Abstract
Previous studies have suggested the recovery of phosphocreatine (PCr) after exercise is at least second-order in some conditions. Possible explanations for higher-order PCr recovery kinetics include heterogeneity of oxidative capacity among skeletal muscle fibers and ATP production via glycolysis contributing to PCr resynthesis. Ten human subjects (28 ± 3 yr; mean ± SE) performed gated plantar flexion exercise bouts consisting of one contraction every 3 s for 90 s (low-intensity) and three contractions every 3 s for 30 s (high-intensity). In a parallel gated study, the sciatic nerve of 15 adult male Sprague-Dawley rats was electrically stimulated at 0.75 Hz for 5.7 min (low intensity) or 5 Hz for 2.1 min (high intensity) to produce isometric contractions of the posterior hindlimb muscles. [31P]-MRS was used to measure relative [PCr] changes, and nonnegative least-squares analysis was utilized to resolve the number and magnitude of exponential components of PCr recovery. Following low-intensity exercise, PCr recovered in a monoexponential pattern in humans, but a higher-order pattern was typically observed in rats. Following high-intensity exercise, higher-order PCr recovery kinetics were observed in both humans and rats with an initial fast component (τ < 15 s) resolved in the majority of humans (6/10) and rats (5/8). These findings suggest that heterogeneity of oxidative capacity among skeletal muscle fibers contributes to a higher-order pattern of PCr recovery in rat hindlimb muscles but not in human triceps surae muscles. In addition, the observation of a fast component following high-intensity exercise is consistent with the notion that glycolytic ATP production contributes to PCr resynthesis during the initial stage of recovery.
Keywords: oxidative capacity, fiber types, skeletal muscle, magnetic resonance spectroscopy, nonnegative least-squares analysis
the time course of phosphocreatine (PCr) recovery following exercise is often characterized by using a monoexponential model (18, 21, 26, 37). A monoexponential pattern is consistent with a first-order metabolic system in which PCr resynthesis is entirely dependent on ATP produced by oxidative phosphorylation (11, 21) with the inverse of the time constant (tau, τ) or rate constant (1/τ) directly proportional to muscle oxidative capacity (19, 26). Specifically, the rate constant of PCr resynthesis has been shown to be directly proportional to citrate synthase activity (which reflects mitochondrial content) and inversely proportional to total creatine content (20, 26). Nevertheless, multiexponential PCr recovery kinetics have been commonly reported after high-intensity exercise (1, 10, 23, 31, 32, 41). Although the factor(s) contributing to this higher-order response have not been established, one possible explanation is heterogeneity of oxidative capacity among the recruited skeletal muscle fiber types.
In the rat hindlimb, some of the muscles are composed of a predominance of fiber types within a limited range of the spectrum of phenotype profiles. For example, the soleus muscle is composed entirely of more oxidative fiber types within the range of type I to IIA fibers (25, 33). Furthermore, in some rat hindlimb muscles there are distinct regional differences in fiber composition within the muscle. Perhaps most apparent is the more superficial, white region of the gastrocnemius, which is composed entirely of type IID/X and IIB fibers, whereas the deeper, red region of the gastrocnemius is composed of a significant percentage of type I and IIA fibers (5, 33). In addition, distinct differences in oxidative capacity have been shown among different rat hindlimb muscles or among regions within a single muscle (5). For example, the deep, red region of the gastrocnemius has a fourfold greater oxidative capacity than the superficial, white region (5). In contrast to the rat hindlimb, the range of oxidative capacities among the different fiber types is typically narrower in humans (12). For example, in the triceps surae muscles (soleus and medial and lateral gastrocnemius) of humans, succinate dehydrogenase activity was only 38–51% greater in the type I fibers than the type IIX fibers (8). Therefore, if heterogeneity of oxidative capacity among muscle fibers contributes to the higher-order PCr recovery kinetics, then it may be expected that this pattern would be more evident in muscles of the rat hindlimb than the triceps surae muscles of humans.
Higher-order PCr recovery kinetics could also be due, in part, to glycolytic ATP production (i.e., nonoxidative ATP production via glycolysis) contributing to PCr resynthesis. For example, Crowther et al. (4) observed that glycolytic flux remained elevated for a short period of time (< 20 s) following exercise under ischemic conditions. If glycolytic ATP production makes a substantial contribution to ATP supply during the initial stages of recovery, a faster phase of PCr recovery would be expected following high-intensity exercise. Furthermore, if an initial fast component is evident, then this would be consistent with feedback control of glycolysis via allosteric effectors such as ADP and AMP. Therefore, the initial PCr resynthesis rate may reflect contributions from both glycolytic and oxidative metabolism and may confound the use of the initial rate of PCr recovery as a measure of oxidative capacity following high-intensity exercise.
The kinetics of PCr recovery have often been described by using a monoexponential or biexponential model. However, a novel approach to examine PCr recovery kinetics is to utilize nonnegative least squares (NNLS) analysis. NNLS resolves the number of exponential components, the value of their time constants, and their relative contribution to the total PCr recovery time course (42). This method has been widely utilized to evaluate NMR T2 relaxometry (30) and has the advantage of requiring no a priori knowledge of the number of components in the underlying kinetic model.
Accordingly, in the present study we utilize NNLS to analyze PCr recovery data from the human triceps surae and rat posterior hindlimb muscles during gated low-intensity and high-intensity protocols, which were repeated multiple times to enhance the confidence of the kinetic modeling procedure. Specifically, we hypothesize: 1) higher-order PCr kinetics would be more evident in the rat posterior hindlimb muscles due to more discrete differences in oxidative capacity among the recruited fibers compared with human triceps surae muscles and 2) a fast component of PCr recovery would be more prevalent after high-intensity exercise in both humans and rats due to glycolytic ATP production contributing to PCr resynthesis during the initial stages of recovery compared with low-intensity exercise.
METHODS
Subjects.
Ten adult human subjects (age 28 ± 3; 1 female) from the university community volunteered for this study. The subjects were healthy and recreationally active. The study was approved by the University Committee on Research Involving Human Subjects with each subject giving informed, written consent. In a parallel study, 15 adult male Sprague-Dawley rats (300–400 g; Harlan Sprague Dawley, Indianapolis, IN) were examined. This study was approved by the University Committee on Animal Use and Care.
Human experiments.
Dynamic plantar flexion exercise was performed in a GE 3T Excite MR system (GE Medical Systems, Milwaukee, WI). This exercise involved the subjects lying supine in the bore of the magnet with their torso secured to the table with a wide Velcro strap. The subject's right knee was flexed (∼10°) and the right foot secured to a footplate on a custom-built nonmagnetic ergometer by Velcro straps. The footplate was attached to a load cell (model SSM-EV-250; Interface, Scottsdale AZ) via TheraBand Resistive latex tubing aligned approximately parallel to the line of pull. Force was digitally recorded using WinDaq software (version 2.19; DATAQ Instruments, Akron OH; sample rate 60 Hz). Each subject performed three to five maximal voluntary contractions (MVC) prior to the plantar flexion exercise protocols, and the level of resistance (i.e., number of latex tubes) for the protocols was selected to permit contractions to be performed at a peak target force of 20% MVC. Subjects performed a total of 10 cycles (6.5 min each) of plantar flexion exercise and recovery at two contraction frequencies. During the initial five cycles, contractions were done at a frequency of one contraction every 3 s for 90 s (low-intensity) followed by 5 min of resting recovery. The final five cycles were performed at a contraction frequency consisting of three contractions every 3 s for 30 s (high-intensity) followed by 6 min of resting recovery. The same resistance was used in the low-intensity and high-intensity protocols.
During rest (60 s), exercise, and recovery one-shot [31P]-MRS spectra (51.7 MHz, repetition time (TR) 3 s, 2,500 Hz sweep width, 1,024 complex points) of the triceps surae muscles were continuously acquired via a 10-cm diameter circular linear transmit/receive surface coil. The calf was positioned over the surface coil and a three-plane, fast-gradient, echo-imaging sequence was performed to ensure that the center of the surface coil (evident by a vial of phenylphosphonic acid) was positioned under the middle of the triceps surae muscles. The spoiler gradients of the MR system provided an audio cue every 3 s that was used by the subjects for pacing of the gated contractions. Subjects performed a contraction or set of three contractions after each audio cue, which occurred immediately after the acquisition of a free-induction decay (FID). Visual real-time feedback of the force time course was provided to each subject to ensure that contractions were performed at 20% MVC.
The FID's obtained during the 2–5th exercise and recovery cycles for the low-intensity and high-intensity protocols were sorted and combined yielding 130 FIDs. The initial cycle was omitted because there tended to be greater changes in [PCr] and pH than the remaining cycles (see results). The relative phosphate amplitudes of phenylphosphonic acid, phosphomonoesters, inorganic phosphate (Pi), phosphodiesters, PCr, and γ-, α-, and β-ATP were estimated using jMRUI software (version 3.0). This procedure consisted of an initial alignment of the PCr peak to 0 ppm and utilizing the AMARES fitting algorithm with estimated starting values and prior knowledge (38). The intracellular pH was determined from the chemical shift of Pi relative to PCr (24).
Rat experiments.
Adult male Sprague-Dawley rats were housed three per cage in a temperature (∼22°C)- and humidity (∼35% relative humidity)-controlled room on a 12:12-h light-dark cycle. Rats were provided Purina Rat Chow and tap water ad libitum. All experiments were performed during the rats' nocturnal cycle. The rats were divided into two groups that underwent either a low-intensity or high-intensity stimulation protocol. The stimulation frequencies were chosen to be less than (0.75 Hz) or greater than (5.0 Hz), the intensity that can be sustained by oxidative metabolism of rat posterior hindlimb muscle (13). The stimulation and recovery protocols consisted of 5.7-min stimulation at 0.75 Hz (low-intensity) followed by 5.7-min resting recovery or 2.1-min stimulation at 5 Hz (high-intensity) followed by 29.9 min of resting recovery. This stimulation-recovery pattern was repeated 6 to 24 times during the low-intensity protocol and 6 or 7 times during the high-intensity protocol to enable averaging of cycles to produce high signal-to-noise spectra. The resting recovery duration was based on pilot experiments showing that intracellular pH reliably recovered to resting levels prior to the next stimulation period.
Prior to the experiments, rats were anesthetized with pentobarbital sodium (50 mg/kg body mass), and an intraperitoneal catheter was inserted for administering additional anesthetic (5 mg/kg) at ∼30-min intervals. The rats were prepared for in situ stimulation of the right leg as described previously (21, 26). In brief, the right sciatic nerve of the supine rat was ligated and placed in a bipolar platinum electrode. The right knee was fixed between two brass posts with a tungsten pin through the condyles of the femur, and the Achilles tendon was attached to a strain-gauge force transducer mounted on an adjustable support in the probe. Muscle length was adjusted to provide maximum peak isometric twitch force in response to a supramaximal pulse (10–20 V, 2-ms duration using a Grass stimulator, model S48). The probe was then mounted in a Bruker AM400 spectrometer (9.4 T, 7.4-cm vertical-bore magnet). The probe was ventilated with 100% oxygen, and the body temperature was continuously monitored via a rectal thermistor (model 402A YSI probe) and maintained at 37°C by adjusting the magnet bore air temperature. [31P]-MRS spectra of the right posterior hindlimb, which includes the soleus, gastrocnemius, and plantaris, were acquired (162 MHz, TR 1.0–1.3 s, 8 KHz sweep width, 2,000 complex data points) via a 2-cm saddle-shaped surface coil. At the start of each experiment, a single, fully relaxed control spectrum of the resting muscle was acquired (TR = 15 s). Similar to the human experiments, pulse acquisition was gated to contractions to minimize motion artifacts, and 16 subsequent FIDs were summed. At the end of the experiment, the stimulated posterior hindlimb muscle group was removed and weighed so force measurements could be expressed per gram wet muscle.
The summed FIDs were zero-filled to 2K complex data and multiplied by an exponential function corresponding to 20-Hz line broadening before Fourier transformation. Due to the data sets being acquired over an extended period of time, phase and frequency drift were corrected using Principal Component Analysis (2). The spectra were added across cycles beginning with the third cycle. The initial two cycles were not included because the [PCr] and pH changes tended to be greater than the other cycles (see results). For each spectrum the relative peak areas were determined using the method of natural line shapes, and intracellular pH was determined by the chemical shift of Pi relative to PCr (24).
PCr kinetic analysis.
PCr data were initially expressed as a percent of resting levels, and then the PCr recovery data were fit to a sum of exponential components using the NNLS algorithms of Whittall and MacKay (42). The parameters were set at 400 time constants linearly spaced from 1.5 to 600 s plus a constant background which fits the end-exercise PCr level. The amplitude of each component was expressed as a percent of the sum of the amplitudes of all the components. In addition, the recovery of PCr following exercise was fit with a monoexponential model using the formula described by: Y(t) = Y(Bsl) + Amp * (1−e−τ/t), where Y represents PCr at any time (t); Bsl is the baseline Y value; Amp is the amplitude change in Y; and τ is the time constant.
Statistical analyses.
Statistical analyses were performed using SPSS (version 10.0; SPSS, Chicago, IL). Force measurements for the rats and humans were compared separately using one-way ANOVA. Intracellular pH was compared using a three-way ANOVA (human vs. rat, low intensity vs. high intensity, and rest vs. end-exercise vs. recovery). End-exercise PCr was analyzed using two-way ANOVA (human vs. rat and low intensity vs. high intensity). The NNLS-derived parameters of PCr recovery (i.e., τ and amplitude) were compared using three-way ANOVA (human vs. rat, low intensity vs. high intensity, and fast vs. slow1 vs. slow2 component). To compare the contributions of each component to the total PCr recovery the amplitude of each component was set as a percent of the total amplitude (100%). When one of the three components was not evident from the NNLS analysis, the amplitude of that component was assigned zero. When a significant interaction effect was observed, post hoc analysis was performed using Bonferroni-corrected t-tests. Significance was set at the P < 0.05 level. Data are reported as means ± SE.
RESULTS
Preliminary analysis of pH and PCr.
Preliminarily analysis and visual inspection of the repeated exercise and recovery cycles showed that the first cycle in humans and the first two cycles in rats tended to have different pH and PCr responses than the remaining cycles. This was similar to the effect of prior exercise on subsequent bouts of exercise reported previously (6, 29, 34). In the present study, for example, during the human high-intensity protocol there was a significant time effect (P = 0.015) observed when comparing the lowest pH obtained during each repeated cycle, with the first cycle having a lower pH compared with the subsequent repeated bouts (cycle 1: pH 6.77 ± 0.05; cycles 2–5: pH range 6.86 ± 0.02 to 6.89 ± 0.02). Similarly, in the rats, there was a significant time effect (P = 0.008), with a lower pH obtained at the end of the first two series of high-intensity contractions compared with the remaining cycles (cycle 1: pH 6.21 ± 0.02; cycle 2: pH 6.58 ± 0.03; cycles 3–8: pH range 6.73 ± 0.03 to 6.78 ± 0.03). Therefore, the first exercise cycle in the human protocols and the first two cycles in the rat protocols were omitted prior to averaging the repeated trials to produce a single data set for each subject.
Force during low- and high-intensity exercise.
During the human plantar flexion exercise protocols, the average peak force obtained during the contractions was similar between the low-intensity (20.7 ± 2.1% of MVC) and high-intensity (20.6 ± 2.0% of MVC) exercise, and therefore the only difference between protocols was the duty cycle. Furthermore, the average peak force obtained during the contractions was maintained throughout both the low-intensity (initial 5 contractions: 146 ± 10 N; final 5 contractions: 159 ± 7 N) and high-intensity (initial 5 contractions: 150 ± 6 N; final 5 contractions 155 ± 7 N) protocols.
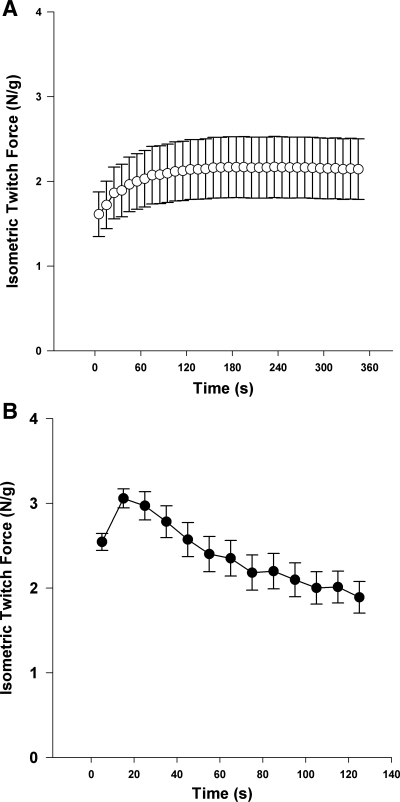
Similar to the human protocols, the duty cycle was also altered with the stimulation of the rat hindlimb muscle. Peak force was lower (P = 0.007) during the low-intensity exercise (2.50 ± 0.16 N/g wet muscle) compared with the high-intensity exercise (3.11 ± 0.12 N/g wet muscle) (Fig. 1). During the low-intensity exercise, force increased and then was maintained at a constant level throughout the remainder of the stimulation. During the high-intensity exercise protocol force levels increased to a peak level, and then decreased throughout the remainder of the stimulation (Fig. 1).
Fig. 1.
Isometric twitch force during low-intensity (0.75 Hz; A) and high-intensity (5 Hz; B) stimulation of rat hindlimb muscle. Force measurements are expressed as Newtons per gram wet muscle. Each data point represents an average over 10 s. Data presented are the means ± SE of each rat's average force produced during its repeated cycles, excluding the first 2 cycles in each protocol.
[31P]-MRS data.
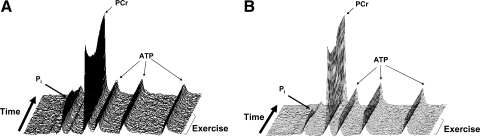
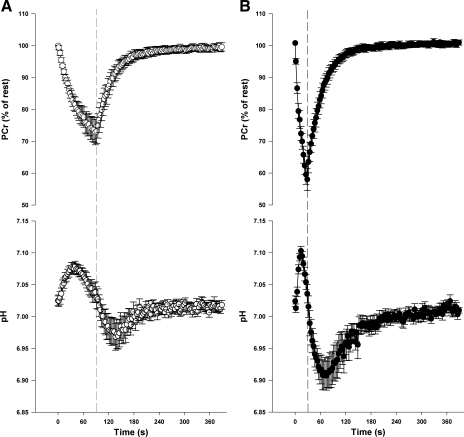
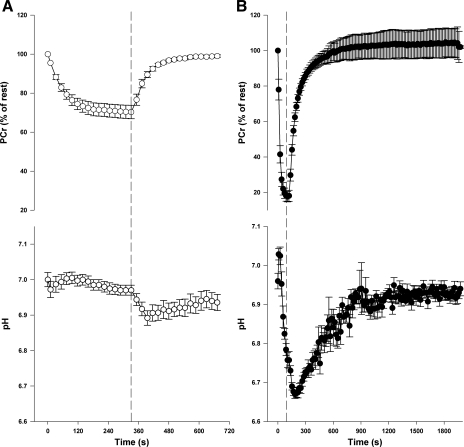
Representative [31P]-MRS spectra from human triceps surae and rat hindlimb muscle are shown in Fig. 2. In humans, intracellular pH was similar between the low-intensity and high-intensity exercise protocols at rest and at the end of exercise. As expected, the minimum pH obtained during the resting recovery was lower (P = 0.005) after the high-intensity compared with the low-intensity exercise (Table 1, Fig. 3). In the rat hindlimb muscle, pH was similar at rest in the low-intensity and high-intensity protocols, but was lower at end-exercise (P < 0.001) and during recovery (P < 0.001) after high-intensity compared with the low-intensity protocol (Table 1, Fig. 4). In both humans and rats the relative [PCr] was lower (P < 0.001) at end of exercise after high-intensity compared with low-intensity exercise (Table 1, Figs. 3 and 4).
Fig. 2.
Sample series of [31P]-MRS spectra acquired during and after low-intensity (0.33 Hz) voluntary plantar flexion exercise in humans (A) and electrically stimulated posterior hindlimb muscle at low-intensity (0.75 Hz) in rats (B). Spectra are a sum of repeated cycles beginning with the second cycle in the human subject (i.e., sum of cycles 2–5) and the third cycle in the rat (i.e., sum of cycles 3–7). PCr, phosphocreatine; Pi, inorganic phosphate.
Table 1.
Phosphocreatine (PCr) and intracellular pH at rest and following plantar flexion contractions at 0.33 Hz (low-intensity) and 1 Hz (high-intensity) in humans and following electrical stimulation of the rat hindlimb at 0.75 Hz (low-intensity) and 5 Hz (high-intensity)
|
Humans |
Rats
|
|||
|---|---|---|---|---|
| Low-Intensity | High-Intensity | Low-Intensity | High-Intensity | |
| End-exercise PCr, % of rest | 73±4 | 60±3* | 70±3 | 18±3*# |
| Resting pH | 7.03±0.01 | 7.02±0.01 | 7.00±0.02 | 6.96±0.02 |
| End-exercise pH | 7.04±0.02 | 7.04±0.01 | 6.97±0.01 | 6.76±0.02*# |
| Lowest pH obtained during recovery | 6.96±0.02 | 6.89±0.08* | 6.89±0.02# | 6.66±0.01*# |
Values are means ± SE
Significantly different than low-intensity within humans or rats;
significantly different than corresponding intensity in humans.
Fig. 3.
PCr and pH changes during and following human plantar flexion exercise performed at low-intensity (A) and high-intensity (B). Dashed line represents end of exercise. Data are expressed as means ± SE.
Fig. 4.
PCr and pH changes during and following contractions of the posterior hindlimb of a rat at low-intensity (0.75 Hz; A) and high-intensity (5 Hz; B) exercise. Dashed line represents end of stimulation. Data are expressed as means ± SE.
PCr kinetics analysis.
The time course of PCr recovery and its corresponding NNLS time constant spectrum are shown for a representative response from a human and rat in Figs. 5 and 6, respectively. NNLS analysis revealed one to three exponential components of PCr recovery in each trial with one or two slower components (τ > 15 s) and, in some trials, a fast component (τ < 15 s).
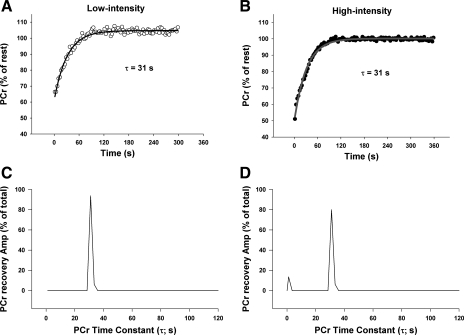
Fig. 5.
Sample PCr changes with “line of best fit” monoexponential curve following low-intensity (A) and high-intensity (B) exercise in humans, and their corresponding nonnegative least-squares (NNLS) spectra (C and D, respectively). Note the initial, fast component (τ < 15 s) following high-intensity exercise.
Fig. 6.
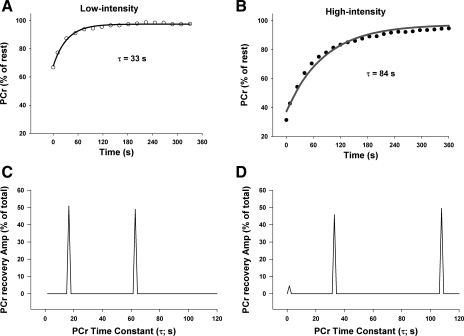
Sample PCr changes with “line of best fit” monoexponential curve following low-intensity (A) and high-intensity (B) exercise in rats, and corresponding NNLS spectra (C and D, respectively). Note the initial, fast component (τ < 15 s) following high-intensity exercise, and two slower components (τ > 15 s) following both low-intensity and high-intensity exercise.
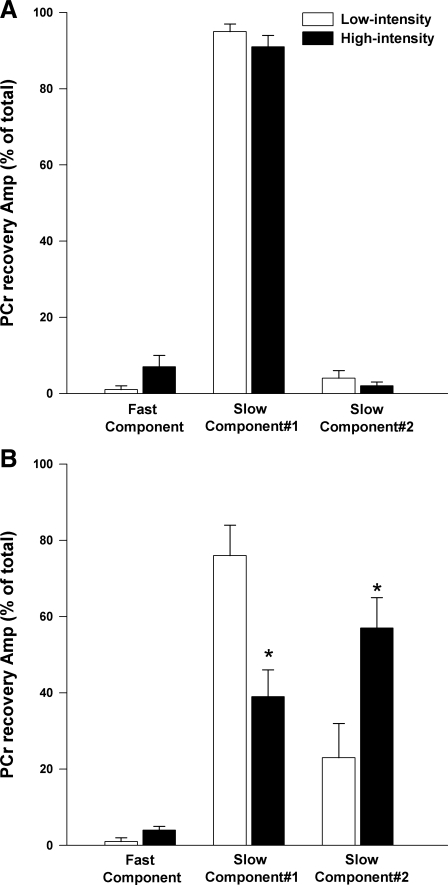
Following low-intensity exercise in humans, a single slow component (τ > 15 s) contributed, on average, 95% of the total PCr recovery response (Table 2, Fig. 7). In contrast, the rats demonstrated two slow components in the majority of cases (Table 2). This difference in number of slow components between the rats and humans was maintained at the high-intensity such that, overall, the rats demonstrated a second slow component more consistently than humans (rats, 12/15; humans, 3/20). In contrast to humans, the relative amplitude of the first slower component was decreased (P = 0.005) and the relative amplitude of the second slower component was increased (P = 0.014) in the high-intensity compared with low-intensity exercise in the rats (Table 2, Fig. 7).
Table 2.
PCr recovery kinetic parameters using nonnegative least squares (NNLS) analyses following plantar flexion contractions at 0.33 Hz (low-intensity) and 1 Hz (high-intensity) in humans and following electrical stimulation of the rat hindlimb at 0.75 Hz (low-intensity) and 5 Hz (high-intensity)
|
Humans |
Rats
|
|||||
|---|---|---|---|---|---|---|
| Fast | Slow1 | Slow2 | Fast | Slow1 | Slow2 | |
| Low-intensity | ||||||
| τ, s | 2±0 | 30±3 | 163±18 | 2±0 | 37±5 | 103±20 |
| % of Total amplitude | 1±1 | 95±2 | 4±2 | 1±1 | 76±8# | 23±9 |
| Number of subjects with component | 2 | 10 | 2 | 2 | 7 | 4 |
| High-intensity | ||||||
| τ, s | 2±1 | 34±3 | 206 | 4±2 | 32±4 | 125±28 |
| % of Total amplitude | 7±3 | 91±3 | 2±1 | 4±1 | 39±7*# | 57±8*# |
| Number of subjects with component | 6 | 10 | 1 | 5 | 8 | 8 |
Data are means ± SE; n = 10 humans; n = 15 rats (n = 7 low, 8 high). NNLS analyses of PCr recovery revealed a fast component (τ <15 s) in some trials and one or two slower components (τ >15 s). The time constant (τ), % total amplitude of the component, and the number of subjects that demonstrated each component are reported; values are presented as means ± SE
Significantly different than low-intensity within humans or rats;
significantly different than corresponding component in humans.
Fig. 7.
The relative amplitudes (Amp) of the exponential components of the PCr recovery data were resolved using NNLS analysis. The relative amplitude of the fast component (τ < 15 s) and two slower components (τ > 15 s) following low-intensity and high-intensity exercise in humans (A) and rats (B) are presented. Data are expressed as mean ± SE. *Significantly different than low-intensity.
In addition, following high-intensity exercise there was an initial, fast component (τ range, 2–11 s) that was observed in the majority of rats and humans. This component tended to account for a greater contribution to the total PCr recovery amplitude following high-intensity compared with low-intensity exercise in humans (7 ± 3% vs. 1 ± 1%, P = 0.060) and rats (4 ± 2% vs. 1 ± 1%, P = 0.095) (Table 2, Fig. 7). When human and rat data were pooled and analyzed separate from the other components, there was a significantly greater (P = 0.012) relative amplitude of the fast component in high-intensity (6 ± 2%) compared with low-intensity (1 ± 1%).
Congruent with the observation of a fast component of PCr recovery, the change in [PCr] during the initial 9 s of recovery was 31 ± 9% (humans, 17 ± 9%; rats, 48 ± 15%) greater than what would be predicted based on the time constants of the NNLS-derived slow component during the high-intensity protocols. When two slow components were evident, the time constant was determined by an amplitude-weighted average. Notably, with the time resolution used in our study, the time constant of the NNLS slow component(s) was similar to the time constant determined by a single a priori monoexponential fit of the data (Table 3).
Table 3.
Time constant (tau, τ) of PCr recovery derived from a monoexponential model and the slow component(s) of NNLS following plantar flexion contractions at 0.33 Hz (low-intensity) and 1 Hz (high-intensity) in humans and following electrical stimulation of the rat hindlimb at 0.75 Hz (low-intensity) and 5 Hz (high-intensity)
|
Humans |
Rats
|
|||
|---|---|---|---|---|
| Low-Intensity | High-Intensity | Low-Intensity | High-Intensity | |
| Monoexponential τ (s) | 35±4 | 36±3 | 48±3 | 89±8*# |
| NNLS, Slow τ (s) | 36±5 | 37±4 | 52±6 | 90±23*# |
Values are means ± SE. When 2 slow components were evident, the time constant (τ) was determined by an amplitude-weighted average.
Significantly different than low-intensity within humans or rats;
significantly different than corresponding intensity in humans. No significant differences were observed between τ derived from the monoexponential model and the NNLS derived slow component(s).
It should be noted that when the first cycle(s) were not omitted from the analyses all of the main outcomes of the study remained the same, although the confidence level of the fitting analysis would likely be reduced due to averaging dissimilar responses. Nonetheless, when the initial cycles were included in the analyses the following three findings were noted: 1) the number of subjects with a fast component was greater following high-intensity than low-intensity exercise in humans (3/10 vs. 7/10) and rats (2/7 vs. 7/8), 2) the amplitude of the fast component increased from low-intensity to high-intensity exercise in humans (3 ± 2% vs. 13 ± 5%) and rats (1 ± 1% vs. 5 ± 1%), and 3) a second slow component was evident more frequently in rats (11/15) than humans (3/20).
DISCUSSION
This is the first study to resolve the number of exponential components, the value of their time constants, and their relative contribution to the total PCr recovery time course in human triceps surae and rat posterior hindlimb muscles. A novel finding of this study was that only a single slower component (τ > 15 s) was observed in the human triceps surae muscles following plantar flexion exercise in the majority of subjects, but two distinct slower components were typically observed in the posterior hindlimb of rats following electrically stimulated contractions. This suggests that the muscle fibers recruited in the rat hindlimb had more discrete differences in oxidative capacity than the triceps surae muscles of humans. Furthermore, this study demonstrated that high-intensity exercise resulted in a more frequent occurrence of an initial, fast component (τ < 15 s) of PCr recovery compared with the low-intensity exercise in both humans and rats. This finding is consistent with the notion that glycolytic ATP production contributes to PCr resynthesis during the initial stages of recovery following intense exercise.
Low-intensity exercise.
Following low-intensity exercise in humans, a single component was observed that accounted for nearly the entire PCr recovery amplitude (95%), indicating that the kinetics would be well characterized with a monoexponential model. This is consistent with the findings of Marsh et al. (18) that showed no improvement in the curve fitting of PCr recovery data when using a higher-order model following moderate-intensity wrist-flexion exercise. This monoexponential response indicates that PCr recovery is entirely dependent on oxidative metabolism (21). It also indicates that the oxidative capacity of the recruited muscle fibers was relatively homogenous and/or dispersed in a Gaussian distribution with no distinct differences in oxidative capacity among populations of fibers. The human plantar flexion exercise was performed with the knee slightly flexed (∼10°), and therefore the medial and lateral gastrocnemius muscles were primarily recruited with the soleus muscles likely active but to a lesser extent (27). Oxidative capacities have been reported to be ∼50% different at the extreme ranges of the fiber-type phenotypes within the gastrocnemius and soleus muscles of recreationally active human subjects (8). In contrast, rat hindlimb muscles have been shown to have more distinct differences (i.e., severalfold) in oxidative capacity among fiber populations (5, 12). Consistent with this, two slower components (τ > 15 s) were observed in most of the rats (12/15) in this study. The electrical stimulation protocol used to induce contractions in the rats would be expected to recruit all muscles of the rat posterior hindlimb. This includes the soleus and both the superficial and the deep regions of the gastrocnemius with the saddle-shaped surface coil enabling measurements from both of these muscles. Therefore, a range of fiber types with large differences in oxidative capacity were recruited, and the two components may represent fibers with distinct differences in oxidative capacity. For example, the initial slow component (τ = 34 ± 5 s) may include fibers from the soleus and deep, red region of the gastrocnemius that contain predominantly oxidative type I and IIA fibers, whereas the second slow component (τ = 118 ± 26 s) may be more representative of the superficial, white gastrocnemius muscle composed of type IID/X and type IIB fibers (5, 33).
High-intensity exercise.
The high-intensity exercise was implemented by performing contractions at a faster rate than the low-intensity exercise. The high-intensity exercise was intended to increase the contribution of ATP production via glycolysis to examine whether it remained elevated during recovery, thereby playing a role in PCr resynthesis. In the present study, although the lowest pH obtained in the high-intensity protocol was lower in the rats compared with humans, in both humans and rats a lower pH was obtained during the high-intensity compared with the low-intensity protocols. This is consistent with glycolytic ATP production making a more significant contribution. Furthermore, despite similar end-exercise pH levels in the low and high-intensity protocols in humans, when the glycolytic flux was calculated during the initial recovery period (10.5 s) as described previously (3, 16), the glycolytic flux was many folds higher following high-intensity (0.098 ± 0.02 mM/s) than low-intensity exercise (0.0087 ± 0.009 mM/s). Following the high-intensity exercise a brief initial fast component of PCr recovery became more evident. The number of occurrences and relative amplitude of the fast component increased in both humans (6/10 subjects, 7% amplitude) and rats (5/8, 4%) compared with the low-intensity exercise in humans (2/10, 1%) and rats (2/7, 1%). Thus, glycolytic ATP production may have contributed to PCr resynthesis during the initial stages of recovery following exercise.
The rate of glycogenolysis and glycolysis is determined by the rate-limiting enzymes glycogen phosphorylase and phosphofructokinase, respectively. The flux through these reactions is controlled, in part, by substrate availability of Pi and allosteric regulation by free cytosolic AMP and ADP, which would both be expected to be elevated following high-intensity exercise. However, some studies (10, 14, 28, 36) have observed no evidence of PCr recovery following exercise in ischemic conditions, suggesting that glycolytic flux terminates abruptly at the end of exercise and that factors more temporally coupled to muscle contraction, such as free cytosolic Ca2+, are required for activation of glycogenolysis/glycolysis. On the other hand, Crowther et al. (4) observed that glycolytic flux remained high at end exercise for 3 s following ischemic exercise and decreased to baseline levels within a 20-s period. Additionally, Lanza et al. (17) demonstrated that PCr recovered partially in between isometric contractions during a graded ischemic exercise protocol, and in vitro and modeling studies show that glycolytic ATP production can drive PCr resynthesis (39). Discrepancies in these findings are difficult to reconcile but may be due, in part, to an enhanced time resolution in the studies demonstrating a glycolytic flux following exercise. Therefore, it seems plausible that glycolytic ATP production may remain elevated for a short period of time after exercise and result in the initial, fast component of PCr recovery observed in the present study.
An alternative explanation for the fast component of PCr recovery is the recruitment of highly oxidative fibers. However, according to the size principle of motor unit recruitment, the most oxidative fibers would be expected to be recruited first, and therefore the fast component would have likely also been prevalent during low-intensity exercise. This was not observed. Furthermore, the time constant of the fast component appears to be too fast (mean τ = 3 s) to be due to oxidative metabolism. This rate of recovery corresponds to an ATP synthesis rate that is many folds greater than typically observed to be the maximal rate of ATP flux via oxidative phosphorylation in skeletal muscle of rats (26) and humans (16). Therefore, the findings of the present study seem to be more consistent with the fast component of PCr resynthesis being due to glycolytic ATP production than the recruitment of fibers with a very high oxidative capacity.
Higher-order PCr recovery kinetics following high-intensity exercise has been observed previously (1, 10, 15, 23, 41). A common explanation for a very slow component of PCr recovery is related to a hydrogen ion (H+)-induced shift in the creatine kinase equilibrium (10, 31, 35). This explanation may appear to be consistent with the slowest component of PCr observed in the rats in our study. However, the amplitude of the slowest component (57%, or ∼15 mM) is more than twofold greater than what could be explained by a [H+]-induced shift of the creatine kinase equilibrium (∼7 mM), when considering the [PCr] and pH changes during recovery and assuming a resting PCr of 32 mM (7). Furthermore, it has been shown that an increased intracellular [H+] via hypercapnia does not result in a predictable reduction in PCr (22). However, it is plausible that acidosis may have contributed to the increase in amplitude of the second slow component in other ways, such as having a direct effect on oxidative phosphorylation. Acidosis has been shown to reduce oxidative capacity in human hand and lower limb muscles (16), cat soleus muscle (9), and in isolated skeletal muscle fibers (40). Furthermore, acidosis has been well established to be associated with an increase in the time constant of PCr recovery (26, 37, 41). For example, Walter et al. (41) demonstrated that acidosis slows PCr recovery by utilizing two different maximal voluntary plantar flexion protocols in which all fibers were presumably recruited but elicited different end-exercise pH levels. Similarly, in the present study, a pronounced acidosis may have had an effect during the high-intensity protocol in the rats. Specifically, the pronounced acidosis may have resulted in a greater proportion of fibers with a lower oxidative capacity, causing an increase in the amplitude of the second slow component.
Perspectives and Significance
In this study, NNLS analysis revealed a single exponential component of PCr recovery following low-intensity exercise in humans, but, in most cases, higher-order PCr recovery kinetics were observed in rat hindlimb muscle. These findings are consistent with greater heterogeneity of oxidative capacity among muscle fibers contributing to higher-order PCr recovery kinetics in rat hindlimb muscles compared with humans. Furthermore, the finding that an initial, fast component (τ < 15 s) was more prevalent during high-intensity exercise compared with low-intensity exercise is consistent with the notion that glycolytic ATP production contributes to PCr resynthesis during the early stages of recovery following intense exercise. This implies that using the change in [PCr] during the initial period of recovery (e.g., 10 s) as a marker of oxidative metabolism could result in significant error if the glycolytic ATP production contribution to PCr resynthesis is not accounted for.
GRANTS
This work was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant R01-AR-043903.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Arnold DL, Matthews PM, Radda GK. Metabolic recovery after exercise and the assessment of mitochondrial function in vivo in human skeletal muscle by means of 31P NMR. Magn Reson Med 1: 307–315, 1984. [DOI] [PubMed] [Google Scholar]
- 2.Brown TR, Stoyanova R. NMR spectral quantitation by principal-component analysis. II. Determination of frequency and phase shifts. J Magn Reson B 112: 32–43, 1996. [DOI] [PubMed] [Google Scholar]
- 3.Conley KE, Blei ML, Richards TL, Kushmerick MJ, Jubrias SA. Activation of glycolysis in human muscle in vivo. Am J Physiol Cell Physiol 273: C306–C315, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Crowther GJ, Kemper WF, Carey MF, Conley KE. Control of glycolysis in contracting skeletal muscle. II Turning it off. Am J Physiol Endocrinol Metab 282: E74–E79, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Delp MD, Duan C. Composition and size of type I, IIA, IID/X, and IIB fibers and citrate synthase activity of rat muscle. J Appl Physiol 80: 261–270, 1996. [DOI] [PubMed] [Google Scholar]
- 6.Forbes SC, Raymer GH, Kowalchuk JM, Thompson RT, Marsh GD. Effects of recovery time on phosphocreatine kinetics during repeated bouts of heavy-intensity exercise. Eur J Appl Physiol 103: 665–675, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Golding EM, Teague WE, Dobson GP. Adjustment of K′ to varying pH and pMg for the creatine kinase, adenylate kinase and ATP hydrolysis equilibria permitting quantitative bioenergetic assessment. J Exp Biol 198: 1775–1782, 1995. [DOI] [PubMed] [Google Scholar]
- 8.Gregory CM, Vandenborne K, Dudley GA. Metabolic enzymes and phenotypic expression among human locomotor muscles. Muscle Nerve 24: 387–393, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Harkema SJ, Meyer RA. Effect of acidosis on control of respiration in skeletal muscle. Am J Physiol Cell Physiol 272: C491–C500, 1997. [DOI] [PubMed] [Google Scholar]
- 10.Harris RC, Edwards RH, Hultman E, Nordesjö LO, Nylind B, Sahlin K. The time course of phosphorylcreatine resynthesis during recovery of the quadriceps muscle in man. Pflügers Arch 367: 137–142, 1976. [DOI] [PubMed] [Google Scholar]
- 11.Haseler LJ, Hogan MC, Richardson RS. Skeletal muscle phosphocreatine recovery in exercise-trained humans is dependent on O2 availability. J Appl Physiol 86: 2013–2018, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Hintz CS, Lowry CV, Kaiser KK, McKee D, Lowry OH. Enzyme levels in individual rat muscle fibers. Am J Physiol Cell Physiol 238: C58–C65, 1980. [DOI] [PubMed] [Google Scholar]
- 13.Hood DA, Gorski J, Terjung RL. Oxygen cost of twitch and tetanic isometric contractions of rat skeletal muscle. Am J Physiol Endocrinol Metab 250: E449–E456, 1986. [DOI] [PubMed] [Google Scholar]
- 14.Hsu AC, Dawson MJ. Muscle glycogenolysis is not activated by changes in cytosolic P-metabolites: A 31P and 1H MRS demonstration. Magn Reson Med 49: 626–631, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Iotti S, Gottardi G, Clementi V, Barbiroli B. The mono-exponential pattern of phosphocreatine recovery after muscle exercise is a particular case of a more complex behaviour. Biochim Biophys Acta 1608: 131–139, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Jubrias SA, Crowther GJ, Shankland EG, Gronka RK, Conley KE. Acidosis inhibits oxidative phosphorylation in contracting human skeletal muscle in vivo. J Physiol 553: 589–599, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lanza IR, Wigmore DM, Befroy DE, Kent-Braun JA. In vivo ATP production during free-flow and ischaemic muscle contractions in humans. J Physiol 577: 353–367, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marsh GD, Paterson DH, Potwarka JJ, Thompson RT. Transient changes in muscle high-energy phosphates during moderate exercise. J Appl Physiol 75: 648–656, 1993. [DOI] [PubMed] [Google Scholar]
- 19.McCully KK, Fielding RA, Evans WJ, Leigh JS Jr, Posner JD. Relationships between in vivo and in vitro measurements of metabolism in young and old human calf muscles. J Appl Physiol 75: 813–819, 1993. [DOI] [PubMed] [Google Scholar]
- 20.Meyer RA Linear dependence of muscle phosphocreatine kinetics on total creatine content. Am J Physiol Cell Physiol 257: C1149–C1157, 1989. [DOI] [PubMed] [Google Scholar]
- 21.Meyer RA A linear model of muscle respiration explains monoexponential phosphocreatine changes. Am J Physiol Cell Physiol 254: C548–C553, 1988. [DOI] [PubMed] [Google Scholar]
- 22.Meyer RA, Adams GR, Fisher MJ, Dillon PF, Krisanda JM, Brown TR, Kushmerick MJ. Effect of decreased pH on force and phosphocreatine in mammalian skeletal muscle. Can J Physiol Pharmacol 69: 305–310, 1991. [DOI] [PubMed] [Google Scholar]
- 23.Meyer RA, Terjung RL. Differences in ammonia and adenylate metabolism in contracting fast and slow muscle. Am J Physiol Cell Physiol 237: C111–C118, 1979. [DOI] [PubMed] [Google Scholar]
- 24.Moon RB, Richards JH. Determination of intracellular pH by 31P magnetic resonance. J Biol Chem 248: 7276–7278, 1973. [PubMed] [Google Scholar]
- 25.Nemeth P, Pette D. Succinate dehydrogenase activity in fibres classified by myosin ATPase in three hind limb muscles of rat. J Physiol 320: 73–80, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paganini AT, Foley JM, Meyer RA. Linear dependence of muscle phosphocreatine kinetics on oxidative capacity. Am J Physiol Cell Physiol 272: C501–C510, 1997. [DOI] [PubMed] [Google Scholar]
- 27.Price TB, Kamen G, Damon BM, Knight CA, Applegate B, Gore JC, Eward K, Signorile JF. Comparison of MRI with EMG to study muscle activity associated with dynamic plantar flexion. Magn Reson Imaging 21: 853–861, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Quistorff B, Johansen L, Sahlin K. Absence of phosphocreatine resynthesis in human calf muscle during ischaemic recovery. Biochem J 291: 681–686, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rico-Sanz J Progressive decrease of intramyocellular accumulation of H+ and Pi in human skeletal muscle during repeated isotonic exercise. Am J Physiol Cell Physiol 284: C1490–C1496, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Saab G, Thompson RT, Marsh GD. Multicomponent T2 relaxation of in vivo skeletal muscle. Magn Reson Med 42: 150–157, 1999. [DOI] [PubMed] [Google Scholar]
- 31.Sahlin K, Harris RC, Hultman E. Resynthesis of creatine phosphate in human muscle after exercise in relation to intramuscular pH and availability of oxygen. Scand J Clin Lab Invest 39: 551–558, 1979. [DOI] [PubMed] [Google Scholar]
- 32.Slade JM, Towse TF, Delano MC, Wiseman RW, Meyer RA. A gated 31P NMR method for the estimation of phosphocreatine recovery time and contractile ATP cost in human muscle. NMR Biomed 19: 573–580, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Staron RS, Kraemer WJ, Hikida RS, Fry AC, Murray JD, Campos GER. Fiber type composition of four hindlimb muscles of adult Fisher 344 rats. Histochem Cell Biol 111: 117–123, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Stary CM, Hogan MC. Intracellular pH during sequential, fatiguing contractile periods in isolated single Xenopus skeletal muscle fibers. J Appl Physiol 99: 308–312, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi H, Inaki M, Fujimoto K, Katsuta S, Anno I, Niitsu M, Itai Y. Control of the rate of phosphocreatine resynthesis after exercise in trained and untrained human quadriceps muscles. Eur J Appl Physiol Occup Physiol 71: 396–404, 1995. [DOI] [PubMed] [Google Scholar]
- 36.Taylor DJ, Bore PJ, Styles P, Gadian DG, Radda GK. Bioenergetics of intact human muscle. A 31P nuclear magnetic resonance study. Mol Biol Rep 1: 77–94, 1983. [PubMed] [Google Scholar]
- 37.van den Broek NMA, De Feyter HMML, de Graaf L, Nicolay K, Prompers JJ. Intersubject differences in the effect of acidosis on phosphocreatine recovery kinetics in muscle after exercise are due to differences in proton efflux rates. Am J Physiol Cell Physiol 293: C228–C237, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Vanhamme L, van den Boogaart A, Van Huffel S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson 129: 35–43, 1997. [DOI] [PubMed] [Google Scholar]
- 39.Vinnakota K, Kemp ML, Kushmerick MJ. Dynamics of muscle glycogenolysis modeled with pH time course computation and pH-dependent reaction equilibria and enzyme kinetics. Biophys J 91: 1264–1287, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walsh B, Tiivel T, Tonkonogi M, Sahlin K. Increased concentrations of Pi and lactic acid reduce creatine-stimulated respiration in muscle fibers. J Appl Physiol 92: 2273–2276, 2002. [DOI] [PubMed] [Google Scholar]
- 41.Walter G, Vandenborne K, McCully KK, Leigh JS. Noninvasive measurement of phosphocreatine recovery kinetics in single human muscles. Am J Physiol Cell Physiol 272: C525–C534, 1997. [DOI] [PubMed] [Google Scholar]
- 42.Whittall KP, MacKay AL. Quantitative Interpretation of NMR Relaxation Data. J Magn Reson 84: 134–152, 1989. [Google Scholar]