Abstract
CCK and apolipoprotein AIV (apo AIV) are gastrointestinal satiety signals whose synthesis and secretion by the gut are stimulated by fat absorption. Intraperitoneally administered CCK-8 is more potent in suppressing food intake than a similar dose administered intravenously, but the reason for this disparity is unclear. In contrast, both intravenous and intraperitoneally administered apo AIV are equally as potent in inhibiting food intake. When we compared the lymphatic concentration of CCK-8 and apo AIV, we found that neither intraperitoneally nor intravenously administered CCK-8 or apo AIV altered lymphatic flow rate. Interestingly, intraperitoneal administration of CCK-8 produced a significantly higher lymphatic concentration at 15 min than did intravenous administration. Intraperitoneal injection of apo AIV also yielded a higher lymphatic concentration at 30 min than did intravenous administration. Intraperitoneal administration of CCK-8 and apo AIV also resulted in a much longer period of elevated CCK-8 and apo AIV peptide concentration in lymph than intravenous administration. Furthermore, enzymatic activity of dipeptidyl peptidase IV (DPPIV) and aminopeptidase was higher in plasma than in lymph during fasting, and so, satiation peptides, such as CCK-8 and apo AIV in the lymph, are protected from degradation by the significantly lower DPPIV and aminopeptidase activity levels in lymph than in plasma. Therefore, the higher potency of intraperitoneally administered CCK-8 compared with intravenously administered CCK-8 in inhibiting food intake may be explained by both its higher concentration in lymph and the prolonged duration of its presence in the lamina propria.
Keywords: lymph, plasma, dipeptidyl peptidase IV, aminopeptidase
obesity is widespread in developed countries, and the prevalence of obesity continues to increase rapidly in the United States (3). Obesity develops when caloric intake exceeds energy expenditure over time, and the excess energy is stored as fat (10). Gastrointestinal hormones, namely CCK and apolipoprotein AIV (apo AIV), contribute to energy homeostasis by curtailing food intake. CCK-8 and CCK-22 are the predominant forms in rats and mice, whereas larger molecular forms (CCK-33 and CCK-58) are present in human plasma (32, 35, 47). Intestinal endocrine cells secrete a mixture of medium-sized CCK forms, whereas central and peripheral neurons mainly release sulfated forms of CCK-8 (45, 46). Intestinal I cells produce and secrete CCK in response to ingestion of either lipid or protein (34). Peripheral CCK is involved in modulating intestinal motility, stimulating pancreatic enzyme secretion, inducing gallbladder contraction, and regulating food intake (8, 18, 22, 25, 41, 44, 50).
Apolipoprotein AIV (apo AIV) is a glycoprotein found in many species; the human form has a molecular weight of 46 kDa (3, 16, 20). Serum apo AIV concentration levels in rats have been demonstrated to fluctuate, like food intake, according to circadian rhythms, increasing during the dark cycle to significantly higher levels, (∼148.8 μg/ml) than exhibited during the light cycle (∼121.1 μg/ml) (16). The jejunum is the major site of apo AIV synthesis, and its synthesis and secretion are associated with chylomicron formation (12, 23, 27). Once chylomicrons enter the circulation, apo AIV rapidly dissociates from chylomicron remnants, with the result that the majority of apo AIV in the circulation exists as free protein, while the remainder is associated with circulating high-density lipoproteins (17). Our laboratory has reported that intravenous administration of apo AIV purified from lymph reduces food intake (15). Later studies have also demonstrated that central administration of both native and recombinant apo AIV inhibit food intake (15, 36).
The fact that intraperitoneally administered CCK is able to reduce food intake is well established (31, 49). In contrast, intraportal administration of CCK-8 of up to 8 μg/kg has been shown to exert little satiation effect in rats (19, 21, 24); however, further study has shown that continuous injections of synthetic CCK-8 in pigs through various injection sites induced varying satiation effects (24). The reason for this apparent difference in efficacy is unclear. We hypothesized that intraperitoneally administered CCK-8 would result in a higher concentration of the peptide in the lamina propria of the gut than would intravenously administered CCK-8. In this study, we used the lymph fistula rat model to test our hypothesis that the concentration of lymphatic CCK-8 (a reflection of lamina propria concentration) would be higher as a result of intraperitoneal administration rather than intravenous administration. We believe this is why intraperitoneal administration of CCK-8 is more effective in reducing meal size than intravenous administration of the peptide. We also included apo AIV in our study because it is another satiation peptide with a larger molecular size than CCK-8, and we wished to determine whether or not the same scenario applied to bigger proteins such as apo AIV.
MATERIAL AND METHODS
Male adult Sprague-Dawley (SD) rats (300–350 g) were fed pelleted standard chow diet (LM-485) obtained from Harlan Sprague Dawley (Indianapolis, IN). Sulfated CCK-8, 4-nitroaniline, and Gly-Pro p-nitroanilide p-toluenesulfonate were obtained from Sigma (St. Louis, MO). Sulfated 125I-CCK-8 was purchased from GE Healthcare Life Sciences (Piscataway, NJ). All chemicals used for the study were of analytical grade.
Preparation of 35S-labeled recombinant apoA-IV. 35S-methionine was used to radioactively label recombinant apo A-IV produced in our laboratory (36). Apo A-IV has six methionine amino acids per protein molecule that can be labeled. Briefly, a pET30 vector (Novagen, Madison, WI) containing a rat apoA-IV cDNA insert was transformed into B834 (DE3) Escherichia coli cells (Novagen), and the transformed cells were plated on M9 minimal media (18.7 mM ammonium chloride, 40.9 mM dibasic sodium phosphate, 22 mM monobasic potassium phosphate, 2 mM magnesium chloride, 0.1 mM calcium chloride, 0.4% glucose/dextrose, 0.0001% thiamine) plates containing kanamycin as the selection agent, and which were supplemented with the amino acids Thr, Leu, Pro, Arg, and Met (Sigma-Aldrich, St. Louis. MO). The following day, a single colony was picked for expansion into 10 ml M9 media supplemented with the same amino acids and kanamycin as described above (Calbiochem, San Diego, CA). The 10-ml culture was incubated overnight, and the next day, 1 ml of the overnight cultures was further expanded into 100 ml of M9 media + amino acids + kanamycin for 3 h or until the OD600 was 0.5. The cells were then pelleted by centrifugation at 8,000 rpm for 10 min, rinsed by resuspension in M9 media alone, repelleted, and resuspended in 100 ml of M9 media plus Thr, Leu, Pro, Arg, and 35S-Met (GE Healthcare, UK). 50 μl of 1 M IPTG (Fisher, Hampton, NH) was added for induction of expression. Cells were incubated with IPTG for 1 h, while shaking at 37°C. The resultant protein was purified by nickel column chromatography (Novagen, Madison, WI) utilizing the protein's N-terminal His tag.
Surgical procedure: 1. surgical and experimental procedure for intravenous and intraperitoneal study.
Male SD rats (n = 5 or 6) per group were used in this study. The animals were fasted overnight prior to surgery. All animal protocols used in this study were approved by the University of Cincinnati Institutional Animal Care and Use Committee. For the lymphatic cannulation, the animals were anesthetized with halothane (Halocarbon Laboratories, River Edge, NJ) administered through a vaporizer. A laparotomy was performed and the superior mesenteric lymph duct was cannulated with a polyvinylchloride tube (medical grade, 0.5 mm ID, 0.8 mm OD; Critchley Products, Silverwater, New South Wales, Australia) according to the procedure described by Bollman et al. (4). The tube was secured with a drop of cyanoacrylate glue (Krazy Glue, Jadow & Sons, New York, NY) and externalized through the right flank. A second cannula (1.02 mm ID, 2.16 mm OD; Silastic medical grade 508–005, Dow Corning Medical Products, Midland, MI) was threaded through a surgical incision of the fundus of the stomach, extended 2 cm into the duodenum, secured with a purse-string suture and a drop of cyanoacrylate glue, and externalized through the right flank. After surgery, the animals were placed in Bollman restraining cages in a temperature-regulated chamber maintained at 28–30°C and allowed to recover overnight. During recovery, the animals received continuous intraduodenal infusion of a glucose-saline solution (145 mM NaCl, 4 mM KCl, and 280 mM glucose) at 3 ml/h to compensate for fluid and electrolyte loss due to lymphatic sampling.
The following day, the glucose/saline solution was replaced with the 0.9% saline used for the duration of the experiments. Fasting lymph samples were collected 1 h prior to administration of either labeled CCK or apo AIV. For the intravenous administration study, a PVC tube was installed in the jugular vein of the rats and threaded into the right atrium during the lymphatic cannulation surgery described above. The cannula was secured in place with sutures, and the other end of the cannula was exteriorized above the animal's neck and plugged. For the intraperitoneal experiment, a tube was introduced into the lower left quadrant of the abdomen, and the cannula exteriorized through the right frank.
Lymphatic labeled CCK-8 study.
All animals were housed in restraining cage and a bolus (245 μl) of a mixture of sulfated CCK-8 (0.6 μg/kg) plus 125I-CCK (0.5 μCi /5 μl) was administered to the rats via either intravenous or intraperitoneal injection. Following either intravenous or intraperitoneal delivery, lymph samples were collected at 15 min intervals throughout the 1st h and then hourly for an additional 5 h. Throughout the experiment, 0.9% saline was infused into duodenum at 3 ml/h to compensate for fluid and electrolyte loss during lymph sampling. Lymphatic flow rates were determined. 125I-CCK specific radioactivity of lymph samples at each time point was determined using a gamma counter, and since the lymph volume was known, we were also able to determine the concentration of labeled CCK. Although the stability of the iodination tag of the CCK-8 might be construed as a potential concern, as will be presented later, the activity of degradative enzymes, such as DPPIV and aminopeptidase, are so much lower in lymph than in plasma that the radiolabeled tags were unlikely to be dissociated from the peptides under these conditions. Therefore, both radiolabeled CCK and apo AIV remained stable and intact probes, as confirmed by previous metabolic studies (5, 13).
Lymphatic labeled apo AIV study.
A bolus injection (245 μl) of 35S-apo AIV (1.5 mg/ml) was administered intravenously or intraperitoneally in lymph fistula rats. In the present study, we used a higher dose of apo AIV per kilogram body wt than we used during the intraperitoneal feeding studies. This higher dose was chosen for a number of reasons, as explained below. First, the labeling of apo AIV is limited because there are six methionines per every molecule of apo AIV, and furthermore, the labeling of the apo AIV is also limited by the specific activity of the 35S methionine as provided by the manufacturer. We used the labeled apo AIV as such without any dilution. The amount of labeled apo AIV that we used was deemed to be the smallest amount that would still provide us with adequate detection sensitivity. To use any lesser amount of 35S-labeled apo AIV would have diminished the ability to detect apo AIV. Second, the plasma apo AIV concentration in the rat is 10–24 mg/dl and increases following lipid feeding (9, 13, 16, 33). In this study, 1.17 mg/kg of recombinant apo AIV was applied to each animal, a small enough dose that it should not impact the amount of circulating apo AIV pool significantly. After delivery, lymph samples were collected at 15-min intervals throughout the 1st h and then hourly for the next 5 h. The lymphatic flow rates of these animals were also determined. 35S-apo AIV radioactivity of lymph samples at each time point was assessed by liquid scintillation counting, and the concentration of lymphatic apo AIV was calculated by dividing the dpm by the lymph volume.
Surgical procedures. 2. surgical and experimental protocol for studying the effect of CCK-8 and apo AIV on food suppression.
For the food intake studies, adult male rats were individually housed in cages with corn cob bedding at an American Association for the Accreditation of Laboratory Animal Care-accredited facility under conditions of controlled illumination (12:12-h light-dark cycle, lights from 0600 to 1800). Each animal was implanted with either an intravenous infusion or intraperitoneal infusion cannula. For the intravenous administration study, a PVC tube was installed in the jugular vein of the rats and threaded into the right atrium during the lymphatic cannulation surgery described above. The cannula was secured in place with sutures and the other end of the cannula was exteriorized above the animal's neck and plugged. For the intraperitoneal experiment, a tube was introduced into the lower left quadrant of the abdomen, and the cannula exteriorized through the right frank.
After recovering for 7 days after surgery, each rat had returned to its initial, presurgery body weight. Animals were transferred to clean bedding cages and were deprived of food for 16 h prior to the beginning of the study at 0930. The fasted animals received a bolus dose, via the intraperitoneal or the intravenous cannula, of 0.25 ml of either saline alone or saline combined with different doses of dissolved sulfated CCK-8 and apo AIV. For the CCK feeding experiments, experimental rats received one of the following doses of sulfated CCK-8 (0.25, 0.6 and 1.0 μg/kg) or saline 5 min prior to refeeding. For the apo AIV study, animals received intraperitoneally or intravenously a bolus dose of 100, 200, or 500 μg/kg 5 min prior to refeeding. Animals were then allowed free access to rodent chow diet and their food intake was measured at 30, 60, and 120 min by weighing their food cups.
Surgical procedures. 3. Surgical and experimental procedure for dipeptidyl peptidase IV (DPPIV) and aminopeptidase activities.
For this study, male SD rats (4–6 per group) were subjected to 16 h of fasting before surgery to determine plasma and lymphatic DPPIV . The next morning, under halothane anesthesia, a PVC tube was introduced into the jugular vein of the rats. A PVC tube was also inserted into the main mesenteric lymph duct of the rats as described above (23), and a soft silicone tube was introduced into duodenum and the incision closed as described above. Postoperative care of the animals was the same as described above and the animals recovered overnight prior to experiment. Fasting plasma and lymph samples were collected at 0900 the morning following surgery.
DPPIV activity.
DPPIV enzyme activity was determined using an enzymatic assay that measures levels of 4-nitroaniline liberated from the DPPIV substrate by DDPIV (42). Briefly, 50 μl of fasting plasma or lymph was incubated with 1 ml of 1.4 mM Gly-Pro-p-nitroanilide p-toluenesulfonate salt as the enzyme substrate in 114 mM Tris buffer, pH 8.0. Standard (150 nmol of 4-nitroaniline in water) and blank (water) samples were assayed within the same plate. After incubating in an oven for 30 min at 37°C, the reaction was stopped by adding 3 ml of 1 M acetate buffer (pH 4.2). Enzyme activity was calculated by measuring the observed increase in specific absorbance at 410 nm and then by multiplying the ratio (absorbance of samples-blank)/(absorbance of standard sample) by 150 nmol to yield data expressed as nanomoles per milliliter per minute (42).
Aminopeptidase activity.
The samples used for DPPIV determination were also used for aminopeptidase enzyme activity determination. The enzymatic assay measured levels of 2-naphthylamine released as a result of aminopeptidase activity (1, 29). Briefly, either 2 μl of fasting lymph or 2 μl of 2 × diluted plasma was incubated with 100 μl of 0.1 mg/ml l-tyrosine β-naphthylamide (Sigma) as the substrate in 50 mM HCl-Tris buffer (pH 7.6, Fisher Scientific, Fair Lawn, NJ) at 37°C for 2 h. The reaction was stopped by adding 100 μl of 0.1 M acetate buffer (pH 4.2). Standard samples (2 μl of 1.4E-06 to 1.4 μg 2-naphthylamine in 50 mM Tris buffer) and the blank (ethanol or 50 mM Tris buffer) were assayed within the same plate. The amount of 2-naphthylamine released was measured fluorometrically at an excitation wavelength of 360 nm with an emission wavelength of 412 nm. Specific aminopeptidase activity for tyrosine was expressed as picomole of tyrosine-naphthylamide hydrolyzed per minute per milliliter of either plasma or lymph.
Statistical analysis.
Results are presented as means ± SE. Parametric statistical analyses were performed for comparison of all groups of animals subjected to the study treatments. Two-way ANOVA was used for comparison of feeding studies via the two routes of administration and the different doses tested. Statistical analyses were performed using GraphPad Prism (ver. 3.0, San Diego, CA), and differences were considered significant if P values were <0.05.
RESULTS
Effect of CCK-8 on food suppression.
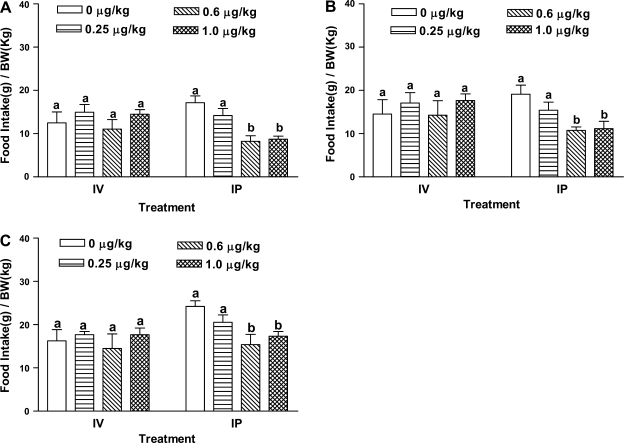
Fasted rats intravenously injected with CCK-8 dosed at 0.25, 0.6, and 1.0 μg/kg did not show suppressed food intake levels at 30, 60, or 120 min (Fig. 1, A–C). In contrast, intraperitoneal administration of CCK at 0.6 μg/kg or above inhibited food intake at 30, 60, and 120 min (Fig. 1, A–C). There was no significant difference in food intake levels between rats dosed with CCK-8 intraperitoneally vs. intravenously at 0.25 μg/kg for all the time points. In contrast, at 30 min, intraperitoneally administered CCK-8 (0.6 or 1.0 μg/kg) yielded a 52% reduction in food consumption compared with the saline group, while intravenous injection of CCK-8 did not reduce food intake compared with the saline group. At 60 min, CCK-treated rats (0.6 or 1.0 μg/kg) showed a 40% reduction of food intake compared with the saline-treated animals (P < 0.006). These data clearly showed that intraperitoneal administration of CCK-8 is more effective than intravenous injection in inhibiting food intake.
Fig. 1.
CCK-8 on food intake. Overnight fasted rats (n = 5 or 6) received a bolus dose either via intraperitoneal or intravenous route, 0.25 ml of either saline alone or one dose of sulfated CCK-8 (0.25, 0.6, or 1 μg/kg) in 0.9% saline 5 min before refeeding. Food intake was measured at 30 (A), 60 (B), and 120 min (C). Data are presented as means ± SE. a,bDiffering letters indicate significance of P < 0.05 compared with the control group at same time point.
Effect of apo AIV on the control of food intake.
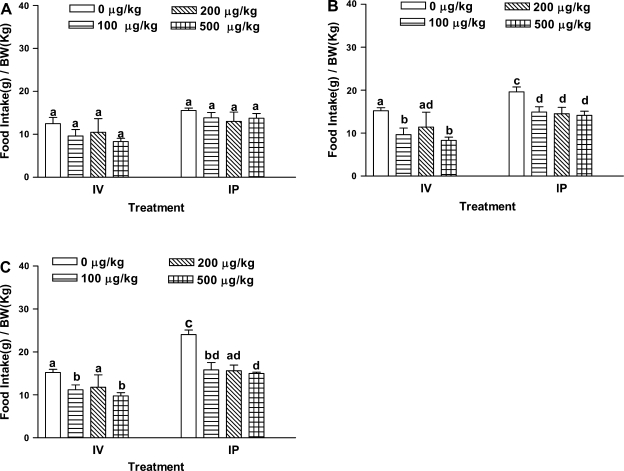
As shown in Fig. 2, intraperitoneal injection, as well as intravenous administration failed to inhibit food consumption significantly at 30 min compared with saline controls. At 60 and 120 min, intraperitoneal injection of recombinant apo AIV (100 μg/kg or above) reduced food intake. In contrast, apo AIV (100 or 500 μg/kg) administered intravenously suppressed food intake at 60 and 120 min; however, intravenous injection of 200 μg/kg apo AIV did not inhibit food intake at any time points. We have repeated this dose several times but still obtained the same result. We do not have an explanation for the lack of effect of the 200 μg/kg dose. Both intravenous and intraperitoneal administration of apo AIV was effective in inhibiting food intake at 60 and 120 min.
Fig. 2.
Recombinant apo AIV on food intake. Fasted rats (n = 5 or 6) received a bolus dose either via intraperitoneal or intravenous route, 0.25 ml of either saline alone or one dose of recombinant apo AIV (100, 200, or 500 μg/kg) in 0.9% saline 5 min before refeeding. Food intake was measured at 30 (A), 60 (B), and 120 (C) min. Data are presented as means ± SE. a,b,c,dDiffering letters indicate significance of P < 0.05 compared with the control group at same time point.
Intraperitoneal and intravenous administration of CCK-8 treatment.
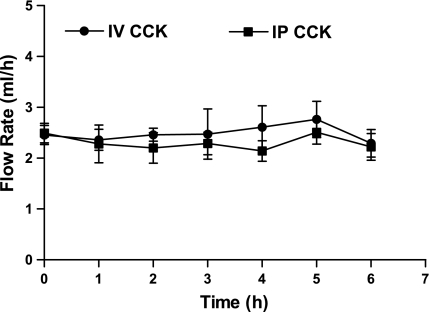
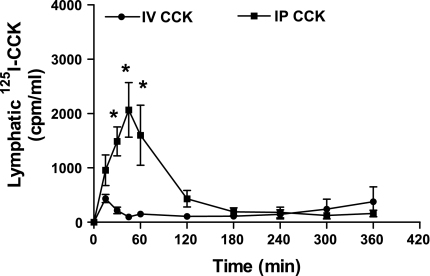
As shown in Fig. 3, neither the intraperitoneal nor the intravenous injection of CCK-8 (0.6 μg/kg) had an effect on fasting lymph flow, and there appears to have been no difference in lymphatic flow rate between animals injected intraperitoneally vs. intravenously (P > 0.05) (Fig. 3). Intraperitoneal injection of CCK-8 (0.6 μg/kg) plus 125I-CCK (0.5 μCi) induced a later peak in lymphatic output but also resulted in a significantly higher lymphatic output of 125I-CCK during first 3 h than did intravenous injection of the same dose (Fig. 4). Rats treated with an intraperitoneal injection of CCK-8 exhibited a significantly higher level of lymphatic 125I-CCK (1,488.4 ± 265.9 cpm/ml) than those rats treated with an intravenous injection (218.9 ± 60.3 cpm/ml) at 30 min (P < 0.0001). At 60 min, the lymphatic 125I-CCK concentration of intraperitoneally treated rats was 1,603.1 ± 553.5 cpm/ml, whereas intravenously treated animals had a lymphatic concentration of 152.8 ± 11.9 cpm/ml CCK. After 120 min, the lymphatic radioactive CCK-8 output of intraperitoneally treated rats decreased significantly but was still higher than that of animals treated intravenously (P > 0.05). At 180 min following administration, lymphatic output of CCK in the intraperitoneally treated rats did not differ from that of the intravenously injected animals.
Fig. 3.
Lymphatic flow rate. Lymph flow rate of different groups of rats receiving either intraperitoneal or intravenous administration (n = 5) of a bolus of sulfated CCK-8 (0.6 μg/kg). Lymph samples were collected hourly. Data are presented as means ± SE for lymph flow rate.
Fig. 4.
Lymphatic output of radioactive CCK. 5 rats received either intraperitoneal or intravenous administration of a bolus of sulfated CCK-8 (0.6 μg/kg) plus 125I-CCK (0.5 μCi/5μl). Radioactivity was measured by a gamma counter, and the results were expressed as means ± SE of radioactive counts were evaluated every 15 min. *P < 0.05 compared with the intravenous group at the same time point.
Intraperitoneal and intravenous administration of apo AIV treatment.
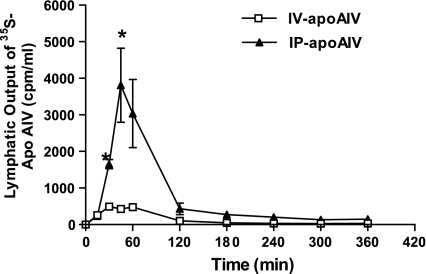
Intraperitoneal administration of 35S-apo AIV (1.17 mg/kg) to rats yielded a significantly higher concentration of lymphatic apo AIV than did intravenous injection of the compound during the first 30 min (P < 0.0001) (Fig. 5). At 15 min following administration, intraperitoneally administered rats exhibited a concentration of apo AIV (231.9 ± 77.0 cpm/ml) comparable to intravenously administered animals (257.4 ± 22.2 cpm/ml). Lymphatic concentration of radiolabeled apo AIV was 1,653.7 ± 125.1 cpm/ml in rats following intraperitoneal treatment at 30 min, compared with 494.9 ± 45.4 cpm/ml observed in the intravenously treated animals at 30 min (P < 0.01). Intraperitoneally treated rats had 3,035.1 ± 933.9 cpm/ml apo AIV in the lymph after the first 60 min, whereas the lymphatic apo AIV concentration of the intravenously treated animals was 476.7 ± 44.5 cpm/ml at 60 min. At 120 min, lymphatic apo AIV concentration in intraperitoneally administered rats had fallen to 430.0 ± 159.3 cpm/ml, while the lymphatic apo AIV concentration for intravenously treated animals was only 102.7 ± 7.3 cpm/ml at the same time point. There was no difference in the lymphatic concentration of apo AIV at 180 min between the intraperitoneally treated rats vs. those injected intravenously.
Fig. 5.
Lymphatic output of radioactive apo AIV. Five rats received either intraperitoneal or intravenous administration of a bolus of 35S methionine- labeled apo AIV (1.17 mg/kg). Radioactive apo AIV was measured by liquid scintillation counting, and means ± SE of radioactive counts were evaluated every 15 min. *P < 0.05 compared with the intravenous group at the same time point.
Enzymatic activity of DPPIV and aminopeptidase in the plasma and lymph.
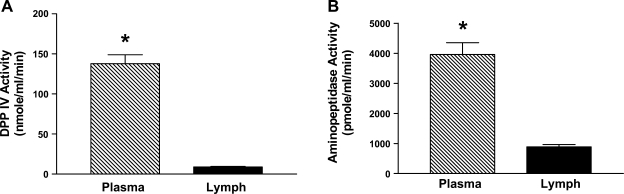
As shown in Fig. 6A, the enzymatic activity of DPPIV during fasting was significantly higher in plasma than in lymph (P < 0.0001); the enzymatic activity of plasma DPPIV was 137.8 ± 11.2 nmol·ml−1·min−1, while that in lymph was 8.9 ± 0.8 nmol·ml−1·min−1. Aminopeptidase activity was also significantly different between the fasting plasma and fasting lymph (P < 0.0001) (Fig. 6B). The tyrosyl aminopeptidase activity in fasting plasma was 3,962.0 ± 389.2 pmol·ml−1·min−1 compared with fasting lymphatic activity of 888.5 ± 74.1 pmol·ml−1·min−1. This suggests that the higher enzymatic activity of DPPIV and aminopeptidase in the plasma than in the intestinal lymph may further contribute to the difference in efficacy of satiation peptides such as CCK-8 administered intraperitoneally vs. intravenous administration of the peptide.
Fig. 6.
Enzymatic activity of DPPIV and tyrosyl aminopeptides in fasting plasma and lymph. Fasting plasma and lymph were collected from rats (n = 4) after recovery from surgery from the previous day. During overnight recovery, the animals were infused intraduodenally with a 5% glucose-saline solution. Fasting plasma and lymph samples were used for DPPIV (A) and tyrosyl aminopeptidase activity (B) determination, and the data are presented as means ± SE. *P < 0.05 between the concentration in fasting lymph and the plasma.
DISCUSSION
In our study, we confirmed previous findings that intraperitoneally injected CCK-8 and apo AIV both significantly inhibited food intake (15, 19, 37), an effect that lasted for 120 min in the present study. The inhibitory effect of CCK-8 on food intake is manifested by a reduction of meal size via CCK1 receptor, while the intermeal interval remained the same (19, 26, 31, 49, 51). The present study is also in agreement with previous studies in that intravenous injection of CCK-8 did not suppress food intake in rats at low doses (21).
Intraperitoneal administration of recombinant apo AIV in the present study inhibited food intake at doses above 100 μg/kg (37). However, the inhibition of food intake by intraperitoneal injection of apo AIV started at 60 min after refeeding in the present study, and the inhibition was different from what was observed in the previous study at 30 min, which could possibly be attributed to either varying fasting periods or different batches of recombinant apo AIV with different efficacy. The present study has demonstrated that intravenous administration of recombinant apo AIV, as well as purified apo AIV from lymph as demonstrated in a previous study (15), suppressed food intake. Therefore, intraperitoneal administration, as well as intravenous injection of either recombinant apo AIV or purified native apo AIV, inhibits food intake. As mentioned earlier, we do not have a good explanation for the apparent lack of efficacy to inhibit food intake of the intravenously infused recombinant apo AIV at 200 μg/kg compared with the doses of 100 or 500 μg/kg at 60 min. We have repeated this study numerous times, and because we tested all of the doses at the same time, it does not seem to be an isolated incident.
Animals treated with CCK-8 intraperitoneally display a significant increase in neuronal activation (c-Fos expression) in the myenteric plexus of the duodenum and jejunum (48). In a subsequent study, the investigators showed that intraperitoneal injection of CCK-8 stimulated more neurons (as reflected by c-fos activation) in the myenteric plexus of duodenum and jejunum than did a similar dose administered intravenously (52). Of course, stimulation of the neurons in the duodenum and jejunum by CCK-8 does not prove that this neural stimulation is involved in the satiation effect of CCK-8. Additional studies will have to be performed to prove this link directly. Villous enteric nerve plexus is distributed throughout the villi and is connected to nerve cell bodies in ganglia on submucous plexus and myenteric plexus (6). Lymph from the lacteals (initial lymphatics) originating at the center of the lamina propria of the villi eventually drain into the mesenteric lymph duct, then into the cisternae chyle, and then subsequently into the thoracic lymph duct, which eventually empty into the left subclavian vein (2). Administered peptides move by diffusion and convection from the peritoneal interstitium to the lymphatic and portal circulation before draining into the systemic circulation (14, 38, 43). Intraperitoneally administered CCK-8 possibly activates neurons in the villous plexus, probably submucous plexus and myenteric plexus, which relay these peripheral satiating signals via vagus afferent nerves to the nucleus of the solitary tract, the dorsomedial hypothalamic nucleus, and the paraventricular nucleus of the hypothalamus (4, 28). We speculate that compounds such as CCK-8 administered via different routes may activate neurons in the lamina propria of the small intestine to a different degree, thereby resulting in their varying potency for the inhibition of food intake. Intestinal lymph is derived from the central lacteals of intestinal villi, thus reflecting the concentration of these peptides to which the enteric neurons are exposed.
Radiolabeled CCK-8 was found in the lymph at 15 min following injections, and the lymphatic concentration of CCK-8 in the intraperitoneally treated rats was significantly higher than that of animals treated with an intravenous injection at 30 min. The maximum concentration of lymphatic CCK-8 and apo AIV was achieved at 45 min. Our findings are in agreement with observations that high levels of 125I-CCK-8 are present in the intestine of both intravenously and intraperitoneally treated animals at 30 min (7). These data would suggest that the neurons in the gut (e.g., in the lamina propria) are exposed to a higher concentration of CCK-8 when these molecules are administered intraperitoneally vs. those administered intravenously. CCK-induced satiation is relayed via the enteric neurons and the vagal afferent nerves to hindbrain (40, 48, 52). Thus, it is not surprising that CCK-8 administered intraperitoneally is more effective than a similar dose of CCK-8 administered intravenously, observed both in this study and other studies (19, 21). Another potential explanation for the more potent satiating effect of intraperitoneally administered CCK-8 is that the level of CCK-8 in the lymph remains elevated for a much longer period than when CCK-8 is administered intravenously. After 3 h of treatment, animals with intraperitoneally injected CCK had detectable CCK-8 levels in the lymph, but there was no detectable CCK-8 in the lymph of intravenously treated rats.
Apo AIV is a larger protein (46 kDa) than CCK-8 (3, 16, 30). Similar to the lymphatic CCK-8 study, lymphatic apo AIV concentration of the intraperitoneally injected group was higher than that of the intravenously treated animals at 30, 45, and 60 min following the injections. This suggests that the intraperitoneal route, just as with CCK-8, may deliver a higher concentration of apo AIV in the lamina propria than the intravenous route. However, unlike CCK-8, there was a smaller elevated level of lymphatic apo AIV even with the intravenous administration between 15 and 120 min. This elevated level of lymphatic apo AIV with intravenous infusion may be responsible for the inhibition of food intake that we observed.
Dipeptidyl peptidase IV (DPPIV) is a serine protease that cleaves N-terminal dipeptides from polypeptides with l-proline or l-alanine at the penultimate position (39). DPPIV has been implicated in the degradation of several hormones and peptides such as peptide YY, glucose-dependent insulinotropic polypeptide, and glucagon-like peptide-1 (53). Degradation of CCK-8 and smaller CCK fragments by plasma aminopeptidases has been studied by Deschodt-Lanckman et al. (11). Tyrosine residues and the amino acids methionine and glycine in the sequence of CCK are cleaved by aminopeptidases at a higher rate than tryptophan (30). We hypothesize that satiating peptides may also remain more effective in the lamina propia than in blood because of the lower degrading enzyme levels in the lamina propria (as reflected by the concentration in lymph) than in the blood. Our present study certainly supports such a notion because the DPPIV and aminopeptidase levels in fasting lymph are significantly lower than those observed in fasting plasma. Thus, low proteolytic degradation could be another contributing factor to the efficacy of satiating peptides in the lamina propria when administered peritoneally compared with intravenous administration.
GRANTS
This work was supported, in part, by National Institutes of Health Grant DK38180, HL082734, DK 17844, DK 56910, DK 56390, DK 70992, and by a postdoctoral fellowship from American Heart Association, Ohio Valley Affiliate.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Banegas I, Prieto I, Vives F, Alba F, Duran R, Segarra AB, de Gasparo M, Ramirez M. Plasma aminopeptidase activities in rats after left and right intrastriatal administration of 6-hydroxydopamine. Neuroendocrinology 80: 219–224, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Barrowman JA, Tso P. Gastrointestinal lymphatics. In: Handbook of Physiology: The Gastrointestinal System. Motility and Circulation. Bethesda, MD: Am. Physiol. Soc., 1989, sect. 6, vol. I, pt. 2, chapt. 48, p. 1733–1777.
- 3.Beisiegel U, Utermann G. An apolipoprotein homolog of rat apolipoprotein A-IV in human plasma. Isolation and partial characterisation. Eur J Biochem 93: 601–608, 1979. [DOI] [PubMed] [Google Scholar]
- 4.Cano V, Ezquerra L, Ramos MP, Ruiz-Gayo M. Characterization of the role of endogenous cholecystokinin on the activity of the paraventricular nucleus of the hypothalamus in rats. Br J Pharmacol 140: 964–970, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cantor P, Rehfeld JF. Radioimmunoassay of cholecystokinin: comparison of different tracers. J Immunol Methods 82: 47–55, 1985. [DOI] [PubMed] [Google Scholar]
- 6.Costa M, Furness JB, Llewellyn-Smith IJ. Histochemistry of the enteric nervous system. In: Physiology of the Gastrointestinal Tract, edited by Johnson LR, Christensen J, Jackson MJ, Jacobson ED, and Walsh JH. New York: Raven Press, 1987, p. 1–3.
- 7.Curry SH, McCarthy D, Morris CF, Simpson-Heren L. Whole body autoradiography of CCK-8 in rats. Regul Pept 55: 179–188, 1995. [DOI] [PubMed] [Google Scholar]
- 8.Deiling DA Mechanism of pancreatic exocrine secretion. Am J Gastroenterol 52: 17–24, 1969. [PubMed] [Google Scholar]
- 9.DeLamatre JG, Roheim PS. The response of apolipoprotein A-IV to cholesterol feeding in rats. Biochim Biophys Acta 751: 210–217, 1983. [DOI] [PubMed] [Google Scholar]
- 10.Deschenes RJ, Lorenz LJ, Haun RS, Roos BA, Collier KJ, Dixon JE. Cloning and sequence analysis of a cDNA encoding rat preprocholecystokinin. Proc Natl Acad Sci USA 81: 726–730, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deschodt-Lanckman M, Bui ND. Cholecystokinin octa- and tetrapeptide degradation by synaptic membranes. I. Evidence for competition with enkephalins for in vitro common degradation pathways. Peptides 2 Suppl 2: 113–118, 1981. [DOI] [PubMed] [Google Scholar]
- 12.Doi T, Liu M, Seeley RJ, Woods SC, Tso P. Effect of leptin on intestinal apolipoprotein AIV in response to lipid feeding. Am J Physiol Regul Integr Comp Physiol 281: R753–R759, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Fidge NH The redistribution and metabolism of iodinated apolipoprotein-A-4 in rats. Biochim Biophys Acta 619: 129–141, 1980. [DOI] [PubMed] [Google Scholar]
- 14.Flessner MF, Dedrick RL, Reynolds JC. Bidirectional peritoneal transport of immunoglobulin in rats: tissue concentration profiles. Am J Physiol Renal Fluid Electrolyte Physiol 263: F15–F23, 1992. [DOI] [PubMed] [Google Scholar]
- 15.Fujimoto K, Cardelli JA, Tso P. Increased apolipoprotein A-IV in rat mesenteric lymph after lipid meal acts as a physiological signal for satiation. Am J Physiol Gastrointest Liver Physiol 262: G1002–G1006, 1992. [DOI] [PubMed] [Google Scholar]
- 16.Fukagawa K, Gou HM, Wolf R, Tso P. Circadian rhythm of serum and lymph apolipoprotein AIV in ad libitum-fed and fasted rats. Am J Physiol Regul Integr Comp Physiol 267: R1385–R1390, 1994. [DOI] [PubMed] [Google Scholar]
- 17.Ghiselli G, Crump WL, III, Gotto AM Jr. Binding of apoA-IV-phospholipid complexes to plasma membranes of rat liver. Biochem Biophys Res Commun 139: 122–128, 1986. [DOI] [PubMed] [Google Scholar]
- 18.Gibbs J, Smith GP. Cholecystokinin and satiety: problems in brain-gut interactions. Brain-Gut Interaction, edited by Tache Y and Wingate D, Boca Raton, FL: CRC, 1991, p 255–262.
- 19.Gibbs J, Young RC, Smith GP. Cholecystokinin decreases food intake in rats. J Comp Physiol Psychol 84: 488–495, 1973. [DOI] [PubMed] [Google Scholar]
- 20.Green PH, Glickman RM, Saudek CD, Blum CB, Tall AR. Human intestinal lipoproteins. Studies in chyluric subjects. J Clin Invest 64: 233–242, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenberg D, Smith GP, Gibbs J. Infusion of CCK-8 into hepatic-portal vein fails to reduce food intake in rats. Am J Physiol Regul Integr Comp Physiol 252: R1015–R1018, 1987. [DOI] [PubMed] [Google Scholar]
- 22.Harper AA, Raper HS. Pancreozymin, a stimulant of the secretion of pancreatic enzymes in extracts of the small intestine. J Physiol 102: 115–125, 1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayashi H, Nutting DF, Fujimoto K, Cardelli JA, Black D, Tso P. Transport of lipid and apolipoproteins A-I and A-IV in intestinal lymph of the rat. J Lipid Res 31: 1613–1625, 1990. [PubMed] [Google Scholar]
- 24.Houpt TR The sites of action of cholecystokinin in decreasing meal size in pigs. Physiol Behav 31: 693–698, 1983. [DOI] [PubMed] [Google Scholar]
- 25.Ivy AC, Oldberg E. A hormone mechanism for gallbladder contraction and evacuation. Am J Physiol 86: 599–613, 1928. [Google Scholar]
- 26.Kadar T, Penke B, Kovacs K, Telegdy G. Depression of rat feeding in familiar and novel environment by sulfated and nonsulfated cholecystokinin octapeptide. Physiol Behav 34: 395–400, 1985. [DOI] [PubMed] [Google Scholar]
- 27.Kalogeris TJ, Fukagawa K, Tsuchiya T, Qin X, Tso P. Intestinal synthesis and lymphatic secretion of apolipoprotein A-IV after cessation of duodenal fat infusion: mediation by bile. Biochim Biophys Acta 1436: 451–466, 1999. [DOI] [PubMed] [Google Scholar]
- 28.Kobelt P, Paulitsch S, Goebel M, Stengel A, Schmidtmann M, van dV, I, Tebbe JJ, Veh RW, Klapp BF, Wiedenmann B, Tache Y, Monnikes H. Peripheral injection of CCK-8S induces Fos expression in the dorsomedial hypothalamic nucleus in rats. Brain Res 1117: 109–117, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Kolehmainen L, Mikola J. Partial purification and enzymatic properties of an aminopeptidase from barley. Arch Biochem Biophys 145: 633–642, 1971. [DOI] [PubMed] [Google Scholar]
- 30.Koulischer D, Moroder L, Deschodt-Lanckman M. Degradation of cholecystokinin octapeptide, related fragments and analogs by human and rat plasma in vitro. Regul Pept 4: 127–139, 1982. [DOI] [PubMed] [Google Scholar]
- 31.Kraly FS, Carty WJ, Resnick S, Smith GP. Effect of cholecystokinin on meal size and intermeal interval in the sham-feeding rat. J Comp Physiol Psychol 92: 697–707, 1978. [DOI] [PubMed] [Google Scholar]
- 32.Lacourse KA, Friis-Hansen L, Samuelson LC, Rehfeld JF. Altered processing of procholecystokinin in carboxypeptidase E-deficient fat mice: differential synthesis in neurons and endocrine cells. FEBS Lett 436: 61–66, 1998. [DOI] [PubMed] [Google Scholar]
- 33.Lefevre M, Roheim PS. Metabolism of apolipoprotein A-IV. J Lipid Res 25: 1603–1610, 1984. [PubMed] [Google Scholar]
- 34.Liddle RA, Goldfine ID, Rosen MS, Taplitz RA, Williams JA. Cholecystokinin bioactivity in human plasma. Molecular forms, responses to feeding, and relationship to gallbladder contraction. J Clin Invest 75: 1144–1152, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liddle RA, Goldfine ID, Williams JA. Bioassay of plasma cholecystokinin in rats: effects of food, trypsin inhibitor, and alcohol. Gastroenterology 87: 542–549, 1984. [PubMed] [Google Scholar]
- 36.Liu M, Maiorano N, Shen L, Pearson K, Tajima D, Zhang DM, Woods SC, Seeley RJ, Davidson WS, Tso P. Expression of biologically active rat apolipoprotein AIV in Escherichia coli. Physiol Behav 78: 149–155, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Lo CM, Zhang DM, Pearson K, Ma L, Sun W, Sakai RR, Davidson WS, Liu M, Raybould HE, Woods SC, Tso P. Interaction of apolipoprotein AIV with cholecystokinin on the control of food intake. Am J Physiol Regul Integr Comp Physiol 293: R1490–R1494, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Lukas G, Brindle SD, Greengard P. The route of absorption of intraperitoneally administered compounds. J Pharmacol Exp Ther 178: 562–564, 1971. [PubMed] [Google Scholar]
- 39.Mentlein R Dipeptidyl-peptidase IV (CD26)–role in the inactivation of regulatory peptides. Regul Pept 85: 9–24, 1999. [DOI] [PubMed] [Google Scholar]
- 40.Moran TH, Smith GP, Hostetler AM, McHugh PR. Transport of cholecystokinin (CCK) binding sites in subdiaphragmatic vagal branches. Brain Res 415: 149–152, 1987. [DOI] [PubMed] [Google Scholar]
- 41.Muller K, Hsiao S. Specificity of cholecystokinin satiety effect: reduction of food but not water itnake. Pharmacol Biochem Behav 6: 643–646, 1977. [DOI] [PubMed] [Google Scholar]
- 42.Nagatsu T, Hino M, Fuyamada H, Hayakawa T, Sakakibara S. New chromogenic substrates for X-prolyl dipeptidyl-aminopeptidase. Anal Biochem 74: 466–476, 1976. [DOI] [PubMed] [Google Scholar]
- 43.Nagy JA Lymphatic and nonlymphatic pathways of peritoneal absorption in mice: physiology versus pathology. Blood Purif 10: 148–162, 1992. [DOI] [PubMed] [Google Scholar]
- 44.Raybould HE, Meyer JH, Tabrizi Y, Liddle RA, Tso P. Inhibition of gastric emptying in response to intestinal lipid is dependent on chylomicron formation. Am J Physiol Regul Integr Comp Physiol 274: R1834–R1838, 1998. [DOI] [PubMed] [Google Scholar]
- 45.Rehfeld JF Immunochemical studies on cholecystokinin. II. Distribution and molecular heterogeneity in the central nervous system and small intestine of man and hog. J Biol Chem 253: 4022–4030, 1978. [PubMed] [Google Scholar]
- 46.Rehfeld JF, Hansen HF. Characterization of preprocholecystokinin products in the porcine cerebral cortex. Evidence of different processing pathways. J Biol Chem 261: 5832–5840, 1986. [PubMed] [Google Scholar]
- 47.Rehfeld JF, Sun G, Christensen T, Hillingso JG. The predominant cholecystokinin in human plasma and intestine is cholecystokinin-33. J Clin Endocrinol Metab 86: 251–258, 2001. [DOI] [PubMed] [Google Scholar]
- 48.Sayegh AI, Ritter RC. Cholecystokinin activates specific enteric neurons in the rat small intestine. Peptides 24: 237–244, 2003. [DOI] [PubMed] [Google Scholar]
- 49.Schneider LH, Murphy RB, Smith GP. Two proglumide analogues are equipotent antagonists of the inhibition of food intake by CCK-8. Peptides 9 Suppl 1: 207–214, 1988. [DOI] [PubMed] [Google Scholar]
- 50.Stein LJ, Woods SC. Cholecystokinin and bombesin act independently to decrease food intake in the rat. Peptides 2: 431–436, 1981. [DOI] [PubMed] [Google Scholar]
- 51.Torregrossa AM, Smith GP. Two effects of high-fat diets on the satiating potency of cholecystokinin-8. Physiol Behav 78: 19–25, 2003. [DOI] [PubMed] [Google Scholar]
- 52.Webb T, Gulley S, Pruitt F, Esdaile AR, Sharma SK, Cox JE, Smith GP, Sayegh AI. Cholecystokinin-8 increases Fos-like immunoreactivity in myenteric neurons of the duodenum and jejunum more after intraperitoneal than after intravenous injection. Neurosci Lett 389: 157–162, 2005. [DOI] [PubMed] [Google Scholar]
- 53.Zhu L, Tamvakopoulos C, Xie D, Dragovic J, Shen X, Fenyk-Melody JE, Schmidt K, Bagchi A, Griffin PR, Thornberry NA, Sinha RR. The role of dipeptidyl peptidase IV in the cleavage of glucagon family peptides: in vivo metabolism of pituitary adenylate cyclase activating polypeptide-(1-38). J Biol Chem 278: 22418–22423, 2003. [DOI] [PubMed] [Google Scholar]