1) in intact rat livers. J Biol Chem 267: 17483–17486, 1992. [PubMed] [Google Scholar]
- 84.Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature 437: 556–559, 2005. [DOI] [PubMed] [Google Scholar]
- 85.Harris GC, Wimmer M, Randall-Thompson JF, Aston-Jones G. Lateral hypothalamic orexin neurons are critically involved in learning to associate an environment with morphine reward. Behav Brain Res 183: 43–51, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Harvey J Leptin regulation of neuronal excitability and cognitive function. Curr Opin Pharmacol 7: 643–647, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Havrankova J, Roth J. Insulin receptors are widely distributed in the central nervous system of the rat. Nature 272: 827–829, 1978. [DOI] [PubMed] [Google Scholar]
- 88.Havrankova J, Roth J, Brownstein M. Concentrations of insulin and of insulin receptors in the brain are independent of peripheral insulin levels: studies of obese and streptozotocin-treated rodents. J Clin Invest 64: 636–642, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Heidenreich KA Insulin and IGF-I receptor signaling in cultured neurons. Ann NY Acad Sci 692: 72–88, 1993. [DOI] [PubMed] [Google Scholar]
- 90.Heidenreich KA Insulin receptors mediate growth effects in cultured fetal neurons. II. Activation of a protein kinase that phosphorylates ribosomal protein S6. Endocrinology 125: 1458–1463, 1989. [DOI] [PubMed] [Google Scholar]
- 91.Hervieu G Melanin-concentrating hormone functions in the nervous system: food intake and stress. Expert Opin Ther Targets 7: 495–511, 2003. [DOI] [PubMed] [Google Scholar]
- 92.Hileman S, Pierroz D, Masuzaki H, Bjorbaek C, El-Haschimi K, Banks W, Flier J. Characterization of short isoforms of the leptin receptor in rat cerebral microvessels and of brain uptake of leptin in mouse models of obesity. Endocrinology 143: 775–783, 2002. [DOI] [PubMed] [Google Scholar]
- 93.Hill JO, Wyatt HR, Reed GW, Peters JC. Obesity and the environment: where do we go from here? Science 299: 853–855, 2003. [DOI] [PubMed] [Google Scholar]
- 94.Hnasko TS, Sotak BN, Palmiter RD. Cocaine-conditioned place preference by dopamine-deficient mice is mediated by serotonin. J Neurosci 27: 12484–12488, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, Thurmon JJ, Marinelli M, DiLeone RJ. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron 51: 801–810, 2006. [DOI] [PubMed] [Google Scholar]
- 96.Horn CC, Addis A, Friedman MI. Neural substrate for an integrated metabolic control of feeding behavior. Am J Physiol Regul Integr Comp Physiol 276: R113–R119, 1999. [DOI] [PubMed] [Google Scholar]
- 97.Hsu R, Taylor JR, Newton SS, Alvaro JD, Haile C, Han G, Hruby VJ, Nestler EJ, Duman RS. Blockade of melanocortin transmission inhibits cocaine reward. Eur J Neurosci 8: 2233–2242, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hull CL, Hovland CI, Ross RT, Hall MP, Perkins DT, Fitch FB. Mathematico-Deductive Theory of Rote Learning: A Study of Scientific Methodology. New Haven, CT: Yale University Press, 1940.
- 99.Hull CL Principles of Behavior. New York: Appleton-Century, 1943, p. 422.
- 100.Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 88: 131–141, 1997. [DOI] [PubMed] [Google Scholar]
- 101.Ikemoto S Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev 56: 27–78, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ikemoto S, Wise RA. Mapping of chemical trigger zones for reward. Neuropharmacology 47: 190–201, 2004. [DOI] [PubMed] [Google Scholar]
- 103.Inui A Feeding and body-weight regulation by hypothalamic neuropeptides—mediation of the actions of leptin. Trends Neurosci 22: 62–67, 1999. [DOI] [PubMed] [Google Scholar]
- 104.Irani BG, Dunn-Meynell AA, Levin BE. Altered hypothalamic leptin, insulin, and melanocortin binding associated with moderate-fat diet and predisposition to obesity. Endocrinology 148: 310–316, 2007. [DOI] [PubMed] [Google Scholar]
- 105.Ishiwari K, Weber SM, Mingote S, Correa M, Salamone JD. Accumbens dopamine and the regulation of effort in food-seeking behavior: modulation of work output by different ratio or force requirements. Behav Brain Res 151: 83–91, 2004. [DOI] [PubMed] [Google Scholar]
- 106.Israel PA, Park CR, Schwartz MW, Green PK, Sipols AJ, Woods SC, Porte D Jr, Figlewicz DP. Effect of diet-induced obesity and experimental hyperinsulinemia on insulin uptake into CSF of the rat. Brain Res Bull 30: 571–575, 1993. [DOI] [PubMed] [Google Scholar]
- 107.Jaber M, Jones S, Giros B, Caron MG. The dopamine transporter: a crucial component regulating dopamine transmission. Mov Disord 12: 629–633, 1997. [DOI] [PubMed] [Google Scholar]
- 108.Kaiyala KJ, Prigeon RL, Kahn SE, Woods SC, Schwartz MW. Obesity induced by a high-fat diet is associated with reduced brain insulin transport in dogs. Diabetes 49: 1525–1533, 2000. [DOI] [PubMed] [Google Scholar]
- 109.Kastin A, Pan W. Dynamic regulation of leptin entry into brain by the blood-brain barrier. Regul Pept 92: 37–43, 2000. [DOI] [PubMed] [Google Scholar]
- 110.Kelley AE, Bakshi VP, Haber SN, Steininger TL, Will MJ, Zhang M. Opioid modulation of taste hedonics within the ventral striatum. Physiol Behav 76: 365–377, 2002. [DOI] [PubMed] [Google Scholar]
- 111.Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci 22: 3306–3311, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kenner KA, Heidenreich KA. Insulin and insulin-like growth factors stimulate in vivo receptor autophosphorylation and tyrosine phosphorylation of a 70K substrate in cultured fetal chick neurons. Endocrinology 129: 301–311, 1991. [DOI] [PubMed] [Google Scholar]
- 113.Kenner KA, Kusari J, Heidenreich KA. cDNA sequence analysis of the human brain insulin receptor. Biochem Biophys Res Commun 217: 304–312, 1995. [DOI] [PubMed] [Google Scholar]
- 114.Kim J, Petersen KE, Scanlon KS, Fitzmaurice GM, Must A, Oken E, Rifas-Shiman SL, Rich-Edwards JW, Gillman MW. Trends in overweight from 1980 through 2001 among preschool-aged children enrolled in a health maintenance organization. Obesity 14: 1107–1112, 2006. [DOI] [PubMed] [Google Scholar]
- 115.King GL, Johnson S. Receptor-mediated transport of insulin across endothelial cells. Science 227: 1583–1586, 1985. [DOI] [PubMed] [Google Scholar]
- 116.Koch L, Wunderlich FT, Seibler J, Konner AC, Hampel B, Irlenbusch S, Brabant G, Kahn CR, Schwenk F, Bruning JC. Central insulin action regulates peripheral glucose and fat metabolism in mice. J Clin Invest 118: 2132–2147, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Krügel U, Schraft T, Kittner H, Kiess W, Illes P. Basal and feeding-evoked dopamine release in the rat nucleus accumbens is depressed by leptin. Eur J Pharmacol 482: 185–187, 2003. [DOI] [PubMed] [Google Scholar]
- 118.Leinninger GM, Myers MG Jr. LRb signals act within a distributed network of leptin-responsive neurones to mediate leptin action. Acta Physiol (Oxf) 192: 49–59, 2008. [DOI] [PubMed] [Google Scholar]
- 119.Leshan RL, Bjornholm M, Munzberg H, Myers MG Jr. Leptin receptor signaling and action in the central nervous system. Obesity 14 Suppl: 208S–212S, 2006. [DOI] [PubMed] [Google Scholar]
- 120.Lin L, Martin R, Schaffhauser AO, York DA. Acute changes in the response to peripheral leptin with alteration in the diet composition. Am J Physiol Regul Integr Comp Physiol 280: R504–R509, 2001. [DOI] [PubMed] [Google Scholar]
- 121.Maness L, Banks W, Kastin A. Persistence of blood-to-brain transport of leptin in obese leptin-deficient and leptin receptor-deficient mice. Brain Res 873: 165–167, 2000. [DOI] [PubMed] [Google Scholar]
- 122.Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanigasawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol 435: 6–25, 2001. [DOI] [PubMed] [Google Scholar]
- 123.Maresh G, Maness L, Zadina J, Kastin A. In vitro demonstration of a saturable transport system for leptin across the blood-brain barrier. Life Sci 69: 67–73, 2001. [DOI] [PubMed] [Google Scholar]
- 124.Mayer J, Thomas DW. Regulation of food intake and obesity. Science 156: 328–337, 1967. [DOI] [PubMed] [Google Scholar]
- 125.McGowan MK, Andrews KM, Kelly J, Grossman SP. Effects of chronic intrahypothalamic infusion of insulin on food intake and diurnal meal patterning in the rat. Behav Neurosci 104: 373–385, 1990. [DOI] [PubMed] [Google Scholar]
- 126.McNay EC Insulin and ghrelin: peripheral hormones modulating memory and hippocampal function. Curr Opin Pharmacol 7: 628–632, 2007. [DOI] [PubMed] [Google Scholar]
- 127.Mirshamsi S, Laidlaw HA, Ning K, Anderson E, Burgess LA, Gray A, Sutherland C, Ashford ML. Leptin and insulin stimulation of signaling pathways in arcuate nucleus neurons: PI3K dependent actin reorganization and KATP channel activation. BMC Neurosci 5: 54, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mizuno TM, Makimura H, Silverstein J, Roberts JL, Lopingco T, Mobbs CV. Fasting regulates hypothalamic neuropeptide Y, agouti-related peptide, and proopiomelanocortin in diabetic mice independent of changes in leptin or insulin. Endocrinology 140: 4551–4557, 1999. [DOI] [PubMed] [Google Scholar]
- 129.Mokdad A, Bowman B, Ford E, Vinicor F, Marks J, Koplan J. The continuing epidemics of obesity and diabetes in the United States. JAMA 286: 1195–1200, 2001. [DOI] [PubMed] [Google Scholar]
- 130.Mokdad A, Serdula M, Dietz W, Bowman B, Marks J, Koplan J. The spread of the obesity epidemic in the United States, 1991–1998. JAMA 282: 1519–1522, 1999. [DOI] [PubMed] [Google Scholar]
- 131.Mondal MS, Nakazato M, Matsukura S. Orexins (hypocretins): novel hypothalamic peptides with divergent functions. Biochem Cell Biol 78: 299–305, 2000. [PubMed] [Google Scholar]
- 132.Morgan K, Obici S, Rossetti L. Hypothalamic responses to long-chain fatty acids are nutritionally regulated. J Biol Chem 279: 31139–31148, 2004. [DOI] [PubMed] [Google Scholar]
- 133.Moroo I, Yamada T, Makino H, Tooyama I, McGeer PL, McGeer EG, Hirayama K. Loss of insulin receptor immunoreactivity from the substantia nigra pars compacta neurons in Parkinson's disease. Acta Neuropathol (Berl) 87: 343–348, 1994. [DOI] [PubMed] [Google Scholar]
- 134.Morton GJ, Matsen M, Kim F, Schwartz MW, Figlewicz DP. Leptin action in the ventral tegmental area reduces food intake via mechanisms independent of IRS-PI3K and mTOR signaling (Abstract). Diabetes 57 Suppl 1: A435, 2008. [Google Scholar]
- 135.Morton GJ, Schwartz MW. The NPY/AgRP neuron and energy homeostasis. Int J Obes Relat Metab Disord 25 Suppl 5: S56–S62, 2001. [DOI] [PubMed] [Google Scholar]
- 136.Mountjoy KG, Wong J. Obesity, diabetes and functions for proopiomelanocortin-derived peptides. Mol Cell Endocrinol 128: 171–177, 1997. [DOI] [PubMed] [Google Scholar]
- 137.Munzberg H Differential leptin access into the brain—a hierarchical organization of hypothalamic leptin target sites? Physiol Behav 94: 664–669, 2008. [DOI] [PubMed] [Google Scholar]
- 138.Munzberg H, Flier JS, Bjorbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology 145: 4880–4889, 2004. [DOI] [PubMed] [Google Scholar]
- 139.Munzberg H, Myers MG Jr. Molecular and anatomical determinants of central leptin resistance. Nat Neurosci 8: 566–570, 2005. [DOI] [PubMed] [Google Scholar]
- 140.Narita M, Nagumo Y, Hashimoto S, Narita M, Khotib J, Miyatake M, Sakurai T, Yanagisawa M, Nakamachi T, Shioda S, Suzuki T. Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J Neurosci 26: 398–405, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nicolaidis S, Rowland N. Metering of intravenous versus oral nutrients and regulation of energy balance. Am J Physiol 231: 661–668, 1976. [DOI] [PubMed] [Google Scholar]
- 142.Niswender KD, Baskin DG, Schwartz MW. Insulin and its evolving partnership with leptin in the hypothalamic control of energy homeostasis. Trends Endocrinol Metab 15: 362–369, 2004. [DOI] [PubMed] [Google Scholar]
- 143.Niswender KD, Morrison CD, Clegg DJ, Olson R, Baskin DG, Myers MG Jr, Seeley RJ, Schwartz MW. Insulin activation of phosphatidylinositol 3-kinase in the hypothalamic arcuate nucleus: a key mediator of insulin-induced anorexia. Diabetes 52: 227–231, 2003. [DOI] [PubMed] [Google Scholar]
- 144.Niswender KD, Morton GJ, Stearns WH, Rhodes CJ, Myers MG Jr, Schwartz MW. Key enzyme in leptin-induced anorexia. Nature 413: 794–795, 2001. [DOI] [PubMed] [Google Scholar]
- 145.Obici S, Feng Z, Karkanias G, Baskin DG, Rossetti L. Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. Nat Neurosci 5: 566–572, 2002. [DOI] [PubMed] [Google Scholar]
- 146.Obici S, Rossetti L. Nutrient sensing and the regulation of insulin action and energy balance. Endocrinology 144: 5172–5178, 2003. [DOI] [PubMed] [Google Scholar]
- 147.Olszewski PK, Levine AS. Central opioids and consumption of sweet tastants: when reward outweighs homeostasis. Physiol Behav 91: 506–512, 2007. [DOI] [PubMed] [Google Scholar]
- 148.Papp M Different effects of short- and long-term treatment with imipramine on the apomorphine- and food-induced place preference conditioning in rats. Pharmacol Biochem Behav 52: 889–893, 1988. [DOI] [PubMed] [Google Scholar]
- 149.Patterson T, Brot MD, Zavosh A, Schenk JO, Szot P, Figlewicz DP. Fasting decreases mRNA and activity of the rat dopamine transporter. Neuroendocrinology 68: 11–20, 1998. [DOI] [PubMed] [Google Scholar]
- 150.Petrovich GD, Gallagher M. Amygdala subsystems and control of feeding behavior by learned cues. Ann NY Acad Sci 985: 251–262, 2003. [DOI] [PubMed] [Google Scholar]
- 151.Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci 18: 9996–100015, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pocai A, Morgan K, Buettner C, Gutierrez-Juarez R, Obici S, Rossetti L. Central leptin acutely reverses diet-induced hepatic insulin resistance. Diabetes 54: 3182–3189, 2005. [DOI] [PubMed] [Google Scholar]
- 153.Porte D, Woods SC. Regulation of food intake and body weight by insulin. Diabetologia 20 Suppl: 274–280, 1981. [PubMed] [Google Scholar]
- 154.Pothos EN, Creese I, Hoebel BG. Restricted eating with weight loss selectively decreases extracellular dopamine in the nucleus accumbens and alters dopamine response to amphetamine, morphine, and food intake. J Neurosci 15: 6640–6650, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Richardson NR, Gratton A. Behavior-relevant changes in nucleus accumbens dopamine transmission elicited by food reinforcement: an electrochemical study. J Neurosci 16: 8160–8169, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Robbins TW, Everitt BJ. Neurobehavioural mechanisms of reward and motivation. Curr Opin Neurobiol 6: 228–236, 1996. [DOI] [PubMed] [Google Scholar]
- 157.Robertson SA, Leinninger GM, Myers MJ Jr. Molecular and neural mediators of leptin action. Physiol Behav 94: 637–642, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rollins BL, King BM. Amygdala-lesion obesity: what is the role of the various amygdaloid nuclei? Am J Physiol Regul Integr Comp Physiol 279: R1348–R1356, 2000. [DOI] [PubMed] [Google Scholar]
- 159.Roseberry AG, Painter T, Mark GP, Williams JT. Decreased vesicular somatodendritic dopamine stores in leptin-deficient mice. J Neurosci 27: 7021–7027, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Schneider LH, Davis JD, Watson CA, Smith GP. Similar effect of raclopride and reduced sucrose concentration on the microstructure of sucrose sham feeding. Eur J Pharmacol 186: 61–70, 1990. [DOI] [PubMed] [Google Scholar]
- 161.Schultz W Getting formal with dopamine and reward. Neuron 36: 241–263, 2002. [DOI] [PubMed] [Google Scholar]
- 162.Schwartz M, Figlewicz D, Kahn S, Baskin D, Greenwood M, Porte D Jr. Insulin binding to brain capillaries is reduced in genetically obese, hyperinsulinemic Zucker rats. Peptides 11: 467–472, 1990. [DOI] [PubMed] [Google Scholar]
- 163.Schwartz M, Peskind E, Raskind M, Boyko E, Porte D Jr. Cerebrospinal fluid leptin levels: relationship to plasma levels and to adiposity in humans. Nat Med 2: 589–593, 1996. [DOI] [PubMed] [Google Scholar]
- 164.Schwartz MW, Figlewicz DP, Baskin DG, Woods SC, Porte D Jr. Insulin in the brain: a hormonal regulator of energy balance. Endocr Rev 13: 387–414, 1992. [DOI] [PubMed] [Google Scholar]
- 165.Schwartz MW, Sipols AJ, Kahn SE, Lattemann DF, Taborsky G Jr, Bergman RN, Woods SC, Porte D Jr. Kinetics and specificity of insulin uptake from plasma to cerebrospinal fluid. Am J Physiol Endocrinol Metab 259: E378–E383, 1990. [DOI] [PubMed] [Google Scholar]
- 166.Schwartz MW, Sipols AJ, Marks JL, Sanacora G, White JD, Scheurink A, Kahn SE, Baskin DG, Woods SC, Figlewicz DP, Porte D Jr. Inhibition of hypothalamic neuropeptide Y gene expression by insulin. Endocrinology 130: 3608–3616, 1992. [DOI] [PubMed] [Google Scholar]
- 167.Seeley RJ, Schwartz MW. Neuroendocrine regulation of food intake. Acta Paediatr Suppl 88: 58–61, 1999. [DOI] [PubMed] [Google Scholar]
- 168.Seeley RJ, Yagaloff KA, Fisher SL, Burn P, Thiele TE, van Dijk G, Baskin DG, Schwartz MW. Melanocortin receptors in leptin effects. Nature 390: 349, 1999. [DOI] [PubMed] [Google Scholar]
- 169.Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev 54: 1–42, 2002. [DOI] [PubMed] [Google Scholar]
- 170.Shalev U, Yap J, Shaham Y. Leptin attenuates food deprivation-induced relapse to heroin seeking. J Neurosci 21: RC129, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Shemer J, Adamo M, Raizada MK, Heffez D, Zick Y, LeRoith D. Insulin and IGF-I stimulate phosphorylation of their respective receptors in intact neuronal and glial cells in primary culture. J Mol Neurosci 1: 3–8, 1989. [DOI] [PubMed] [Google Scholar]
- 172.Simon G, Von Korff M, Saunders K, Miglioretti DL, Crane PK, van Belle G, Kessler RC. Association between obesity and psychiatric disorders in the US adult population. Arch Gen Psychol 63: 824–830, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sipols AJ, Baskin DG, Schwartz MW. Effect of intracerebroventricular insulin infusion on diabetic hyperphagia and hypothalamic neuropeptide gene expression. Diabetes 44: 147–151, 1995. [DOI] [PubMed] [Google Scholar]
- 174.Sipols AJ, Bayer J, Bennett R, Figlewicz DP. Intraventricular insulin decreases κ-opioid-mediated sucrose intake in rats. Peptides 23: 2181–2187, 2002. [DOI] [PubMed] [Google Scholar]
- 175.Sipols AJ, Stuber GD, Klein SN, Higgins MS, Figlewicz DP. Insulin and raclopride combine to decrease short-term intake of sucrose solutions. Peptides 21: 1361–1367, 2000. [DOI] [PubMed] [Google Scholar]
- 176.Smith GP Dopamine and food reward. Prog Psychobiol Physiol Psychol 16: 83–143, 1995. [Google Scholar]
- 177.Smith GP, Gibbs J. Postprandial satiety. Prog Psychobiol Physiol Psychol 8: 179–242, 1979. [Google Scholar]
- 178.Stein LJ, Dorsa DM, Baskin DG, Figlewicz DP, Porte D Jr, Woods SC. Reduced effect of experimental peripheral hyperinsulinemia to elevate cerebrospinal fluid insulin concentrations of obese Zucker rats. Endocrinology 121: 1611–1615, 1987. [DOI] [PubMed] [Google Scholar]
- 179.Stratford TR, Kelley AE. Evidence of a functional relationship between the nucleus accumbens shell and lateral hypothalamus subserving the control of feeding behavior. J Neurosci 19: 11040–11048, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Takahashi M, Yamada T, Tooyama I, Moroo I, Kimura H, Yamamoto T, Okada H. Insulin receptor mRNA in the substantia nigra in Parkinson's disease. Neurosci Lett 204: 201–204, 1996. [DOI] [PubMed] [Google Scholar]
- 181.Unger J, Livingston JN, Moss AM. Insulin receptors in the central nervous system: localization, signaling mechanisms and functional aspects. Prog Neurobiol 36: 343–362, 1991. [DOI] [PubMed] [Google Scholar]
- 182.Vaughan RA, Huff RA, Uhl GR, Kuhar MJ. Protein kinase C-mediated phosphorylation and functional regulation of dopamine transporters in striatal synaptosomes. J Biol Chem 272: 15541–15546, 1997. [DOI] [PubMed] [Google Scholar]
- 183.Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, Netusil N, Fowler JS. Brain dopamine and obesity. Lancet 357: 354–357, 2001. [DOI] [PubMed] [Google Scholar]
- 184.Wang J, Obici S, Morgan K, Barzilai N, Feng Z, Rossetti L. Overfeeding rapidly induces leptin and insulin resistance. Diabetes 50: 2786–2791, 2001. [DOI] [PubMed] [Google Scholar]
- 185.Warren M, Frost-Pineda K, Gold M. Body mass index and marijuana use. J Addict Dis 24: 95–100, 2005. [DOI] [PubMed] [Google Scholar]
- 186.Weng Z, Signore AP, Gao Y, Wang S, Zhang F, Hastings T, Yin XM, Chen J. Leptin protects against 6-hydroxydopamine-induced dopaminergic cell death via mitogen-activated protein kinase signaling. J Biol Chem 282: 34479–34491, 2007. [DOI] [PubMed] [Google Scholar]
- 187.Werther GA, Hogg A, Oldfield BJ, McKinley MJ, Figdor R, Allen AM, Mendelsohn FAO. Localization and characterization of insulin receptors in rat brain and pituitary gland using in vitro autoradiography and computerized densitometry. Endocrinology 121: 1562–1570, 1987. [DOI] [PubMed] [Google Scholar]
- 188.White JD, Olchovsky D, Kershaw M, Berelowitz M. Increased hypothalamic content of preproneuropeptide-Y messenger ribonucleic acid in streptozotocin-diabetic rats. Endocrinology 126: 765–772, 1990. [DOI] [PubMed] [Google Scholar]
- 189.Will MJ, Franzblau EB, Kelley AE. The amygdala is critical for opioid-mediated binge eating of fat. Neuroreport 15: 1857–1860, 2004. [DOI] [PubMed] [Google Scholar]
- 190.Wilson C, Nomikos GG, Collu M, Fibiger HC. Dopaminergic correlates of motivated behavior: importance of drive. J Neurosci 15: 5169–5178, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wise RA Brain reward circuitry: insights from unsensed incentives. Neuron 36: 229–240, 2002. [DOI] [PubMed] [Google Scholar]
- 192.Wise RA, Rompre PP. Brain dopamine and reward. Annu Rev Psychol 40: 191–225, 1989. [DOI] [PubMed] [Google Scholar]
- 193.Woods SC, Lotter EC, McKay LD, Porte D Jr. Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature 282: 503–505, 1979. [DOI] [PubMed] [Google Scholar]
- 194.Woods SC, Schwartz MW, Baskin DG, Seeley RJ. Food intake and the regulation of body weight. Annu Rev Psychol 51: 255–277, 2000. [DOI] [PubMed] [Google Scholar]
- 195.Zahniser NR, Goens MB, Hanaway PJ, Vinych JV. Characterization and regulation of insulin receptors in rat brain. J Neurochem 42: 1354–1362, 1984. [DOI] [PubMed] [Google Scholar]
- 196.Zhang M, Gosnell BA, Kelley AE. Intake of high-fat food is selectively enhanced by μ-opioid receptor stimulation within the nucleus accumbens. J Pharmacol Exp Ther 284: 908–914, 1998. [PubMed] [Google Scholar]
- 197.Zhang Y, Proenca R, Maffie M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature 372: 425–432, 1994. [DOI] [PubMed] [Google Scholar]
- 198.Zheng H, Patterson LM, Berthoud HR. Orexin signaling in the ventral tegmental area is required for high-fat appetite induced by opioid stimulation of the nucleus accumbens. J Neurosci 27: 11075–11082, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]