Abstract
The aim of this study was to investigate the effects and mechanisms of intestinal electrical stimulation (IES) on gastric tone, antral and pyloric contractions, and gastric emptying in dogs. Female hound dogs were equipped with a duodenal or gastric cannula, and one pair of serosal electrodes was implanted in the small intestine. The study consisted of five different experiments. Liquid gastric emptying was assessed by collection of chyme from the duodenal cannula in a number of sessions with and without IES and with and without N-nitro-l-arginine (l-NNA). Postprandial antral and pyloric contractions were measured with and without IES and in the absence and presence of l-NNA or phentolamine by placement of a manometric catheter into the antrum and pylorus via the duodenal cannula. Gastric tone was assessed by measurement of gastric volume at a constant pressure. Gastric emptying was substantially and significantly delayed by IES or l-NNA compared with the control session. IES-induced delay of gastric emptying became normal with addition of l-NNA. IES reduced gastric tone, which was blocked by l-NNA. IES also inhibited antral contractions (frequency and amplitude), and this inhibitory effect was not blocked by l-NNA but was blocked by phentolamine. IES alone did not affect pyloric tone or resistance, but IES + l-NNA decreased pyloric tone. In conclusion, IES reduces gastric tone via the nitrergic pathway, inhibits antral contractions via the adrenergic pathway, does not affect pyloric tone, and delays liquid gastric emptying. IES-induced delay of gastric emptying is attributed to its inhibitory effects on gastric motility.
Keywords: gastrointestinal motility, gastric pacing
electrical stimulation as a potential modality for treatment of morbid obesity is gaining more and more attention (15, 16), since the conventional behavior modifications and pharmacotherapies have not been effective in the long term (11, 29) and surgical interventions result in a high rate of mortality and morbidity (4, 9, 21, 42, 46). Gastric electrical stimulation (GES) has been under clinical investigation for the treatment of morbid obesity, and preliminary data from studies of the effects of GES on food intake and weight loss have been encouraging but inconclusive (15, 16).
The proximal small intestine plays an important role in regulating gastric emptying (30), optimizing nutrient absorption (26), and signaling satiety in the central nervous system (20). Because intestinal electrical stimulation (IES) may have multiple effects on gastrointestinal functions, including gastric emptying, small bowel transit, nutrient absorption, and feedback signaling of satiety to the central nervous system, it is a very attractive alternative option for treatment of obesity. In 1977, Kelly and Code (28) showed that distal duodenal pacing caused duodenal-gastric reflux of BaSO4 in dogs and a 25% reduction of the rate of liquid gastric emptying. However, there has been a lack of follow-up studies of the inhibitory effects of IES on gastrointestinal motility and related mechanisms.
Although the finding may not be conclusive, a number of studies showed accelerated gastric emptying in obesity patients (5, 6, 49, 52). The accelerated gastric emptying is believed to shorten the satiety period, i.e., time to ingestion of the next meal. The critical factors that control gastric emptying are gastric tone, antral peristalsis, pyloric resistance, and duodenal feedback control. Dysfunction of any of these factors results in impairment of gastric emptying (13, 19, 22, 24, 25, 27, 36, 38, 45, 47, 48). Effective peristaltic antral contractions play a major role in solid gastric emptying (13, 27). Coordination of the antropyloroduodenal region is another key factor that controls solid and liquid gastric emptying (2, 3, 17, 25), and any factors that affect coordination of the antropyloroduodenal region will delay gastric emptying (28, 52). Although a few previous studies reported the inhibitory effect of IES on gastric emptying, the underlying mechanisms are unknown. It is not clear whether the IES-induced delay of gastric emptying is attributed to a reduction of gastric tone and/or antral contractions. It is unknown whether the pylorus is also involved in the IES-induced delay of gastric emptying. Nitric oxide, released from the nonadrenergic noncholinergic nerve, is known to be involved in relaxation of the gastric fundus. GES was previously reported to inhibit gastric tone via the nitrergic pathway and to decrease antral contractility via the sympathetic (α- and β-adrenergic) pathway. We believed that the effects and mechanisms of IES on fundic tone and antral contractions might be similar to those of GES. Accordingly, we hypothesized that IES would inhibit gastric motility via the nitrergic and adrenergic pathways.
Therefore, the aim of the present study was to systematically and comprehensively investigate the effects and mechanisms of IES on gastric motility, including gastric tone, antral contractions, pyloric tone, and gastric emptying in dogs.
MATERIALS AND METHODS
Animal preparation.
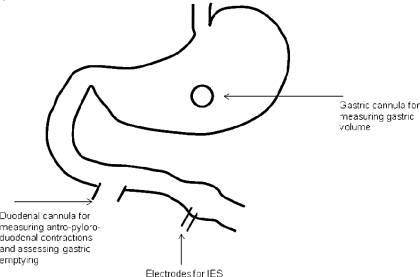
The procedures were approved by the Institutional Animal Care and Use Committee at the University of Texas Medical Branch at Galveston. Fourteen healthy female hound dogs (17.4–25.2 kg body wt) were included in the study. The operation was performed under general anesthesia after an overnight fast. Seven of the dogs were surgically prepared with a chronic duodenal fistula located 20 cm beyond the pylorus (Fig. 1) ; the cannula was brought out through the abdominal wall and fixed to prevent rotation. In the other seven dogs, which were used for the study of gastric tone, a chronic gastric cannula was placed 10 cm above the pylorus (Fig. 1). In all dogs, two 28-gauge cardiac pacing electrodes (A & E Medical, Farmingdale, NJ) were implanted 1 cm apart on the serosal surface of the small intestine 30 cm distal to the duodenal cannula. The electrode wires were subcutaneously tunneled through the anterior abdominal wall along the right side of the trunk and placed outside the skin around the right hypochondrium for attachment to the electrical stimulator. The study was initiated after the dogs had completely recovered from the surgery, usually after 2 wk.
Fig. 1.
Surgical preparation. In 7 dogs, a gastric cannula was placed 10 cm proximal to the pylorus for assessment of gastric tone; in 7 other dogs, a duodenal cannula was placed 20 cm distal to the pylorus for assessment of antral contractions, pyloric tone, and gastric emptying. Each dog was implanted with 1 pair of electrodes on the small intestine 30 cm distal to the pylorus. IES, intestinal electrical stimulation.
Experimental protocols.
Experiment 1, which was performed on the seven dogs with the duodenal fistula, was designed to study the effects and mechanisms of IES on gastric emptying of liquid. Each dog was studied randomly in four sessions on 2 separate days (≥2 days apart) after a 12-h fast. During each study session, the dog was fed a liquid meal consisting of 237 ml of Ensure (Ross Products Division, Abbott Laboratories, Columbus, OH) mixed with 100 mg of phenol red. The meal had 250 calories (6 g of fat, 40 g of carbohydrate, and 9 g of protein). Gastric emptying was assessed using an established method (41). Experiment 1 consisted of four sessions: 1) control without IES, 2) IES for the entire 90 min, 3) continuous infusion of N-nitro-l-arginine (l-NNA, 2.5 mg·kg−1·h−1; Sigma, St. Louis, MO), and 4) IES + l-NNA.
Experiment 2, which was performed on the seven dogs with the duodenal cannula, was designed to study the effects and mechanisms of IES on antral contractions and pyloric tone after a solid meal. Experiment 2 consisted of three randomized sessions: 1) control, 2) l-NNA, and 3) phentolamine. At the beginning of each session, the dog was fed 375 g of standard canned dog food. A manometric catheter with a sleeve (Dentsleeve) was placed into the stomach and pylorus for assessment of antral contractions and pyloric tone during four consecutive 20-min postprandial periods: baseline (without intervention), treatment (administration of drug or vehicle), IES (continuous IES throughout the 20-min period), and recovery (no IES or medication). In session 1, intravenous normal saline was continuously infused during the second and third 20-min periods. Session 2 was the same as session 1, except saline was replaced with l-NNA (2.5 mg·kg−1·h−1). In session 3, phentolamine (Sigma; 1 mg/kg bolus followed by perfusion with 1 mg·kg−1·h−1 iv) was administered. Antral contractions were measured in sessions 1–3, and pyloric tone was measured in sessions 1 and 2.
Experiment 3, which was performed in the seven dogs with the duodenal cannula, was designed to study the effect of IES on antral contractions after a liquid meal. The procedure was the same as that described for experiment 2, except the solid meal was replaced with the same liquid meal used in experiment 1. Antral contractions were measured for 20 min immediately after the test meal and then for an additional 20 min with IES.
Experiment 4, which was performed in the seven dogs with the gastric cannula, was designed to investigate the effects and mechanism of IES on gastric tone. Experiment 4 consisted of three randomized sessions: 1) control, 2) IES, and 3) IES + l-NNA. Gastric volume was measured by a barostat using an established method described elsewhere (50). Fasting gastric volume was recorded at an operating pressure 2 mmHg higher than the minimal distending pressure for 20 min at baseline and 20 min with IES. The l-NNA session was the same, expect l-NNA was infused for 40 min after the 20-min baseline and IES was performed throughout the l-NNA infusion. Gastric volume was calculated as the mean value during each 20-min period. Gastric tone was determined from gastric volume; that is, an increase in gastric volume represents a decrease in gastric tone, and vice versa.
IES.
IES consisted of a series of square-wave pulses with a frequency of 20 cycles or pulses per minute (cpm), pulse amplitude of 10 mA, and pulse width of 200 ms. These stimulation parameters were previously shown to inhibit intestinal contractions in the fed state (33). An electrical stimulator (model A310, World Precision Instruments, Sarasota, FL) was connected to the connection wires of the electrodes in the small intestine with a constant-current mode.
Gastric emptying.
The liquid test meal contained 237 ml of Ensure mixed with 100 mg of phenol red, which was used as a marker, and gastric emptying was determined by assessment of the amount of phenol red in each collection of gastric effluent using an established method described elsewhere (41). During the study, the volume of each collection was recorded every 15 min for 90 min. A spectrophotometer was used to detect the amount of phenol red in each sample. Gastric emptying was assessed by calculation of the amount of phenol red recovered from each collection of gastric content.
Measurement of contractile activity and tone of the antrum and pylorus.
Antral and pyloric contractions were measured using the manometric catheter, which consisted of a 4-cm-long sleeve sensor, one sensor (or side hole) at the distal end, and two sensors, spaced 2 cm apart, at the proximal side (Synectics Medical, Stockholm, Sweden). The catheter was inserted via the duodenal cannula into the pylorus and antrum immediately after the test meal. The position of the sleeve portion astride the pylorus was confirmed by the pull-through technique by assessment of the waveform of the antral contractions (at a frequency of 5 cpm) measured by the sensor distal to the sleeve and the waveform of the duodenal contractions (at a frequency of 10–15 cpm) measured from the two sensors located proximal to the sleeve.
Analysis of contractile active and tonic pressure of the antrum and pylorus.
The manometric trace of each 20-min period consisted of 1) the baseline level of the trace (tonic pressure) and 2) the phasic oscillations of the trace above the baseline level (contractions). A phasic contraction was defined as a >10-mmHg increase in pressure over the baseline level. This threshold of 10 mmHg was used to exclude possible pressure changes attributed to respiration artifacts. The area under the curve (AUC) was used to represent the summation of contractile activities over a given period. It was calculated by summing all contractile points over each of the four 20-min periods. The sum was then divided by 1,200 (s) to avoid large values. The number of contractions in each 20-min period was visually counted.
The tonic pressures were calculated from the baseline level of the manometric recording. The average of the pressure values in the absence of phasic contractions and the lowest pressures of each phasic contraction was considered the tonic pressure.
Statistics.
Values are means ± SD. ANOVA was used to compare data among three or more different interventions or periods. Student's paired t-test was used to investigate the differences between pairs at P < 0.05 (by ANOVA). P < 0.05 was considered statistically significant.
RESULTS
Effects and nitrergic mechanism of IES on gastric tone.
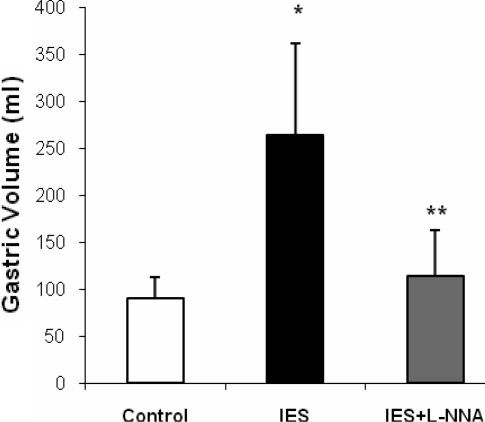
l-NNA sessions (P < 0.001 by 1-way ANOVA). Gastric volume was 90.9 ± 22.4 ml in the control session and substantially increased to 263.6 ± 98.0 ml during IES (P = 0.001), suggesting a reduction of gastric tone. In the presence of l-NNA, IES failed to increase gastric volume (113.9 ± 49.5 ml, P = 0.2 vs. control and P = 0.003 vs. IES), suggesting involvement of the nitrergic pathway in the IES-induced gastric relaxation (Fig. 2).
Fig. 2.
Effect of IES on gastric volume. IES significantly increased gastric volume, represented by decreased gastric tone (P = 0.001), and its inhibitory effect was blocked by N-nitro-l-arginine (l-NNA). *P = 0.2 vs. control. **P = 0.003 vs. IES.
Effects and mechanism of IES on antral contractions.
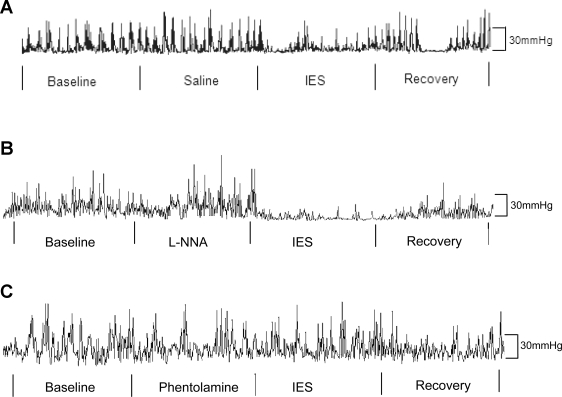
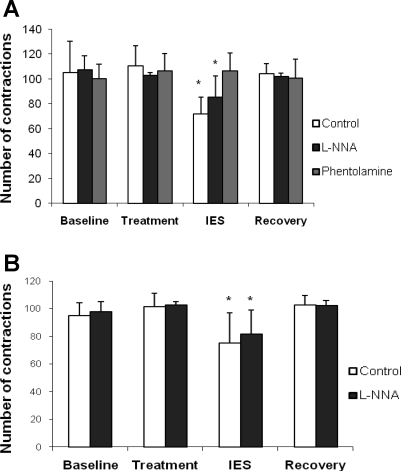
IES significantly inhibited phasic contractions in the distal antrum after the solid meal (Fig. 3A). As shown in Fig. 4A, the total number of contractions in the antrum during the 20-min IES period was significantly different, regardless of the test conditions [saline infusion vs. intravenous l-NNA, P < 0.0001 by 2-way ANOVA across 4 experimental periods (baseline vs. treatment vs. IES vs. recovery) and 2 study sessions (saline infusion as control vs. intravenous l-NNA)]. Specifically, the number of contractions in the distal antrum was significantly inhibited during the IES periods compared with the 20-min period immediately before or after IES (“treatment” period or recovery period), regardless of test conditions (saline infusion vs. intravenous l-NNA, P < 0.05 by paired t-test). These data also indicate that l-NNA did not block the inhibitory effects of IES on the number of contractions in the antrum. Similar results were noted for the AUC of the contractile activity (Table 1). The data obtained from the session with phentolamine are also presented in Table 1 and Fig. 4A. The inhibitory effect of IES on the postprandial contractions was blocked by phentolamine, suggesting a sympathetic pathway (Fig. 4A). The effects of IES on pyloric contractions were similar (Fig. 4B).
Fig. 3.
Typical antral manometric traces after a solid meal. A: control with infusion of saline. B: infusion of l-NNA. C: infusion of phentolamine.
Fig. 4.
Effect of IES on antral and pyloric contractions induced by a solid meal. A: IES significantly inhibited antral contractions (P < 0.05). This inhibition was not blocked by l-NNA but was blocked by phentolamine, suggesting involvement of the sympathetic pathway. B: IES significantly inhibited pyloric contractions (P < 0.05). This inhibition was not blocked by l-NNA.
Table 1.
Effects of IES on AUC of contractions after a solid meal during baseline, treatment, IES, and recovery in the antropyloroduodenal region
|
AUC, mmHg/s |
||||
|---|---|---|---|---|
| Baseline | Treatment | IES | Recovery | |
| Antrum | ||||
| Control | 6.7±3.4 | 6.8±2.9 | 4.8±2.3 | 5.4±2.7 |
| l-NNA iv | 10.9±5.8 | 11.9±5.4 | 5.5±1.0 | 11.4±11.7 |
| Phentolamine | 10.1±3.8 | 12.3±1.6 | 12.2±1.5 | 10.3±3.1 |
| Pylorus | ||||
| Control | 7.1±4.2 | 11.31±6.4 | 4.7±3.7 | 11.3±8.7 |
| l-NNA iv | 10.5±5.0 | 32.3±3.5† | 4.0±2.0* | 33.6±15.0† |
Values (means ± SD) are expressed as area under the curve (AUC) during 4 consecutive 20-min periods [baseline, treatment, intestinal electrical stimulation (IES), and recovery]. Saline (control) or N-nitro-l-arginine (l-NNA) was administered during treatment period.
P < 0.05 vs. treatment or recovery.
P < 0.05 vs. control.
The liquid meal did not induce obvious antral contractions. The AUC of the postprandial antral contractions was 1.9 ± 0.5, which was not different from the fasting data. Accordingly, the inhibitory effect of IES could not be determined because of the absence of antral contractions.
Effects and nitrergic mechanism of IES on pyloric tone.
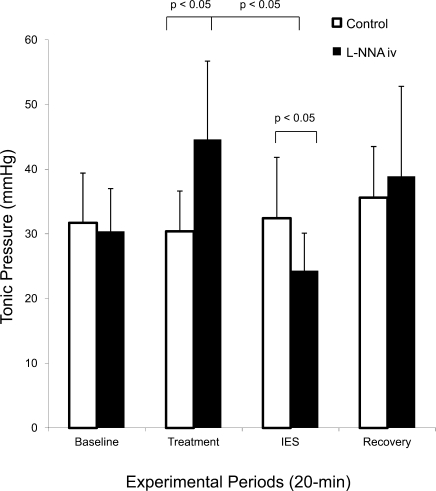
The effects of IES and l-NNA are presented in Fig. 5. In the control session (without l-NNA), IES did not affect pyloric tone, which was 31.7 ± 7.7 mmHg at baseline, 30.4 ± 6.6 mmHg during saline infusion, 32.4 ± 9.4 mmHg during IES, and 35.6 ± 7.9 mmHg during recovery (P > 0.05). No significant difference was noted. In another session, pyloric tone was 30.4 ± 6.6 mmHg at baseline, increased to 44.6 ± 12.4 mmHg with administration of l-NNA (P < 0.05 vs. the same period in the saline session), decreased to 24.3 ± 5.8 mmHg during IES (P < 0.05 vs. l-NNA infusion period or IES without l-NNA), and recovered to 38.9 ± 13.9 mmHg during recovery.
Fig. 5.
Effects of IES on tonic pressure of the pylorus with and without l-NNA. Neither saline nor IES affected pyloric tone. l-NNA increased pyloric tone, whereas IES reduced pyloric tone compared with the period immediately before IES in the same session and the same period in the control session, suggesting an inhibitory effect of IES on pyloric tone in the presence of l-NNA.
These data indicate that although IES did not affect pyloric tone in the absence of l-NNA, it significantly reduced pyloric tone in the presence of l-NNA.
Effects and mechanisms of IES on gastric emptying.
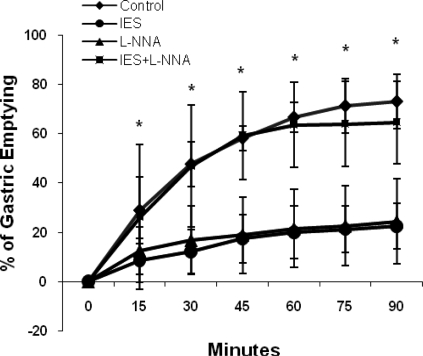
IES significantly delayed gastric emptying, and this inhibitory effect was blocked by l-NNA. IES alone or l-NNA alone delayed gastric emptying, whereas gastric emptying in the IES + l-NNA session was almost normal compared with the control session and significantly faster than in the IES-only session (Fig. 6). Gastric emptying at 90 min was 72.9 ± 10.9% in the control session, 22.4 ± 9.1% with IES (P < 0.001 vs. control), 24.4 ± 17.2% with administration of l-NNA (P < 0.01 vs. control), and 64.6 ± 16.8% with IES + l-NNA (P = 0.4 vs. control, P < 0.01 vs. IES).
Fig. 6.
Effect of IES on liquid gastric emptying at 0–90 min. IES and l-NNA delayed gastric emptying at all time points (P < 0.01); however, in the presence of l-NNA, IES failed to delay gastric emptying (P < 0.01 vs. IES + l-NNA), suggesting involvement of the nitrergic pathway in the inhibitory effect of IES on gastric emptying.
DISCUSSION
In this study, we found that IES reduced gastric tone, inhibited antral contractions, did not affect pyloric tone, and delayed liquid gastric emptying. The mechanistic studies indicated that the inhibitory effects of IES on gastric tone, antral contractions, and gastric emptying were blocked by l-NNA, phentolamine, and l-NNA, respectively. These data suggest that the inhibitory effect of IES on gastric tone is mediated by the nitrergic pathway, the inhibitory effect of IES on antral contractions is mediated by the sympathetic pathway, and the inhibitory effect of IES on gastric emptying is attributed to the reduced gastric tone.
Electrical stimulation of the duodenum was first reported to delay gastric emptying of nonnutrient liquid meals in dogs by Kelly and Code in 1977 (28). Liquid gastric emptying was delayed by 25% during electrical stimulation of the small intestine. Subsequently, IES was successfully used to treat dumping syndrome after gastrectomy by slowing gastric emptying (18, 34, 35). However, the mechanism of IES-induced delay of gastric emptying had not been previously investigated. In the present study, we confirmed the previous findings of IES-induced delay of gastric emptying and also found that l-NNA restored the IES-induced delay of gastric emptying. This suggested that the IES-mediated reduction of gastric tone played a major role in the IES-induced delay of gastric emptying: IES reduced gastric tone and led to a delay of gastric emptying, whereas l-NNA blocked the inhibitory effect of IES on gastric tone, and, therefore, gastric emptying was not delayed.
Nitric oxide is a well-known inhibitory neurotransmitter of gastrointestinal motility. It has been shown that nitric oxide synthase is present in the enteric nervous system (3, 10, 12). Electrical field stimulation induced the release of nitric oxide in muscle strips of the small intestine in vitro (1). This finding leads to the logical assumption that electrical stimulation of the small intestine induces the production of nitric oxide, which might be responsible for suppressed gastric motility and delayed gastric emptying. Furthermore, previous studies showed that l-NNA increased contractile activity and tonic pressure in the antrum (37), pylorus (7), and duodenum (8). However, liquid and solid gastric emptying was delayed by l-NNA (23, 44). It has been postulated that l-NNA delays gastric emptying by blocking the relaxation effect on the pylorus, which results in increased pyloric resistance. In addition, it abolishes the inhibitory effects on duodenal contractions, which results in increased duodenal brake. An increase in pyloric tone was noted with l-NNA compared with saline. We were surprised to find that pyloric tone was significantly reduced by IES in the presence of l-NNA. This phenomenon has not been reported in the literature and deserves further investigation.
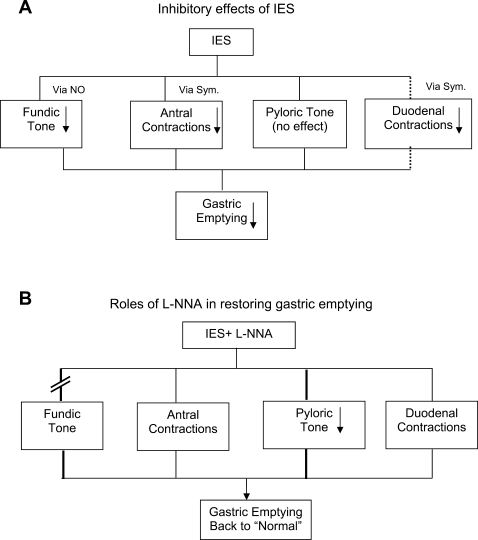
Gastric emptying is controlled by various motility components working in concert: the propulsive components for gastric emptying (fundic tone and antral contractions) vs. the resistive components (pyloric tone and duodenal resistive contractions). However, studying motility in the antropyloroduodenal region is a technical challenge. In our unique dog model, a catheter with a 4-cm-long sleeve was inserted into the pylorus via a duodenal cannula with a known fixed length. Using the push-through technique, we were able to accurately position the sleeve astride the pylorus. Only an ∼20-cm segment of the catheter was inserted. The catheter was fixed to the cannula to prevent dislocation of the sleeve from the pylorus. New findings reported in the present study are illustrated in Fig. 7. The inhibitory effect of IES on gastric emptying was mediated via 1) inhibition of fundic tone via the nitrergic pathway, 2) inhibition of antral contractions via the sympathetic pathway, and 3) inhibition of duodenal contractions via the sympathetic pathway (Fig. 7A), as shown in a previous study (33). The reduction of duodenal motility slows intestinal transit and, therefore, increases duodenal resistance, contributing to delay of gastric emptying. The role of l-NNA in prevention of IES-induced delay of gastric emptying is illustrated in Fig. 7B: 1) l-NNA blocks the nitrergic pathway and, therefore, blocks the inhibitory effect of IES on gastric tone and 2) l-NNA + IES reduces pyloric tone (resistance). Regarding the role of sympathetic mechanisms of IES in antral contractions, it should be noted that, in a previous study, GES was found to inhibit antral contractions mediated through α- and β-adrenergic pathways (39). Although it was not investigated, involvement of the β-adrenergic pathway could not be ruled out. It is reasonable to speculate that the mechanisms involved in IES are the same as those involved in GES.
Fig. 7.
Mechanisms involved in the inhibitory effect of IES on gastric emptying. A: IES reduced fundic tone via the nitrergic [nitric oxide (NO)] pathway, reduced antral contractions via the sympathetic (Sym) pathway, had no effect on pyloric tone, and reduced duodenal contractions via the sympathetic pathway. All these inhibitory effects resulted in delayed gastric emptying. B: l-NNA blocked the inhibitory effect of IES on gastric tone, reduced pyloric tone, and almost restored gastric emptying.
The physiological observations and mechanistic findings of the present study are of great clinical importance. In a previous clinical study in healthy volunteers (32), IES applied via ring electrodes attached to the tip of a nasojejunal feeding tube placed in the duodenum resulted in a decrease in water intake and delay of solid gastric emptying. On the basis of the findings of the present study, the decreased water intake with IES might be attributed to a decrease of gastric tone or an increase of gastric volume with IES, inasmuch as an increase of gastric volume was reported to be correlated with a decrease of food intake in dogs (40). The findings of the present study suggest that the delay of gastric emptying of solids with IES might be caused by a decrease of antral contractions.
The inhibitory effects of IES on various gastric functions observed in the present study support the concept of IES therapy for obesity, although we provide no direct data. Limitation of food intake and reduction of absorption are two major goals in the development of obesity treatment modalities. The induction of gastric distension (or reduced gastric tone) and delay of gastric emptying have been reported to reduce food intake (51). In addition, a few recent animal and human studies have shown an inhibitory effect of IES on fat absorption. In a rodent study, IES accelerated intestinal transit and reduced fat absorption (43). Similar acceleration of intestinal transit and reduction of fat absorption were noted in a clinical study in healthy volunteers (31). Taken together, these findings suggest that IES might be a better therapy for obesity than GES, which has been shown to delay gastric emptying and reduce food intake but to have no effects on intestinal transit or nutrient absorption. However, systematic studies are needed to compare the efficacy of GES and IES in the treatment of obesity.
In conclusion, IES reduces gastric tone via a nitrergic mechanism, inhibits antral contractions via the adrenergic pathway, exerts no effects on pyloric tone, and delays liquid gastric emptying. The IES-induced delay of gastric emptying is attributed to its inhibitory effects on gastric motility.
Perspectives and Significance
The present study was designed to investigate the effects of IES on gastric motility, including gastric tone, antral contractions, pyloric tone, liquid gastric emptying, and possible mechanisms. We have found that IES reduced gastric tone via the nitrergic pathway, inhibited postprandial antral contractions via the sympathetic pathway, and delayed liquid gastric emptying.
GRANTS
This work was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-063733.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Allescher HD, Tougas G, Vergara P, Lu S, Daniel EE. Nitric oxide as a putative nonadrenergic noncholinergic inhibitory transmitter in the canine pylorus in vivo. Am J Physiol Gastrointest Liver Physiol 262: G695–G702, 1992. [DOI] [PubMed] [Google Scholar]
- 2.Andersson S, Nylander B. Identification of the gastroduodenal junction by potential difference measurements. Scand J Gastroenterol Suppl 35: 83–87, 1975. [PubMed] [Google Scholar]
- 3.Anvari M, Paterson CA, Daniel EE. Role of nitric oxide mechanisms in control of pyloric motility and transpyloric flow of liquids in conscious dogs. Dig Dis Sci 43: 506–512, 1998. [DOI] [PubMed] [Google Scholar]
- 4.Arcila D, Velazquez D, Gamino R, Sierra M, Salin-Pascual R, Gonzalez-Barranco J, Herrera MF. Quality of life in bariatric surgery. Obes Surg 12: 661–665, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Asakawa A, Inui A, Ueno N, Makino S, Fujino MA, Kasuga M. Urocortin reduces food intake and gastric emptying in lean and ob/ob obese mice. Gastroenterology 116: 1287–1292, 1999. [DOI] [PubMed] [Google Scholar]
- 6.Asakawa A, Inui A, Ueno N, Makino S, Uemoto M, Fujino MA, Kasuga M. ob/ob Mice as a model of delayed gastric emptying. J Diabetes Complications 17: 27–28, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Bayguinov O, Sanders K. Role of nitric oxide as an inhibitory neurotransmitter in the canine pyloric sphincter. Am J Physiol Gastrointest Liver Physiol 264: G975–G983, 1993. [DOI] [PubMed] [Google Scholar]
- 8.Bayguinov O, Vogalis F, Morris B, Sanders K. Patterns of electrical activity and neural responses in canine proximal duodenum. Am J Physiol Gastrointest Liver Physiol 263: G887–G894, 1992. [DOI] [PubMed] [Google Scholar]
- 9.Blachar A, Federle MP. Gastrointestinal complications of laparoscopic roux-en-Y gastric bypass surgery in patients who are morbidly obese: findings on radiography and CT. Am J Roentgenol 179: 1437–1442, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Boeckxstaens GE, Pelckmans PA, Ruytijens IF, Bult H, De Man JG, Herman AG, Van Maerche YM. Bioassay of nitric oxide released upon stimulation of non-adrenergic non-cholinergic nerves in the canine ileocolonic junction. Br J Pharmacol 103: 1085–1091, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bray GA, Tartaglia LA. Medicinal strategies in the treatment of obesity. Nature 404: 672–677, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Bult H, Boechxstaens GE, Pelckmans PA, Jordaens FH, Van Maerche YM, Herman AG. Nitric oxide as an inhibitory non-adrenergic non-cholinergic neurotransmitter. Nature 345: 346–347, 1990. [DOI] [PubMed] [Google Scholar]
- 13.Camilleri MM, Brown ML, Becher G, Zingsmeister AR. Relation between antral motility and gastric emptying of solids and liquids in humans. Am J Physiol Gastrointest Liver Physiol 249: G580–G585, 1985. [DOI] [PubMed] [Google Scholar]
- 14.Chiloiro M, Caroli M, Guerra V, Lodadea Piepoli A, Riezzo G. Gastric emptying in normal weight and obese children—an ultrasound study. Int J Obes Relat Metab Disord 23: 1303–1306, 1999. [DOI] [PubMed] [Google Scholar]
- 15.Cigaina V Gastric pacing as therapy for morbid obesity: preliminary results. Obes Surg 12 Suppl 1: 12S–16S, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Cigaina V, Hirschberg AL. Gastric pacing for morbid obesity: plasma levels of gastrointestinal peptides and leptin. Obes Res 11: 1456–1462, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Dent J, Sun WM, Anvari M. Modulation of pumping function of gastric body and antropyloric contractions. Dig Dis Sci 39: 28S–31S, 1994. [DOI] [PubMed] [Google Scholar]
- 18.Eagon JC, Miedema BW, Kelly KA. Postgastrectomy syndromes. Surg Clin North Am 72: 445–465, 1992. [DOI] [PubMed] [Google Scholar]
- 19.Gilja OH, Heimdal A, Hausken T, Gregersen H, Matre K, Berstad A, Odegaard S. Strain during gastric contractions can be measured using Doppler ultrasonography. Ultrasound Med Biol 28: 1457–1465, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Glatzle J, Kalogeris TJ, Zittel TT, Guerrini S, Tso P, Raybould HE. Chylomicron components mediate intestinal lipid-induced inhibition of gastric motor function. Am J Physiol Gastrointest Liver Physiol 282: G86–G91, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Goldner WS, O'Dorisio TM, Dillon JS, Mason EE. Severe metabolic bone disease as a long-term complication of obesity surgery. Obes Surg 12: 685–692, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Gregersen H, Gilja OH, Hausken T, Heimdal A, Gao C, Matre K, Odegaard S, Berstad A. Mechanical properties in the human gastric antrum using B-mode ultrasonography and antral distension. Am J Physiol Gastrointest Liver Physiol 283: G368–G375, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Haba T, Sarna SK. Regulation of gastroduodenal emptying of solids by gastropyloroduodenal contractions. Am J Physiol Gastrointest Liver Physiol 264: G261–G271, 1993. [DOI] [PubMed] [Google Scholar]
- 24.Hausken T, Mundt M, Samsom M. Low antroduodenal pressure gradients are responsible for gastric emptying of a low-caloric liquid meal in humans. Neurogastroenterol Motil 14: 97–105, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Heddle R, Miedema BW, Kelly KA. Integration of canine proximal gastric, antral, pyloric, and proximal duodenal motility during fasting and after a liquid meal. Dig Dis Sci 38: 856–869, 1993. [DOI] [PubMed] [Google Scholar]
- 26.Huge A, Weber E, Ehrlein HJ. Effects of enteral feedback inhibition on motility, luminal flow, and absorption of nutrients in proximal gut of minipigs. Dig Dis Sci 40: 1024–1034, 1995. [DOI] [PubMed] [Google Scholar]
- 27.Indireshkumar K, Brasseur JG, Faas H, Hebbard GS, Kunz P, Dent J, Feinle C, Li M, Boesiger P, Fried M, Schwizer W. Relative contributions of “pressure pump” and “peristaltic pump” to gastric emptying. Am J Physiol Gastrointest Liver Physiol 278: G604–G616, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Kelly KA, Code CF. Duodenal-gastric reflux and slowed gastric emptying by electrical pacing of the canine duodenal pacesetter potential. Gastroenterology 72: 429–433, 1977. [PubMed] [Google Scholar]
- 29.Klein S Outcome success in obesity. Obes Res Suppl 4: 354S–358S, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Lin HC, Doty JE, Reedy TJ, Meyer JH. Inhibition of gastric emptying by sodium oleate depends on length of intestine exposed to nutrient. Am J Physiol Gastrointest Liver Physiol 259: G1031–G1036, 1990. [DOI] [PubMed] [Google Scholar]
- 31.Liu JS, Qiao X, Zha H, Song GQ, Zhang Y, Gao Z, Hou XH, Chen JDZ. Effects of intestinal pacing on small bowel transit and nutrient absorption in healthy volunteers. Obes Surg. In press. [DOI] [PubMed]
- 32.Liu S, Hou XH, Chen JDZ. Therapeutic potential of duodenal electrical stimulation for obesity: effects on satiety and gastric emptying. Am J Gastroenterol 100: 792–796, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Liu S, Liu JS, Chen JDZ. Neural mechanisms involved in the inhibition of intestinal motility induced by intestinal electrical stimulation in conscious dogs. Neurogastroenterol Motil 18: 62–68, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Miedema BW, Kelly KA. The Roux stasis syndrome. Treatment by pacing and prevention by use of an “uncut” Roux limb. Arch Surg 127: 295–300, 1992. [DOI] [PubMed] [Google Scholar]
- 35.Morrison PD, Kelly KA. Increasing antidumping effect of intestinal pacing with motor-active agents. Dig Dis Sci 31: 422–427, 1986. [DOI] [PubMed] [Google Scholar]
- 36.Mundt MW, Hausken T, Samsom M. Effect of intragastric barostat bag on proximal and distal gastric accommodation in response to liquid meal. Am J Physiol Gastrointest Liver Physiol 283: G681–G686, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Ohta D, Lee CW, Sarna SK, Condon RE, Lang IM. Central inhibition of nitric oxide synthase modulates upper gastrointestinal motor activity. Am J Physiol Gastrointest Liver Physiol 272: G417–G424, 1997. [DOI] [PubMed] [Google Scholar]
- 38.Okada S, Onai T, Kilroy G, York DA, Bray GA. Adrenalectomy of the obese Zucker rat: effects on the feeding response to enterostatin and specific mRNA levels. Am J Physiol Regul Integr Comp Physiol 265: R21–R27, 1993. [DOI] [PubMed] [Google Scholar]
- 39.Ouyang H, Xing J, Chen JDZ. Tachygastria induced by gastric electrical stimulation is mediated via α- and β-adrenergic pathway and inhibits antral motility in dogs. Neurogastroenterol Motil 17: 846–853, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Ouyang H, Yin J, Chen JDZ. Gastric or intestinal electrical stimulation-induced increase in gastric volume is correlated with reduced food intake. Scand J Gastroenterol 41: 1261–1266, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Ouyang H, Yin J, Wang Z, Pasricha PJ, Chen JD. Electroacupuncture accelerates gastric emptying in association with changes in vagal activity. Am J Physiol Gastrointest Liver Physiol 282: G390–G396, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Podnos YD, Jimenez JC, Wilson SE, Stevens CM, Nguyen NT. Complications after laparoscopic gastric bypass: a review of 3,464 cases. Arch Surg 138: 957–961, 2003. [DOI] [PubMed] [Google Scholar]
- 43.Sun Y, Chen JDZ. Intestinal electrical stimulation decreases fat absorption in rats: therapeutic potential for obesity. Obesity 12: 1235–1242, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanaka T, Mizumoto A, Haga N, Itoh Z. A new method to measure gastric emptying in conscious dogs: a validation study and effect of EM523 and l-NNA. Am J Physiol Gastrointest Liver Physiol 272: G909–G915, 1997. [DOI] [PubMed] [Google Scholar]
- 45.Tefera S, Gilja OH, Olafsdottir E, Hausken T, Hatlebakk JG, Berstad A. Intragastric maldistribution of a liquid meal in patients with reflux oesophagitis assessed by three-dimensional ultrasonography. Gut 50: 153–158, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tolonen P, Victorzon M. Quality of life following laparoscopic adjustable gastric banding—the Swedish band and the Moorehead-Ardelt questionnaire. Obes Surg 13: 424–426, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Treacy PJ, Jamieson GG, Dent J. Pyloric motor function during emptying of a liquid meal from the stomach in the conscious pig. J Physiol 422: 523–538, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Treacy PJ, Jamieson GG, Dent J. The effect of duodenal distension upon antro-pyloric motility and liquid gastric emptying in pigs. Aust NZ J Surg 66: 37–40, 1996. [DOI] [PubMed] [Google Scholar]
- 49.Wright RA, Krinsky S, Fleeman C, Trujillo J, Teague E. Gastric emptying, obesity. Gastroenterology 84: 747–751, 1983. [PubMed] [Google Scholar]
- 50.Yin J, Ouyang H, Chen JDZ. Therapeutic potential of intestinal electrical stimulation for obesity. A preliminary canine study. Obesity 15: 1133–1138, 2007. [DOI] [PubMed] [Google Scholar]
- 51.Yin J, Zhang J, Chen JD. Inhibitory effects of intestinal electrical stimulation on food intake, weight loss, and gastric emptying in rats. Am J Physiol Regul Integr Comp Physiol 293: R78–R82, 2007. [DOI] [PubMed] [Google Scholar]
- 52.Zahorska-Markiewicz B, Jonderko K, Lelek A, Skrzypek D. Gastric emptying in obesity. Hum Nutr Clin Nutr 40: 309–313, 1986. [PubMed] [Google Scholar]