Abstract
Maternal obesity is becoming more prevalent. We used borderline hypertensive rats (BHR) to investigate whether a high-fat diet at different stages of development has adverse programming consequences on metabolic parameters and blood pressure. Wistar dams were fed a high- or low-fat diet for 6 wk before mating with spontaneously hypertensive males and during the ensuing pregnancy. At birth, litters were fostered to a dam from the same diet group as during gestation or to the alternate diet condition. Female offspring were weaned on either control or “junk food” diets until about 6 mo of age. Rats fed the high-fat junk food diet were hyperphagic relative to their chow-fed controls. The junk food-fed rats were significantly heavier and had greater fat pad mass than those rats maintained on chow alone. Importantly, those rats suckled by high-fat dams had heavier fat pads than those suckled by control diet dams. Fasting serum leptin and insulin levels differed as a function of the gestational, lactational, and postweaning diet histories. Rats gestated in, or suckled by high-fat dams, or maintained on the junk food diet were hyperleptinemic compared with their respective controls. Indirect blood pressure did not differ as a function of postweaning diet, but rats gestated in the high-fat dams had lower mean arterial blood pressures than those gestated in the control diet dams. The postweaning dietary history affected food-motivated behavior; junk food-fed rats earned less food pellets on fixed (FR) and progressive (PR) ratio cost schedules than chow-fed controls. In conclusion, the effects of maternal high-fat diet during gestation or lactation were mostly small and transient. The postweaning effects of junk food diet were evident on the majority of the parameters measured, including body weight, fat pad mass, serum leptin and insulin levels, and operant performance.
Keywords: developmental programming, blood pressure, metabolic syndrome, motivation
the 1999–2002 national health and Nutrition Examination Survey (NHANES III) found that >50% of nonpregnant women of child-bearing age (20–39 yr of age) in the United States of America were overweight or obese (body mass index >25 kg/m2), and >30% of girls between the ages of 12–19 were either at risk of being overweight or were overweight (22). These statistics imply that almost half of all babies are born to mothers who are either overweight or obese during pregnancy, and this fraction is likely to increase in the future.
Adverse effects of obesity on mothers have been well-studied; these include gestational diabetes, preeclampsia, prolonged delivery, and delayed wound healing postdelivery, all of which significantly increase health care costs (18). Babies born to obese women have a higher incidence of congenital abnormalities (31), are large for their gestational age, and are at a greater risk of developing metabolic syndrome later in childhood (9). It is not clear whether this poor metabolic prognosis develops directly as a result of the in utero or gestational experience (fetal programming) or is a result of poor feeding habits learned during childhood. The term fetal programming was developed in part to explain enduring effects on offspring of maternal undernutrition that has been reported in both epidemiological and laboratory investigations (5, 27, 38, 48).
A handful of laboratory studies has reported the effects of maternal obesity and/or high-fat diet on the development of the offspring in rats (1). One of the objectives of this project, results of which will be reported here for females and elsewhere for males (30) is to examine the cardiovascular consequence; we thus elected to use a Wistar-derived strain of rats that is genetically susceptible to hypertension triggered by environmental stress (20). Borderline hypertensive rats (BHR) were produced by mating female rats of the Wistar strain with spontaneously hypertensive (SHR) males. We know of no published work on food intake or diet-induced obesity in BHR. As in other studies (1, 26), we simulated a prospectively obese in utero environment by feeding Wistar female rats a diet high in saturated fat, or a low-fat control diet, for 6–8 wk before impregnation and through gestation. At parturition, to isolate prenatal from postnatal effects, litters were cross-fostered to another dam in either the same or the alternate dietary condition compared with their gestational dam.
Obesity is caused by an excess of food consumption relative to energy expenditure (12). Consumption of calorically dense high-fat diets often produces hyperphagia and/or obesity in rodents (50) and so, after weaning, the offspring in this experiment were fed either a standard low-fat chow (Purina 5001) only or were fed a “junk food” diet, a rotating regimen of high-fat foods similar to those consumed by humans. To measure behavioral parameters in the offspring, we recorded food intake throughout the study. In addition, toward the end of the study, we assessed food motivation using fixed (FR) and progressive (PR) ratio operant cost schedules of reinforcement for small food pellets. In FR schedules, the cost of each pellet is fixed at a specified number of responses, lever presses in this case. In PR schedules, which are particularly sensitive to motivational differences (2, 22, 28), the cost of successively earned pellets is increased according to a programmed function. Physiological parameters that we measured relevant to obesity and metabolic syndrome were blood pressure, fasting and nonfasting serum plasma leptin concentrations, and fasting serum insulin concentrations.
We hypothesized that rats gestated in a high-fat-fed dam, cross-fostered to a high-fat dam and weaned on the junk food diet (HHJ rats) would be most susceptible to developing the metabolic syndrome while rats in the control condition that were gestated in and fostered to dams fed the control diet, and subsequently weaned on a standard chow diet (LLC rats) would be the least susceptible.
METHODS
Animals and housing.
Primiparous Wistar rats, purchased from Harlan Laboratories (Indianapolis, IN), weighed 290–320 g and were ∼5 mo of age at the beginning of the study. They were housed individually in polycarbonate cages with stainless steel wire mesh lids in a controlled environment (21–24°C, 45–55% relative humidity, 12:12-h light-dark cycle, with lights off 1000–2200). These females were maintained on one of two semipurified pellet diets (Research Diets, New Brunswick, NJ) for 6–8 wk before mating. The high-fat diet (D12492) contained 34.9% fat (mostly saturated) by weight, which yields ∼60% calories from fat and has a caloric density of 5.24 kcal/g. The control diet (D01060501) contained 4.3% fat by weight, which yields ∼10% calories from fat and has a caloric density of 3.85 kcal/g. The protein and micronutrient contents are matched in these diets. When initially placed on these diets, the rats' food intake and body weight were monitored every day for the first 6 days, and then every 5 days through week 6. Before the start of the experiments, animals were handled frequently to minimize stress during the experiments.
All procedures were in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals and were approved by the University of Florida Animal Care and Use Committee.
Synchronous mating and cross-fostering.
After they had been fed the above diets for 6–8 wk, Wistar dams were mated with proven breeder SHR males (Harlan Laboratories). Dams were weighed throughout gestation, at parturition, and at weaning. At parturition, the litters were weighed and culled to 10 pups of which 6 were males and 4 were females, whenever possible. All litters were cross-fostered such that they were housed with a dam in the same or in the alternate dietary condition compared with their gestational mother. The cross-fostering procedure thus produced four dietary groups (Table 1). Litters were weighed on postnatal days (PD) 0, 5, 10, 15, and 21. The litters were weaned at PD21, and littermates were separated based on sex. Dams were killed at weaning, and their principal fat pads (subcutaneous, mesenteric, periovarian, retroperitoneal, and perirenal) were dissected and weighed.
Table 1.
Maternal diet, cross-fostering procedures, and dietary conditions of the different litter types
| Maternal Diet During Week 1 (adaptation) | Maternal Diet During Week 2-Week 8 and Through Gestation | Litter Type Received at Cross-Fostering | Litter Condition at Weaning (PD21) | Diet From PD21-PD61 |
|---|---|---|---|---|
| Chow | High-fat diet (H) | High-fat litter | HH (6 litters; n = 24 female rats) | Rotating junk food diet n = 12 rats Condition: HHJ |
| Standard chow diet n = 12 rats Condition: HHC | ||||
| Low-fat litter | HL (6 litters; n = 22 female rats) | Rotating junk food diet n = 12 rats Condition: HLJ | ||
| Standard chow diet n = 10 rats Condition: HLC | ||||
| Low-fat diet (L) | High-fat litter | LH (6 litters; n = 24 female rats) | Rotating junk food diet n = 12 rats Condition: LHJ | |
| Standard chow diet n = 12 rats Condition: LHC | ||||
| Low-fat litter | LL (6 litters; n = 22 female rats) | Rotating junk food diet n = 10 rats Condition: LLJ | ||
| Standard chow diet n = 12 rats Condition: LLC |
PD, postnatal day. See text for group definitions.
Postweaning maintenance.
Female offspring (4/litter) were weaned at PD21 on either standard chow (Purina 5001; n = 2/litter) or a rotating junk food diet (n = 2/litter) generating eight experimental groups (Table 1). Offspring designated as HHJ received gestational high-fat diet, were fostered to a dam fed a high-fat diet, and received postweaning junk food diet. Offspring designated as LLC received gestational low-fat diet, were fostered to a dam fed a low-fat diet, and received postweaning chow. The other six groups are HHC, HLJ, HLC, LLJ, LHJ, and LHC. The junk food regimen consisted of presentation of one of the following six high-fat foods: cookie dough (4.98 kcal/g, made from 22% flour, 54% sugar, 24% shortening, and vanilla essence); peanut butter chow (4.78 kcal/g, 50% powdered Purina 5001 + 50% smooth peanut butter); Vienna sausages (2.83 kcal/g); processed cheese product (2.85 kcal/g); condensed-milk/chow (3.32 kcal/g, 50% powdered Purina 5001 + 50% sweetened condensed milk); and the high-fat semisynthetic diet D12492 (5.24 kcal/g). With the exception of the standard chow and D12492, all of these ingredients were generic brands from a local supermarket. Each of these diets was presented for 2 days separated by 2 days of standard chow (Purina 5001; 3.34 kcal/g). Because the protein content or quality of some junk foods may be suboptimal for growth in rats, chow periods were included to allow adequate-quality protein consumption. Food intake and body weights were monitored every 2 days from PD21 through PD189. To manage the large numbers of rats involved, at PD45, the offspring were moved to an adjacent vivarium maintained at a similar temperature and relative humidity as the original room, but with a normal light cycle (lights on 0800–2000). No changes in intake or growth were noted after this change. Rats were in this new light cycle for ∼4 mo before physiological measures were commenced.
Blood pressure measures.
Blood pressure was measured using a Volume Pressure Recording (VPR) system (CODA 6+; Kent Scientific, Torrington, CT). The principle of the VPR method is similar to tail cuff inflation but uses two tail cuffs. The proximal occlusion cuff (O-cuff) constricts the tail artery while the distal VPR cuff measures the change in tail artery volume when blood flow resumes as the O-cuff deflates. This system has been validated in a number of other studies (3, 17, 43). These tests were performed in a room maintained at ∼31°C to ensure adequate blood flow through the tail to improve the signal at the VPR transducer. Rats were habituated to restraint tubes for 6 days (PD165-170) during which time four sets of blood pressure measurements were taken over ∼20 min; each set was comprised of five 1-min inflation-deflation cycles. Averaged data from the last 2 days were used for statistical analysis.
Operant behavior.
All operant behavioral procedures were conducted in nonfasted rats. Rats were placed in operant chambers measuring 30 × 24 × 21 cm with a steel rod floor (Med Associates, St. Albans, VT). One fixed lever protruded through one wall of the chamber, to the right of a recessed food trough. A cue light was placed directly above the lever and was illuminated for 1 s when the rats received reinforcement. To test for possible differences in motivation as a function of the different diet histories, rats were first maintained on a FR1 schedule of reinforcement and then on a PR schedule. Rats were tested on alternate days over a 48-day period (PD225-273); the first 11 sessions used FR1, and the next 12 sessions used the PR. Cue lights and pellet delivery were controlled by a computer running Med-PC software (Med Associates) that also recorded the number of lever presses emitted and the number of reinforcements received. Body weights were measured on the 1st day of the first FR1 session and on the last day of the last PR session.
Rats received one session of training on the FR1 program. The FR1 program lasted for a total of 60 min, and each time the rats successfully depressed the lever they received a single 45-mg pellet of similar composition to chow (Purina no. 1811155). After their last FR1 session, they were placed on two consecutive PR training sessions. The ratio requirement for successive rewards within a PR session increased by a factor of 1.05, rounded to the nearest integer. Thus the cumulative presses needed for the 10th, 20th, 30th, 40th, 50th, and 60th pellets in a session were 12, 34, 69, 135, 237, and 422 presses. This program terminated when 15 min elapsed since the last pellet was received, and the number of pellets received at that time defined the breaking point for that session. There were no evident changes in behavior across days in either series, so for statistical analysis the numbers of rewards earned were averaged across FR or PR sessions for each animal.
Physiological measures.
On PD200, after an 18-h fast, rats were sedated with isoflurane, and blood was collected via heart puncture. On PD224, the same rat from which the fasting blood had been collected was terminally anesthetized with pentobarbital sodium (100 mg/kg), and nonfasted blood was collected from the aorta. At this time, organs (heart, kidneys, adrenals, and spleen) and fat pads (mesenteric, perirenal, and periovarian combined and subcutaneous) were dissected and weighed. Blood collected was allowed to coagulate, after which it was centrifuged at 3,000 revolutions/min for 20 min; the serum was aspirated and stored at −60°C until future analyses of insulin and leptin were performed. Commercially available RIA kits (Leptin RL-83K; Insulin RL-RI-13K; Linco, St. Charles, MO) were used for these assays; the manufacturer's protocol was followed, and the assay tubes were counted for 1 min using a Beckman 8000 γ-detector. The serum concentrations of the hormones were determined from a calibration curve constructed using standards supplied in the kits. Each sample was run in duplicate, and the average value was taken for calculation; duplicate counts typically varied by <5%.
Data analysis.
One-way ANOVA (SPSS, Chicago, IL) and post hoc Tukey tests (where appropriate) were used to examine for differences in maternal caloric intake, maternal body weights, maternal fat pads, mean litter weights, and general litter statistics. Three-way ANOVAs were conducted to examine for significant differences in body weight, organ weight, fat pad mass, serum leptin and insulin levels, and blood pressure of the rats as a function of gestational, lactational, and postweaning environments. For the FR and PR lever press studies, three-way ANOVAs were conducted on the mean pellets earned, with post hoc Tukey tests. In all cases, significance levels were set at P < 0.05.
RESULTS
Characteristics of the mothers and litters.
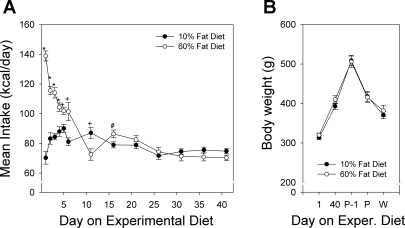
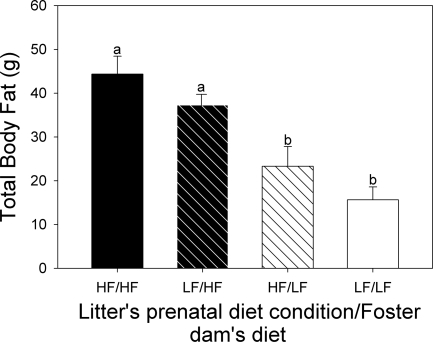
Caloric intake was significantly (P < 0.001) greater in the high-fat compared with the low-fat group during the 1st wk of exposure to the experimental diets (Fig. 1A). After the 1st wk, the intake of the two diets did not differ at any time before or during gestation; since the food intake was similar, the mean body weight of the dams (Fig. 1B) did not differ between the two dietary conditions. However, the total fat pad mass (sum of mesenteric, subcutaneous, perirenal, and periovarian fat pads) of the high-fat-fed dams at the time the pups were weaned (Fig. 2) was higher than in the control diet dams (P < 0.001) regardless of the type of foster litter they had suckled during the lactation period.
Fig. 1.
A: caloric intake of adult female rats before impregnation. Rats placed on the high-fat diet ate significantly more than the low-fat diet rats until about day 8, after which there were no systematic differences in their total daily caloric intake (*P < 0.001, +P < 0.01, #P < 0.05). B: body weights of adult female rats on days 1 and 40 of the experimental diets before pregnancy, the day before parturition (P-1), the day of parturition (P), and the day of weaning (W). Regardless of diet type, there were no significant differences in body weight between the 2 dietary groups. Data are shown as means ± SE.
Fig. 2.
Total body fat (subcutaneous + mesenteric + periovarian + perirenal fat pads) of mother rats after litters were weaned. Foster dams fed the high-fat (HF) diet had significantly heavier fat mass compared with the dams maintained on the low-fat (LF) diet (P < 0.001). There was no effect of the prenatal diet condition of the litter that the dam suckled during the lactation period. Bars marked with different letters are significantly different from each other (P < 0.05). Data are shown as means ± SE.
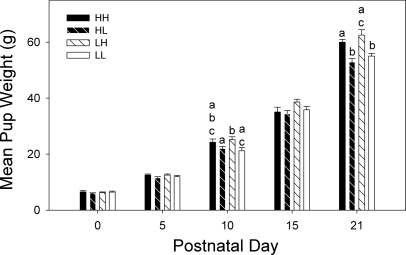
There were no significant differences in mean litter size (15.4 ± 0.7 vs. 14.1 ± 0.4, P > 0.05), mean number of male pups (7.4 ± 0.7 vs. 7.6 ± 0.5, P > 0.05), mean number of female pups (7.6 ± 0.8 vs. 6.5 ± 0.5, P > 0.05), or in mortality rates (<5%) between litters born to high-fat-fed or control diet dams. Mean litter weights across days are shown in Fig. 3. After culling to 10 pups, mean litter weight did not differ significantly between the four groups (HH, HL, LH, and LL) at either PD0 or PD5. By PD10, LH litters were significantly heavier than HL and LL litters. At PD15, a similar trend was apparent but was not significant. At PD21, the litters that had suckled from a high-fat dam (HH and LH litters) were significantly heavier than those that had suckled from a dam fed control diet (HL and LL litters).
Fig. 3.
Weights of rat pups at ∼5-day intervals from birth [postnatal day (PD) 0] through weaning (PD21). There were no group differences at PD0 or PD5. From PD10 onward, the LH pups (gestated in low-fat-diet dams, fostered to high-fat dams) were significantly heavier than HL (gestated in high-fat dams, fostered to low-fat dams) or LL (gestated and fostered with low-fat-diet dams) groups. Bars marked with different letters are significantly different from each other (P < 0.05). Data are shown as means ± SE.
Postweaning growth.
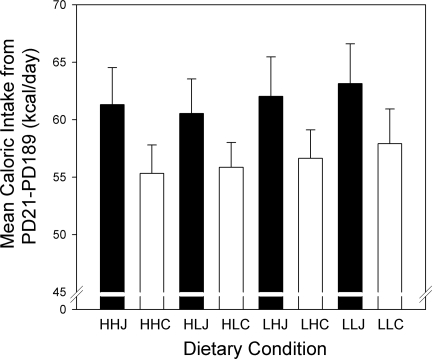
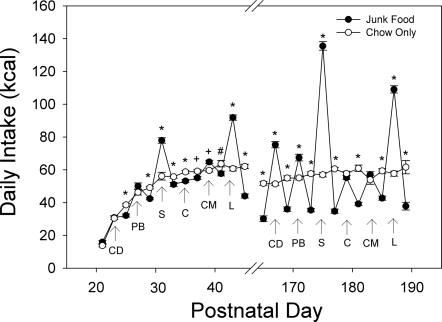
The average daily caloric intakes of the offspring from PD21 to PD191 are shown in Fig. 4. During this time, there were no significant differences in caloric intake as a function of their gestational or lactational histories. In contrast, there was a significant effect of postweaning diet (P < 0.01): the junk food-fed rats consumed ∼10% more calories overall than the chow-fed controls. Junk food-fed rats also consumed more calories from junk foods than from chow (available in alternation with the junk food items), and chow intake was reduced on these days relative to the chow-fed groups whose intakes showed little day-to-day variation (Fig. 5). A comparison of the differences in mean overall intake as a function of the different diets provided to the junk food rats is presented in Table 2.
Fig. 4.
Total energy intake of female offspring averaged from PD21 to PD191. The rats on the junk food postweaning diets (J: filled bars) consumed more calories on average compared with chow-fed groups (C: open bars), regardless of their gestational or lactational histories (P < 0.01 comparing energy intake based on postweaning diet). The three letter group designations refer to gestational, lactational, and postweaning diet. Data are shown as means ± SE.
Fig. 5.
Detail of energy intake (kcal/day) of female offspring from PD21 to PD45 and then from PD165 to PD189. The rats on the junk food postweaning diets (filled circles) consumed a larger proportion of their calories from the junk food options and compensated partially by reducing chow intake (*P < 0.001, +P < 0.01, #P < 0.05). The intakes between PD45 and PD165 were measured and were very similar to those from PD165-189 but are not shown for clarity. CD, cookie dough; PB, peanut butter + chow; S, Vienna sausage; C, processed cheese product; CM, condensed milk + chow; L, high-fat diet (D12492). Data are shown as means ± SE.
Table 2.
Energy consumed from PD21 to PD191 for the junk food-fed and standard chow-fed rats
| Caloric Intake from PD21 to PD191 (kcal) of Junk Food-Fed Rats | HHJ | HLJ | LHJ | LLJ |
|---|---|---|---|---|
| Chow | 39.7±2.7 | 42.7±2.4 | 41.4±2.8 | 42.1±2.7 |
| Cookie dough | 73.4±3.3 | 70.2±2.6 | 77.4±3.9 | 76.8±3.6 |
| Peanut butter + Chow | 72.8±5.6 | 70.8±3.6 | 67.0±4.2 | 77.4±5.0 |
| Sausage | 123.7±4.2 | 117.5±4.1 | 123.4±5.5 | 120.4±4.7 |
| Processed cheese product | 62.5±3.1 | 57.4±2.3 | 60.8±3.7 | 63.4±3.3 |
| Condensed milk + chow | 62.3±2.2 | 60.1±2.3 | 59.9±2.9 | 61.4±4.2 |
| High-fat semisynthetic D12492 | 106.2±3.8 | 101.5±3.8 | 110.4±4.0 | 109.0±4.7 |
| Caloric Intake from PD21-191 (kcal) of Chow-Fed Rats | HHC | HLC | LHC | LLC |
| Chow intake (corresponding to chow intake of junk food rats) | 55.6±2.6 | 56.2±2.1 | 57.0±2.7 | 59.0±2.6 |
| Chow intake (corresponding to cookie dough intake of junk food rats) | 52.4±1.9 | 52.4±1.7 | 53.6±2.1 | 53.6±2.6 |
| Chow intake (corresponding to PB + chow intake of junk food rats) | 53.2±1.2 | 54.6±1.4 | 55.1±2.1 | 57.2±3.6 |
| Chow intake (corresponding to sausage intake of junk food rats) | 55.3±1.6 | 56.1±2.3 | 56.3±2.4 | 57.5±3.7 |
| Chow intake (corresponding to processed cheese intake of junk food rats) | 56.4±2.1 | 57.6±2.2 | 58.0±2.6 | 58.6±2.7 |
| Chow intake (corresponding to condensed milk + chow intake of junk food rats) | 55.8±2.3 | 55.5±3.6 | 57.5±2.0 | 57.9±2.4 |
| Chow intake (corresponding to D12492 intake of junk food rats) | 55.2±3.5 | 57.0±2.2 | 57.6±2.2 | 55.4±5.8 |
Values are means ± SE. See text for group definitions.
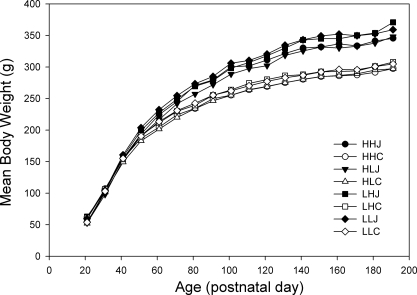
As noted above, body weights at PD21 were significantly heavier in rats suckled by high fat compared with control diet dams (P < 0.001), but this difference proved to be transient and disappeared after PD25. Postweaning diet influenced body weight of the offspring (Fig. 6), with the junk food-fed groups weighing more than the chow-fed groups. This first became evident at PD45 (P < 0.01) and, with the exception of PD49, remained statistically significant through PD191 (P < 0.001). In addition to being heavier, those rats weaned on the junk food diet were ∼4% longer, as measured by terminal (PD224) nose-to-anus length (23.9 ± 0.14 vs. 22.9 ± 0.15, P < 0.001).
Fig. 6.
Body weights of female offspring every 10 days from PD21 to PD191. The body weights began to diverge as a function of postweaning diet from PD45 (P < 0.01), and this difference increased with age (P < 0.00001 at PD191). Data are shown as means; SE are not shown for clarity but were typically <2.5% of the means.
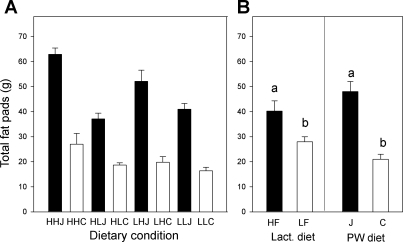
Complementing the body weight data, those rats that were weaned on the junk food diets had significantly heavier terminal fat pad masses than the chow-fed groups, with significant (P < 0.001) differences in total harvested fat mass (Fig. 7) as well as in the component pads (subcutaneous, mesenteric, periovarian, perirenal; separate data not shown). Interestingly, an effect of the lactational dam's diet was also seen on the combined mesenteric + periovarian + perirenal fat pads (P < 0.001) and the total harvested fat (P < 0.001): rats suckled by high-fat dams had heavier fat pads compared with those that had been suckled by low-fat dams (Fig. 7B). A significant interaction was noted between postweaning diet and the lactational dam's diet (P < 0.05).
Fig. 7.
A: total harvested fat pad mass (subcutaneous, mesenteric, periovarian, perirenal) of female offspring on PD224. Main effects of postweaning diet and lactational diet were observed (P < 0.0001). There was also an interaction between lactational and postweaning diet (P < 0.05). B: data from A combined and replotted to show effects of suckling condition. Total fat pad mass was significantly greater in rats that had been suckled by HF compared with those suckled by LF dams (P < 0.0001). Rats maintained postweaning (PW) on the junk food diet had significantly heavier fat pad masses compared with the chow groups (P < 0.0001). Bars marked with different letters are significantly different (P < 0.0001). Data are shown as means ± SE.
The absolute heart (1.20 ± 0.02 vs. 1.12 ± 0.02 g, P < 0.001) and kidney (1.20 ± 0.02 vs. 1.06 ± 0.02 g, P < 0.001) weights of the rats fed junk food after weaning were significantly greater than those from chow-fed groups, although this difference paralleled their greater body weights.
Physiological measures.
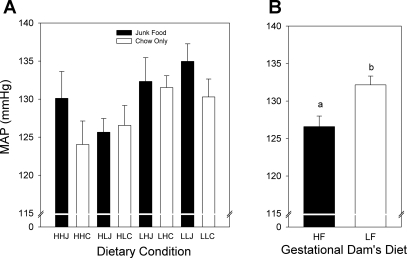
Mean arterial blood pressure (MAP; Fig. 8) and diastolic blood pressure (DBP; data not shown) differed as a function of the gestational dams' diet (P < 0.01). Rats gestated in control diet dams had consistently higher (by ∼5 mmHg) MAP (Fig. 8B) and DBP than those gestated in high-fat diet-fed dams. There was no effect on blood pressure of either lactational or postweaning dietary histories.
Fig. 8.
A: mean arterial blood pressure (MAP) of female offspring measured on PD170. B: data from A combined and replotted to show effects of gestational condition. Rats gestated in the LF dams had higher MAP than those gestated in HF dams (P < 0.01). Data are shown as means ± SE.
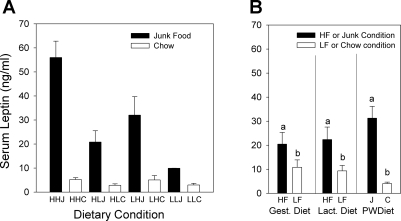
Fasting serum leptin (7.09 ± 0.94 vs. 1.26 ± 0.11 ng/ml, P < 0.001) and insulin (0.56 ± 0.06 vs. 0.28 ± 0.03 ng/ml, P < 0.001) levels were significantly higher on PD200 as a function of postweaning diet: junk food-fed rats had elevated serum levels of both hormones compared with their chow-fed controls. Nonfasting serum leptin levels (collected on PD224) (Fig. 9) differed significantly as a function of the gestational dams' diets (P < 0.01), the lactational dams' diets (P < 0.0001), and the postweaning diets (P < 0.0001). Specifically, those rats that had either been gestated in or suckled by a high-fat dam had significantly higher nonfasting serum leptin levels, and those rats that had been weaned on the junk food diet also had significantly higher nonfasting serum leptin levels (Fig. 9B). Additionally, significant interactions were observed for nonfasting leptin between the postweaning and lactational diets (P < 0.0001), as well as the postweaning and gestational diets (P < 0.01).
Fig. 9.
A: nonfasting serum leptin concentrations of female offspring harvested on PD224. Junk food-fed groups had significantly higher nonfasting (P < 0.00001) leptin levels compared with the chow-fed groups. B: data from A combined and replotted to show effects of gestational, lactational, and postweaning dietary condition. Leptin levels were significantly higher in rats gestated in HF compared with LF dams (P < 0.001) and in those suckled by HF dams compared with LF diet dams (P < 0.001). Serum leptin levels were significantly higher in rats fed junk food diet postweaning than those fed chow. Bars denoted with different letters are significantly different (P < 0.0001). Data are shown as means ± SE.
Food-motivated behavior.
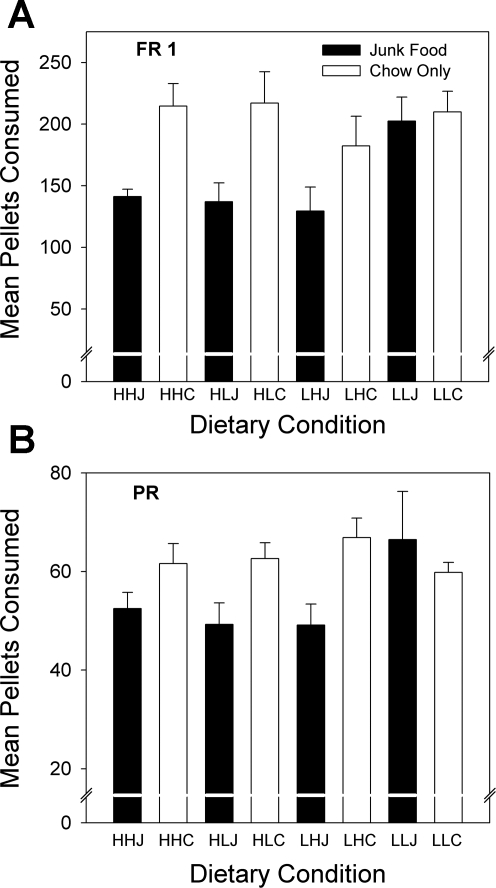
The average performances across the FR1 and PR sessions are shown in Fig. 10. Three-way ANOVA of the pellets consumed on the FR1 schedule revealed a significant effect of the postweaning diet (P < 0.0001): junk food-fed rats pressed fewer times and so received fewer pellet reinforcements compared with the chow-fed rats. On the PR, a similar effect of postweaning diet (P < 0.01) was observed. There were, however, no main or interactive effects of gestational or lactational diet on either of these tasks. The overall number of pellets consumed on the PR was far less than on the FR (P < 0.001) although the cumulative number of presses on the PR (∼250 for the junk food groups, 450 for the chow groups) were some twofold higher than the corresponding number of presses on the FR.
Fig. 10.
Food-motivated behavior of female offspring. Shown are the number of 45-mg pellets consumed averaged across fixed ratio (FR) 1 (A) and progressive ratio (PR, B) sessions. Junk food-fed rats consumed fewer pellets (and pressed the lever fewer times) than the rats maintained on chow. Rats obtained fewer pellets on the PR schedule compared with the FR1 schedule. Data are shown as means ± SE.
DISCUSSION
These experiments characterized the effects of manipulating fat content in the maternal diet (60 vs. 10%) on intrauterine and early postnatal life in terms of growth parameters, as well as differences in maternal white adipose tissue mass measured at weaning. We additionally investigated putative programming effects of different combinations of gestational, lactational, and postweaning environments on the development of metabolic syndrome in female BHR.
We had hoped that the maternal high-fat diet introduced well before impregnation might lead to overeating and obesity, but it did not. The future mothers instead showed a short-lived hyperphagia, followed by a return to normal intake, as reported by others (7, 26). A more varied high-fat diet might have been more effective (37).
Given the similar caloric intakes of the high- and low-fat groups, it is not surprising that there were no differences in body weight after 6 wk on their respective diets before mating, or at parturition or weaning. Some studies have found that high-fat diets can produce weight gain in rats without hyperphagia (17, 44), whereas others have not (24). Factors such as age, strain, or the particular diet may influence this outcome. However, despite no difference in body weight, our data do indicate a change in body energy partitioning in the two dietary groups. Total fat pad mass, measured when the dams were killed at weaning, was almost twofold higher in the high-fat than the low-fat groups, regardless of the litter type they had nursed. These findings agree with those of Boozer et al. (10) who found that male rats fed 48% fat diet had more body fat than those fed a 12% fat diet for 6 wk.
There were no differences in mean pup birth weight as a result of the different gestational dietary environments, consistent with previous reports (23). There were also no differences in the number of pups per litter or ratio of male to female offspring as a consequence of being gestated in a dam fed a high- vs. low-fat diet. By PD10, those pups that had been gestated in a low-fat-fed mother but cross-fostered to a high-fat dam (group LH) were significantly heavier than the other groups of pups. At PD21, pups that had been nursed by high-fat-fed dams (HH and LH) were significantly heavier than those that had been nursed by the dams fed the low-fat diet (HL and LL). It is noteworthy that pups' weights began to diverge as a function of maternal diet as early as PD10, which is before the age at which the pups begin to consume solid food. This may be because dams fed a high-fat diet produce milk of a higher fat content in the milk or have increased milk production (4, 14, 45). Differences in the type of fat (saturated vs. unsaturated) in maternal diet are known to influence membrane composition in the pups; we used a saturated fat diet that has previously been linked to adverse physiological consequences (26, 43).
After weaning, we found no differences in energy intake as a function of either gestational or lactational dietary conditions. There is apparent disagreement in the literature regarding programming of hyperphagia; some studies have reported hyperphagia as a consequence of maternal obesity or high-fat diet (6, 39), whereas others have not found this effect (41). It should be noted that the former studies used elective intake of palatable diets, whereas the latter were force-fed a liquid diet. These findings could be interpreted to mean that palatable and putatively more rewarding diets in the mothers may preferentially program appetite regulation to favor excess consumption. Although high-fat diets are often equated with high palatability, our results did not cause overconsumption by the biological mothers or foster dams and did not program hyperphagia. In contrast, there seems to be more agreement among studies investigating the programming effects of maternal undernutrition (15, 47) and/or protein deprivation (8); however, because these manipulations are associated with lower body weight, the hyperphagia in these reports depends on expressing food intake relative to body weight. The “predictive adaptive response” (PAR) hypothesis (19) proposes that the fetus makes adaptations in utero based on the predicted postnatal environment. A hypercaloric or high-fat environment in utero or early in postnatal life should, according to PAR, make animals less likely to experience the deleterious effects of eating a high-fat diet, including overeating and obesity. However, our rats fed high-fat junk food after weaning were hyperphagic relative to those fed chow only; they took more of their energy from the junk food diets and compensated by reducing their chow intake on intervening days. However, this compensation was imperfect (cf. Refs. 29, 36, 49), and their overall intake was higher than the chow-only groups. As a result, junk food groups were heavier than chow groups by PD45, and this difference increased with age. It should be noted that the junk food-fed rats were not only heavier (∼17%) and had more fat (∼118%) than chow-fed rats, but also had slightly enhanced linear growth (∼4%).
Significant differences in adiposity were found not only as a function of the postweaning diet but also as a function of the lactational dams' diets. Rats that had either been suckled by a high-fat-fed dam or were weaned on a high-fat diet had greater total body adiposity than their low-fat counterparts. The effect of the postweaning diet was also found in male offspring that were not the topic of this paper (30). This emphasizes the importance of the suckling environment on the development of obesity. Work in humans (34) has shown that the breast-fed children of mothers that had diabetes during lactation were at increased risk of becoming overweight in adolescence. The significance of the lactational period is demonstrated by a multitude of studies that overwhelmingly point to an increase in either adiposity, insulin resistance, hyperphagia, or hyperleptinemia as a result of either hypernutrition by reducing litter size (32, 35, 46) or by fostering pups to a dam fed a high-fat diet as was done in the present experiment. How the suckling environment influences systems development is presently still unknown; possible explanations include an increase in preference for high-fat food, since lipid levels in milk correlate with dietary fat (4, 14, 45), a programmed increase in intake due either to reduced litter size (32, 35, 46) or increased milk production in high-fat-fed dams (14), or a permanent change in neural orexigenic pathways due to absent (11) or inappropriate timing of the leptin surge, or due to a permanent increase in galanin expression that has been demonstrated to induce a preference for fat ingestion in neonatally overfed rats (33). It should be noted that these effects from the suckling period are contrary to the “protective” predictions of the PAR hypothesis. The significant interaction that we observed between the lactational dam's diet and the postweaning diet points to the idea that the high-fat suckling environment may have induced changes in metabolic rate or energy efficiency, resulting in the increased adiposity. In addition to the elevated adiposity, gross heart and kidney weights of the junk food-fed rats were greater than those of chow-fed counterparts.
There is some evidence that factors such as maternal obesity (26, 39) and high-fat diets (46) increase the likelihood of developing hypertension, but this was not found in the present study. The low-fat diet on which the dams were maintained in this experiment contained almost 60% of the calories as corn starch, so it is possible that high-carbohydrate diets may also be problematic. Informally, we noted that low-fat-fed dams tended to be more agitated than high-fat-fed dams when picked up for weighing or cage changes. Fetal stress and elevated prenatal glucocorticoid exposure has been linked to the development of hypertension (40), so if dams fed the low-fat diet were more stress responsive, increased corticosterone may have programmed their offspring toward hypertension and so obfuscated any effect of the high-fat diet in the other cohort.
Consistent with increased adiposity in the junk food-fed rats, they also had increased serum concentrations of leptin and insulin compared with their chow-fed counterparts. Nonfasting serum leptin levels differed not only as a function of the postweaning diet but also as a function of the gestational and lactational dams' diets. This was also found in male offspring (30) and points to the possibility that the perinatal maternal environment programs long-term changes in metabolic function. Given that caloric intake of the junk food-fed rats was higher than that of the chow-fed rats, and that there were no differences in intake as a function of the gestational or lactational conditions, the hyperleptinemic condition of the rats that were either gestated in a high-fat dam, suckled by a high-fat dam, or weaned on the junk food diet may indicate reduced sensitivity to circulating leptin in critical receptor regions (e.g., hypothalamus). Hyperinsulinemia was also noted in the fasted junk food-fed rats, suggesting a reduced insulin sensitivity that has often been associated with metabolic syndrome (25).
The motivation of rats to obtain food was assessed using both FR and PR schedules. The PR method of assessing motivational state is well-validated and has been used for assessing the incentive value of drug (2) and food rewards (22, 28). On the FR, junk food-fed rats took fewer pellets that those maintained on chow. This suggests that the incentive value of the reward was less in the junk food groups compared with chow-fed groups. This may be a successive negative contrast effect (13) whereby prior access to a palatable option (junk food) reduces the incentive value of a second option (45-mg pellets) that may otherwise be considered palatable. On the PR, the junk food-fed rats showed reduced break points (fewer pellets earned) and about one-half the number of presses compared with chow-fed rats, indicative of reduced motivation. No programming effects of gestational or lactational diet were evident in any of these measures.
Perspectives
These data suggest that the maternal environment (both prenatal and perinatal) exerts programming effects on energy-related systems involving adipogenesis and sensitivity to insulin and leptin. It is important, however, to note that the postweaning diet had significant effects on nearly all parameters measured: caloric intake, adiposity, gross organ weights, circulating serum and insulin levels, and motivation to obtain food. This would serve to emphasize the importance of the dietary choices made in adulthood and suggest that those choices may well override programming effects of a suboptimal maternal environment. Our expectation that the BHR model might show greater effects on cardiovascular parameters than more usual rat strains (e.g., Refs. 1, 26) was not evident in any of the measures.
GRANTS
This work was supported, in part, by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-064712.
Acknowledgments
We thank Melissa Chaney and Nora Ekeanya for assistance in preparing the diets used in these experiments.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Armitage JA, Taylor PD, Poston L. Experimental models of developmental programming: consequences of exposure to an energy rich diet during development. J Physiol 565: 3–8, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnold JM, Roberts DC. A critique of fixed and progressive ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacol Biochem Behav 57: 441–447, 1997. [DOI] [PubMed] [Google Scholar]
- 3.Aukes AM, Vitullo L, Zeeman GG, Cipolla MJ. Pregnancy prevents hypertensive remodeling and decreases myogenic reactivity in posterior cerebral arteries from Dahl salt-sensitive rats: a role in eclampsia? Am J Physiol Heart Circ Physiol 292: H1071–H1076, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Averette LA, Odle J, Monaco MH, Donovan SM. Dietary fat during pregnancy and lactation increases milk fat and insulin-like growth factor I concentrations and improves neonatal growth rates in swine. J Nutr 129: 2123–2129, 1999. [DOI] [PubMed] [Google Scholar]
- 5.Barker DJ Fetal nutrition and cardiovascular disease in later life. Br Med Bull 53: 96–108, 1997. [DOI] [PubMed] [Google Scholar]
- 6.Bayol SA, Farmington SJ, Stickland NC. A maternal ‘junk food’ diet in pregnancy and lactation promotes an exacerbated taste for ‘junk food’ and a greater propensity for obesity in rat offspring. Br J Nutr 98: 843–851, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Beck B, Richy S. Differential long-term dietary regulation of adipokines, ghrelin, or corticosterone: impact on adiposity. J Endocrinol 196: 171–179, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Bellinger L, Lilley C, Langley-Evans SC. Prenatal exposure to a maternal low-protein diet programmes a preference for high-fat foods in the young adult rat. Br J Nutr 92: 513–520, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 115: 290–296, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Boozer CN, Schoenbach G, Atkinson RL. Dietary fat and adiposity: a dose-response relationship in adult male rats fed isocalorically. Am J Physiol Endocrinol Metab 268: E546–E550, 1995. [DOI] [PubMed] [Google Scholar]
- 11.Bouret SG, Draper SJ, Simerly RB. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science 304: 108–110, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Bray GA, Paeratakul S, Popkin BM. Dietary fat and obesity: a review of animal, clinical and epidemiological studies. Physiol Behav 83: 549–555, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Crespi LP Amount of reinforcement and level of performance. Psychol Rev 51: 341–357, 1944. [Google Scholar]
- 14.Del Prado M, Delgado G, Villalpando S. Maternal lipid intake during pregnancy and lactation alters milk composition and production and litter growth in rats. J Nutr 127: 458–462, 1997. [DOI] [PubMed] [Google Scholar]
- 15.Desai M, Gayle D, Han G, Ross MG. Programmed hyperphagia due to reduced anorexigenic mechanisms in intrauterine growth-restricted offspring. Reprod Sci 14: 329–337, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Euser AG, Cipolla MJ. Cerebral blood flow autoregulation and edema formation during pregnancy in anesthetized rats. Hypertension 49: 334–340, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Férézou-Viala J, Roy AF, Sérougne C, Gripois D, Parquet M, Bailleux V, Gertler A, Delplanque B, Djiane J, Riottot M, Taouis M. Long-term consequences of maternal high-fat feeding on hypothalamic leptin sensitivity and diet-induced obesity in the offspring. Am J Physiol Regul Integr Comp Physiol 293: R1056–R1062, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Galtier-Dereure F, Boegner C, Bringer J. Obesity and pregnancy: complications and cost. Am J Clin Nutr 71: 1242S–1248S, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Gluckman PD, Hanson MA. The developmental origins of the metabolic syndrome. Trends Endocrinol Metab 15: 183–187, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Hatton DC, DeMerritt J, Coste SC, McCarron DA. Stress-induced hypertension in the borderline hypertensive rat: stimulus duration. Physiol Behav 53: 635–41, 1993. [DOI] [PubMed] [Google Scholar]
- 21.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA 291: 2847–2850, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Hodos W Progressive ratio as a measure of reward strength. Science 134: 943–944, 1961. [DOI] [PubMed] [Google Scholar]
- 23.Holemans K, Caluwaerts S, Poston L, Van Assche FA. Diet-induced obesity in the rat: a model for gestational diabetes mellitus. Am J Obstet Gynecol 190: 858–865, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Johnson JA, Trasino SE, Ferrante AW Jr, Vasselli JR. Prolonged decrease of adipocyte size after rosiglitazone treatment in high- and low-fat-fed rats. Obesity 15: 2653–2663, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 4444: 840–846, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Khan IY, Taylor PD, Dekou V, Seed PT, Lakasing L, Graham D, Dominiczak AF, Hanson MA, Poston L. Gender-linked hypertension in offspring of lard-fed pregnant rats. Hypertension 41: 168–175, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Langley-Evans SC Fetal programming of cardiovascular function through exposure to maternal undernutrition. Proc Nutr Soc 60: 505–513, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Low MJ, Hayward MD, Appleyard SM, Rubinstein M. State-dependent modulation of feeding behavior by proopiomelanocortin-derived beta-endorphin. Ann NY Acad Sci 994: 192–201, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Lucas F, Ackroff K, Sclafani A. High-fat diet preference and overeating mediated by postingestive factors in rats. Am J Physiol Regul Integr Comp Physiol 275: R1511–R1522, 1998. [DOI] [PubMed] [Google Scholar]
- 30.Mitra A Effect of perinatal high fat diet on stress responsivity, motivation, and the induction of metabolic syndrome in offspring using a borderline hypertensive rodent model. (Ph.D. Dissertation). Gainesville, Florida. University of Florida, 2008.
- 31.Naeye RL Maternal body weight and pregnancy outcome. Am J Clin Nutr 52: 273–279, 1990. [DOI] [PubMed] [Google Scholar]
- 32.Oscai LB, McGarr JA. Evidence that the amount of food consumed in early life fixes appetite in the rat. Am J Physiol Regul Integr Comp Physiol 235: R141–R144, 1978. [DOI] [PubMed] [Google Scholar]
- 33.Plagemann A, Harder T, Rake A, Voits M, Fink H, Rohde W, Dörner G. Perinatal elevation of hypothalamic insulin, acquired malformation of hypothalamic galaninergic neurons, and syndrome x-like alterations in adulthood of neonatally overfed rats. Brain Res 836: 146–155, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Plagemann A, Harder T. Breast feeding and the risk of obesity and related metabolic diseases in the child. Metab Syndr Relat Disord 3: 222–232, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Plagemann A, Heidrich I, Götz F, Rohde W, Dörner G. Obesity and enhanced diabetes and cardiovascular risk in adult rats due to early postnatal overfeeding. Exp Clin Endocrinol 99: 154–158, 1992. [DOI] [PubMed] [Google Scholar]
- 36.Reed DR, Tordoff MG, Friedman MI. Sham-feeding of corn oil by rats: sensory and postingestive factors. Physiol Behav 47: 779–781, 1990. [DOI] [PubMed] [Google Scholar]
- 37.Ribot J, Rodríguez AM, Rodríguez E, Palou A. Adiponectin and resistin response in the onset of obesity in male and female rats. Obesity 16: 723–730, 2008. [DOI] [PubMed] [Google Scholar]
- 38.Roseboom TJ, van der Meulen JH, Ravelli AC, Osmond C, Barker DJ, Bleker OP. Effects of prenatal exposure to the Dutch famine on adult disease in later life: an overview. Mol Cell Endocrinol 185: 93–98, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Samuelsson AM, Matthews PA, Argenton M, Christie MR, McConnell JM, Jansen EH, Piersma AH, Ozanne SE, Twinn DF, Remacle C, Rowlerson A, Poston L, Taylor PD. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension 51: 383–392, 2008. [DOI] [PubMed] [Google Scholar]
- 40.Seckl JR Prenatal glucocorticoids and long-term programming. Eur J Endocrinol 151: U49–U62, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Shankar K, Harrell A, Liu X, Gilchrist JM, Ronis MJ, Badger TM. Maternal obesity at conception programs obesity in the offspring. Am J Physiol Regul Integr Comp Physiol 294: R528–R538, 2008. [DOI] [PubMed] [Google Scholar]
- 42.Starr A, Graepel R, Keeble J, Schmidhuber S, Clark N, Grant A, Shah AM, Brain SD. A reactive oxygen species-mediated component in neurogenic vasodilatation. Cardiovasc Res 78: 139–147, 2008. [DOI] [PubMed] [Google Scholar]
- 43.Taylor PD, Khan IY, Lakasing L, Dekou V, O'Brien-Coker I, Mallet AI, Hanson MA, Poston L. Uterine artery function in pregnant rats fed a diet supplemented with animal lard. Exp Physiol 88: 389–398, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Torre-Villalvazo I, Tovar AR, Ramos-Barragán VE, Cerbón-Cervantes MA, Torres N. Soy protein ameliorates metabolic abnormalities in liver and adipose tissue of rats fed a high fat diet. J Nutr 138: 462–468, 2008. [DOI] [PubMed] [Google Scholar]
- 45.Trottier G, Koski KG, Brun T, Toufexis DJ, Richard D, Walker CD. Increased fat intake during lactation modifies hypothalamic-pituitary-adrenal responsiveness in developing rat pups: a possible role for leptin. Endocrinology 139: 3704–3711, 1998. [DOI] [PubMed] [Google Scholar]
- 46.Velkoska E, Cole TJ, Morris MJ. Early dietary intervention: long-term effects on blood pressure, brain neuropeptide Y, and adiposity markers. Am J Physiol Endocrinol Metab 288: E1236–E1243, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Vickers MH, Breier BH, Cutfield WS, Hofman PL, Gluckman PD. Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. Am J Physiol Endocrinol Metab 279: E83–E87, 2000. [DOI] [PubMed] [Google Scholar]
- 48.Vickers MH, Gluckman PD, Coveny AH, Hofman PL, Cutfield WS, Gertler A, Breier BH, Harris M. Neonatal leptin treatment reverses developmental programming. Endocrinology 146: 4211–4216, 2005. [DOI] [PubMed] [Google Scholar]
- 49.Warwick ZS, Synowski SJ, Bell KR. Dietary fat content affects energy intake and weight gain independent of diet caloric density in rats. Physiol Behav 77: 85–90, 2002. [DOI] [PubMed] [Google Scholar]
- 50.Warwick ZS, Synowski SJ. Effect of food deprivation and maintenance diet composition on fat preference and acceptance in rats. Physiol Behav 68: 235–239, 1999. [DOI] [PubMed] [Google Scholar]